Abstract
Background
Insomnia is common in primary care. Cognitive behavioural therapy for insomnia (CBT-I) is effective but requires more time than is available in the general practice consultation. Sleep restriction is one behavioural component of CBT-I.
Aim
To assess whether simplified sleep restriction (SSR) can be effective in improving sleep in primary insomnia.
Design and setting
Randomised controlled trial of patients in urban general practice settings in Auckland, New Zealand.
Method
Adults with persistent primary insomnia and no mental health or significant comorbidity were eligible. Intervention patients received SSR instructions and sleep hygiene advice. Control patients received sleep hygiene advice alone. Primary outcomes included change in sleep quality at 6 months measured by the Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), and sleep efficiency (SE%). The proportion of participants reaching a predefined ‘insomnia remission’ treatment response was calculated.
Results
Ninety-seven patients were randomised and 94 (97%) completed the study. At 6-month follow-up, SSR participants had improved PSQI scores (6.2 versus 8.4, P<0.001), ISI scores (8.6 versus 11.1, P = 0.001), actigraphy-assessed SE% (difference 2.2%, P = 0.006), and reduced fatigue (difference −2.3 units, P = 0.04), compared with controls. SSR produced higher rates of treatment response (67% [28 out of 42] versus 41% [20 out of 49]); number needed to treat = 4 (95% CI = 2.0 to 19.0). Controlling for age, sex, and severity of insomnia, the adjusted odds ratio for insomnia remission was 2.7 (95% CI = 1.1 to 6.5). There were no significant differences in other outcomes or adverse effects.
Conclusion
SSR is an effective brief intervention in adults with primary insomnia and no comorbidities, suitable for use in general practice.
Keywords: behaviour therapy, general practice, sleep initiation and maintenance disorders
INTRODUCTION
The symptom of insomnia affects approximately 40% of adults in the general population, with between 7% and 22% meeting the criteria for an insomnia disorder.1–3 Insomnia is often chronic,4,5 and is associated with an increased risk of depression and anxiety,6,7 cardiovascular disease,8,9 and reduced quality of life.10 GPs are consulted more frequently than other health professionals for sleep problems,11 and patients typically prefer non-pharmacological treatment strategies.12 In general practice, approximately 12% of adults with insomnia experience primary insomnia.13,14 Primary insomnia is diagnosed when there is no other identified cause, such as obstructive sleep apnoea or other contributing medical condition.15 In 2014, the third edition of the International Classification of Sleep Disorders combined primary and secondary insomnia under the single diagnosis of chronic insomnia disorder;16 although the current study only included those with primary insomnia as defined above.
Cognitive behavioural therapy for insomnia (CBT-I) is effective but its use in general practice is limited because of the time and training required for its delivery.17,18 Recent research has focused on briefer, more accessible treatments. These include studies by Buysse et al using the behavioural components of CBT-I (sleep restriction and stimulus control),19 Edinger and Sampson using an abbreviated form of CBT-I,20 and Espie et al using CBT-I delivered by nurses in the primary care setting.21 The results have been promising.
The sleep restriction component of CBT-I consolidates fragmented sleep by reducing the time allowed in bed (the sleep opportunity); thereby inducing mild sleep deprivation to enhance the endogenous sleep drive.22 The aim of the current study was to assess whether an even shorter intervention than those mentioned above (simplified sleep restriction) designed to fit into two GP consultations could improve sleep among patients with primary insomnia.
METHOD
Study setting and patients
A parallel design randomised controlled trial was conducted in Auckland, New Zealand. Fourteen general practices participated. Primary care patients were eligible if they were between 16 and 75 years old with primary insomnia lasting >6 months, did not have obstructive sleep apnoea, a mental health or other significant comorbidity that may have led to secondary insomnia, and were not taking hypnotic medication for at least 2 weeks prior to baseline assessment.23 All enrolled adult patients were sent a form to screen for insomnia and to invite potentially eligible patients to participate. Those who responded were further assessed using a longer questionnaire (to determine the cause of the sleep disorder), physical examination (blood pressure, pulse, body mass index, neck circumference, inspection of face, nose, tongue, tonsils, and palate, and auscultation of heart and lungs) and a sleep diary (requiring difficulty sleeping on at least 3 nights a week) to ensure they had primary insomnia and were fit for participation. Patients on prescription sleep medication and unwilling or unable to discontinue regular use during the study, were excluded.
How this fits in
Insomnia is a common health problem seen in general practice. Cognitive behavioural therapy for insomnia (CBT-I) is effective but its use has been limited by the time and expense required for delivery. Sleep restriction, or restricting the time in bed, is one component of CBT-I. This trial found that simplified sleep restriction advice, delivered in two GP consultations, is a practical, effective intervention for chronic primary insomnia.
Intervention
After randomisation, participants in both the simplified sleep restriction (SSR) and control groups received verbal advice about sleep hygiene, including information such as avoiding caffeine, relaxing before bedtime, and creating a pre-bed routine,24 at a visit with the study GP. Those in the SSR group also received verbal and written instructions (sleep prescription) setting both bedtime and wake-up times based on a preceding 2-week daily sleep diary.
The study GP (one of the authors) developed the intervention design and algorithm used in the trial after reviewing the literature and existing evidence for CBT-I and sleep restriction. All intervention instructions were delivered by the study GP. The total time in bed allowed in the sleep prescription was equal to the average total sleep duration plus 50% of the total time spent awake in bed (therefore reducing the total additional ‘wake time’ by half), with a minimum time in bed of 5 hours. Actual bedtime and wake-up times were negotiable. Participants were asked to continue with a sleep diary until the next visit.
Participants attended a second visit 2 weeks later. If participants were sleeping for <85% of the time they spent in bed (according to their diary), the time allowed in bed was further reduced to total sleep duration plus 30 minutes. If patients felt excessively sleepy, they were advised to spend 30 minutes more in bed each night. Wherever possible, wake-up time was kept constant, and any changes required were made to bedtime. Participants were also given a written flowchart summarising change options (available from authors on request) and asked to self-adjust their sleep every 2 weeks thereafter. Control participants also attended a second visit after 2 weeks where their general sleep progress according to sleep hygiene guidelines was discussed. Scripts were used for the delivery of instructions to both groups.
Randomisation and blinding
Computer-generated block randomisation was performed by a biostatistician not involved in recruitment or assessment. A central telephone system was used to implement the random allocation sequence following enrolment and baseline assessment, maintaining allocation concealment. Because of the nature of the intervention, participants could not be completely blinded to the intervention; however, they were only aware that they would receive some advice about their sleep but were not told the exact nature. For the first 40% of participants (37 out of 91), a research assistant blind to allocation assessed follow-up measures. However, because of funding constraints, the study GP, who was not blind to allocation, followed up the remaining 60% of participants (54 out of 91). Standard scripts, objective measures, and self-completed questionnaires were used wherever possible to minimise the risk of assessment bias. The study GP gave no assistance to the participants when completing their questionnaires.
Outcome measures
Sleep quality was measured by the Pittsburgh Sleep Quality Index (PSQI)25 and Insomnia Severity Index (ISI)26 at baseline, 3 months, and 6 months, and change in sleep efficiency from baseline to 6 months (total sleep time/time in bed [from lights out] × 100% from 2-week diaries and actigraphy).27 Secondary outcomes measured at baseline and 6 months included sleep-onset latency, wakefulness after sleep-onset, and total sleep time, assessed from 2-week sleep diaries27 and actigraphy (Minimitter Actiwatch®-64; Minimitter, Bend, Oregon);28–30 sleepiness using the Epworth Sleepiness Scale;31 fatigue using the Flinders Fatigue Scale;32 depression using the Patient Health Questionnaire (PHQ-9),33 and anxiety using the Generalised Anxiety Disorders (GAD-7) questionnaire.34,35 Actigraphy used 30-second epochs, analysed with Actiware software (version 5.70.1; medium wake threshold).
Demographic information was collected by self-administered questionnaire. Medical status, medication use, hypnotic use, and caffeine or other substance use, were evaluated using interviewer-administered questionnaires. Potential adverse effects were assessed at 2 weeks, 3 months, and 6 months, including blood pressure, history of accidents, angina, myocardial infarction, stroke or other condition resulting in hospitalisation, and extreme sleepiness (such as while driving, operating machinery, cooking, or in other unsafe situations).
Statistical tests
Sample size calculations used data from a 6-week pilot trial (n = 45) of sleep restriction.36 To detect an absolute difference in the proportion with a treatment response between intervention and control of 30%, at least 45 participants in each group would be required (α = 0.05, 80% power)
The results were analysed using SAS software (version 9.3). All available data were used and no missing data imputation was performed. Mixed models for repeated measures were used for PSQI and ISI with two post-baseline time points (3 and 6 months), and multiple linear regression was used to analyse continuous outcomes with one post-baseline time point (6 months). All analyses used an intention-to-treat approach and were adjusted for age, sex, and baseline insomnia severity (ISI score).
The definition of treatment effect described by Buysse et al19 was used to determine treatment responses of remission, response, partial response, and non-response. These four categorical outcomes were then collapsed into two categories: ‘remission’ or ‘response’ versus ‘partial response’ or ‘non-response’. The absolute risk reduction, number needed to treat (NNT), and the corresponding 95% confidence intervals (CIs) were calculated using unadjusted data. The four and two category outcomes were then analysed using ordinal and logistic regression, respectively, adjusting for age, sex, and baseline insomnia severity. From this modelling, adjusted odds ratios and 95% CIs were obtained. Complete case sensitivity analysis and analysis adjusting for hypnotic use at 6 months were also performed.
RESULTS
Participant flow and recruitment
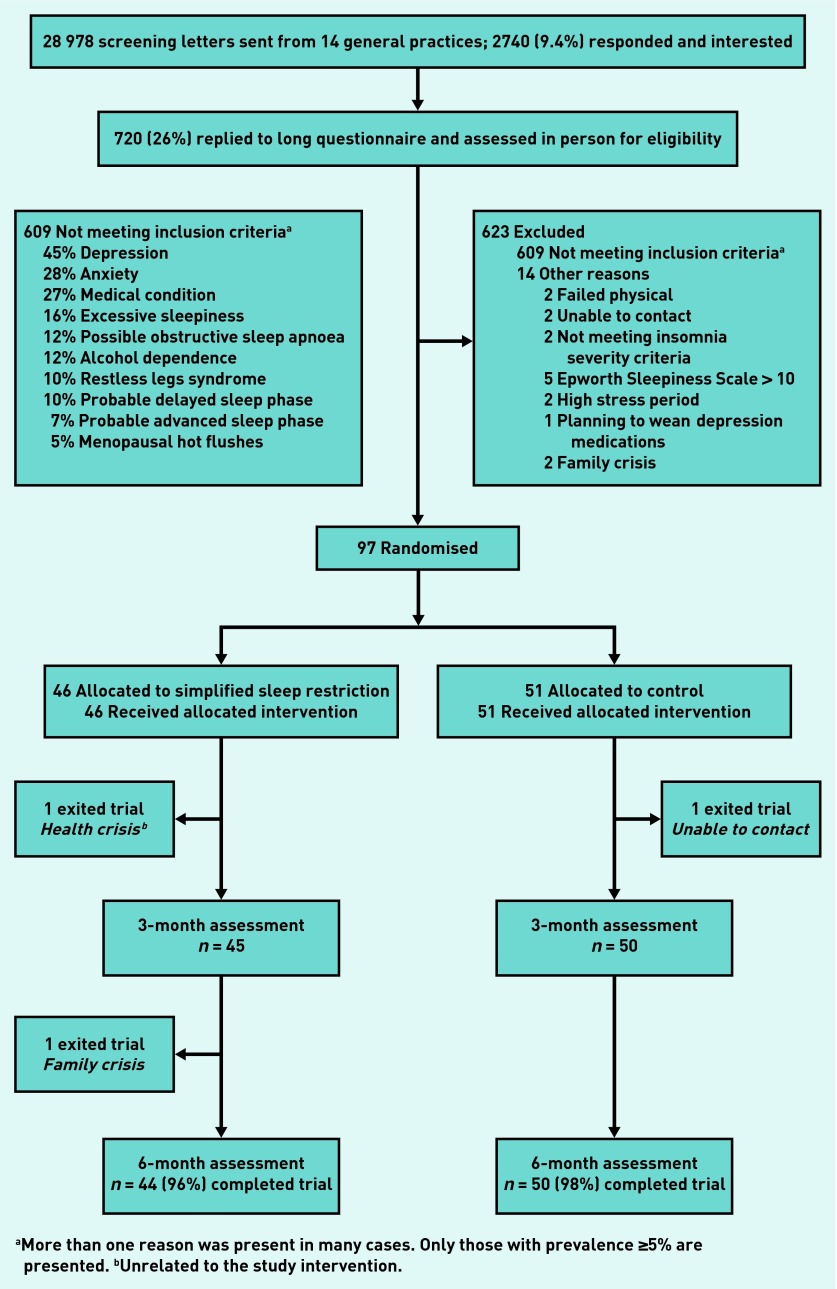
Of the 28 978 screening letters sent out, 2740 responses were received from those potentially eligible and interested in participating in the trial. Of these, 1302 (48%) met general criteria for insomnia and were sent the comprehensive sleep disorders screening questionnaire to identify those with possible primary insomnia, and of these, 720 (55%) completed the second questionnaire and were potentially eligible. Ninety seven (13%) participants fulfilled all inclusion and no exclusion criteria and were randomised (Figure 1). Recruitment and follow-up occurred between March 2009 and November 2012.
Figure 1.
CONSORT diagram showing flow of study participants through the randomised controlled trial of simplified sleep restriction for primary insomnia.
aMore than one reason was present in many cases. Only those with prevalence ≥5% are presented. bUnrelated to the study intervention.
Baseline data
There were no obvious differences between groups in baseline characteristics (Table 1). The overall attrition rate during the trial was 3% (two lost to follow-up from SSR, one from control).
Table 1.
Baseline characteristics of participants
| Characteristic | Sleep restriction (n= 46) Mean (SD) | Control (n= 51) Mean (SD) |
|---|---|---|
| Age, years | 55.4 (12.7) | 51.8 (13.4) |
|
| ||
| Female, n (%) | 39 (85) | 36 (71) |
|
| ||
| Duration of insomnia, years | 15 (14) | 14 (13) |
|
| ||
| Primary outcomes | ||
|
| ||
| Pittsburgh Sleep Quality Index25 | 10.43 (3.08) | 10.29 (3.02) |
|
| ||
| Insomnia Severity Index26 | 14.76 (3.85) | 14.51 (3.36) |
|
| ||
| Sleep efficiency [sleep diary],a % | 73.32 (11.80) | 74.15 (11.05) |
|
| ||
| Sleep efficiency [actigraphy], % | 82.68 (4.66) | 82.37 (4.67) |
|
| ||
| Secondary outcomes — sleep | ||
|
| ||
| Epworth Sleepiness Scale31 | 5.17 (3.70) | 6.45 (3.29) |
|
| ||
| Flinders Fatigue Scale32 | 12.74 (6.83) | 12.53 (5.11) |
|
| ||
| Sleep-onset latency, minutes | ||
| Sleep diary | 31.50 (23.00) | 29.55 (22.48) |
| Actigraphy | 16.10 (14.47) | 14.66 (12.42) |
|
| ||
| Wake after sleep onset, minutes | ||
| Sleep diary | 44.82 (28.92) | 47.84 (30.14) |
| Actigraphy | 47.59 (13.88) | 51.81 (19.51) |
|
| ||
| Total sleep time, minutes | ||
| Sleep diary | 369.59 (65.27) | 376.93 (61.08) |
| Actigraphy | 415.68 (34.17) | 418.36 (39.36) |
|
| ||
| Secondary outcomes — mental health | ||
|
| ||
| PHQ-9 depression score33 | 4.98 (2.76) | 5.31 (2.80) |
|
| ||
| GAD-7 anxiety score34 | 3.33 (3.06) | 3.41 (2.45) |
|
| ||
| Physiological measures | ||
|
| ||
| Systolic blood pressure, mmHg | 122.24 (13.98) | 123.88 (13.20)b |
|
| ||
| Diastolic blood pressure, mmHg | 75.52 (10.35) | 75.62 (8.98)b |
|
| ||
| Heart rate, beats per minute | 68.91 (9.82) | 73.29 (10.86) |
|
| ||
| Body mass index, kg/m2 | 25.84 (3.89) | 25.16 (4.28) |
Using data averaged from sleep diary recordings for a 2-week period. Sleep efficiency is calculated as total sleep time/time spent in bed × 100%.
n = 50 due to equipment malfunction. PHQ-9 = Patient Health Questionnaire-9. GAD-7 = Generalised Anxiety Disorders-7.
The mean duration of the advice given at the initial visit was 20 minutes for SSR and 11 minutes for control participants. The duration of the advice given at the second visit was 14 and 11 minutes, respectively.
Outcomes and estimation
Compared with control, the SSR intervention led to statistically significant improved PSQI scores (−2.14, 95% CI = −3.15 to −1.13); improved ISI scores (−2.50, 95% CI = −3.97 to −1.03); improved sleep efficiency (2.22%, 95% CI = 0.65 to 3.79), and improved sleep-onset latency (−6.13 minutes, 95% CI = −11.82 to −0.44) as measured by actigraphy; and a reduction in feelings of fatigue (−2.27, 95% CI = −4.42 to −0.13) (Table 2 and Table 3). Using the two treatment outcome categories,19 the NNT was four (95% CI = 2.0 to 19.0). There were no statistically significant differences between the groups in change in sleep efficiency (sleep diary), in the other sleep parameters, perceived sleepiness, depression, anxiety, or blood pressure (Table 3 and Table 4). However, heart rate reduced significantly more in the control than in the intervention group (P = 0.02), although baseline heart rate was higher in the control group. Similar results to the main analysis were obtained for the primary outcome measures when adjusted for hypnotic use at 6 months and with a complete cases analysis for PSQI and ISI. By 6 month follow-up, two participants in the sleep restriction group and 10 in the control group reported using hypnotic medication at least 3 times a week.
Table 2.
Pittsburgh Sleep Quality Index and Insomnia Severity Index for simplified sleep restriction versus control at baseline, 3 months, and 6 months. Data presented as mean (SD)
| Primary outcomes | Intervention (simplified sleep restriction) | Control | Difference between the groups (95% CI), P-valueb | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline scorean= 46 | 3-month scorean= 45 | 6-month scorean= 43 | Baseline scorean= 51 | 3-month scorean= 49 | 6-month scorean= 50 | ||
| Pittsburgh Sleep Quality Index25 | 10.43 (3.08) | 6.24 (3.11) | 6.49 (3.52) | 10.29 (3.02) | 8.71 (3.32) | 8.10 (3.49) | −2.14 (−3.15 to −1.13) <0.0001 |
| Insomnia Severity Index26 | 14.76 (3.85) | 8.47 (4.45) | 8.28 (4.41) | 14.51 (3.36) | 11.51 (3.94) | 10.50 (3.72) | −2.50 (−3.97 to −1.03) 0.001 |
Statistically significant results are in bold.
Observed (raw) data.
Mixed model for repeated measures adjusted for age, sex, and baseline insomnia severity (using ISI score).
Table 3.
Sleep results for simplified sleep restriction versus control at baseline and 6 months. Data presented as mean (SD)
| Intervention (Simplified sleep restriction) | Control | Difference between the groups,b,d (95% CI) P-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline scorean= 46 | 6-month scorean= 43 | Mean change (95% CI)b,c | Baseline scorean= 51 | 6-month scorean= 50 | Mean change (95% CI)b,c | ||
| Primary outcomes | |||||||
|
| |||||||
| Sleep efficiency % (sleep diary) | 73.32 (11.80) | 80.46 (10.66)e | 6.72 (3.90 to 9.54) | 74.15 (11.05) | 78.24 (10.52)f | 4.28 (−6.70 to 1.86) | 2.44 (−0.96 to 5.84) 0.200 |
|
| |||||||
| Sleep efficiency % (actigraphy) | 82.68 (4.66) | 83.74 (4.94)g | 1.75 (0.42 to 3.09) | 82.37 (4.67) | 81.96 (5.38)f | −0.46 (−1.58 to 0.65) | 2.22 (0.65 to 3.79) 0.006 |
|
| |||||||
| Secondary outcomes | |||||||
|
| |||||||
| Epworth Sleepiness Scale | 5.17 (3.70) | 3.70 (2.93) | −1.62 (−2.72 to −0.53) | 6.45 (3.29) | 4.86 (3.28) | −1.55 (−2.50 to −0.61) | −0.07 (−1.40 to 1.27) 0.900 |
|
| |||||||
| Flinders Fatigue Scale | 12.74 (6.83) | 7.30 (5.49) | −5.39 (−7.16 to −3.63) | 12.53 (5.11) | 9.08 (4.96) | −3.12 (−4.65 to −1.59) | −2.27 (−4.42 to −0.13) 0.040 |
|
| |||||||
| Sleep onset latency, minutes | |||||||
| Sleep diary | 31.50 (23.00) | 22.77 (19.60) | −6.29 (−12.75 to 0.16) | 29.55 (22.48) | 25.21 (19.38)e | −2.84 (−8.38 to 2.70) | −3.45 (−11.23 to 4.32) 0.400 |
| Actigraphy | 16.10 (14.47) | 13.37(7.91)f | −5.08 (−9.90 to −0.26) | 14.66 (12.42) | 15.61 (12.74)e | 1.06 (−2.97 to 5.08) | −6.13 (−11.82 to −0.44) 0.040 |
|
| |||||||
| Wake after sleep onset, minutes | |||||||
| Sleep diary | 44.82 (28.92) | 29.47 (31.71) | −13.37 (−22.69 to −4.05) | 47.84 (30.14) | 40.02 (27.00)e | −7.61 (−15.60 to 0.39) | −5.76 (−17.00 to 5.47) 0.300 |
| Actigraphy | 47.59 (13.88) | 44.33 (15.44)f | −4.54 (−8.99 to −0.08) | 51.81(19.51) | 51.59 (17.50)e | −1.46 (−5.18 to 2.27) | −3.08 (−8.35 to 2.18) 0.200 |
|
| |||||||
| Total sleep time, minutes | |||||||
| Sleep diary | 369.59 (65.27) | 397.30 (63.29) | 23.71 (9.51 to 37.90) | 376.93 (61.08) | 398.17 (64.91)e | 18.99 (6.81 to 31.67) | 4.72 (−12.39 to 21.83) 0.600 |
| Actigraphy | 415.68 (34.17) | 407.20 (36.92)f | −7.00 (−15.94 to 1.95) | 418.36 (39.36) | 415.05 (38.45)e | −7.10 (−14.58 to 0.38) | 0.10 (−10.47 to 10.67) 0.990 |
Statistically significant results are in bold.
Observed (raw) data.
Multiple linear regression adjusted for age, sex, and baseline insomnia severity (using Insomnia Severity Index score26).
Least square mean for change from baseline to 6-month score.
Least square mean difference for change from baseline to 6-month score between sleep restriction and control group.
n = 43.
n = 49.
n = 42.
Table 4.
Mental health and physiological outcomes for simplified sleep restriction versus control at baseline and 6 months. Data presented as mean (SD)
| Simplified sleep restriction | Control | Difference between the groupsb,d,e (95% CI) P-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline score,an= 46 | 6-month score,an= 43 | Mean change (95% CI)b,c,d | Baseline score,an= 51 | 6-month score,an= 50 | Mean change (95% CI)b,c,d | ||
| Mental health outcomes | |||||||
| PHQ-9 depression score | 4.98 (2.76) | 2.93 (2.39) | 2.19 (−3.21, −1.17) | 5.31 (2.80) | 3.62 (2.69) | −1.66 (−2.54 to −0.78) | −0.53 (−1.77 to 0.71) 0.40 |
| GAD-7 anxiety score | 3.33 (3.06) | 1.47 (1.88) | −1.76 (−2.59 to −0.92) | 3.41 (2.45) | 2.56 (2.35) | −0.84 (−1.56 to −0.12) | −0.92 (−1.93 to 0.10) 0.08 |
| Physiological measures | |||||||
| Systolic blood pressure, mmHg | 122.24 (2.06) | 121.21 (2.15) | −1.33 (−4.28 to 1.63) | 123.88 (1.87)f | 125.06 (1.97)g | 0.25 (−3.48 to 3.98)h | −1.58 (−6.34 to 3.19) 0.50 |
| Diastolic blood pressure, mmHg | 75.52 (1.53) | 74.51 (1.42) | −1.30 (−3.29 to 0.68) | 75.62 (1.27) | 74.92 (1.48)g | −1.46 (−3.97 to 1.05)h | 0.16 (−3.05 to 3.37) 0.90 |
| Heart rate, beats per minute | 68.91 (1.45) | 69.47 (1.57) | 0.72 (−1.98 to 3.43) | 73.29 (1.52) | 69.61 (1.37)g | −3.82 (−6.45 to−1.19)g | 4.54 (0.81 to 8.27) 0.02 |
Statistically significant results are in bold.
Observed (raw) data.
Multiple linear regression adjusted for age, sex, and baseline insomnia severity (using Insomnia Severity Index score26).
Least square mean for change from baseline to 6-month score.
Differences in physiological measures calculated from t-test procedure in SAS.
Least square mean difference for change from baseline to 6-month score between sleep restriction and control group.
n = 50 due to machine error.
n = 49.
n = 48. PHQ-9 = Patient Health Questionnaire-9. GAD-7 = Generalized Anxiety Disorders-7.34
Adverse events
No adverse events were recorded in the SSR group in the initial 2 weeks and there were no obvious differences between groups over time. Statistical testing was not performed because of the small number of events (Table 5).
Table 5.
Adverse events experienced by participants
| Adverse event n(%) | Baselinea | Week 3b | 6 monthsc | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Sleep restriction n= 46 | Control n= 51 | Sleep restriction n= 45 | Control n= 51 | Sleep restriction n= 43 | Control n= 48 | |
| Motor vehicle accident | 3 (7) | 4 (8) | 0 | 1 (2) | 2 (5) | 5 (10) |
|
| ||||||
| Injury | ||||||
| Medical attention | 9 (20) | 5 (10) | 0 | 1 (2) | 4 (9) | 6 (13) |
| No medical attention | 7 (15) | 5 (10) | 0 | 2 (4) | 2 (5) | 8 (17) |
|
| ||||||
| Worsening angina | 0 | 0 | 0 | 1 (2) | 0 | 0 |
|
| ||||||
| Myocardial infarction | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Stroke | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Hospitalisation | 3d(7) | 1e(2) | 0 | 0 | 3f(7) | 3g(6) |
|
| ||||||
| Sleepiness | ||||||
| Driving | 1(2) | 3 (6) | 1 (2) | 0 | 0 | 2 (4) |
| Childcare | 0 | 3 (6) | 1 (2) | 0 | 0 | 0 |
Baseline questionnaire asks about occurrence of adverse events over preceding 6 months.
Week-3 questionnaire asks about sleepiness in the preceding 2 weeks (that is, from time of treatment initiation).
6-month questionnaires asks about sleepiness in the preceding 6 months (that is, the duration of the trial).
Panic attack, faint, pacemaker insertion.
MRI, deafness, and loss of balance.
Finger surgery, knee replacement, gallstones.
Broken arm, ankle surgery, pneumonia.
DISCUSSION
Summary
The simplified sleep restriction (SSR) intervention delivered over two brief visits by a GP was effective in improving primary insomnia over 6 months, compared with modified sleep hygiene advice alone. There were improvements in patients’ perceptions of sleep quality and fatigue, and objectively measured sleep efficiency.
The difference in change of the PSQI score between participants in the SSR and control groups over 6 months was statistically significant, with the intervention group improving by an average of 3.94 PSQI points and 6.48 ISI points. An improvement of 3 PSQI points and 6 ISI points are considered clinically significant improvements in primary insomnia.19,37 The clinical meaningfulness of the results is also demonstrated by 67% (28 out of 42) in the SSR group experiencing insomnia remission or treatment response at 6 months compared with 41% (20 out of 49) in the control group (NNT = 4, 95% CI = 2.0 to 19.0). The adjusted odds of getting an insomnia remission or treatment response for those in the SSR group was 2.7 times more likely (95% CI = 1.12 to 6.48) than for those in the control group.
There were no statistically significant differences between groups for sleep efficiency or sleep-onset latency as measured by sleep diary, or for wake after sleep-onset and total sleep time measured by both actigraphy and sleep diary. The reason for this is not entirely clear. Possibly, measures of global sleep quality such as the PSQI and the ISI are more sensitive to change, or represent a more meaningful reflection of a subjective insomnia complaint than minutes spent asleep or awake as recorded by a sleep diary or actigraphy.
Strengths and limitations
Strengths of this study included recruitment from general practice and delivery of the intervention by a GP. Although the study GP had an interest in insomnia, no specialised training in behavioural sleep medicine was undertaken. Instructions were delivered within the time frame of two slightly extended GP consultations meaning the time and expense for both patient and practitioner may be realistic for primary care. The written sleep prescription and the sleep self-adjustment algorithm were two novel components, which may have enhanced adherence. While standardised delivery of the intervention by one GP ensured good fidelity of the intervention, further research is needed to assess the generalisability of this effect when delivered by a wide range of GPs.
Limitations included a large number of exclusion criteria to define a primary insomnia population. Future trials should consider having fewer exclusion criteria to extend the generalisability of the results. Information regarding socioeconomic status, education, and literacy levels was not collected. This may also limit the generalisability of the results. Most follow-up outcome assessments were not blinded. However, the use of objective measures where possible, countersigning digital data readouts and self-report questionnaires, was used to minimise the risk of assessment bias. As insomnia is a chronic condition, a longer follow-up time would have been informative about the durability of treatment effect. Polysomnography was not used to screen participants; therefore it is possible that those who did not respond to the sleep restriction intervention may have been suffering from obstructive sleep apnoea, although attempts were made to screen for this using interviews and physical examination.
The outcomes used in this trial were those recommended for insomnia trials.28 With multiple outcomes there is a risk of a type 1 error. However, only two primary outcomes were defined, both of which found significant improvement, so type 1 error is unlikely. As sample size calculations were not carried out on the basis of all the outcomes, the trial may have been under-powered to detect small differences as statistically significant in outcomes such as the sleep diary measures.
Comparison with existing literature
The current study found slightly lower treatment response rates than were seen in the pilot trial, which involved volunteers recruited by advertisement, who may have been more motivated than those routinely screened in primary care.
The results are consistent with other research into brief interventions for insomnia. In the study by Edinger and Sampson,20 participants attended two individual 25-minute sessions and were followed up after 3 months. The authors found significant improvements compared with control in awakenings after sleep onset and sleep efficiency measured by sleep diaries, as well as insomnia symptoms measured by the Insomnia Symptom Questionnaire. However, this was a small study (n = 20) and, in contrast to most other insomnia research trials, consisted mostly of males (90%), which may limit the generalisability. In the study by Espie et al21 participants attended five group sessions and were followed up after 6 months. Significant improvements in wake after sleep onset (measured by actigraphy) were found compared with control.
The results found in this trial were also similar (but of smaller magnitude) to those found by Buysse et al,19 who compared sleep restriction therapy plus stimulus control (brief behavioural treatment for insomnia) to an ‘information only’ control; although follow-up was only 6 weeks.
Although not all outcome measures improved significantly, Aikens and Rouse38 suggested that patients may be satisfied by an improved functional outcome even if their sleep is not perfectly normalised by treatment. When viewed from this perspective, the current trial demonstrated that SSR was superior to control on several measures of importance including improved global sleep quality, reduced insomnia severity, and reduction in daytime fatigue.
Implications for research and practice
This trial showed that SSR can produce clinically meaningful improvements in insomnia symptoms at 6 months. It has the potential for primary care delivery (by a GP or nurse) to a significant number of people who have chronic primary insomnia and are seen in general practice. Treating insomnia could improve quality of life and potentially reduce associated morbidity.39 Patients who have an inadequate response could still be referred for specialist assessment if desired. Future research should also examine delivery within the real-world practice setting, and include those with comorbid insomnia. Larger trials could assess whether the use of SSR also reduces the use or need for hypnotic medication.
Acknowledgments
Anisoara Jardim (Department of Anaesthesiology, University of Auckland) conducted blinded actigraphy analysis, Alistair Stewart (Department of Biostatistics and Epidemiology, University of Auckland) generated the randomisation sequence and was the safety assessor, the late Marion Upsdell-Dodd and Ka Soh Eng (research assistants), and Angela Robinson (Department of General Practice and Primary Health Care, administrative support). Finally and most importantly, thank you to the doctors, practice staff, and participants who took part in this study.
Funding
The Health Research Council of New Zealand (reference 08/057), the Royal NZ College of General Practitioners (RNZCGP), the RNZCGP Auckland Faculty Charitable Trust, The School of Medicine Foundation University of Auckland, and the Kate Edger Educational Charitable Trust. Trial registration: anzctr.org.au ACTRN12609000127202.
Ethical approval
The study protocol was approved by the New Zealand Regional ‘Northern X’ Health and Disability Ethics Committee (NTX/08/02/003). All participants provided written informed consent at enrolment.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Ohayon MM, Reynolds CF., III Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD) Sleep Med. 2009;10(9):952–960. doi: 10.1016/j.sleep.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69(6):592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Léger D, Poursain B, Neubauer D, Uchiyama M. An international survey of sleeping problems in the general population. Curr Med Res Opin. 2008;24(1):307–317. doi: 10.1185/030079907x253771. [DOI] [PubMed] [Google Scholar]
- 4.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–453. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 5.Buysse DJ, Angst J, Gamma A, et al. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 8.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45(6):344–350. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Liao D, Bixler EO, et al. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Léger D, Morin CM, Uchiyama M, et al. Chronic insomnia, quality-of-life, and utility scores: comparison with good sleepers in a cross-sectional international survey. Sleep Med. 2012;13(1):43–51. doi: 10.1016/j.sleep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Morin CM, Gaulier B, Barry T, Kowatch RA. Patients’ acceptance of psychological and pharmacological therapies for insomnia. Sleep. 1992;15(4):302–305. doi: 10.1093/sleep/15.4.302. [DOI] [PubMed] [Google Scholar]
- 13.Leger D, Guilleminault C, Dreyfus JP, et al. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res. 2000;9(1):35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 14.Arroll B, Fernando A, 3rd, Falloon K, et al. Prevalence of causes of insomnia in primary care: a cross-sectional study. Br J Gen Pract. 2012 doi: 10.3399/bjgp12X625157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Sleep Disorders Association . International Classification of Sleep Disorders: Diagnostic and coding manual. 2nd edn. Westchester, IL: American Sleep Disorders Association; 2005. [Google Scholar]
- 16.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd edn. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 17.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004) Sleep. 2006;29(11):1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 18.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 21.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30(5):574–584. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 22.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- 23.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 24.Riedel BW. Sleep hygiene. In: Lichstein KL, Morin CM, editors. Treatment of late-life insomnia. Thousand Oaks, CA: Sage Publications, Inc; 2000. pp. 125–146. [Google Scholar]
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 27.Morin CM. Insomnia: psychological assessment and management. New York, NY: The Guilford Press; 1993. [Google Scholar]
- 28.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 29.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 30.Brooks JO, 3rd, Friedman L, Bliwise DL, Yesavage JA. Use of the wrist actigraph to study insomnia in older adults. Sleep. 1993;16(2):151–155. doi: 10.1093/sleep/16.2.151. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Grasidar M, Lack L, Richards H, et al. The Flinders Fatigue Scale: preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med. 2007;3(7):722–728. [PMC free article] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 36.Fernando A, 3rd, Arroll B, Falloon K. A double-blind randomised controlled study of a brief intervention of bedtime restriction for adult patients with primary insomnia. J Prim Health Care. 2013;5(1):5–10. [PubMed] [Google Scholar]
- 37.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487–2494. doi: 10.1185/03007990903167415. [DOI] [PubMed] [Google Scholar]
- 38.Aikens JE, Rouse ME. Help-seeking for insomnia among adult patients in primary care. J Am Board Fam Pract. 2005;18(4):257–261. doi: 10.3122/jabfm.18.4.257. [DOI] [PubMed] [Google Scholar]
- 39.Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4):227–247. doi: 10.1207/S15402010BSM0104_5. [DOI] [PubMed] [Google Scholar]