Abstract
We reported previously that a signaling pathway consisting of Gi-Ras-NF-κB mediates lysophosphatidic acid (LPA)-induced urokinase plasminogen activator (uPA) upregulation in ovarian cancer cells. However, it is not clear what signaling components link Ras to nuclear factor (NF)-κB for this LPA-induced event. In the present study, we found that treatment of protein kinase C (PKC) inhibitors including conventional PKC (cPKC) inhibitor Gö6976 abolished LPA-induced uPA upregulation in ovarian cancer cell lines tested, indicating the importance of cPKC activity in this LPA-induced event. Indeed, LPA stimulation led to the activation of PKCα and Ras–PKCα interaction. Although constitutively active mutants of PKCα (a cPKC), PKCθ (a novel PKC (nPKC)) and PKCζ (an atypical PKC (aPKC)) were all able to activate NF-κB and upregulate uPA expression, only dominant-negative PKCα mutant attenuated LPA-induced NF-κB activation and uPA upregulation. These results suggest that PKCα, rather than PKC isoforms in other PKC classes, participates in LPA-induced NF-κB activation and uPA upregulation in ovarian cancer cells. To determine the signaling components downstream of PKCα mediating LPA-induced uPA upregulation, we showed that forced expression of dominant-negative CARMA3 or silencing CARMA3, Bcl10 and MALT1 with specific siRNAs diminished these LPA-induced events. Furthermore, we demonstrated that PKCα/CARMA3 signaling axis is important in LPA-induced ovarian cancer cell in vitro invasion.
Keywords: ovarian cancer, LPA, uPA, NF-κB
Introduction
Lysophosphatidic acid (LPA) elicits diverse cellular responses such as cell proliferation, cell migration, cell survival and ion transport (Birgbauer and Chun, 2006). Recent study has linked LPA/LPA receptors to the development of various cancers, in particular ovarian cancer (Fang et al., 2002). LPA is present at elevated levels in the ascites of ovarian cancer patients (Xu et al., 1998) and produced by ovarian cancer cells but not normal ovarian epithelial cells (Eder et al., 2000). LPA2 and LPA3 are overexpressed in most ovarian cancer cells (Wang et al., 2007b). Laboratory investigation demonstrates that (1) LPA is capable of enhancing cell growth/survival, cell motility and the production/activation of many proteases (Fishman et al., 2001; Do et al., 2007); (2) inducing the expression of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and interleukin (IL)-8 (Hu et al., 2001; Schwartz et al., 2001) and (3) removing LPA by lipid phosphate phosphatase inhibits tumor development (Tanyi et al., 2003). Taken together, these findings suggest that LPA may impact cancer progression through an autocrine system.
The levels of urokinase plasminogen activator (uPA) expression and activity are low in benign ovarian tumors but increased significantly in advanced ovarian tumors (Murthi et al., 2004). High concentrations of uPA in the ascites/plasma correlate with the poor prognosis and response to chemotherapy (Schmalfeldt et al., 1995; Konecny et al., 2001). Furthermore, uPA induces ovarian cancer cell proliferation and migration (Fischer et al., 1998; Kjoller and Hall, 2001) and inhibiting uPA function with specific inhibitors significantly decrease ovarian cancer invasion and metastasis (Suzuki et al., 2004). Previous studies show that LPA induces uPA expression (Pustilnik et al., 1999) and we further demonstrate that inhibiting uPA expression blocks LPA-induced invasion (Li et al., 2005). These findings strongly indicate that uPA may play a role in LPA-associated ovary oncogenesis.
Protein kinase C (PKC) belongs to a serine/threonine kinase family that include three classes of isoenzymes—conventional, novel and atypical PKCs. Conventional PKCs (cPKCs) are dependent on calcium and diacylglycerol (DAG) for their activity, whereas novel PKCs (nPKCs) are calcium-independent and atypical PKCs (aPKCs) are both calcium- and DAG-independent (Corbalan-Garcia and Gomez-Fernandez, 2006). Despite their functional specificity, all three classes can activate nuclear factor (NF)-κB. For instance, PKCβ and PKCθ mediate B- and T-cell antigen receptor-induced NF-κB activation, respectively (Matsumoto et al., 2005; Sommer et al., 2005). PKCζ is required for IL-1-induced NF-κB activation in articular chondrocytes (LaVallie et al., 2006). Several recent studies have identified key signaling molecules downstream of PKCs to mediated T- and B-cell antigen receptor-mediated NF-κB activation:(1) CARMA1, a scaffolding protein which serves to integrate the upstream signal of PKCs with downstream effectors (Wang et al., 2002); (2) Bcl10, an intermediate bridging protein (Willis et al., 1999) and (3) MALT1, an effector which can stimulate I-Kappa B kinase (IκB kinase) through interaction with Bcl10 (Lucas et al., 2001). Although CARMA1 expression is restricted to hematopoietic cells, CARMA3, Bcl10 and MALT1 are present in almost all tissue/cells. It is of interest to determine whether CARMA1/3-Bcl10-MALT1 signaling complex can facilitate PKC-mediated NF-κB activation in ovarian cancer cells.
LPA was reported to upregulate uPA expression in human ovarian cancer cells but not in normal ovarian epithelial cells (Pustilnik et al., 1999). We further showed that a Gi-Ras-NF-κB-dependent signaling pathway mediates LPA-induced uPA upregulation in ovarian cancer cells (Li et al., 2005). However, it is unknown what signaling components link Ras to NF-κB for this LPA-induced event. Using specific inhibitors and dominant-negative PKC mutants, we found that PKCα is necessary for LPA-induced NF-κB activation and uPA upregulation. To reveal the functional link between Ras and PKCα, we found that LPA stimulation led to PKCα activation, but this activation was abrogated by dominant-negative Ras mutant and Ras inhibitor farnesyltransferase inhibitor (FTI-277). Furthermore, LPA induced Ras and PKCα interaction and this interaction was sensitive to the inhibitors of both farnesyltransferase and cPKC. These results indicate that PKCα is a signaling component downstream of Ras mediating LPA-induced cellular event. In this study, we also investigated the role of newly characterized signaling complex CARMA3-Bcl10-MALT1 in LPA-induced NF-κB activation/uPA upregulation. Forced expression of dominant-negative CARMA3 mutant or treatment of cells with siRNAs specifically targeting CARMA3, Bcl10 or MALT1 abrogated these LPA-induced events, implicating the importance of CARMA3-Bcl10-MALT1 in LPA-associated signaling in ovarian cancer cells. Finally, we provide evidence that PKCα-CARMA3 signaling axis plays an essential role in LPA-induced ovarian cancer cell in vitro invasion.
Results
PKC inhibitor inhibits LPA-induced NF-κB activation/uPA upregulation in ovarian cancer cells
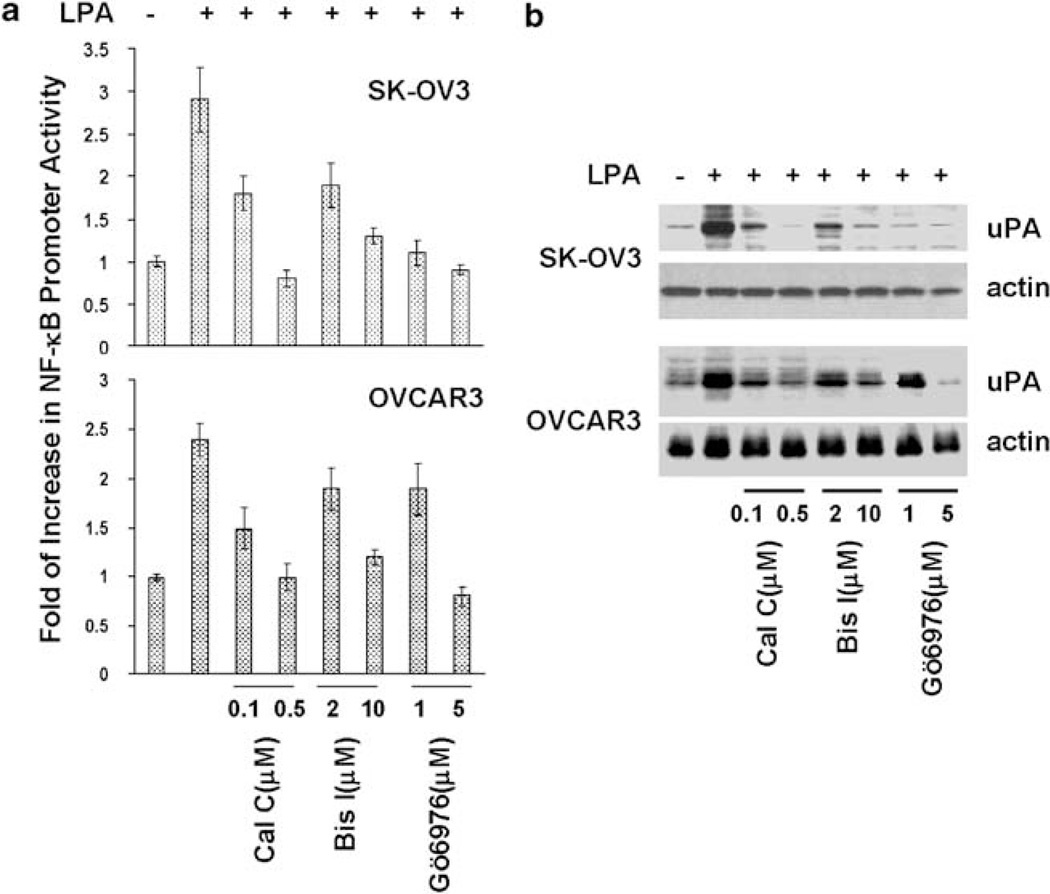
We previously show that Ras works downstream of Gi to mediate LPA-induced NF-κB activation and uPA upregulation (Li et al., 2005). As PKCs can be activated by Ras (Dlugosz et al., 1994; Perletti et al., 1996; Pal et al., 2001) and PKCs are involved in LPA-induced NF-κB activation in bronchial epithelial cells (Cummings et al., 2004), we examined whether PKC activity is needed for LPA-induced NF-κB activation and uPA upregulation in ovarian cancer cells. Ovarian cancer OVCAR3 and SK-OV3 cells were treated with calphostin C, bisindolylmaleimide I or Gö6097 for 2 h and then stimulated with 10 µm LPA. LPA gave rise two- to threefold increase in NF-κB promoter activity and five- to eightfold increase in the level of uPA protein over the unstimulated cells (Figures 1a and b). Pretreatment of cells with pan-PKC inhibitor calphostin C and bisindolylmaleimide abolished LPA-induced NF-κB activation and LPA upregulation (Figures 1a and b). Interestingly, Gö6097, an inhibitor of cPKC, inhibited these LPA-induced events equally well as the pan-PKC inhibitors (Figures 1a and b). To determine the specificity of these inhibitors in LPA-associated action, we examined how these inhibitors affected tumor necrosis factor-α (TNFα)-induced events. TNFα significantly increased both NF-κB promoter activity and uPA level (Supplementary Figures S1A and B). In contrary to LPA-induced events, TNFα-induced events were not sensitive to these inhibitors (Supplementary Figures S1A and B). These results suggest that cPKC activity is specifically required for LPA-induced NF-κB activation and uPA upregulation.
Figure 1.
PKC inhibitors inhibit lysophosphatidic acid (LPA)-induced NF-κB activation/urokinase plasminogen activator (uPA) upregulation. SK-OV3 and OVCAR3 cells were treated with calphostin C (0.1 and 0.5 µm), bisindolylmaleimide I (2 and 10 µm) or Gö6976 (1 and 5 µm) for 2 h, and then stimulated with 10 µm LPA for 4 or 16 h. (a) Cells were analysed for NF-κB promoter activity. (b) Cells were subjected to immunoblotting to detect uPA and actin with the respective antibodies.
PKCα mediates LPA-induced NF-κB activation/uPA upregulation
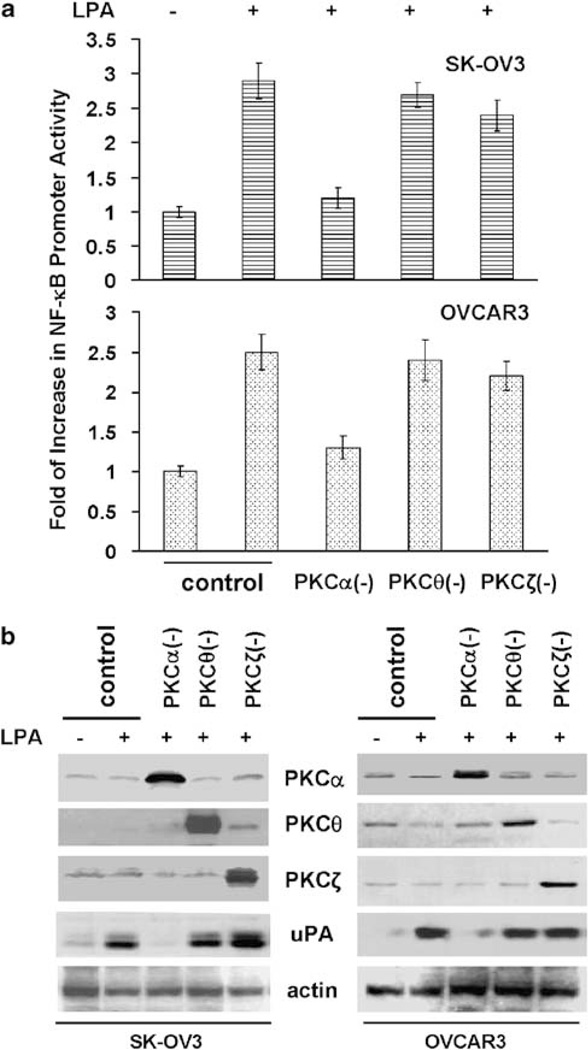
To identify the PKC isoform involved in LPA action, we first examined the presence of PKC isoforms by immunoblotting with subtype-specific antibodies and found that PKCα was the only cPKC member in SK-OV3 and OVCAR3 lines (Supplementary Figure S2A). In addition, PKCθ (an nPKC) and PKCζ (an aPKC) were also seen (Supplementary Figure S2A). In subsequent experiments, cells were transfected with NF-κB promoter reporter gene construct and then infected with Ad containing dominant-negative PKCα, θ or ζ. After serum starvation, cells were stimulated with LPA for 4 h. While the overexpression of all three dominant-negative PKC mutants was readily seen (Figure 2b), only dominant-negative PKCα attenuated LPA-induced NF-κB activation (Figure 2a). In parallel, we expressed the same PKC mutants in these cells and subsequently determined how their expression affected uPA upregulation. Dominant-negative PKCα, but not dominant-negative PKCθ or PKCζ, diminished LPA-induced uPA upregulation in both lines (Figure 2b). As over-expression of dominant-negative PKCα did not inhibit TNFα-induced NF-κB activation and uPA upregulation (Supplementary Figures S3A and B), these results suggest that PKCα specifically mediates these LPA-induced events in ovarian cancer cells.
Figure 2.
Protein kinase C (PKC)α is involved in lysophosphatidic acid (LPA)-induced nuclear factor (NF)-κB activation/urokinase plasminogen activator (uPA) upregulation. (a) pNF-κB-luc-transfected SK-OV3 and OVCAR3 cells were infected with control Ad or Ad containing dominant-negative PKCα, PKCθ or PKCζ for 24 h followed by overnight starvation. Cells were stimulated with 10 µm LPA for 4 h and then assayed for NF-κB promoter activity. (b) Cells were infected with control Ad or Ad containing dominant-negative PKCα, PKCθ or PKCζ for 24 h followed by overnight starvation. Cells were stimulated with 10 µm LPA for 16 h and then subjected to immunoblotting to detect uPA and actin with the respective antibodies.
LPA activates PKCα in a Ras-dependent manner
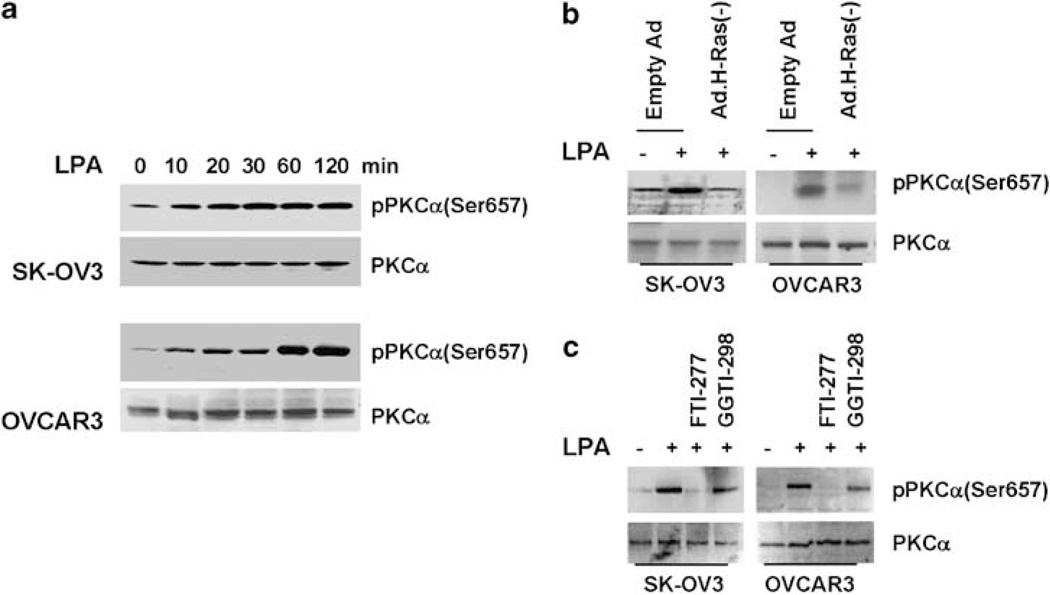
Our previous studies demonstrate the importance of Ras in LPA-induced uPA upregulation (Li et al., 2005). Others also show the ability of Ras to activate PKC (Dlugosz et al., 1994; Perletti et al., 1996; Pal et al., 2001). We hypothesized that PKCα might act downstream of Ras to facilitate LPA action. To test this hypothesis, we first determined the effect of LPA on PKCα activation in SK-OV3 and OVCAR3 lines. Overnight-starved cells were stimulated with 10 µm LPA for various times and then harvested for immunoblotting. As PKCα activation accompanies with serine657 phosphorylation, we determined PKCα activation by immunoblotting with anti-phospho-PKCα(Ser657) antibody. The serine657 phosphorylation of PKCα was low in unstimulated cells, but increased significantly and peaked between 30 and 60 min (Figure 3a). These results demonstrate the ability of LPA to activate PKCα.
Figure 3.
Lysophosphatidic acid (LPA) activates protein kinase C (PKC)α in a Ras-dependent manner. (a) Overnight-starved SK-OV-3 or OVCAR3 cells were stimulated with 10 µm LPA for various times, then lysed and lysates subjected to immunoblotting to detect phosphor-PKCα(Ser657) and PKCα with the respective antibodies. (b) Cells were infected with control Ad or Ad containing dominant-negative Ras for 24 h followed by overnight starvation. Cells were stimulated with 10 µm LPA for 1 hr and then subjected to immunoblotting to detect phosphor-PKCα(Ser657) and PKCα with the respective antibodies. (c) Overnight-starved cells were treated with 1 µm FTI-277 or GGTI-298 for 2 h and then stimulated with 10 µm LPA for 1 hr followed by immunoblotting to detect phospho-PKCα(Ser657) and PKCα with the respective antibodies.
We next examined the potential involvement of Ras in LPA-induced PKCα activation. Cells were infected with Ad containing dominant-negative H-Ras followed by 1-hr LPA stimulation. LPA induced PKCα activation in cells infected with control Ad (Figure 3b), but this induction was reduced in cells expressing dominant-negative H-Ras (Figure 3b). As Ras activation requires farnesylation, we examined how blocking Ras activity with farnesyltransferase inhibitor would affect LPA-induced PKCα activation. Cells were treated with farnesyltransferase inhibitor (FTI-277) or geranylgeranyltransferase inhibitor (GGTI-298) for 2 h prior to LPA-stimulation. FTI-277, but not GGTI-298, abrogated PKCα activation (Figure 3c). These results suggest that LPA induces PKCα activation in a Ras-dependent manner.
LPA stimulation facilitates PKCα–Ras interaction
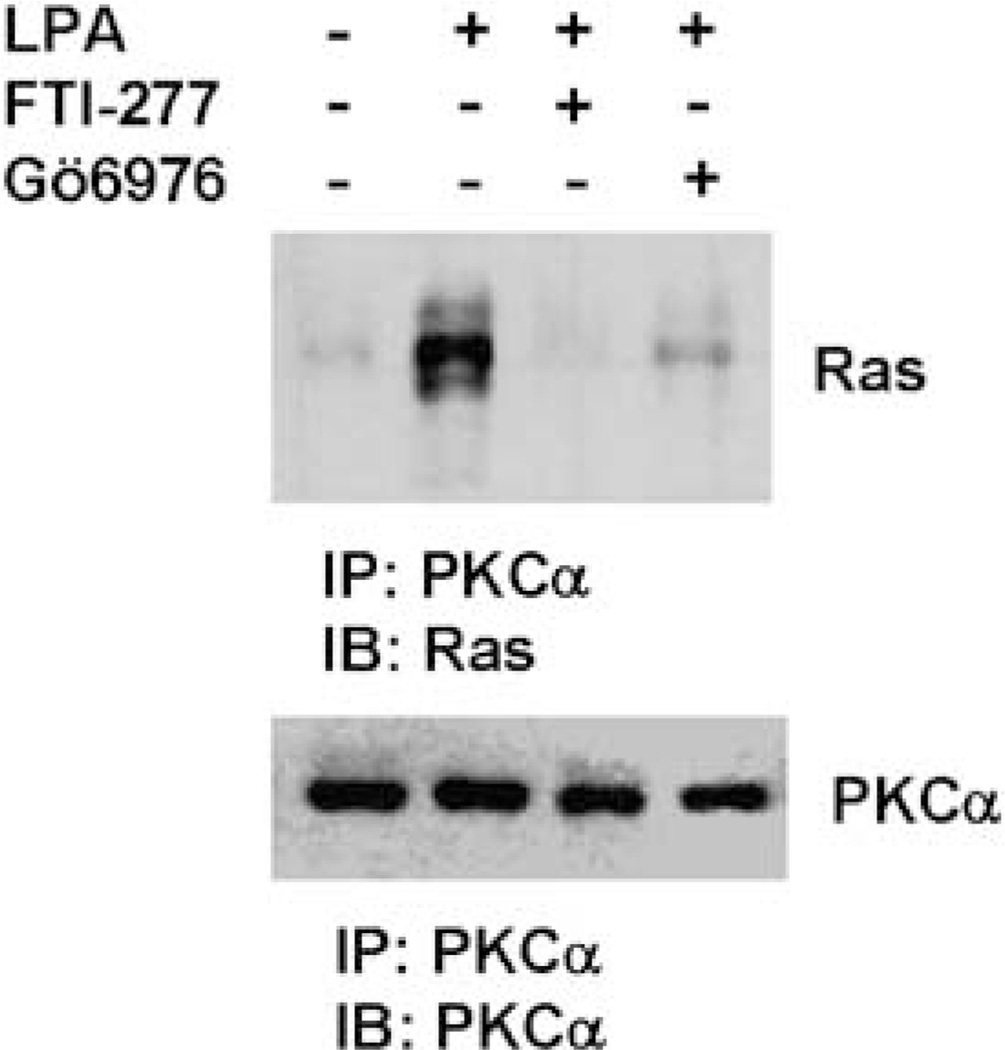
As PKCα action leads to PKCα translocation to the plasma membrane and active Ras is known to locate on the plasma membrane, we hypothesized that LPA stimulation may lead both Ras and PKCα in the close proximity thus allowing Ras to activate PKCα. To test this hypothesis, SK-OV3 cells were stimulated with LPA for 10 min, then lysed for immunoprecipitation with PKCα mAb. Immunoblotting with the immunoprecipitates showed that little Ras was detected in PKCα immunoprecipitate of the unstimulated cells while significantly more Ras observed in LPA-stimulated cells (Figure 4). FTI-277 or Gö6976 treatment prevented Ras–PKCα interaction in LPA-stimulated cells as indicated by significant less Ras detected in PKCα immunoprecipitates (Figure 4). These results suggest that (1) LPA stimulation leads to Ras–PKCα interaction and (2) the activities of Ras and PKCα are necessary for their interaction.
Figure 4.
Lysophosphatidic acid (LPA) induces Ras–protein kinase C (PKC)α interaction. SK-OV3 cells were treated with 1 µm FTI-277 or 5 µm Gö6976 or left untreated for 2 h and LPA then added to cells for 10 min followed by immunoprecipitation with PKCα mAb. Immunoprecipitates were analysed by immunoblotting to detect Ras by anti-Ras polyclonal antibody. The membrane was stripped and reprobed with-PKCα polyclonal antibody.
CARMA3-Bcl10-MALT1 signaling cascade is required for LPA-induced uPA upregulation in ovarian cancer cells
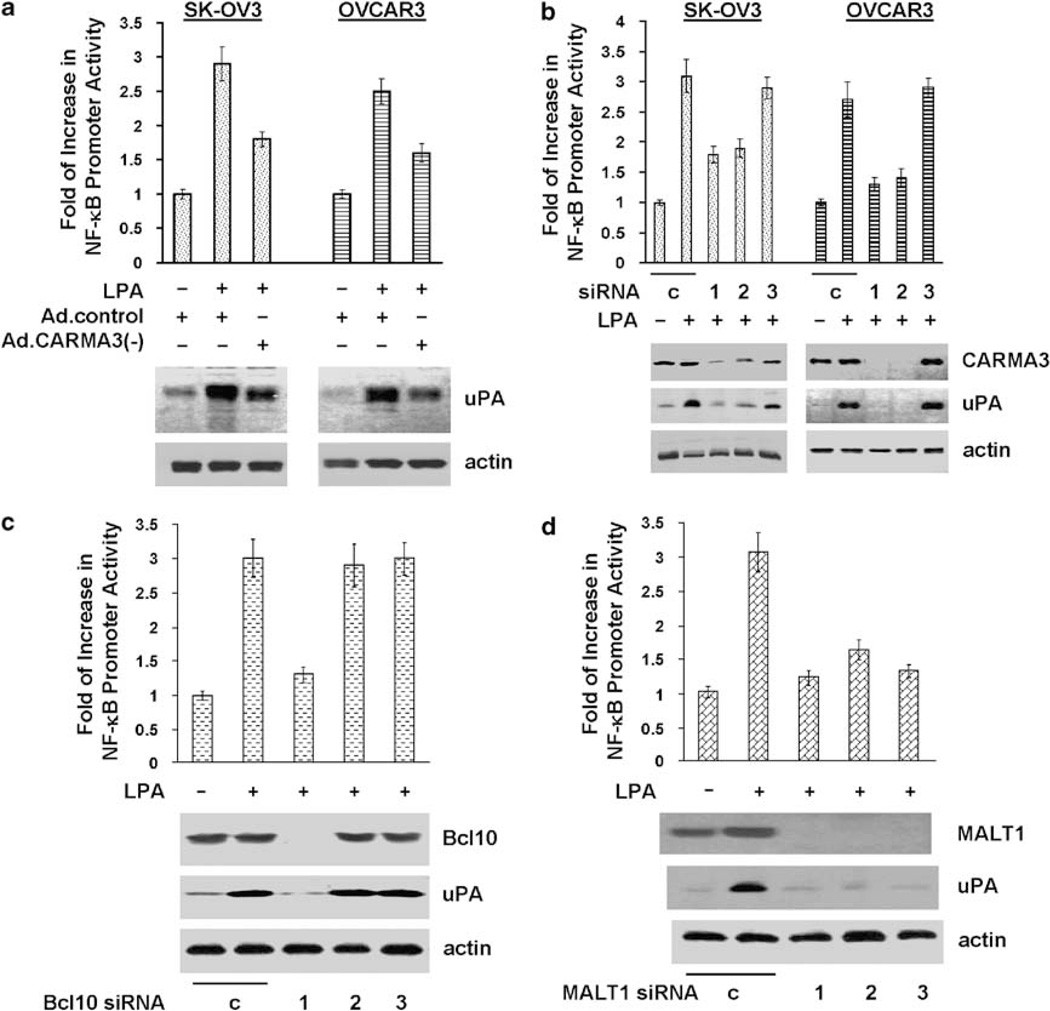
CARMA1/3-Bcl10-MALT1 play an essential role in PKC-mediated NF-κB activation (Wang et al., 2002, 2007a; Klemm et al., 2007). A recent study further shows that CARMA3 is important for LPA-induced NF-κB activation in murine embryonic fibroblasts (Grabiner et al., 2007). To investigate whether such signaling complex was involved in LPA-induced uPA upregulation in ovarian cancer cells, we first performed immunoblotting with specific CARMA antibodies and detected only CARMA3 in OVCAR3 and SK-OV3 cells (data not shown). To address the role of CARMA3, we chose a dominant-negative CARMA3 mutant for our studies. This mutant, CARMA3ΔCARD, contains a deletion of CARD, a protein–protein interaction domain that recruits the downstream signaling protein, Bcl10, through CARD–CARD interaction and thus interrupting signal transmission from PKC to Bcl10/MALT1 (McAllister-Lucas et al., 2001). Expression of this CARMA3 mutant inhibited LPA-induced NF-κB activation and uPA upregulation in both SK-OV3 and OVCAR3 cells (Figure 5a), while it displayed no effect on TNFα-induced events (Supplementary Figure S4A). Meanwhile, we also designed three CARMA3 siRNAs (see Supplementary Material) and introduced them into SK-OV3 and OVCAR3 cells. Two siRNAs that silenced CARMA3 expression inhibited LPA-induced (but not TNFα-induced) events (Figure 5b and Supplementary Figure S4B), while the siRNA that did not alter CARMA3 expression displayed no effect (Figure 5b). These results thus demonstrate the importance and specificity of CARMA3 in LPA-induced uPA upregulation in ovarian cancer cells.
Figure 5.
CARMA3, Bcl10 and MALT1 are involved in lysophosphatidic acid (LPA)-induced nuclear factor (NF)-κB activation/uPA upregulation. (a) pNF-κB-luc-transfected SK-OV3 or OVCAR3 cells were infected with control Ad or Ad containing CARD-deleted CARMA3 (Ad.CARMA3(−)) for 24 h followed by overnight starvation. Cells were stimulated with 10 µm LPA for 4 or 16 h followed by the analyses of NF-κB promoter activity and uPA expression as described in the ‘Materials and methods’ section. (b–d) SK-OV3 cells were transfected with 100nM control, CARMA3, Bcl10 or MALT1 siRNAs for 2 days. Part of the cells was transfected with pNF-κB-luc for analysing LPA-induced NF-κB promoter activation as described in the ‘Materials and methods’ section. Remaining cells were analysed for LPA-induced uPA expression as described in the ‘Materials and methods’ section.
We next examined the requirement of Bcl10 and MALT1 in LPA action by silencing their expression with siRNAs in SK-OV3 cells. Similar to CARMA3 siRNAs, siRNAs that diminished Bcl10 (siRNA-1) or MALT1 (all three siRNAs) expression inhibited LPA-induced (but not TNFα-induced) events (Figures 5c and d; Supplementary Figures S4C and D). Our studies clearly demonstrate the essential role of CARMA3-Bcl10-MALT1 signaling complex in LPA-induced uPA upregulation in ovarian cancer cells.
PKCα-CARMA3 signaling cascade is essential for LPA-stimulated in vitro cell invasion
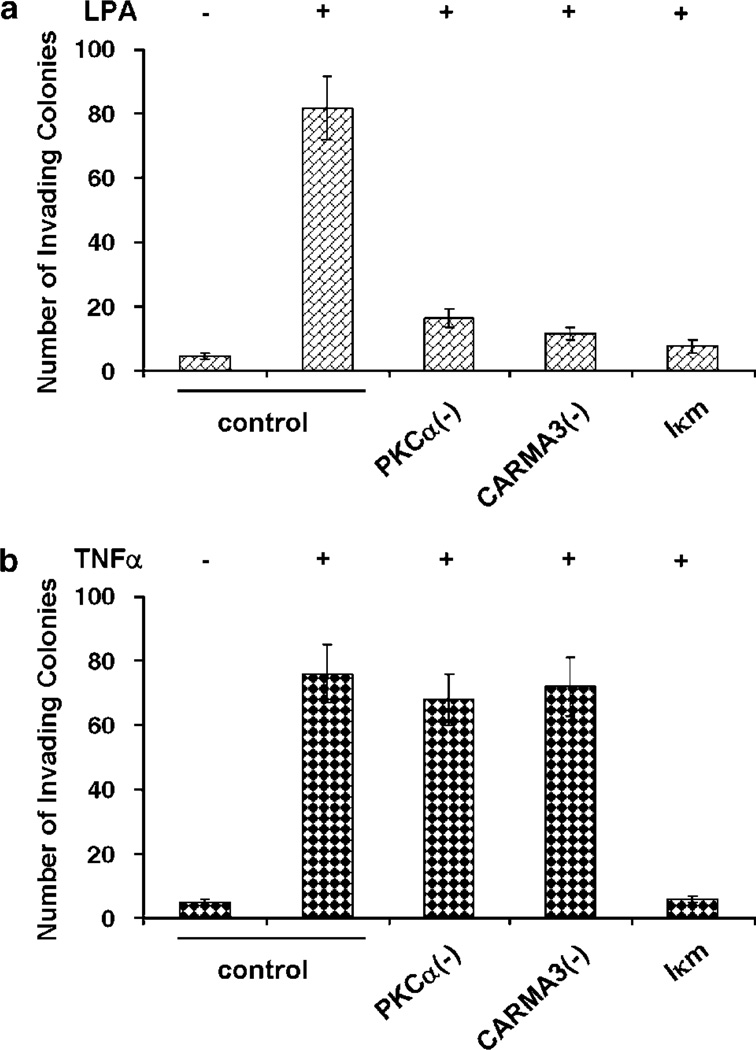
As uPA is important for LPA-induced invasion (Li et al., 2005), we investigated whether PKCα-CARMA3 signaling axis was similarly important for LPA-induced invasion. SK-OV3 cells were infected with Ad containing dominant-negative PKCα, CARMA3 (CARMA3ΔCARD) or non-phosphorylable IκB (Iκm) and then analysed for LPA or TNFα-stimulated in vitro invasion. Both LPA and TNFα induced significant invasion in cells infected with control Ad (Figure 6). Dominant-negative PKCα, CARMA3ΔCARD and Iκm all inhibited LPA-induced invasion (Figure 6a). However, only the expression of Iκm inhibited TNFα-induced invasion (Figure 6b). These results suggest that PKCα-CARMA3 axis is essential for LPA-induced (but not TNFαinduced) ovarian cancer cell invasion although NF-κB activity is required for both.
Figure 6.
Intercepting PKCα-CARMA3 axis inhibits SK-OV3 in vitro invasion. SK-OV3 cells were infected with control Ad or Ad containing dominant-negative PKCα, CARMA3ΔCARD or Iκm, and then added into matrigel invasion chambers to analyse in vitro invasion as described in Supplementary Material. Lysophosphatidic acid (LPA, 10 µm) (a) or TNFα (50 ng ml−1) (b) was added in the underwells to stimulate cell invasion.
Discussion
An early study reported that LPA significantly induced uPA expression in ovarian cancer cells but not in normal ovary epithelial cells (Pustilnik et al., 1999), we further found that a signaling pathway consisting Gi-Ras-NF-κB mediated this LPA-induced event (Li et al., 2005). However, it was unknown how signal was transmitted from Ras to NF-κB. Using specific PKC inhibitors and dominant-negative PKC mutants, we found that Gö6876 (a cPKC inhibitor) and dominant-negative PKCα diminished both LPA and Ras-induced NF-κB activation/uPA upregulation (Figure 1 and Supplementary Figure S5). Although PKC isoforms from all three classes can activate NF-κB and upregulate uPA (Supplementary Figure S2), only dominant-negative PKCα blocked these LPA-induced events (Figure 2). These findings suggest that PKCα is specifically involved in LPA-induced NF-κB activation/uPA upregulation in ovarian cancer cells. A recent study reported that PKCδ mediates LPA-induced NF-κB activation/IL-8 secretion in bronchial epithelial cells (Cummings et al., 2004). However, the results from our experiments ruled out the role of PKCδ in LPA-induced events in ovarian cancer cells (Supplementary Figure S6). It is likely that a particular PKC isoform may mediated LPA-induced NF-κB activation in a particular cell type.
Ras activates PKCα in primary keratinocytes and PKCα activity is required for Ras-induced keratin K1 and K10 expression (Dlugosz et al., 1994). PKCε is activated by Ras and promotes Ras-mediated transformation of rat colon epithelial cells (Perletti et al., 1996). In fibrosarcoma HT1080 cells, Ras associates with and phosphorylates PKCζ and PKCζ is essential for Ras-mediated VEGF expression (Pal et al., 2001). We showed that LPA activated PKCα (Figure 3) and induced Ras–PKCα interaction (Figure 4). Blocking Ras activity abolished PKCα activation (Figure 3) and Ras–PKCα interaction (Figure 4). In fact, ovarian cancer cells expressing active Ras displayed elevated PKCα activity and this elevated activity was diminished by Ras inhibitor FTI-277 (Supplementary Figure S5). Our observation thus provides another example of functional link between Ras and PKC.
CARMA1, Bcl10 and MALT1 function as part of a signaling complex to bridge PKC to IKKs, thus facilitating NF-κB activation in lymphocytes (Matsumoto et al., 2005; Sommer et al., 2005). In our study, we found that intercepting CARMA3 function with a CARD-deleted CARMA3 or silencing CARMA3 expression with specific siRNAs attenuated LPA-induced NF-κB activation and uPA upregulation (Figure 5). Moreover, knocking down Bcl10 or MALT1 also inhibited these LPA-induced events (Figure 5). These findings suggest a general role of CARMA1/3-Bcl10-MALT1 in NF-κB activation. Our conclusions are supported by recent studies that reported defective NF-κB activation in CARMA3, Bcl10 or MALT1-defective murine embryonic fibroblasts by LPA (Grabiner et al., 2007; Klemm et al., 2007; Wang et al., 2007a).
In conclusion, we have characterized PKCα-CAR-MA3 axis as part of a signaling pathway mediating LPA-induced uPA upregulation in ovarian cancer cells. As uPA plays a critical role in ovary neoplasias, we speculate that PKCα or CARMA3 may represent attractive therapeutic targets for ovary malignancies.
Materials and methods
Immunoblotting
To determine the effect of PKC inhibitors, overnight-starved OVCAR3 and SK-OV3 cells were treated with calphostin C (0.1 and 0.5 µm), bisindolylmaleimide I (2 and 10 µm) or Gö6097 (1 and 5 µm) for 2 h prior to 16-hr 10 µm LPA stimulation. Cells were lysed and lysates analysed by immunoblotting to detect uPA. To determine the effect of dominant-negative mutants of signaling components, cells were infected with 103 viral particles per cell control Ad or Ad encoding CARMA3ΔCARD, dominant-negative PKCα, PKCθ or PKCζ for 24 h, serum-starved for 24 h and then stimulated with 10 µm LPA for 16 h followed by immunoblotting to detect uPA expression. To determine the effect of CARMA3, Bcl10 and MALT1 siRNAs, cells were transfected with 100nM CARMA3, Bcl10 or MALT1 siRNAs using Lipofectamine 2000 for 48 h and then serum-starved for another 24 h. Cells were stimulated with 10 µm LPA for 16 h followed by immunoblotting to detect uPA.
To determine PKCα activity, overnight-starved cells were stimulated with 10 µm LPA for varying times, then lysed and lysates subjected to immunoblotting to detect phospho-PKCα(Ser657) and PKCα. To determine the effect of dominant-negative Ras mutant, cells were infected with control Ad or Ad containing H-Ras(N17) at 103 viral particles per cell for 24 h and then starved for another 24 h. Cells were stimulated with 10 µm LPA for 60 min and then analysed for phospho-PKCα(Ser657). To determine the effect of FTI-277 and GGTI-298, overnight-starved cells were treated 1 µm FTI-277 or 1 µm GGTI-298 for 2 h and then stimulated 10 µm LPA for 60 min followed by immunoblotting to detect phospho-PKCα(Ser657).
Analysis of NF-κB activity
SK-OV3 or OVCAR3 cells were transfected with pNF-κB-Luc plasmid (Stratagene, San Diego, CA, USA) and pAc-RLuc (Promega, Madison, WI, USA) at 30:1 ratio for 24 h using Lipofectamine2000. To determine the effect of PKC inhibitors, transfected cells were starved for 24 h and then treated with calphostin C (0.1 and 0.5 µm), bisindolylmaleimide I (2 and 10 µm) or Gö6097 (1 and 5 µm) for 2 h followed by 4-hr stimulation of 10 µm LPA. Cells were lysed and lysates measured for luciferase activities using Dual-luciferase Reporter Assay System (Promega). To determine the effect of dominant-negative forms of CARMA3, PKCα, θ and ζ, transfected cells were infected with control Ad or Ad encoding CARMA3ΔCARD, dominant-negative PKCα, θ or ζ (103 viral particles per cell) for 24 h, and then starved for 24 h followed by 4-hr LPA stimulation. To determine the effect of CARMA3, Bcl10 and MALT1 siRNAs, cells were first transfected with 100 nm CARMA3, Bcl10 or MALT1 siRNAs for 48 h and then again transfected with pNF-κB-Luc/pAc-Rluc for 24 h. Cells were then starved for 24 h followed by 4-hr LPA stimulation. The Renilla luciferase activity was used to normalize NF-κB promoter activity.
Immunoprecipitation
Overnight-starved SK-OV3 cells were treated with 1 µm FTI-277 or 5 µm Gö6976 for 2 h or left untreated, and then stimulated with 10 µm LPA for 10 min. Cells were lysed, lysates immunoprecipitated with PKCα mAb and immunoprecipitates subjected to immunoblotting with anti-Ras polyclonal antibody.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Health Grant R01 CA93926.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63:2695–2701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Gomez-Fernandez JC. Protein kinase C regulatory domains: the art of decoding many different signals in membranes. Biochim Biophys Acta. 2006;1761:633–654. doi: 10.1016/j.bbalip.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, et al. Protein kinase Cδ mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Cheng C, Williams EK, Dharia AG, Denning MF, Yuspa SH. Alterations in murine keratinocyte differentiation induced by activated rasHa genes are mediated by protein kinase Cα. Cancer Res. 1994;54:6413–6420. [PubMed] [Google Scholar]
- Do TV, Symowicz JC, Berman DM, Liotta LA, Petricoin EF, III, Stack MS, et al. Lysophosphatidic acid down-regulates stress fibers and up-regulates pro-matrix metalloproteinase-2 activation in ovarian cancer cells. Mol Cancer Res. 2007;5:121–131. doi: 10.1158/1541-7786.MCR-06-0319. [DOI] [PubMed] [Google Scholar]
- Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6:2482–2491. [PubMed] [Google Scholar]
- Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- Fischer K, Lutz V, Wilhelm O, Schmitt M, Graeff H, Heiss P, et al. Urokinase induces proliferation of human ovarian cancer cells: characterization of structural elements required for growth factor function. FEBS Lett. 1998;438:101–105. doi: 10.1016/s0014-5793(98)01279-4. [DOI] [PubMed] [Google Scholar]
- Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61:3194–3199. [PubMed] [Google Scholar]
- Grabiner BC, Blonska M, Lin PC, You Y, Wang D, Sun J, et al. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-{kappa}B activation. Genes Dev. 2007;21:984–996. doi: 10.1101/gad.1502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93:762–768. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- Kjoller L, Hall A. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J Cell Biol. 2001;152:1145–1157. doi: 10.1083/jcb.152.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm S, Zimmermann S, Peschel C, Mak TW, Ruland J. Bcl10 and Malt1 control lysophosphatidic acid-induced NF-kappaB activation and cytokine production. Proc Natl Acad Sci USA. 2007;104:134–138. doi: 10.1073/pnas.0608388103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny G, Untch M, Pihan A, Kimmig R, Gropp M, Stieber P, et al. Association of urokinase-type plasminogen activator and its inhibitor with disease progression and prognosis in ovarian cancer. Clin Cancer Res. 2001;7:1743–1749. [PubMed] [Google Scholar]
- LaVallie ER, Chockalingam PS, Collins-Racie LA, Freeman BA, Keohan CC, Leitges M, et al. Protein kinase Czeta is upregulated in osteoarthritic cartilage and is required for activation of NF-kappaB by tumor necrosis factor and interleukin-1 in articular chondrocytes. J Biol Chem. 2006;281:24124–24137. doi: 10.1074/jbc.M601905200. [DOI] [PubMed] [Google Scholar]
- Li H, Ye X, Mahanivong C, Bian D, Chun J, Huang S. Signaling mechanisms responsible for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. J Biol Chem. 2005;280:10564–10571. doi: 10.1074/jbc.M412152200. [DOI] [PubMed] [Google Scholar]
- Lucas PC, Yonezumi M, Inohara N, McAllister-Lucas LM, Abazeed ME, Chen FF, et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-kappa B signaling pathway. J Biol Chem. 2001;276:19012–19019. doi: 10.1074/jbc.M009984200. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B, et al. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-kappaB activation. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- McAllister-Lucas LM, Inohara N, Lucas PC, Ruland J, Benito A, Li Q, et al. Bimp1, a MAGUK family member linking protein kinase C activation to Bcl10-mediated NF-kappaB induction. J Biol Chem. 2001;276:30589–30597. doi: 10.1074/jbc.M103824200. [DOI] [PubMed] [Google Scholar]
- Murthi P, Barker G, Nowell CJ, Rice GE, Baker MS, Kalionis B, et al. Plasminogen fragmentation and increased production of extracellular matrix-degrading proteinases are associated with serous epithelial ovarian cancer progression. Gynecol Oncol. 2004;92:80–88. doi: 10.1016/j.ygyno.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Pal S, Datta K, Khosravi-Far R, Mukhopadhyay D. Role of protein kinase Czeta in Ras-mediated transcriptional activation of vascular permeability factor/vascular endothelial growth factor expression. J Biol Chem. 2001;276:2395–2403. doi: 10.1074/jbc.M007818200. [DOI] [PubMed] [Google Scholar]
- Perletti GP, Folini M, Lin HC, Mischak H, Piccinini F, Tashjian AH., Jr Overexpression of protein kinase C epsilon is oncogenic in rat colonic epithelial cells. Oncogene. 1996;12:847–854. [PubMed] [Google Scholar]
- Pustilnik TB, Estrella V, Wiener JR, Mao M, Eder A, Watt MA, et al. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5:3704–3710. [PubMed] [Google Scholar]
- Schmalfeldt B, Kuhn W, Reuning U, Pache L, Dettmar P, Schmitt M, et al. Primary tumor and metastasis in ovarian cancer differ in their content of urokinase-type plasminogen activator, its receptor, and inhibitors types 1 and 2. Cancer Res. 1995;55:3958–3963. [PubMed] [Google Scholar]
- Schwartz BM, Hong G, Morrison BH, Wu W, Baudhuin LM, Xiao YJ, et al. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol Oncol. 2001;81:291–300. doi: 10.1006/gyno.2001.6124. [DOI] [PubMed] [Google Scholar]
- Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL, et al. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity. 2005;23:561–574. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kobayashi H, Kanayama N, Saga Y, Suzuki M, Lin CY, et al. Inhibition of tumor invasion by genomic down-regulation of matriptase through suppression of activation of receptor-bound pro-urokinase. J Biol Chem. 2004;279:14899–14908. doi: 10.1074/jbc.M313130200. [DOI] [PubMed] [Google Scholar]
- Tanyi JL, Morris AJ, Wolf JK, Fang X, Hasegawa Y, Lapushin R, et al. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 2003;63:1073–1082. [PubMed] [Google Scholar]
- Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS, et al. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nat Immunol. 2002;3:830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- Wang D, You Y, Lin PC, Xue L, Morris SW, Zeng H, et al. Bcl10 plays a critical role in NF-kappaB activation induced by G protein-coupled receptors. Proc Natl Acad Sci USA. 2007a;104:145–150. doi: 10.1073/pnas.0601894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wu X, Chen W, Liu J, Wang X. The lysophosphatidic acid (LPA) receptors their expression and significance in epithelial ovarian neoplasms. Gynecol Oncol. 2007b;104:714–720. doi: 10.1016/j.ygyno.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:35–45. doi: 10.1016/s0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.