Abstract
As the most important interface between human body and external environment, skin acts as an essential barrier preventing various environmental damages, among which DNA-damaging UV radiation from the sun remains the major environmental risk factor causing various skin diseases. It has been well documented that wavelengths in the UVB radiation range (290–320 nm) of the solar spectrum can be absorbed by skin and lead to cutaneous injury and various other deleterious effects. During process such as wound healing, the orchestrated movement of cells in a particular direction is essential and highly regulated, integrating signals controlling adhesion, polarity and the cytoskeleton. Cell adhesion and migration are modulated through both of actin and microtubule (MT) cytoskeletons. However, little was known about how UVB affects skin wound healing and migration of epidermal keratinocytes. Here, we demonstrate that UVB can delay the wound healing progress in vivo with a murine model of full thickness skin wound. In addition, UVB significantly inhibited keratinocyte motility by altering focal adhesion turnover and cytoskeletal dynamics. Our results provide new insights into the etiology of UVB exposure-induced skin damages.
INTRODUCTION
Skin is one of the largest organs in the human body, which is located at the interface with external environment and acts as a barrier preventing various damages including ultraviolet radiation (UVR) (1). UV radiation is divided into three distinct bands in order of decreasing wavelength and increasing energy: UVA (320–400 nm), UVB (290–320 nm), and UVC (200–290 nm). Different wavelengths and energy associated with UV subdivision correspond to distinctly different effects on living tissue. It has been shown that UVB can lead to various pathological outcomes in skin, including sunburns, wrinkles, diminished immunity against infections, premature aging, and cancer (2–4). Although it is well established that UVB exposure can lead to skin tissue damage, little is known about its effects on skin wound repair and keratinocyte cell migration.
Repairing of skin wound is a complex process entailing multiple tissue and cell lineages. Mobilization of quiescent epidermal keratinocytes to proliferate and migrate is a key process during skin wound healing. The intricate, multi-step process of cell migration requires integrated activities of the cytoskeleton, membrane, and cell/ECM adhesions (5, 6). Focal adhesions are multi-molecular complexes that provide a structural link between the intracellular cytoskeleton and the extracellular matrix (7). So far, more than 150 components of focal adhesions have been identified (8). Assembly and disassembly of focal adhesions are crucial processes during cell migration. Although molecular mechanisms underlying focal adhesion dynamics are not completely understood, microtubules have emerged as important regulators of focal adhesion turnover. They target focal adhesions and multiple targeting of focal adhesions by microtubules leads to the disassembly of focal adhesions (9). Recently, the spectraplakin ACF7 was identified as a molecule that crosslinks microtubules and F-actin filaments, guiding microtubules towards focal adhesions before their disassembly (10). Besides, a mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4) was identified as an FA regulator that associates with MTs, knockout of which stabilizes focal adhesions and impairs cell migration (11).
In the current study, we set out experiments to determine the role of UVB on skin wound healing and migration of skin keratinocytes. Our results demonstrated that UVB exposure can significantly inhibit wound healing in murine model with a full-thickness cutaneous wound by inhibition of keratinocyte movement through its role in regulation of focal adhesion dynamics.
Materials and Methods
Antibodies, Reagents, and Plasmid DNA Constructions
Mouse monoclonal Vinculin antibody were obtained from Sigma (St. Louis, MO). Rabbit polyclonal β-tubulin antibody were obtained from Abcam (Cambridge, MA). Other chemicals or reagents were obtained from Sigma, unless otherwise indicated.
Plasmids encoding DsRed-Zyxin, GFP-Paxillin have been described (12, 13).
Skin UVB irradiation and Wound Healing
All procedures involving mouse experiments and tissue handling were approved by the IACUC at the UChicago, Chicago IL. Seven week-old male/female C57BL/6 mice were used in the experiment. Animals were housed under specific pathogen-free conditions. Mice were shaved one day prior to the first UVB irradiation. Irradiated mice were exposed to UVB dorsally again two days later.
For skin wound healing assays, same sex littermates of ~ 12 wk old mice were anesthetized, and two full-thickness excisional wounds were made on both sides of the dorsal midline (12). Mice were housed separately, and no self-induced trauma was observed in mice. Tissue was collected 2–6 days after wounding, and wound reepithelialization was evaluated by histological analyses. Hyperproliferative epidermis (HE) was identified by hematoxylin and eosin staining, and the length of HE that extended into the wounds was measured and quantified.
Histology and Immunofluorescence
Skin or wound samples were embedded in OCT, frozen, sectioned, and fixed in 4% formaldehyde. For paraffin sections, samples were incubated in 4% formaldehyde at 4°C overnight, dehydrated with a series of increasing concentrations of ethanol and xylene, and then embedded in paraffin. Paraffin sections were rehydrated in decreasing concentrations of ethanol and subjected to antigen unmasking in 10 mM Citrate, pH 6.0. Sections were subjected to hematoxylin and eosin staining or immunofluorescence staining as described (12). Antibodies were diluted according to manufacturer’s instruction, unless indicated.
Cell Culture
Primary mouse keratinocytes were isolated from the epidermis of newborn mice using trypsin, after prior separation of the epidermis from the dermis by an overnight dispase treatment. Keratinocytes were plated on mitomycin C–treated 3T3 fibroblast feeder cells until passage 3. Cells were cultured in E-media supplemented with 15% serum and a final concentration of 0.05 mM Ca2+. All experiments were performed using primary cells with less than 10 passages.
Cell Migration Assays and Time-lapse Videomicroscopy
Scratch-wound healing assay was performed essentially as described (14). Briefly, keratinocytes were plated on 35mm tissue culture dish coated with fibronectin. After cells reached confluency, wounds were created by manual scraping of the cell monolayer with a pipette tip. The dishes were then washed with PBS, replenished with media, and photographed using a phase contrast microscope. Afterwards, dishes were placed in the tissue culture incubator, and the matched wound regions were photographed 24 and 36 hours after wounding.
To trace the movement of individual keratinocytes, cells were plated on fibronectin-coated dishes and imaged with an Olympus vivaview microscope (20X) for 2 hours at 1 frame/minute and manually tracked in Image J.
TRIF Microscopy
TIRF imaging was done using a Leica GSD/TIRFM ground state depletion superresolution microscope. Solid-state laser light (405, 488, 532, and 642 nm) was focused at the back focal plane of a 160× numerical aperture 1.43 objective. Signals were recorded by using an Andor iXon3 897 high-speed electron-multiplying charge-coupled device camera. For TIRF imaging, the stained cells were mounted in an open chamber filled with 1× PBS.
Quantification of focal adhesion size and relative staining intensity
For focal adhesion size analysis, keratinocytes were fixed and stained with mouse anti-Vinculin and corresponding secondary antibody to label focal adhesions. Pictures were acquired on a fluorescence microscope on a 16-bit scale. Focal adhesion characteristics were quantified using Image J. An integrated morphometric analysis was performed on thresholded images to select classified objects of a size greater than 10 pixel2 as focal adhesions based on the anti-Vin staining (10). Non-specific nuclear staining was removed manually.
FRAP and Focal Adhesion Assembly/Disassembly Measurements
Kinetics of focal adhesion assembly and disassembly were performed essentially as previously described (15, 12). Keratinocytes were plated on fibronectin-coated dishes and transfected with plasmid encoding DsRed-Zyxin. Time series of images were acquired on a 3i Marianas Yokogawa-type spinning-disc confocal microscope equipped with a 100x α-plane (1.45 oil) lens and an EM charge- coupled device camera. The rate constants for focal adhesion assembly and disassembly were obtained by calculating the slope of relative fluorescence intensity increases or decreases of individual focal adhesion on a semilogarithmic scale against time.
FRAP assays were performed essentially as described (12). Briefly, cells were plated on fibronectin-coated 3.5-cm dishes and transfected with plasmid encoding DsRed-zyxin. Three or two prebleach events were performed followed by a 1-s bleach event. Fluorescence recovery was recorded for 100 seconds after photobleaching events, and data from these photokinetic experiments were analyzed using DeltaVision software.
Skin Explant
To monitor focal adhesion dynamics ex vivo3 mm biopsies were taken from back skin of newborn CD1 mice. Biopsies were coated with a thin layer of matrigel for adhesion and immediately transferred to a coverslip coated with 10 µg/ml fibronectin. Skins were then incubated at room temperature for 10 minutes to solidify the matrigel and then exposed to rich E-media containing 15% serum and 0.3 mM calcium (16), which promoted keratinocytes outgrowth within 2–3 days, analogous to a wound-response.
Statistical analysis
Statistical analysis was performed using Excel or OriginLab software. Box plots are used to describe the entire population without assumptions on the statistical distribution. A student t test was used to assess the statistical significance (P value) of differences between two experimental conditions.
RESULTS
UVB treatment affects wound healing in mice
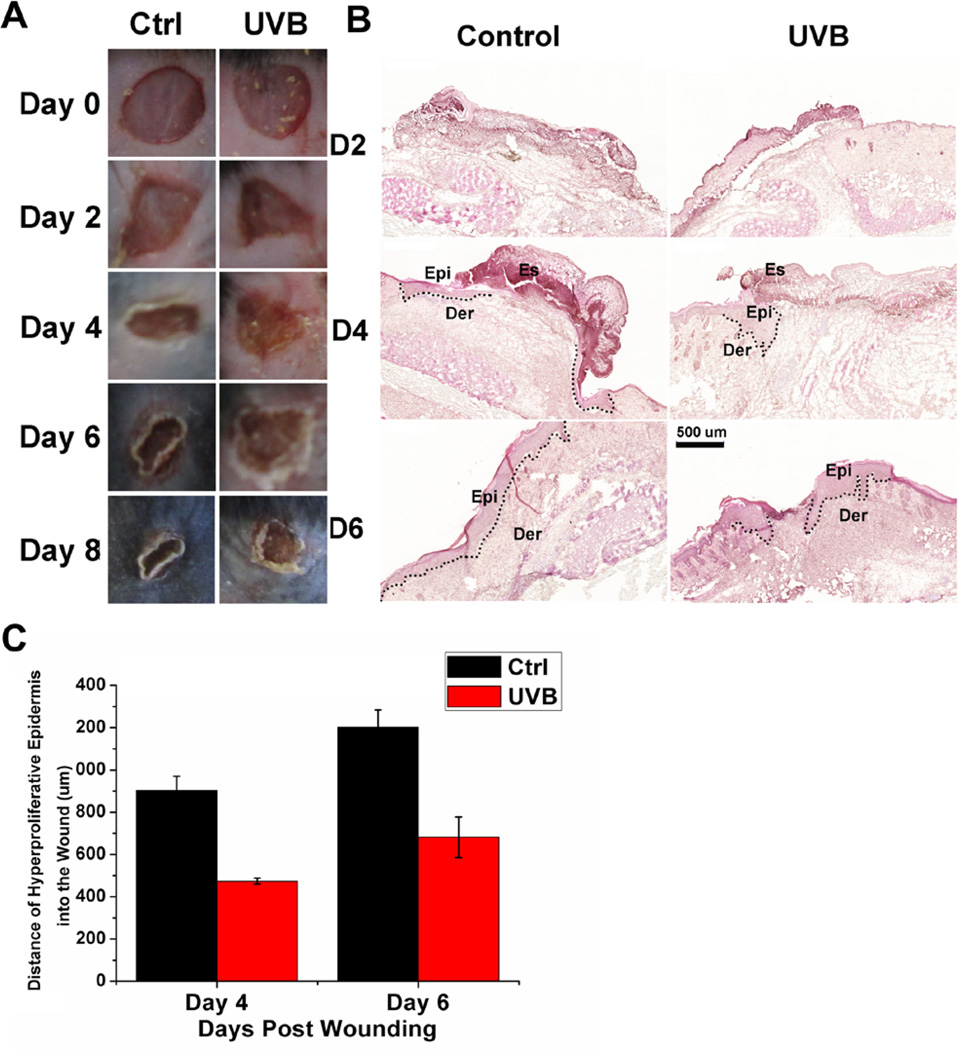
Mouse skin serves as a robust system to investigate cell migration in vivo (17). Thus, UVB’s effect on wound healing in vivo was evaluated using full-thickness excisional wound introduced to dorsal skin of adult C57/B6J mice. The time course observations indicated that UVB (100 mj/cm2) treatment can significantly delay wound closure, compared with mice untreated (Figure 1A). Histological analysis (Figure 1B) further confirmed delayed wounds repair upon UVB treatment. Quantification revealed that the area of hyperproliferative epithelium (HE) that typically proliferates and migrates into the wound site was diminished by more than 50% over 4 and 6 days following injury (Figure 1C).
Figure 1. Wound healing and histopathological examination in UVB-treated mice.
(A) Macroscopic view of wound healing on day 0, 2, 4, 6 and 8. A 6-mm wound was created on the back of mice and wound closure was monitored with or without UVB treatment. (B) Wound healing as monitored by histological staining of skin sections at the wound edges 2, 4 and 6 days after injury with or without UVB treatment (100 mj/cm2). Halves of wound sections are shown. Epi: epidermis; der: dermis; Es: eschar. Dotted lines denote dermal–epidermal boundaries. Scale bar represents 500 µm. (C) Quantification of the length of hyperproliferative epidermis generated at times indicated after wounding. All bars represent mean ± SD from three mice.
UVB effects on keratinocyte motility
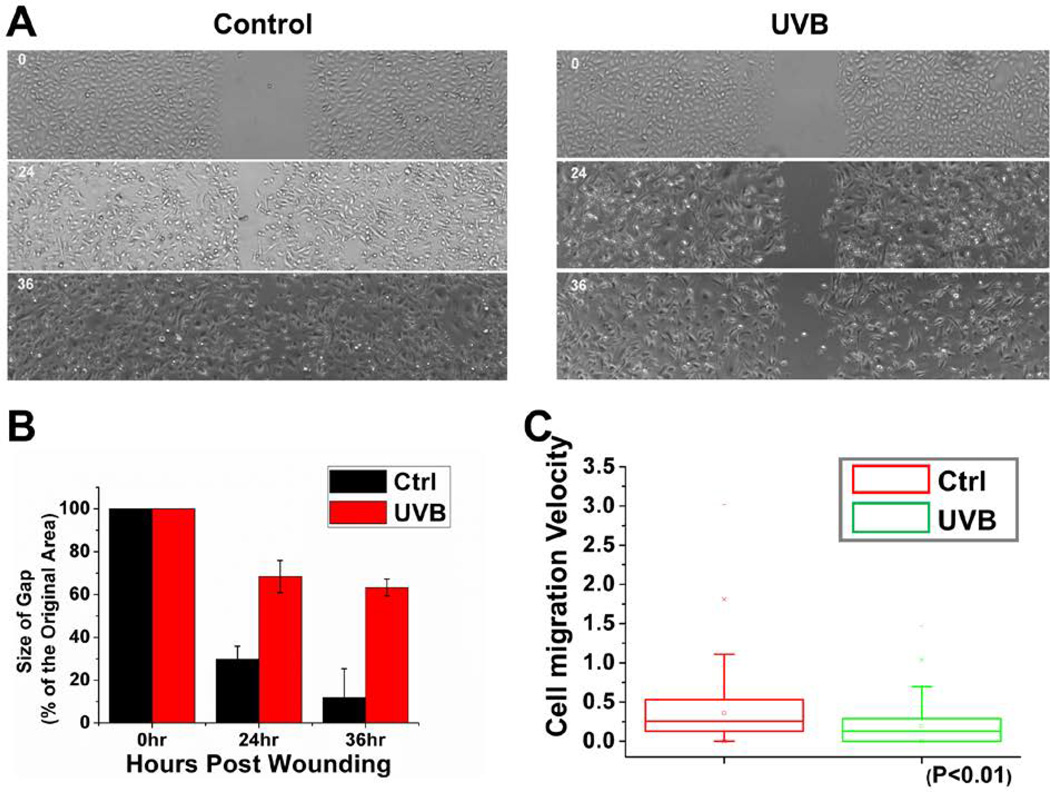
Directional cell movement makes significant contribution to skin wound repair. To determine this effect of UVB treatment on migration of skin epidermal cells, we isolated primary mouse keratinocytes. With cultured keratinocytes, we first examined the polarized rate of cell migration during recovery of ~500 µm scratch wounds introduced into cell monolayers. Cells treated with UVB (20mj/cm2) migrate poorly in vitro. Whereas untreated keratinocytes closed the gap usually within 36 hours, UVB treated keratinocytes moved only ~30% of this distance into the scratch wound (Figures 2A and 2B). Videomicroscopy allowed us to image and monitoring the velocities and directional migration of individual skin cells. Assays of representative keratinocytes indicated significantly slower movements of UVB-treated cells compared to untreated cells. Quantification of these movements revealed a dramatic decrease in average speed of UVB-treated keratinocytes (Figure 2C).
Figure 2. Cell migration defects in UVB-treated keratinocytes.
(A) Migration of confluent monolayers of mouse keratinocytes cultured from control and UVB treatment was assessed by in vitro scratch-wound assays. (B) The kinetics of in vitro wound healing was quantified. Wounding areas were measured using ImageJ software and expressed as the percentage of the original area size with the bar graph (P < 0.01). A representative experiment is shown. (C) Movements of individual keratinocytes were traced by videomicroscopy. Migration tracks of multiple cells for each group (control or UVB treated) are shown here as scatter plots.
UVB effects on focal adhesion dynamics
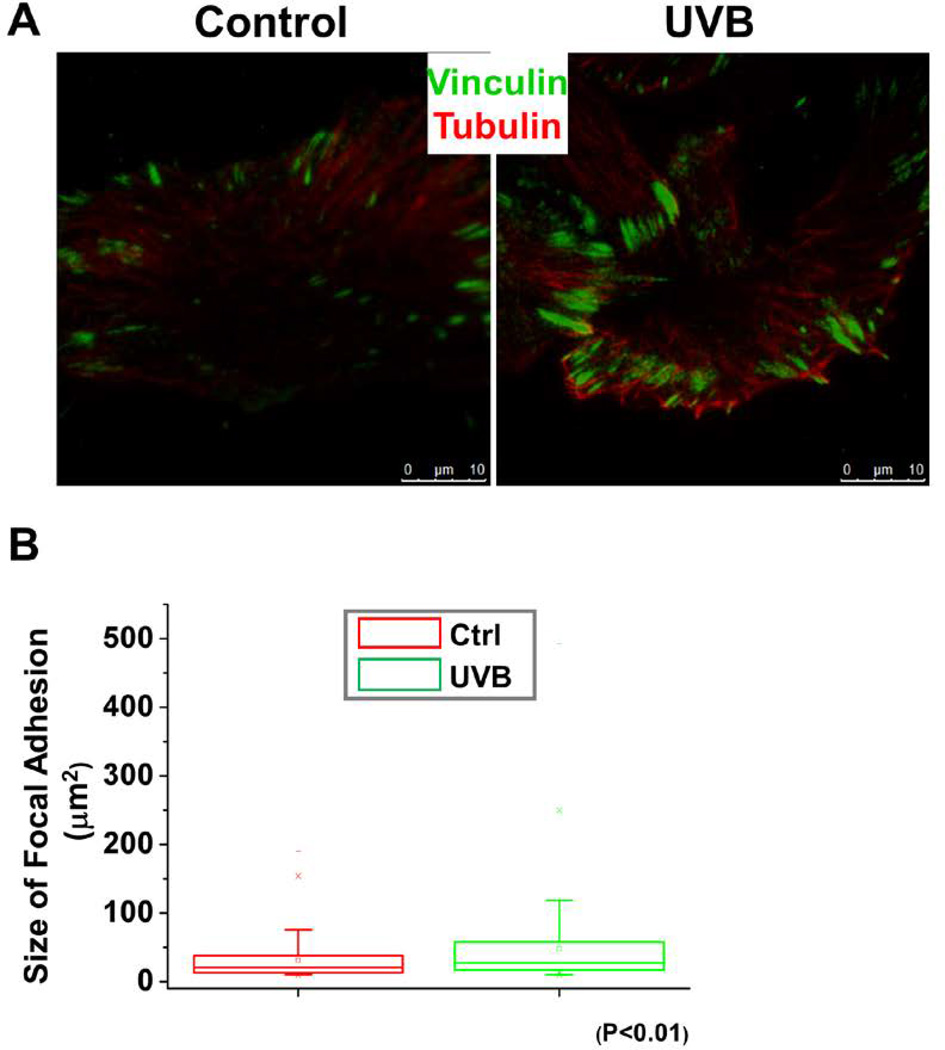
Turnover of focal adhesion is a key step during directional cell movement. To determine the effects of UVB in focal adhesion dynamics, we monitored focal adhesion patterns after UVB treatment. Immunofluorescence staining coupled with TIRF (total internal reflection fluorescence) microscopy revealed greatly enhanced labeling of focal adhesions in UVB treated keratinocytes relative to untreated controls (Figure 3A). Figure 3B shows the quantifications for vinculin, a representative structural component of focal adhesions. Our results showed a significant increase in the size of focal adhesions in UVB treated cells.
Figure 3. UVB affects focal adhesion size.
(A) Immunolabeling of UVB treated and untreated keratinocytes for tubulin (red), and focal adhesion marker vinculin (green). Scale bar represents 10 µm. (B) Box and whisker plot indicating the size distribution of focal adhesions in control and UVB treated cells.
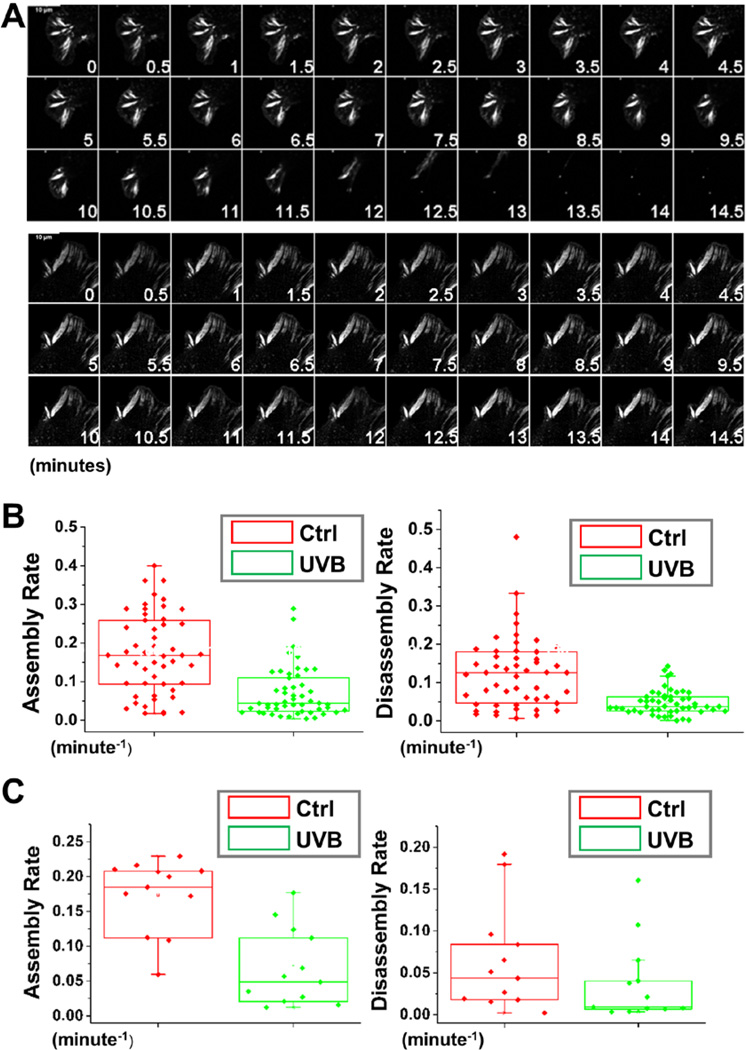
Enlarged focal adhesions at the cell periphery usually suggest potential defects in focal adhesion turnover (18, 19). To explore the nature of this defect in UVB treated cells, we began by employing confocal videomicroscopy to trace and examine the behaviour of individual focal adhesion. To monitor the process, we transfected cells with plasmids encoding DsRed-Zyxin and treated the cells with UVB. Representative examples of the changes in focal adhesions dynamics arising from UVB treatment are shown in complete form in Supplementary Video 1 and 2, and in montages in Figure 4A. Most strikingly, during the 15 minutes interval of observation, focal adhesion in UVB treated cells were significantly more static, while many focal adhesions in non-treated keratinocytes underwent continual bouts of formation, maturation and disassembly. Quantifications of the kinetics of individual focal adhesions revealed dramatic decrease in both the assembly and disassembly rates of focal adhesions in UVB treated cells (Figure 4B). To further determine the role of UVB in focal adhesion dynamics, we carried out skin explant analysis (20) and transfected primary skin keratinocytes outgrown from the skin with plasmid encoding GFP-Paxillin. Consistent with our in vitro analysis, treatment of skin explant with UVB also leads to impaired focal adhesion assembly and disassembly (Figure 4C), strongly suggesting that UVB might directly regulate focal adhesion dynamics in vivo.
Figure 4. UVB affects focal adhesion dynamics.
(A) Representative time-lapse images (montages) of DsRed-Zyxin expressing keratinocytes. Note formation and dissolution of focal adhesions in control cells and very static focal adhesions in UVB treated cells. Scale bar represents 10 µm. (B) Box and whisker plots revealing slow assembly and disassembly rates of focal adhesions in UVB treated cells relative to their control counterparts. (C) Box and whisker plots revealing slow assembly and disassembly rates of focal adhesions in UVB treated skin explants relative to their control counterparts.
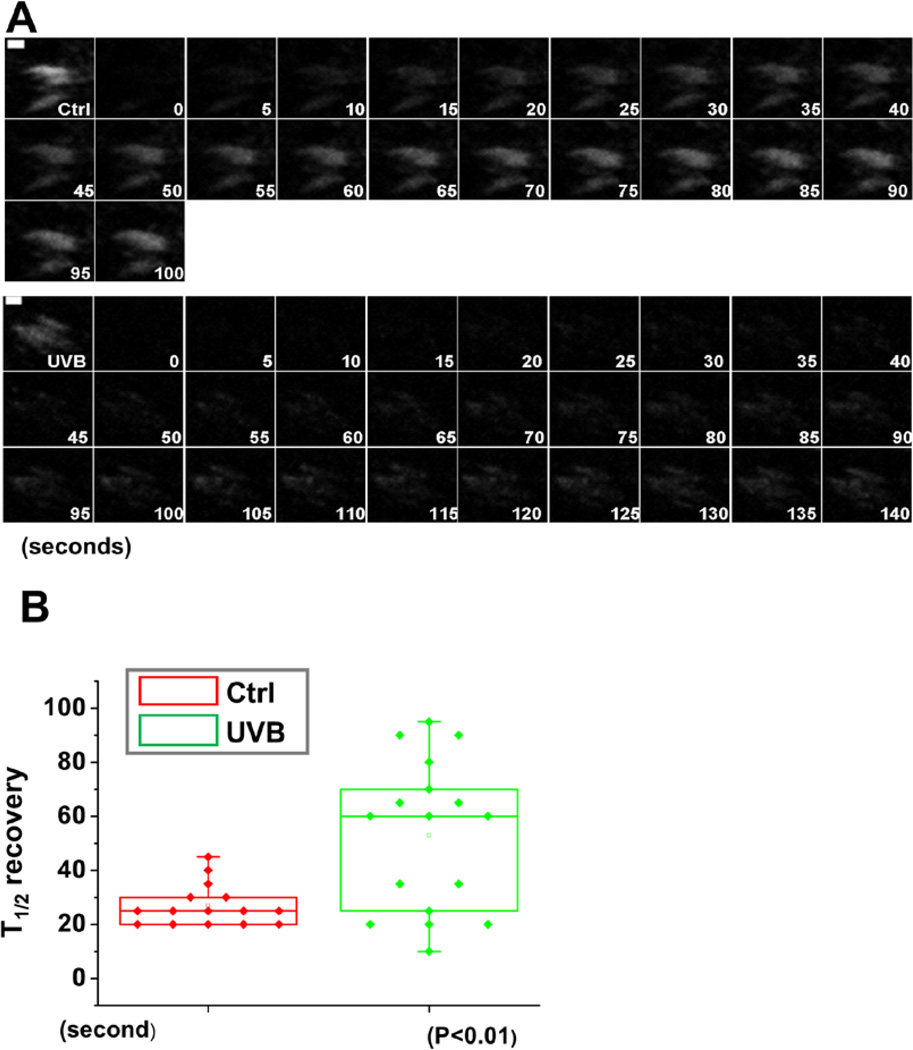
The defects in focal adhesion dynamics were further supported by fluorescence recovery after photobleaching (FRAP) experiments (Figure 5A). By comparing non-treated cells, we found that UVB treatment resulted in a substantial increase in half-times of fluorescence recovery after bleaching (Figure 5B). Together, these data provide compelling evidence that focal adhesions are more stable upon UVB treatment, suggesting that UVB inhibits cell migration at least in part by suppressing focal adhesion dynamics.
Figure 5. UVB effects on FA dynamics by FRAP.
(A) Fluorescence recovery after photobleaching (FRAP) was used to visualize reduced dynamics of focal adhesions in UVB treated vs untreated cells expressing DsRed-Zyxin. Fluorescence recovery was recorded for 100 s after photobleaching. Representative time-lapse images (montage) of focal adhesions are shown. Scale bar represents 1 µm. (B) Box-and-whisker diagram quantifying the differences in half-time (T1/2) of FRAP between control and UVB treated cells.
UVB effects on microtubule (MT) plus end dynamics
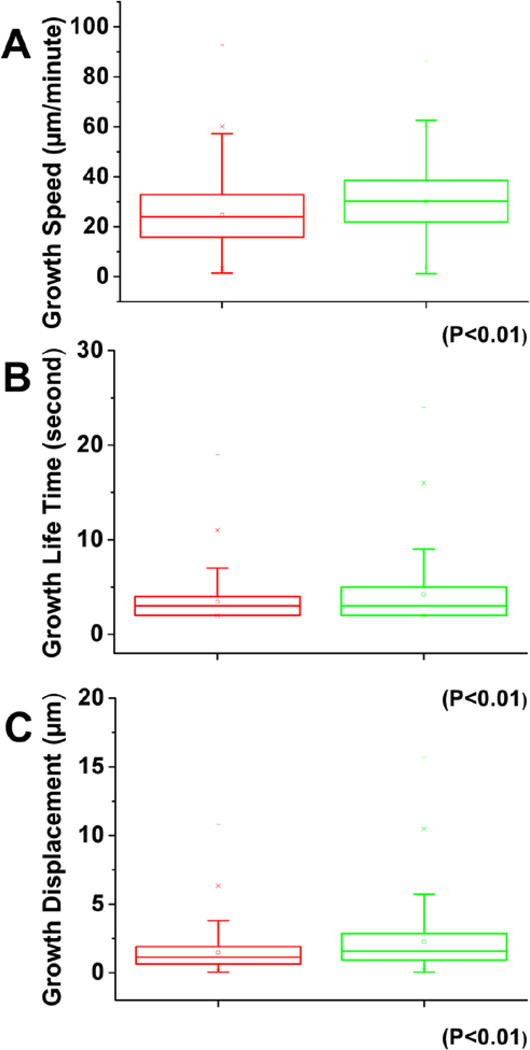
Microtubule targeting is involved in focal adhesion turnover (21, 10). Growing MT ends accumulate a special group of MT interacting proteins, often referred to as MT plus-end tracking proteins (22). Thus we sought to examine whether microtubule plus end dynamics were specifically disrupted in keratinocytes after UVB treatment. To test this, we acquired high-resolution live images of tagged end-binding protein 1 (EB1), which binds all growing microtubule plus ends (Supplemental Video 3 and 4), and we then quantified parameters of microtubule polymerization dynamics using the Matlab-based open-source software plusTipTracker. EB1-TaqRed comet number and morphology were similar in control and UVB treated cells. However, compared with controls, EB1-TaqRed comet velocity was increased by 23% upon UVB treatment and microtubule growth-track lifetime (which measures the number of seconds of microtubule polymerization in a given growth track before pausing or catastrophe) increased by 23%. Microtubule growth-track length (which measures the distance of persistent MT polymerization growth before pausing or catastrophe) also increased after UVB treatment (Figure 6A–C). To summarize, UVB treatment did not significantly altered microtubule dynamic instability, but did significantly increased the growth speed, growth lifetime and growth displacement of microtubule plus ends.
Figure 6. UVB effects on microtubule (MT) dynamics.
Box and whisker plots for EB1 plus end dynamics in control and UVB treated cells. Three parameters of MT plus end dynamics were retrieved from the confocal videos, plus end growth speed (A), growth lifetime (B), and growth displacement (C).
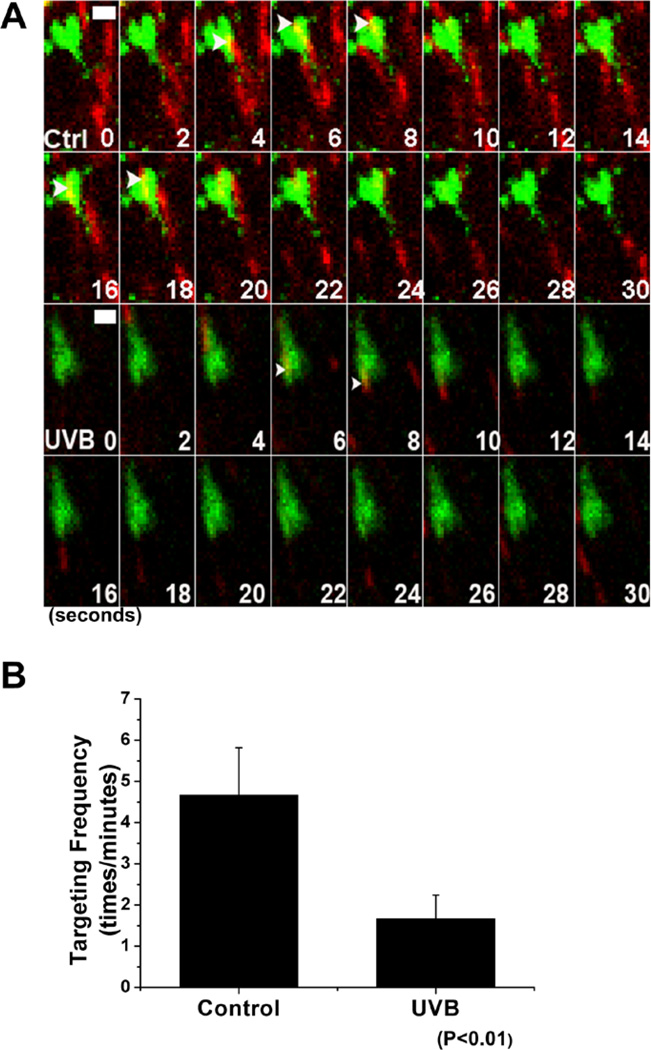
Targeting of microtubule plus ends toward focal adhesions can activate a MAP4K4-dependent signaling cascade, leading to focal adhesion disassembly (11, 23). With multi-fluorescent probe strategy, we monitored microtubule targeting upon UVB treatment. Our quantification demonstrated that microtubule targeting to focal adhesion was significantly less frequent in UVB treated keratinocytes (Figures 7A and 7B). Together, our results suggest that UVB exposure can slow migration of skin keratinocytes by affecting cytoskeletal/focal adhesion dynamics.
Figure 7. UVB effects on MT targeting to FA.
(A) Representative time-lapse images (montages) of keratinocytes expressing GFP-Paxillin and TaqRed-EB1 show MT plus-end growth (red) toward FAs (green). The scale bar represents 1 µm. (B) Behavior of MT targeting to FAs was manually traced, and targeting frequencies to individual FAs were quantified and depicted by bar graph. Note the significant alterations in the ability of MTs targeting to FAs in UVB treatment.
DISCUSSION
Skin is the first barrier between human body and environment while ultraviolet radiation in sunlight is the major environmental factor causing skin damages. Wavelengths in the UVB radiation range (290–320 nm) of the solar spectrum are absorbed by the skin and responsible for causing physical injury of skin and inflammation (24, 2), mutations of chromosomal DNA (25, 3), suppression of immune response (26, 4), and eventually non-melanoma skin cancer; (3). Although the relationship between UVB radiation and skin cancer and the underlying molecular mechanism have been well documented, litter is known about the function of UVB radiation on keratinocyte motility and skin wound healing.
In order to investigate the possible role of UVB exposure on skin wound healing, we carried out experiments in vivo and in vitro. Our results demonstrated that UVB exposure can significantly suppress skin wound repair and migration of keratinocytes.
Cell migration requires the coordinated activity of several modular processes, including formation and turnover of focal adhesion sites, cytoskeletal dynamics, and polarized distribution of adaptor and signaling proteins (27–29). Assembly and disassembly of focal adhesions are crucial processes during cell migration. Our results indicate that UVB treatment can impair focal adhesion dynamics in cultured keratinocytes, suggesting that UVB affects cell migration at least in part through focal adhesion dynamics. Microtubules have also emerged as important regulators of focal adhesion turnover (9, 30). Interestingly, UVB treatment significantly reduces targeting of focal adhesions by microtubules.
Future studies will be required to fully illustrate how UVB irradiation affects keratinocytes cell migration and focal adhesion dynamics. Exposure to DNA-damaging agents such as UVB triggers multiple signal transduction pathways that are thought to play a role in activating repair and recovery processes (31–33). For example, DNA damaging resulting from UVB radiation can activate multiple protein kinases, such as DNA-PK, ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) (34). Despite their well-documented role in phosphorylation of nuclear target proteins and controlling various nuclear processes, including DNA repair, transcription and cell proliferation, emerging evidence now suggest that they also play a role in the cytosol. Recent evidence showed that DNA-PK can phosphorylate GOLPH3 and enhance its interaction with myosin 18A, leading to altered organization of Golgi complex in cytoplasm (35, 36). Thus, it is likely that UVB radiation might change the expression profile or phosphorylation status of certain key proteins controlling cytoskeletal/focal adhesion dynamics in keratinocytes, thus affecting the overall progress of cell migration.
In closing, our study demonstrates the effect of UVB on keratinocytes cell migration and focal adhesion dynamics. It provides novel insight into the issue of how keratinocytes respond to UVB radiation and advance our understanding of UVB-induced skin lesion from a different perspective.
Supplementary Material
ACKNOWLEDGMENTS
We thank Philbert Lee for critical reading and comment of the manuscript. We are very grateful to Dr. Vytas Bindokas at University of Chicago Light Microscopy Core for their excellent technical assistance. Microscopic imaging was carried out at the University of Chicago Integrated Light Microscopy Facility. The animal studies were carried out in the ALAAC-accredited animal research facility at the University of Chicago. This work was supported by a grant R01-AR063630 from the National Institutes of Health (XW), the Research Scholar Grant (RSG-13-198-01) from the American Cancer Society (XW), and the National Institutes of Health Grants AA021434 and AA020265 (S.-YC).
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Supplementary Video 1–2: Focal adhesion dynamics in UVB treated keratinocytes.
Primary keratinocytes expressing DsRed-Zyxin were plated on fibronectin (10 µg/ml)-coated dishes. Cells were treated with (video 2) or without (control, video 1) UVB. Dynamics of focal adhesion were recorded by confocal spinning-disc videomicroscopy. Images were taken at 2 frames per minute.
Supplementary Video 3–4: Microtubule dynamics in UVB treated keratinocytes.
Primary keratinocytes expressing GFP-EB1 were plated on fibronectin (10 µg/ml)-coated dishes. Cells were treated with (video 4) or without (control, video 3) UVB. Dynamics of microtubule plus ends were recorded by confocal spinning-disc videomicroscopy. Images were taken at 1 frame per second.
References
- 1.Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the 'brain-skin connection'. Trends in immunology. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunology and cell biology. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 3.Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutation research. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutation research. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 5.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nature cell biology. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz R, Webb D. Cell migration. Current biology : CB. 2003;13:R756–R759. doi: 10.1016/j.cub.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nature cell biology. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules at early focal adhesions. The Journal of cell biology. 1998;142:181–190. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Kodama A, Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008;135:137–148. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue J, Xie M, Gou X, Lee P, Schneider MD, Wu X. Microtubules regulate focal adhesion dynamics through MAP4K4. Developmental cell. 2014;31:572–585. doi: 10.1016/j.devcel.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–327. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama A, Karakesisoglou I, Wong E, Vaezi A, Fuchs E. ACF7: an essential integrator of microtubule dynamics. Cell. 2003;115:343–354. doi: 10.1016/s0092-8674(03)00813-4. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. Focal adhesion kinase regulation of N-WASP subcellular localization and function. The Journal of biological chemistry. 2004;279:9565–9576. doi: 10.1074/jbc.M310739200. [DOI] [PubMed] [Google Scholar]
- 15.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature cell biology. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 16.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 18.Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Current biology : CB. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- 19.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, Fuchs E. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell. 2011;144:341–352. doi: 10.1016/j.cell.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nature cell biology. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 22.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nature reviews. Molecular cell biology. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 23.Krylyshkina O, Kaverina I, Kranewitter W, Steffen W, Alonso MC, Cross RA, Small JV. Modulation of substrate adhesion dynamics via microtubule targeting requires kinesin-1. The Journal of cell biology. 2002;156:349–359. doi: 10.1083/jcb.200105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavallo J, DeLeo VA. Sunburn. Dermatologic clinics. 1986;4:181–187. [PubMed] [Google Scholar]
- 25.Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. The journal of investigative dermatology. Symposium proceedings / the Society for Investigative Dermatology, Inc. [and] European Society for Dermatological Research. 1996;1:136–142. [PubMed] [Google Scholar]
- 26.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 27.Guan JL. Focal adhesion kinase in integrin signaling. Matrix biology : journal of the International Society for Matrix Biology. 1997;16:195–200. doi: 10.1016/s0945-053x(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 28.Penela P, Nogues L, Mayor F., Jr Role of G protein-coupled receptor kinases in cell migration. Current opinion in cell biology. 2014;27:10–17. doi: 10.1016/j.ceb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Mendoza P, Diaz J, Torres VA. On the role of Rab5 in cell migration. Current molecular medicine. 2014;14:235–245. doi: 10.2174/1566524014666140128111347. [DOI] [PubMed] [Google Scholar]
- 30.Stehbens S, Wittmann T. Targeting and transport: how microtubules control focal adhesion dynamics. The Journal of cell biology. 2012;198:481–489. doi: 10.1083/jcb.201206050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fornace AJ., Jr Mammalian genes induced by radiation; activation of genes associated with growth control. Annual review of genetics. 1992;26:507–526. doi: 10.1146/annurev.ge.26.120192.002451. [DOI] [PubMed] [Google Scholar]
- 32.Herrlich P, Rahmsdorf HJ. Transcriptional and post-transcriptional responses to DNA-damaging agents. Current opinion in cell biology. 1994;6:425–431. doi: 10.1016/0955-0674(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 33.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 34.Shah P, He YY. Molecular Regulation of UV-Induced DNA Repair. Photochemistry and photobiology. 2014 doi: 10.1111/php.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farber-Katz SE, Dippold HC, Buschman MD, Peterman MC, Xing M, Noakes CJ, Tat J, Ng MM, Rahajeng J, Cowan DM, Fuchs GJ, Zhou H, Field SJ. DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3. Cell. 2014;156:413–427. doi: 10.1016/j.cell.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumann K. DNA damage: Dispersing Golgi. Nature reviews. Molecular cell biology. 2014;15:153. doi: 10.1038/nrm3762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.