Abstract
Visuomotor adaptation to gaze-shifting prism glasses requires recalibration of the relationship between sensory input and motor output. Healthy individuals flexibly adapt movement patterns to many external perturbations; however, individuals with cerebellar damage do not adapt movements to the same extent. People with Parkinson disease (PD) adapt normally, but exhibit reduced after-effects, which are negative movement errors following the removal of the prism glasses and are indicative of true spatial realignment. Walking is particularly affected in PD, and many individuals experience freezing of gait (FOG), an episodic interruption in walking, that is thought to have a distinct pathophysiology. Here, we examined how individuals with PD with (PD + FOG) and without (PD − FOG) FOG, along with healthy older adults, adapted both reaching and walking patterns to prism glasses. Participants completed a visually guided reaching and walking task with and without rightward-shifting prism glasses. All groups adapted at similar rates during reaching and during walking. However, overall walking adaptation rates were slower compared to reaching rates. The PD − FOG group showed smaller after-effects, particularly during walking, compared to PD + FOG, independent of adaptation magnitude. While FOG did not appear to affect characteristics of prism adaptation, these results support the idea that the distinct neural processes governing visuomotor adaptation and storage are differentially affected by basal ganglia dysfunction in PD.
Keywords: Prism adaptation, Parkinson disease, Freezing of gait, After-effects
Introduction
Motor adaptation while wearing prism glasses is a form of visuomotor learning in which the nervous system modifies the relationship between a visual input and motor output, resulting in a new movement pattern. Adaptation occurs after multiple trials, during which individuals minimize the error between predicted and actual sensory consequences of a movement. In healthy individuals, adaptation after-effects, defined as movement errors in the opposite direction of initial errors made during adaptation, occur after the visual perturbation is removed (Martin et al. 1996; Weiner et al. 1983). After-effects indicate that the novel movement pattern was stored and retained. To return to baseline performance, individuals must “de-adapt” the new sensorimotor relationship in the same iterative fashion as during adaptation (Bastian 2008).
The cerebellum is a critical structure for normal visuomotor adaptation (Baizer et al. 1999; Bastian 2008; Block and Bastian 2012; Fernandez-Ruiz et al. 2007; Martin et al. 1996; Weiner et al. 1983). Individuals with cerebellar damage require more attempts to adapt, or never adapt their movements and demonstrate little to no after-effect following exposure to prism glasses. These consistent results demonstrate that the cerebellum is required not only to update motor commands based on sensory feedback errors, but also to store transient sensorimotor patterns.
In addition to the cerebellum, other brain regions including the basal ganglia are implicated in visuomotor adaptation. Parkinson disease (PD) is a neurodegenerative disorder that affects dopaminergic cells in the substantia nigra pars compacta, resulting in excessive output of the basal ganglia. Individuals with PD show diminished after-effects following adaptation compared to healthy older adults. This suggests that to some degree, the basal ganglia influence sensorimotor recalibration or spatial realignment (Fernandez-Ruiz et al. 2003; Stern et al. 1988). While after-effect magnitude is reduced in PD, the adaptation process itself is relatively preserved such that adaptation rates are similar between PD and healthy controls (Gutierrez-Garralda et al. 2013; Stern et al. 1988). Collectively, the data from individuals with PD or cerebellar damage suggest that the processes of adaptation and storage are likely controlled by distinct but interconnected neural processes.
Although several studies have compared adaptation in PD to healthy controls, it remains unclear whether individuals with particular PD phenotypes differ in terms of motor adaptation performance. One particularly interesting phenotype is characterized by the presence of freezing of gait (FOG). Defined as “a brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk” (Nutt et al. 2011), FOG is a disabling phenomenon that affects 20–60 % of all individuals with advanced PD (Bloem et al. 2004). Because of the characteristic deficits in limb coordination during gait, and more recently observed differences in brain activity and connectivity, FOG may be considered a distinct phenotype of PD and not simply a result of more advanced or severe disease. Specifically, people with PD who experience freezing (PD + FOG) have difficulties regulating cadence and stride length during complex walking tasks compared to those who do not experience freezing (PD − FOG) (Nanhoe-Mahabier et al. 2011; Nieuwboer et al. 2007; Plotnik et al. 2008). Furthermore, decreased activity in regions of the cerebellum (Peterson et al. 2014) and reduced connectivity in fronto-striatal (Shine et al. 2013; Vercruysse et al. 2014) and visual networks (Tessitore et al. 2012) are reported in PD + FOG. Since these networks are important in not only executing voluntary movement but also visuomotor adaptation, PD + FOG may exhibit different behavior during prism adaptation tasks compared to PD − FOG, distinct from overall deficits in motor control. However, to our knowledge, no study has examined visuomotor adaptation in PD + FOG compared to PD − FOG and healthy older adults. This comparison may provide insight into distinct neural mechanisms that are perturbed by the presence of FOG.
In this study, we compared the rates and magnitudes of prism adaptation and after-effects during two tasks (reaching and walking) to determine the effect of PD and FOG on adaptation. We chose to examine not just reaching, but also walking because it is particularly affected in PD + FOG and as such may reveal differences between PD groups that are not apparent during reaching. Based on previous results, we expected smaller after-effects during both tasks in the two PD groups compared to healthy older adults. Furthermore, because cerebellar-specific deficits in PD + FOG may also affect visuomotor adaptation, we predicted the PD + FOG group would adapt slower and have smaller subsequent after-effects during walking compared to PD − FOG and healthy older adults.
Materials and methods
Participants
Thirteen individuals with PD + FOG (age 68.2 ± 6.04, 13 right-handed), 13 with PD − FOG (age 67.4 ± 11.63, 12 right-handed), and 13 healthy older adults (CTRL; age 69.0 ± 4.03, 11 right-handed) participated. In the PD groups, FOG was assessed using the New Freezing of Gait Questionnaire (NFOGQ) (Nieuwboer et al. 2009). Freezing status is determined by freezing activity in the past month; those who answer yes, indicating that they have experienced freezing in the past month, are asked additional questions about the frequency and severity of freezing to attain a composite NFOGQ score; those who answer no, indicating they have not experienced freezing in the past month, are given a score of zero. PD − FOG and PD + FOG groups were matched for age, sex, and disease severity. PD participants were recruited from the Washington University School of Medicine Movement Disorders Clinic. Each PD participant had a confirmed diagnosis of idiopathic PD according to established criteria (Calne et al. 1992). To avoid confounding effects of dopamine on visuomotor adaptation (Mongeon et al. 2013), participants with PD were studied of any anti-Parkinson medication, defined as a minimum 12-h withdrawal. Control participants were spouses of PD participants or were recruited from a volunteer database, matched to the age and sex of PD participants, and had normal central and peripheral neurological function. Inclusion criteria for all participants were: (1) visual acuity of 20/40 or better, (2) able to walk independently, (3) able to stand and walk for 30 min without rest, (4) normal somatosensory function, (5) no history of vestibular disease, and (6) no evidence of dementia (Mini-Mental Status Examination, MMSE ≥ 26 (Folstein et al. 1975)). Participants were excluded based on the following criteria: (1) serious medical problem, (2) use of neuroleptic or dopamine-blocking drug, (3) use of drug that may affect balance, (4) evidence of abnormality from brain imaging, (5) history of other neurological injury, and (6) history of ocular disease, such as macular degeneration. Motor severity was assessed in the PD groups using the Movement Disorder Society—Unified Parkinson Disease Rating Scale motor subsection III (MDS-UPDRS III). Additionally, balance impairment was quantified using the Mini-Balance Evaluation Systems Test (MiniBEST), a measure of dynamic balance control (Duncan et al. 2013). This study was approved by the Human Research Protection Office at Washington University and is in accord with all national and international policies concerning human subject research. All individuals gave written informed consent prior to participating in the study.
Tasks
Participants performed two tasks while wearing eyeglass frames containing 30-diopter laterally displacing prism lenses (Fresnel Prism and Lens Co, Bloomington, MN): a reaching task requiring participants to reach-and-point to a visual target, and a walking task requiring participants to walk in a straight line to a visual target.
Reaching task
The goal of the reaching task was to reach forward and point to a visual target as accurately as possible. Participants stood 1.6 m in front of a large piece of parcel paper hung on a wall. A 5 cm × 5 cm crosshair positioned in the middle of the paper served as the reaching target, which was vertically aligned at the participant’s shoulder height. Using a laser pointer, participants were asked to keep their eyes closed and flex the shoulder of the dominant arm as quickly as possible, push the button on the laser pointer, and hold this position (i.e., do not attempt to correct). A member of the research team marked the location of the reach-and-point on the paper to provide visual feedback of its endpoint. Participants completed 70 total reach-and point movements in three separate phases: baseline (10 trials), adaptation (40 trials), and post-adaptation (20 trials). During baseline trials, participants reached with eyes closed. After each reach-and-point, they opened their eyes to assess performance and to prepare for any needed adjustments during the next movement. During adaptation trials, participants wore prism glasses and another pair of modified goggles that secured the prism glasses to the head and obscured vision outside of the prism lenses. Here, participants reached with eyes open. Finally, during post-adaptation trials, visual input was again removed (eyes closed) during the reach. The primary reason for eliminating visual input was to minimize on-line movement correction during reaching, which is common during upper-limb movements. While participants did have visual feedback during the adaptation phase, modification of the prism glasses minimized viewing of the arm during the reach. Thus, the majority of visual input during this phase was the target and the end pointing location. We also asked participants to reach as quickly as possible, reducing the potential to use proprioceptive feedback to alter arm trajectory.
Walking task
The goal of the walking task was to walk forward in a straight trajectory, ending with one’s feet on a target. A 3.0 × 0.7 m walkway was marked by tape on the floor, including target lines (0.3 m) located at each end of the path. While beginning at one end of the path, participants walked at a normal pace and stopped when the arches of their feet were directly on the target line. As in the reaching task, 70 walking trials were performed in three phases. During the baseline phase, participants walked to the target with eyes closed. Participants were discouraged from counting their steps while walking with eyes closed, but were encouraged to visualize walking to the target. At the end of each walking trial, participants opened their eyes to assess performance and make adjustments for subsequent trials. Then, a research team member positioned the participant at the center of the walking path before beginning the next trial.
During the adaptation phase, walking was completed while wearing the same prism glasses and modified goggles as worn for the reaching task. In addition, participants were fitted with a platform that rested on the shoulders and sat parallel to the horizontal plane, occluding vision of the ground and the lower half of the body while walking. Preliminary pilot data showed that the magnitude of adaptation was greatly diminished if participants were able to look down (and not through the prism glasses) and use the path lines as visual cues to complete the task. Therefore, we limited participants’ ability to look at the ground or at their feet while walking and instead encouraged them to look straight ahead. The target line was visible over the platform at the start of each trial but would then be obscured as the participant proceeded on the path. At the end the trial, a research team member temporarily moved the platform to allow view of the target line. In this way, we ensured participants were using visual information about their body position relative to the target to complete the task. Finally, in the post-adaptation phase, participants walked to the target with their eyes closed, similar to baseline trials. Consecutive trials were performed in opposite walking directions thus using both ends of the path and minimizing any directional effect on performance.
Data collection and analysis
Performance during reaching was determined by manually measuring the horizontal distance from the endpoint of the reach to the target position to the nearest 0.5 cm. Rightward errors were considered positive, indicating the direction of the prism shift. Lateral distance was converted to angular error using trigonometric calculations. Movement data during the walking task were recorded at 100 Hz using an 8-camera motion capture system (Motion Analysis Inc, Santa Rosa, CA). Reflective markers were placed bilaterally on the greater trochanter and on the left scapula (offset) of each participant. Data in each movement trial were truncated at the trial stopping point, indicated by a trigger pressed when the participant stopped walking. All movement data were processed for discontinuities and digitally low-pass-filtered using a Butterworth filter with cutoff of 6 Hz. The body’s center position was defined as the midpoint of the two trochanter markers. The target line position was determined by the midpoint of two collinear markers set on either side of the walking path.
Analysis was conducted using custom-written MATLAB (The Mathworks Inc., Natick, MA) scripts to determine the absolute endpoint error and angular deviation. We defined the x-direction as the direction of walking and the y-direction as any left/right deviation in laboratory space. Therefore, angular deviation was calculated as the inverse tangent of the change in the y-direction of the body’s midpoint divided by the change in x-direction of the body’s midpoint. Positive angular deviation angles represented rightward errors. Herein, we only report angular errors because they account for the initial position of the body at the start of a trial.
Mean performance during each task was determined for each group. The magnitude of adaptation (MAdap) was defined as the difference between the error of the first trial and the average error of last five trials of the adaptation phase. The magnitude of the after-effect (MPost) was defined as the error first trial during post-adaptation (Fernandez-Ruiz and Diaz 1999). To quantify individual rate of adaptation (Table 2) and group mean rate of adaptation and de-adaptation (Fig. 1), trial-by-trial angular deviation data were fit to exponential functions using MATLAB built-in data fitting functions. For both adaptation and post-adaptation phases, a monotonic decay function in the form y = A * exp(−bt) + C was used, where t is the trial number, A is a scaling constant, b is the rate constant, and C is the horizontal asymptote. Since the value 1/b represents the time constant of the exponential function and thus an index of adaptation rate, we chose to limit the range of b to reflect the task conditions, such that the minimum adaptation rate is one trial and maximum rate is 40 trials. Therefore, upper and lower bounds for the parameter b were set at 1 and 0.025, respectively. Finally, we assessed the goodness of fit using the R2 value of each curve. While an exact cutoff was not used to determine adequate fit, individual data were examined to assess each fit (see Table 2).
Table 2.
Adaptation rates and model fits
| Participant | CTRL | PD − FOG | PD + FOG | |||
|---|---|---|---|---|---|---|
| Rate | R2 | Rate | R2 | Rate | R2 | |
| Reaching | ||||||
| 1 | 1.00 | 0.59 | 1.00 | 0.45 | 1.00 | 0.30 |
| 2 | 4.16 | 0.73 | 2.21 | 0.52 | 1.78 | 0.31 |
| 3 | 1.00a | 0.18 | 3.99 | 0.60 | 1.00 | 0.41 |
| 4 | 1.00a | 0.15 | 1.00 | 0.66 | 1.00 | 0.18 |
| 5 | 1.86 | 0.77 | 1.00 | 0.46 | 1.00 | 0.79 |
| 6 | 3.48 | 0.79 | 1.00a | 0.11 | 1.27 | 0.46 |
| 7 | 2.26 | 0.49 | 1.00 | 0.77 | 40.0b | 0.15 |
| 8 | 1.00 | 0.40 | 1.00 | 0.33 | 4.94a | 0.08 |
| 9 | 1.23 | 0.53 | 19.46 | 0.46 | 1.68 | 0.49 |
| 10 | 1.00 | 0.60 | 1.00 | 0.51 | 1.48 | 0.80 |
| 11 | 1.00a | 0.02 | 1.00 | 0.47 | 1.41 | 0.90 |
| 12 | 4.63 | 0.31 | 1.38a | 0.21 | 1.00 | 0.69 |
| 13 | 1.12 | 0.70 | 1.25a | 0.24 | 40.0c | 0.00 |
| Mean | 1.90 | 0.48 | 2.80 | 0.44 | 7.50 | 0.43 |
| SEM | 1.33 | 0.25 | 1.41 | 0.19 | 4.01 | 0.29 |
| Walking | ||||||
| 1 | 40b | 0.26 | 8.75 | 0.82 | 2.04 | 0.68 |
| 2 | 12.9 | 0.42 | 40.0ac | 0.00 | 8.50a | 0.25 |
| 3 | 1.44 | 0.42 | 1.39 | 0.25 | 3.51 | 0.41 |
| 4 | 12.0 | 0.87 | 1.90 | 0.37 | 6.09 | 0.73 |
| 5 | 3.73 | 0.54 | 14.4 | 0.61 | 6.47 | 0.77 |
| 6 | 14.9 | 0.83 | 15.9 | 0.80 | 40.0 | 0.51 |
| 7 | 15.9 | 0.45 | 1.49 | 0.41 | 40.0a, c | 0.09 |
| 8 | 40.0a, c | 0.01 | 1.41 | 0.40 | 16.2 | 0.83 |
| 9 | 40.0b | 0.38 | 8.04 | 0.47 | 22.0 | 0.68 |
| 10 | 3.46 | 0.70 | 1.51 | 0.58 | 5.07 | 0.58 |
| 11 | 19.3 | 0.66 | 40.0c | 0.01 | 4.86 | 0.77 |
| 12 | 4.99 | 0.43 | 4.52 | 0.94 | 5.70 | 0.50 |
| 13 | 4.43 | 0.47 | 5.61a | 0.16 | 40.0 | 0.79 |
| Mean | 16.4 | 0.49 | 11.1 | 0.45 | 15.4 | 0.58 |
| SEM | 4.03 | 0.23 | 3.79 | 0.30 | 4.16 | 0.23 |
High trial–trial variability;
did not reach steady state;
did not reduce errors
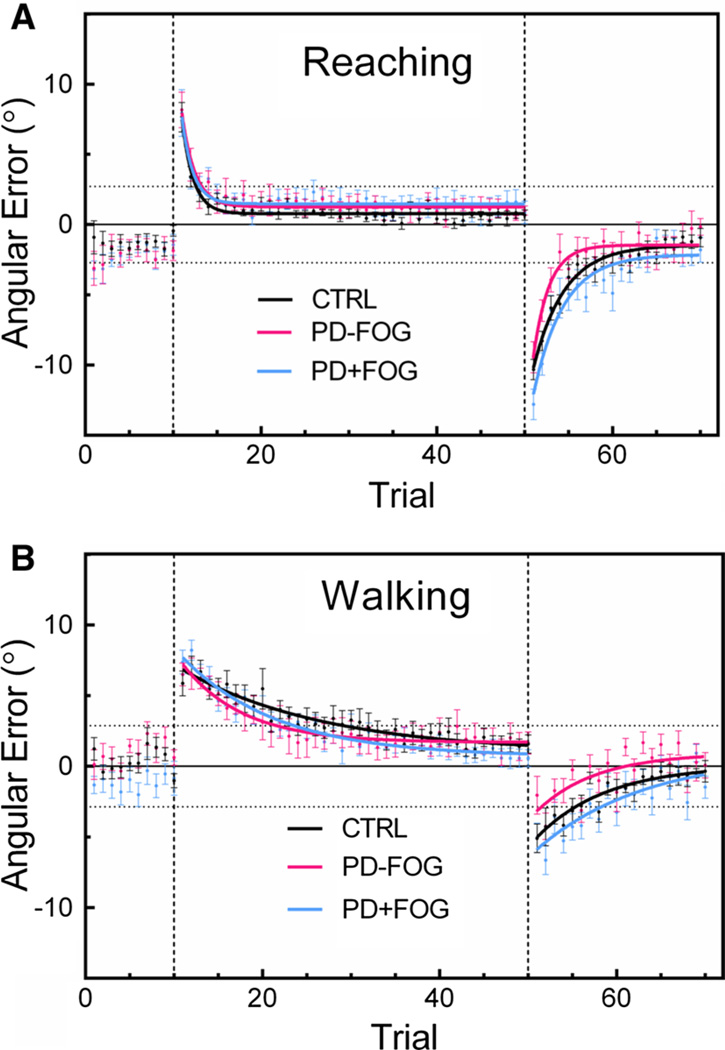
Fig. 1.
Average trial-by-trial angular error during reaching (a) and walking (b) in all groups for baseline, adaptation, and post-adaptation phases. Continuous lines represent the exponential fit to data; vertical dashed lines distinguish the phase (baseline, adaptation, post-adaptation); horizontal dotted lines mark the location of the target edges. Error bars are ±SEM
Statistical analysis
Differences in demographic data (age, MMSE, sex) were evaluated using appropriate comparisons (one-way ANOVA or Chi-square test), and PD-specific variables were compared using independent t tests (MDS-UPDRS III and MiniBEST) or Kruskal-Wallis tests (disease duration and NFOGQ). A repeated-measures ANOVA with between-subject effect of group and within-subject effect of task (Reaching/Walking) was used to determine differences in adaptation rate, MAdap, and MPost. Post hoc comparisons between PD − FOG and PD + FOG were made using Tukey’s tests if main effects were found. In addition, we calculated Pearson and Spearman correlation coefficients to determine linear relationships between measures of adaptation and demographic variables age and MDS-UPDRS III. All statistical analyses were performed using SPSS, version 21 (IBM, Chicago, IL). Statistics were considered significant when p < 0.05.
Results
The PD + FOG group on average had greater, but not significantly different, motor symptom severity and duration of PD compared to PD − FOG (MDS-UPDRS III = 49.23 ± 10.66 and 40.77 ± 12.58, respectively), which is typical given that FOG occurs later in the disease progression (Giladi and Nieuwboer 2008). MiniBEST scores were similar between the PD groups. Finally, groups did not significantly differ by age, sex, or MMSE (Table 1).
Table 1.
Participant demographics
| CTRL (n = 13) | PD − FOG (n = 13) | PD + FOG (n = 13) | p value | |
|---|---|---|---|---|
| Age (year) | 69.00 ± 4.03 | 67.38 ± 11.63 | 68.15 ± 6.04 | 0.84 |
| Sex (M/F) | 7/6 | 7/6 | 6/7 | 1.00 |
| MMSE (0–30) | 28.46 ± 1.39 | 27.88 ± 1.51 | 28.38 ± 1.50 | 0.98 |
| Disease duration (year) | – | 6.43 ± 4.07 | 9.27 ± 5.21 | 0.24 |
| MDS-UPDRS III (0–132) | – | 40.77 ± 12.58 | 49.23 ± 10.66 | 0.07 |
| MiniBEST (0–32) | – | 21.00 ± 3.01 | 20.00 ± 3.80 | 0.29 |
| NFOGQ (0–28) | – | 0 | 11.31 ± 2.35 | <0.01 |
Data are mean ± SD
Range of measures given in parentheses
P Value obtained from one-way ANOVA for age and MMSE, Chi-square test for sex, Kruskal–Wallis test for disease duration and NFOGQ, and Student’s t tests for MDS-UPDRS and MiniBEST
MMSE Mini-Mental Status Examination (lower scores indicate greater cognitive impairment), MDS-UPDRS III Movement Disorder Society version of the Unified Parkinson Disease Rating Scale subsection III (higher scores indicate greater severity), MiniBEST Mini-Balance Evaluation Systems Test (lower scores indicate poorer balance), NFOGQ New Freezing of Gait Questionnaire (higher scores indicate greater severity)
Summary of reaching and walking behavior
Mean angular errors for each trial and exponential fits during reaching and walking are shown in Fig. 1. Overall, performance was consistent with other typical prism adaptation studies. On average, each group gradually decreased movement errors after successive trials during the adaptation phase, eventually reaching a minimal error level. After removing the prisms, all groups showed significant after-effects on the first trial during the post-adaptation phase. Because recalibration is also required during post-adaptation, all groups gradually reduced after-effect errors and returned to baseline performance.
Adaptation rate
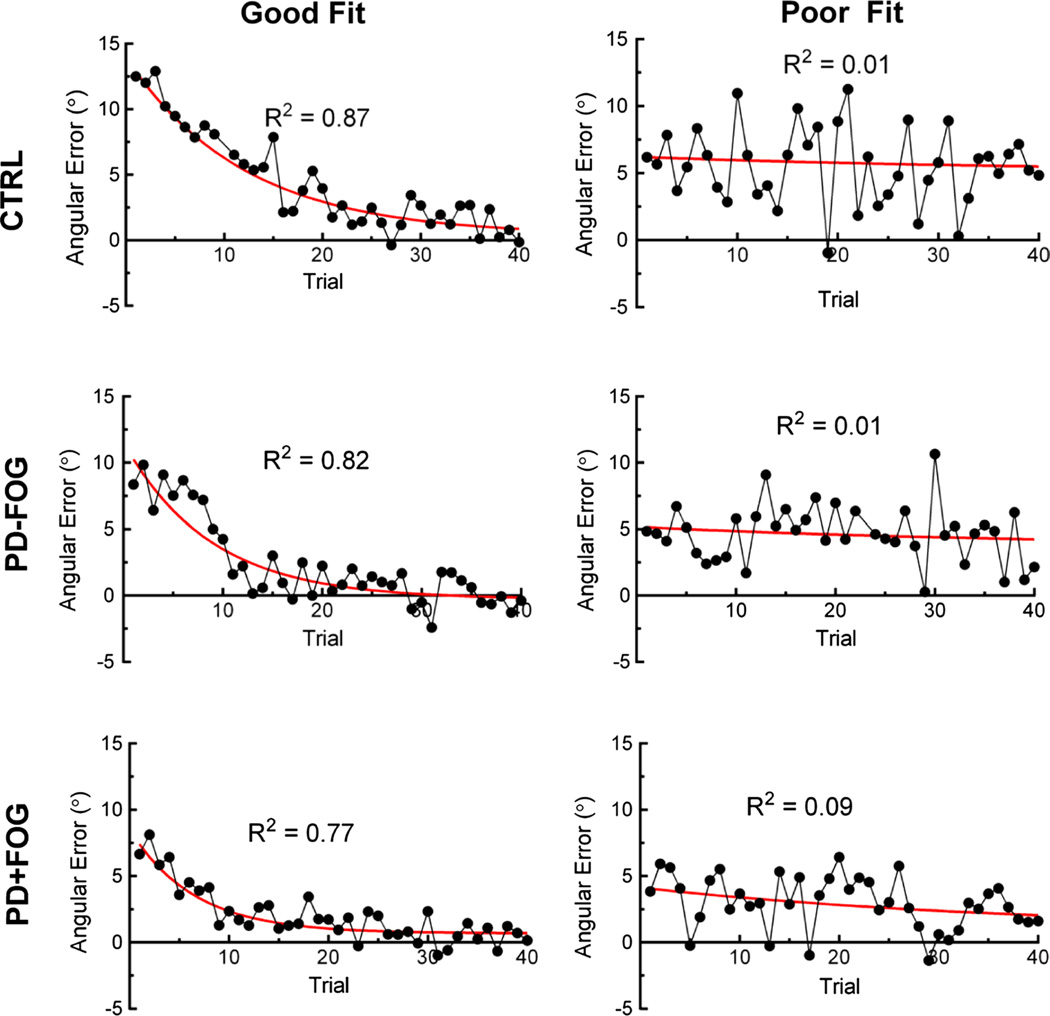
We quantified adaptation rate during reaching and walking as the reciprocal of the time constant b derived from exponential fits of data during the adaptation phase. Individual and group mean values for rate and model fit (R2) are shown in Table 2. During reaching, adaptation rates were fast (CTRL = 1.90 ± 0.37 trials; PD − FOG = 2.79 ± 1.41 trials; PD + FOG = 7.50 ± 4.01 trials) in all groups. One participant in the PD + FOG group did not reduce reaching errors over the 40 trials allotted and thus was assigned the maximal rate of 40. Adaptation rates during walking were slower (CTRL = 16.38 ± 4.03 trials; PD − FOG = 11.15 ± 3.80 trials; PD + FOG = 15.41 ± 4.16 trials) and more variable across groups. Several participants had maximal walking adaptation rates of 40 due to either not reducing walking errors or not reaching a steady state within the 40 trials allotted. We observed that walking errors were also subject to greater trial-to-trial variability, which led to several poor exponential fits (low R2 values). Variability is illustrated in Fig. 2, showing representative good and poor fits of walking adaptation data from each group. We included all data in the current analysis after confirming that results were unchanged even if participants with poor fits were removed. The ANOVA revealed a significant effect of task such that walking adaptations rates were higher (slower) than reaching rates across groups (F36,1 = 19.8, p < 0.001). However, group was not a significant effect in this model (F36,2 = 1.34, p = 0.28). These data confirm that PD + FOG adapted at similar rates to PD − FOG and CTRL and that all groups adapted slower during walking.
Fig. 2.
Representative walking adaptation data showing good (left column) and poor fits (right column) in CTRL (top row), PD − FOG (middle row), and PD + FOG (bottom row). Continuous lines are monotonic exponential fits
Magnitude of adaptation and after-effects
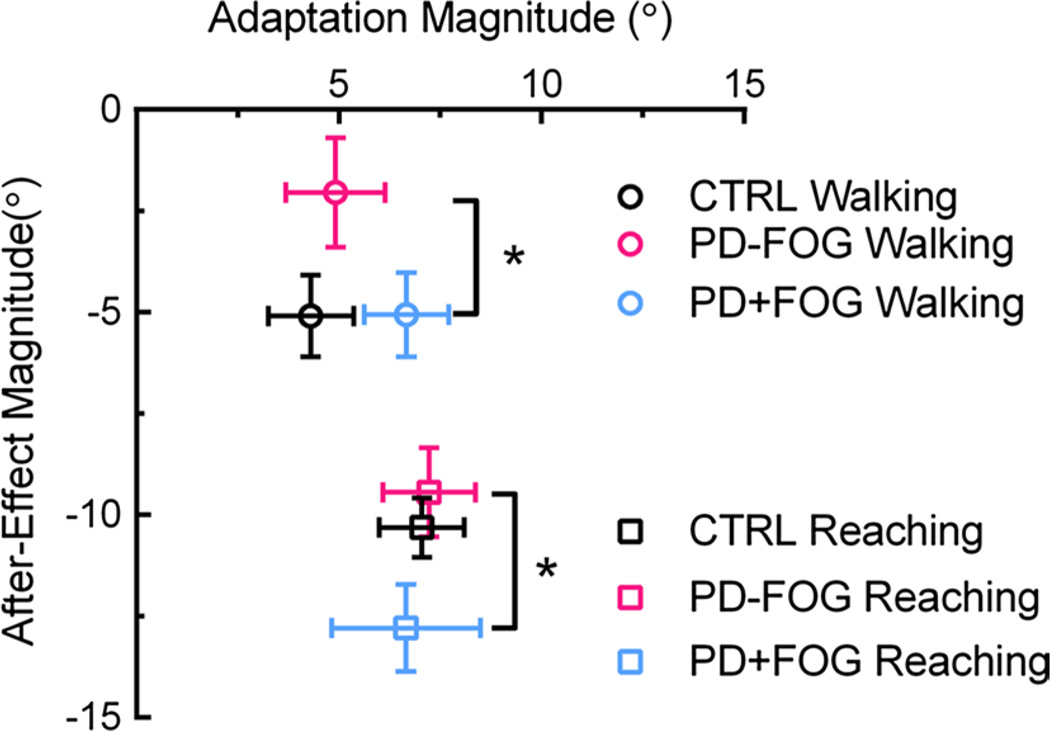
Figure 3 shows the relationship between the MAdap and MPost during each task across groups. MAdap represents the error difference between the first and average of last five trials after donning the prism glasses, while MPost is the error on the first trial after removing the prism glasses. MAdap was not different between tasks (F36,1 = 2.437, p = 0.13) or between groups (F36,2 = 0.345, p = 0.71), indicating the prism glasses produced similar error magnitudes across groups during both tasks. However, MPost was significantly greater during reaching compared to walking (F36,1 = 54.314, p < 0.001). There was also a main group effect for MPost (F36,2 = 5.112, p = 0.011); MPost was significantly less in PD − FOG compared to PD + FOG (post hoc comparison, p = 0.009).
Fig. 3.
Relationship between magnitude of adaptation (abscissa) and after-effect (ordinate) during reaching (squares) and walking (circles) adaptation. Error bars are ±SEM. *Significant post hoc differences in MPost between PD − FOG and PD + FOG (p = 0.009). In addition, MPost was significantly different across tasks (reaching > walking; F36,1 = 54.314, p < 0.001)
Association between adaptation and demographic information
To determine whether adaptation rates, MAdap or MPost, were related to PD severity, we performed linear Pearson (MAdap and MPost) or Spearman (adaptation rates) correlations between experimental and demographic variables. There was a positive relationship between walking adaptation rate and MDS-UPDRS III (ρ = 0.422, p = 0.032), such that those with greater disease severity adapted slower during walking. Since age may also affect rate of visuomotor adaptation (Buch et al. 2003), we then compared adaptation and after-effect measures with age for all participants. No significant relationships between age and these variables were noted; however, one correlation was trending toward significance (age vs. walking MAdap, r = −0.285, p = 0.078). Table 3 summarizes the results of the correlations.
Table 3.
Correlation analyses of demographic and experimental variables
| Age versus | R/ρ | p | UPDRS III versus | R/ρ | p |
|---|---|---|---|---|---|
| Reaching | Reaching | ||||
| Adap ratea | 0.208 | 0.204 | Adap ratea | 0.204 | 0.317 |
| MAdap | −0.083 | 0.615 | MAdap | −0.338 | 0.091 |
| MPost | −0.144 | 0.381 | MPost | −0.273 | 0.178 |
| Walking | Walking | ||||
| Adap ratea | −0.113 | 0.492 | Adap ratea | 0.422 | 0.032 |
| MAdap | −0.285 | 0.078 | MAdap | 0.001 | 0.998 |
| MPost | 0.008 | 0.958 | MPost | −0.185 | 0.367 |
r Pearson correlation coefficient, ρ Spearman correlation coefficient
Spearman correlation used. See text for definitions of variables
Discussion
In this study, we noted similar rates of adaptation in PD + FOG and PD − FOG during both reaching and walking. The primary difference in the PD groups was the magnitude of after-effects, which overall was smaller in PD − FOG compared to PD + FOG. In addition, we noted that for all groups, adaption of walking was slower than adaptation of reaching and after-effects were larger during reaching than during walking.
The rate of adaptation is associated with how quickly one reduces movement errors while wearing prisms, a process known to be regulated by the cerebellum (Martin et al. 1996). Previous studies indicate cerebellar-specific dysfunction in PD + FOG, which we predicted would also affect visuomotor adaptation. Using fMRI to study brain activity during gait, Peterson et al. report decreased activity in the cerebellum during standing in PD + FOG (Peterson et al. 2014). Furthermore, a resting-state fMRI study showed different connectivity patterns between the cerebellum and supplementary motor area in PD + FOG compared to PD − FOG (Fling et al. 2014). Despite these data, we found no significant differences in adaptation rate during reaching or walking between PD + FOG and PD − FOG. One reason for the lack of cerebellar findings in this study could be due to the anatomical specificity of walking control in the cerebellum. During normal walking, the cerebellar vermis regulates upright posture and flexor/extensor activation, while the lateral regions of the cerebellum control walking under external guidance (Morton and Bastian 2004; Thach and Bastian 2004). Thus, adapting walking trajectory to prism glasses is primarily regulated by the lateral cerebellum. In contrast, the neuroimaging studies of PD + FOG mentioned above focused on the vermis (particularly the cerebellar locomotor region) and did not explore other cerebellar regions. Therefore, dysfunction localized in the vermis is unlikely to affect visuomotor adaptation, explaining the similarity in adaptation rates in the PD groups.
Furthermore, both PD groups adapted at similar rates to CTRL participants. These results align with previous reports of normal adaptation in PD (Fernandez-Ruiz et al. 2003; Gutierrez-Garralda et al. 2013; Stern et al. 1988; Weiner et al. 1983). In contrast, Contreras-Vidal and Buch noted that people with PD adapt pointing movements slower when exposed to a large kinematic distortion (Contreras-Vidal and Buch 2003). The conflicting results may be due to the magnitude of the visual perturbation, which for prism adaptation is small. Gradual perturbations may be controlled by primarily cerebellar mechanisms (Robertson and Miall 1999), whereas large perturbations may be corrected using fronto-striatal circuitry. Therefore, the similar adaptation rates in the PD groups relative to CTRLs are reasonable, showing that error correction mechanisms in the cerebellum appear on average to be unaffected by PD. However, we did observe a relationship between global motor function (MDS-UPDRS III) and adaptation rate such that those with worse motor impairment adapted walking trajectories slower. PD progression is associated with increased cerebellar dysfunction (Wu and Hallett 2013), which could explain the greater adaptation rates observed in the more impaired individuals. Additional comparisons using groups of PD participants of various motor impairment levels (e.g., mild vs. moderate) are thus needed to provide information on the relationship between adaptation and disease progression.
The novel result from this study was the difference in after-effect magnitude, independent of adaptation magnitude, in people with PD with and without FOG. The after-effect magnitude reflects the true spatial realignment achieved during adaptation (Redding and Wallace 2002). Our results of smaller after-effects, particularly during walking, in PD confirmed previous studies (Fernandez-Ruiz et al. 2003; Stern et al. 1988; Weiner et al. 1983), but only in the PD − FOG group. One possible explanation of smaller after-effects is smaller adaptation magnitudes (Fernandez-Ruiz and Diaz 1999). However, we noted no difference in MAdap between the groups or between tasks. Thus, the storage of new visuomotor relationships is reduced in PD, supporting the argument that spatial realignment is impaired in PD regardless of adaptation magnitude, providing a role for the basal ganglia in controlling prism adaptation.
The remaining question is why PD + FOG actually showed larger after-effects than PD − FOG, suggesting that PD + FOG achieved a greater level of spatial realignment. The difference in after-effect magnitude between PD + FOG and PD − FOG could be explained by compensatory mechanisms used by PD + FOG that are advantageous for the walking task. One hypothesis regarding walking dysfunction in PD + FOG is that in situations of uncertainty, PD + FOG use more cortical resources because they are unable to recruit automatic mechanisms for movement control (Vandenbossche et al. 2012). In the novel environment created by wearing prism glasses, more cortical (i.e., voluntary) control of walking is required to successfully adapt to the visual perturbation and retain the new movement pattern. This strategy may selectively benefit the PD + FOG group, resulting in the observed larger after-effects. Evidence from other types of walking adaptation paradigms indirectly supports this idea. For example, walking on a split-belt treadmill where one belt is driven faster than the other requires one to recalibrate the stride length and timing of both legs. PD + FOG are unable to regulate their gait while walking on a split-belt treadmill and instead increase their stride length variability compared to PD − FOG and controls (Mohammadi et al. 2015; Nanhoe-Mahabier et al. 2013). Split-belt adaptation may be controlled automatically by subcortical structures including the mesencephalic locomotor region, another region known to be dysfunctional in PD + FOG (Fling et al. 2014; Snijders et al. 2011). Walking control targeted by split-belt treadmill walking differs from the prism adaptation walking task studied herein, for which more voluntary control is needed. Still, the reasons why the after-effect magnitude was actually enhanced by FOG during prism adaptation are unclear and require further investigation.
The results of this study should be interpreted in light of the following limitations. While we classified the PD group as having the presence or absence of FOG, we acknowledge that FOG exists on a continuum, where the severity of FOG varies between individuals. While our sample of PD participants had moderately high motor impairment (MDS-UPDRS III mean = 45), the severity of freezing in the PD + FOG group was relatively mild (mean NFOGQ = 11.31). To add, no PD + FOG participant experienced freezing while walking forward during the walking task (three participants froze while turning after walking to the target). Perhaps including individuals with more severe FOG (i.e., greater NFOGQ score) than those in our sample would reveal greater differences; however, those with more severe FOG would be unable to complete the task because of frequent freezing episodes, especially off medication. Overall, future studies of motor adaptation in PD and FOG should aim to include more severe PD + FOG to study the spectrum of both PD and FOG severity.
One unique finding reported here was that older adults with and without PD took longer to adapt their walking pattern than their reaching pattern while wearing rightward-shifting prism glasses. In turn, the after-effect magnitude was larger following reaching adaptation compared to walking. This result is not surprising given that the number of trials performed after full adaptation increases the magnitude of the after-effect, enriching the sensorimotor recalibration (Fernandez-Ruiz and Diaz 1999). However, the reasons why walking adaptation rates were significantly greater than reaching are less clear. One possibility is the difference in task demands associated with both reaching and walking. For instance, balance and limb coordination require different levels of control during walking than reaching. In addition, the sensory input guiding walking (visual/optic flow, proprioceptive and vestibular) is richer than during reaching. Therefore, there are considerably more parameters to reconcile during spatial realignment of walking compared to reaching, which may account for the slower adaptation rate. Further work should look to examine this distinction in healthy controls, providing insight into the intrinsic properties of the sensorimotor adaptation system [see model proposed by (Redding and Wallace 1988)].
We conclude that prism adaptation rate during reaching or walking is not affected by PD or the presence of FOG. Despite similarities in adaptation, smaller after-effects were observed in the PD group during walking, particularly in PD − FOG. In addition, we observed that all participants adapted slower during walking, which suggests task-dependent effects for adaptation performance. Altogether, these results indicate that cerebellar-dependent deficits in PD + FOG have a minimal effect on visuomotor adaptation. In contrast, basal ganglia dysfunction in PD, without the confounder of FOG, affects the storage of novel visuospatial relationships and overall spatial realignment.
Acknowledgments
The authors thank Ryan Duncan, Martha Hessler, and Richard Nagel for assistance with data collection and evaluation of PD participants and Kendra Cherry, Ryan Duncan, and Marie McNeely for helpful manuscript feedback. This work was supported by grants from the Parkinson’s and Movement Disorder Foundation, The Program in Physical Therapy at Washington University, the National Institutes of Health R01 NS077959, and the Washington University Institute of Clinical and Translational Sciences Grants UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences.
Footnotes
Conflict of interest The authors have no conflicts to report.
References
- Baizer JS, Kralj-Hans I, Glickstein M. Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol. 1999;81:1960–1965. doi: 10.1152/jn.1999.81.4.1960. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21:628–633. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block HJ, Bastian AJ. Cerebellar involvement in motor but not sensory adaptation. Neuropsychologia. 2012;50:1766–1775. doi: 10.1016/j.neuropsychologia.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learn Mem. 2003;10:55–63. doi: 10.1101/lm.50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32(Suppl):S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Buch ER. Effects of Parkinson’s disease on visuomotor adaptation. Exp Brain Res. 2003;150:25–32. doi: 10.1007/s00221-003-1403-y. [DOI] [PubMed] [Google Scholar]
- Duncan RP, et al. Comparative utility of the BESTest, mini-BESTest, and brief-BESTest for predicting falls in individuals with parkinson disease: a cohort study. Phys Ther. 2013;93:542–550. doi: 10.2522/ptj.20120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Diaz R. Prism adaptation and after effect: specifying the properties of a procedural memory system. Learn Mem. 1999;6:47–53. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, et al. Normal prism adaptation but reduced after-effect in basal ganglia disorders using a throwing task. Eur J Neurosci. 2003;18:689–694. doi: 10.1046/j.1460-9568.2003.02785.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, et al. Prism adaptation in spinocerebellar ataxia type 2. Neuropsychologia. 2007;45:2692–2698. doi: 10.1016/j.neuropsychologia.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS ONE. 2014;9:e100291. doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;(Suppl 2):S423–S425. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Garralda JM, Moreno-Briseno P, Boll MC, Morgado-Valle C, Campos-Romo A, Diaz R, Fernandez-Ruiz J. The effect of Parkinson’s disease and Huntington’s disease on human visuomotor learning. Eur J Neurosci. 2013;38:2933–2940. doi: 10.1111/ejn.12288. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119(Pt 4):1183–1198. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- Mohammadi F, Bruijn SM, Vervoort G, van Wegen EE, Kwakkel G, Verschueren S, Nieuwboer A. Motor switching and motor adaptation deficits contribute to freezing of gait in Parkinson’s disease. Neurorehabil Neural Repair. 2015;29:132–142. doi: 10.1177/1545968314545175. [DOI] [PubMed] [Google Scholar]
- Mongeon D, Blanchet P, Messier J. Impact of Parkinson’s disease and dopaminergic medication on adaptation to explicit and implicit visuomotor perturbations. Brain Cogn. 2013;81:271–282. doi: 10.1016/j.bandc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10:247–259. doi: 10.1177/1073858404263517. [DOI] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Snijders AH, Delval A, Weerdesteyn V, Duysens J, Overeem S, Bloem BR. Walking patterns in Parkinson’s disease with and without freezing of gait. Neuroscience. 2011;182:217–224. doi: 10.1016/j.neuroscience.2011.02.061. [DOI] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Snijders AH, Delval A, Weerdesteyn V, Duysens J, Overeem S, Bloem BR. Split-belt locomotion in Parkinson’s disease with and without freezing of gait. Neuroscience. 2013;236:110–116. doi: 10.1016/j.neuroscience.2013.01.038. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Chavret F, Willems A-M, Desloovere K. Does freezing in Parkinson’s disease change limb coordination? J Neurol. 2007;254:1268–1277. doi: 10.1007/s00415-006-0514-3. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Pickett KA, Duncan R, Perlmutter J, Earhart GM. Gait-related brain activity in people with Parkinson disease with freezing of gait. PLoS ONE. 2014;9:e90634. doi: 10.1371/journal.pone.0090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur J Neurosci. 2008;27:1999–2006. doi: 10.1111/j.1460-9568.2008.06167.x. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Components of prism adaptation in terminal and concurrent exposure: organization of the eye-hand coordination loop. Percept Psychophys. 1988;44:59–68. doi: 10.3758/bf03207476. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Strategic calibration and spatial alignment: a model from prism adaptation. J Mot Behav. 2002;34:126–138. doi: 10.1080/00222890209601935. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Miall RC. Visuomotor adaptation during inactivation of the dentate nucleus. NeuroReport. 1999;10:1029–1034. doi: 10.1097/00001756-199904060-00025. [DOI] [PubMed] [Google Scholar]
- Shine JM, Matar E, Ward PB, Bolitho SJ, Pearson M, Naismith SL, Lewis SJ. Differential neural activation patterns in patients with Parkinson’s disease and freezing of gait in response to concurrent cognitive and motor load. PLoS ONE. 2013;8:e52602. doi: 10.1371/journal.pone.0052602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Hermann A, Rosen J. Prism adaptation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:1584–1587. doi: 10.1136/jnnp.51.12.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord. 2012;18:781–787. doi: 10.1016/j.parkreldis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Thach WT, Bastian AJ. Role of the cerebellum in the control and adaptation of gait in health and disease. Prog Brain Res. 2004;143:353–366. doi: 10.1016/s0079-6123(03)43034-3. [DOI] [PubMed] [Google Scholar]
- Vandenbossche J, et al. Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Front Hum Neurosci. 2012;6:356. doi: 10.3389/fnhum.2012.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse S, Spildooren J, Heremans E, Wenderoth N, Swinnen SP, Vandenberghe W, Nieuwboer A. The neural correlates of upper limb motor blocks in Parkinson’s disease and their relation to freezing of gait. Cereb Cortex. 2014;24:3154–3166. doi: 10.1093/cercor/bht170. [DOI] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]