Abstract
Background
The effectiveness of diclofenac versus paracetamol in primary care patients with pain caused by knee osteoarthritis is unclear.
Aim
To assess the effectiveness of diclofenac compared with paracetamol over a period of 2, 4, and 12 weeks in patients with knee osteoarthritis.
Design and setting
Randomised controlled trial in general practice.
Method
There were 104 patients included in the study, they were aged ≥45 years consulting their GP with knee pain caused by knee osteoarthritis. Patients were randomly allocated to diclofenac (n = 52) or paracetamol (n = 52) for at least 2 weeks. Primary outcomes were daily knee pain severity, and knee pain and function measured with the Knee Injury and Osteoarthritis Outcome Score (KOOS).
Results
Over a period of 2- and 4-weeks follow-up, no significant difference in daily knee pain was found between the patient groups: estimated differences of 0.5 (95% CI = −0.2 to 1.3) and −0.2 (95% CI = −1.0 to 0.7), respectively. Over the 12-weeks follow-up, no significant differences were found between both groups for KOOS pain: estimated difference of −2.8 (95% CI = −10.7 to 5.1) and KOOS function of −2.7 (−10.6 to 5.0).
Conclusion
Over a period of 2- and 4-weeks follow-up no significant difference in daily measured knee pain severity was found between primary care patients with knee osteoarthritis taking paracetamol or diclofenac. Also, over a period of 12-weeks follow-up no significant differences were found regarding KOOS pain and KOOS function between both groups. Patients more frequently reported minor adverse events after taking diclofenac (64%) than paracetamol (46%).
Keywords: diclofenac, general practice, knee osteoarthritis, paracetamol, randomised controlled trial
INTRODUCTION
Osteoarthritis (OA) is the most frequent joint disease, which causes pain and functional disability.1 Worldwide estimates are that 9.6% of males and 18.0% of females aged ≥60 years have symptomatic OA.2 Clinical practice guidelines regarding knee OA recommend paracetamol as the medication of first choice when pain medication for OA is needed, because of its better safety profile.3–5 If paracetamol does not provide sufficient pain relief, then non-steroidal anti-inflammatory drugs (NSAIDs) may be considered.6 However, a large cohort study showed that most patients preferred NSAIDs to paracetamol.7 In addition, Ausiello and Stafford8 reported that physicians prescribed paracetamol in 10% of visits relating to OA, while NSAIDs were prescribed in over 30% of the patients. Thus, it seems that patients, and perhaps also GPs, do not consider paracetamol as the first choice medication in OA, because of the believed superior effectiveness of NSAIDs above paracetamol. Summarised evidence for managing OA showed higher effect sizes for NSAIDs than for paracetamol.9 Moreover, paracetamol may not be perceived as a medication, because of its wide availability.10
Most previous trials have compared NSAIDs with paracetamol in highly selected patients already using NSAIDs and needing a wash-out period prior to randomisation.11–13 One study showed that prior use of NSAIDs predicted a better response in favour of NSAIDs versus paracetamol.12 In addition, previous studies mostly included patients recruited in a secondary care setting.12,14,15 Therefore, the aim of this study was to assess the effectiveness of diclofenac compared with paracetamol over a period of 2, 4, and 12 weeks in patients with knee OA in general practice.
METHOD
Study design
A pragmatic open-labelled randomised controlled trial was performed in general practice. Detailed information of the study protocol is published elsewhere.16
Setting and patients
GPs in the south west of the Netherlands recruited patients who consulted them with a new episode of non-traumatic knee pain. A new episode of knee pain was defined as pain presented to the GP for the first time and the patient had not consulted their GP with these symptoms in the previous 3 months.17 Patients were eligible for inclusion if they met all of the following criteria: age ≥45 years; consulted their GP with a new episode of non-traumatic knee pain; knee pain severity of 2 or more (on a 0–10 scale); and fulfilled the clinical criteria of the American College of Rheumatology for knee OA.18 Patients were excluded if they had a contraindication for NSAIDs and/or paracetamol use (for example, myocardial infarction or stroke, or oral use of corticosteroids), an arthroplasty or osteotomy of the knee on the contralateral or unilateral side, if they already took NSAIDs or paracetamol at doses similar to or higher than the study dose, and if they had had surgery, or major trauma of the affected joint.
How this fits in
The effectiveness of diclofenac compared with paracetamol in primary care patients with pain resulting from knee osteoarthritis is not known. In this study, no significant differences in knee pain severity and knee function over a period of 2, 4, and 12-weeks follow-up were found between patients taking diclofenac or paracetamol. These findings support the currently available clinical guidelines recommending paracetamol as the first choice pain medication for knee OA.
Even after extending the planned inclusion period of 18 months by an additional 12 months, only 104 eligible patients could be included in the study. To reinforce the inclusion, the GPs’ electronic medical records were searched for eligible patients. These more prevalent patients were eligible if they fulfilled the study inclusion criteria and had visited their GP in the last 2 months for a new episode of knee complaints.
Randomisation and intervention
Eligible patients were randomly assigned to receive either diclofenac (maximum daily intake of 3 × 50 mg) or paracetamol (maximum daily intake of 3 × 1000 mg) for a period of 2 weeks and, if required, an additional 1–2 weeks.3 During the treatment period, usual care was provided by the GP to all patients. A computer-generated randomisation list was used with random blocks of 4, 6, or 8 made by an independent researcher. The researcher who assigned the patients to diclofenac or paracetamol was blinded for allocation sequence by using sealed envelopes.
Outcomes
Primary outcomes
Primary outcomes were:
Daily knee pain severity assessed with an 11-point numerical rating scale (NRS) in a diary over a period of 2 and 4 weeks, with a score range of 0–10 (0 = no pain; 10 = worst pain ever).
Knee pain and function measured with the Knee Injury and Osteoarthritis Outcome Score (KOOS), with a score range of 0–100 (0 = no pain/function; 100 = worst pain/function ever) assessed at 3-, 6-, 9-, and 12-weeks follow-up.19
Secondary outcomes
All secondary outcomes were assessed using questionnaires at 3-, 6-, 9-, and 12-weeks follow-up. Secondary outcomes were:
patients’ perceived severity of knee pain averaged over the last week using a NRS of 0–10 (0 = no pain; 10 = worst pain ever);
quality of life assessed with the EuroQol instrument EQ-5D;20
compliance with medication intake was measured with the diary. Compliance was dichotomised into compliant (patients who took the maximum daily dose of the allocated medication for ≥10 consecutive days during the first 2 weeks) and not compliant (patients who took less than the maximum daily dose of the allocated medication for ≥5 days during the first 2 weeks);
co-interventions and co-medication or change in medication doses were assessed with the diary. Patients reported daily their used medication and doses;
adverse events (patients self-administered).
Radiographs
At baseline, a weight-bearing antero-posterior radiograph of the affected knee was made. Two independent readers scored the radiographs using the Kellgren and Lawrence (K&L) classification criteria (grades 0–4) (agreement between readers for cut-off K&L ≥2; κ = 0.6).21
Sample size
To detect a clinically relevant and a statistically significant difference of 10 points (diclofenac users: mean 77.7; SD 19.0) on the KOOS pain score between the two treatment groups (diclofenac versus paracetamol) during the 12-weeks follow-up, 73 patients needed to be included per group (power of 80%, α = 0.05, one-sided testing). One-sided testing was used because no inferiority of diclofenac versus paracetamol was expected. A total of 154 patients (2 × 77) was needed to account for an expected 5% loss to follow-up.
Statistical analysis
All analyses were performed according to the intention-to-treat principle; analysing all patients in the treatment group to which they were randomly allocated. Descriptive data of baseline characteristics were presented for both groups to check comparability. To account for the correlation between measurements of the primary outcomes within the same person, generalised estimating equations (GEE) analyses were used to estimate the model using the compound symmetry working correlation structure over a period of 2 and 4 weeks for daily knee pain severity and 12-weeks follow-up for the KOOS pain and KOOS function.
Because of the non-linear relationship between the scores of knee pain severity every day via a diary, a broken-stick model was used (that is, a linear spline function) with interior knots22 at days 3 and 7 for the period of 2-weeks follow-up, and at days 3, 7, 14, and 21 regarding diary data for the period of 4-weeks follow-up. The broken stick model is a form of a spline that represents a function that is linear in each interval but with an inclination that may change constantly.22 GEE analyses were also used for the continuous secondary outcomes: severity of knee pain averaged over the last week and quality of life.
Effect sizes were calculated using the mean differences divided by the pooled SD at baseline of both groups.
Effect sizes of 0.2–0.5 are considered to be small, 0.5–0.8 is a moderate effect, and a score of ≥0.8 indicates a large treatment effect.23 Post-hoc analyses were adjusted for age, sex, BMI, and baseline KOOS pain. Analyses were performed using SPSS software (version 20) and SAS (version 9.2).
RESULTS
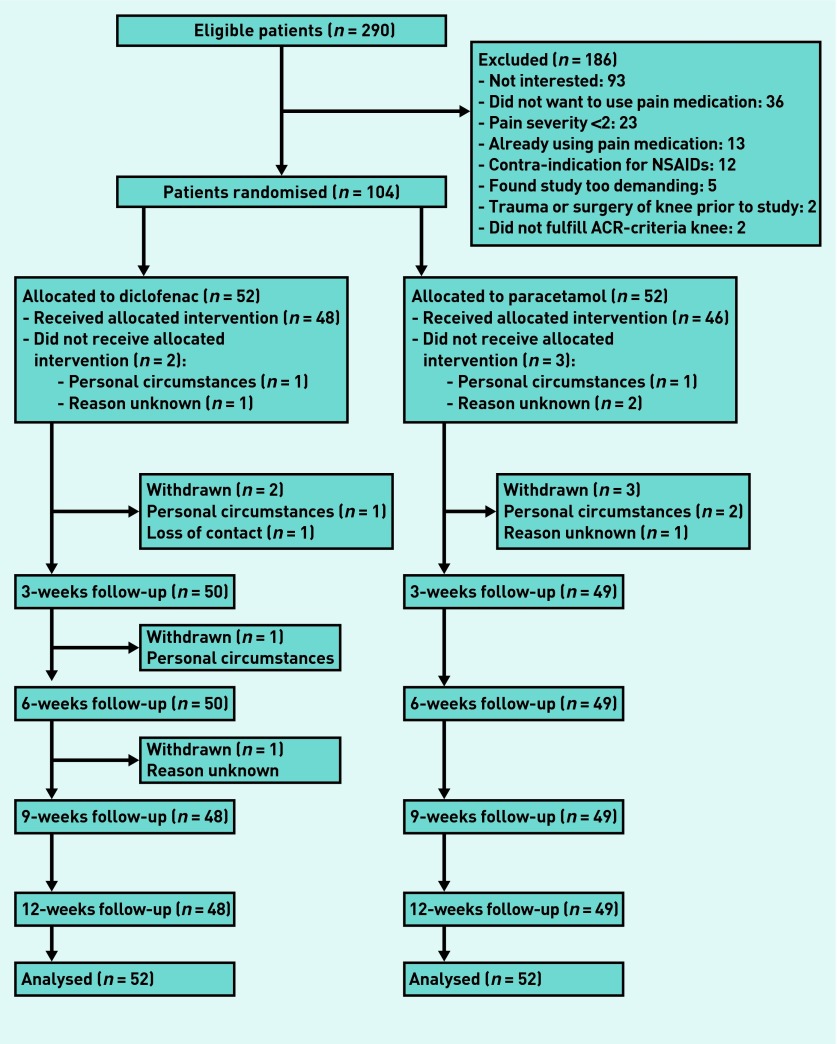
Between April 2009 and September 2011, 104 patients were included and randomised (52 to paracetamol, 52 to diclofenac). Figure 1 presents the flow chart of the present study. Of the 104 randomised patients, 97 (93.3%) participated in the 12-week follow-up assessment. Three patients (5.8%) in the paracetamol group and four patients in the diclofenac group (7.7%) withdrew from the study. Compared with the total study population, patients who withdrew were older and had a lower BMI but had comparable knee pain severity at baseline. Table 1 presents the baseline characteristics of the randomised patients.
Figure 1.
Flowchart of the study. ACR = American College of Rheumatology. NSAIDs = non-steroidal anti-inflammatory drugs.
Table 1.
Baseline patient characteristics (n = 104)
| Paracetamol (n= 52) | Diclofenac (n= 52) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | % | Mean (SD) | n | % | Mean (SD) | |
| Age, years | 64.0 (9.0) | 63.9 (9.2) | ||||
| Female | 32 | 61.5 | 33 | 63.5 | ||
| Low education level | 38 | 77.6 | 43 | 82.7 | ||
| Paid job | 15 | 30.6 | 19 | 36.5 | ||
| Body mass index, kg/m2 | 27.7 (4.0) | 28.6 (4.8) | ||||
|
| ||||||
| Duration of symptoms | ||||||
| <3 weeks | 9 | 18.3 | 10 | 19.2 | ||
| 3 weeks–3 months | 8 | 16.3 | 17 | 32.7 | ||
| ≥3 months | 32 | 65.3 | 25 | 48.1 | ||
|
| ||||||
| Side of osteoarthritis | ||||||
| Left | 17 | 34.7 | 16 | 30.8 | ||
| Right | 21 | 42.9 | 18 | 34.6 | ||
| Bilateral | 11 | 22.4 | 18 | 34.6 | ||
|
| ||||||
| Kellgren and Lawrence score | ||||||
| Grade 0 or 1 | 33 | 63.5 | 33 | 63.5 | ||
| Grade ≥2 | 16 | 30.8 | 19 | 36.5 | ||
| Missing | 3 | 5.7 | 0 | 0 | ||
|
| ||||||
| Clinical ACR criteria | ||||||
| Age >50 years | 50 | 96.2 | 46 | 88.5 | ||
| Stiffness <30 minutes | 36 | 69.2 | 32 | 61.5 | ||
| Crepitus | 34 | 65.4 | 31 | 59.6 | ||
| Bony tenderness | 46 | 88.5 | 42 | 80.8 | ||
| Bony enlargement | 29 | 55.8 | 22 | 42.3 | ||
| No palpable warmth | 45 | 86.5 | 48 | 92.3 | ||
|
| ||||||
| Knee pain severity (NRS, 0–10)a | 5.1 (1.8) | 5.4 (2.1) | ||||
|
| ||||||
| KOOS (0–100)a | ||||||
| Pain | 47.9 (16.8) | 50.7 (16.4) | ||||
| ADL (function) | 42.7 (18.4) | 46.0 (17.7) | ||||
| Symptoms | 51.8 (12.8) | 52.4 (13.0) | ||||
| Sport and Recreation | 75.3 (19.1) | 69.3 (26.1) | ||||
| QoL | 55.6 (11.1) | 56.0 (13.8) | ||||
|
| ||||||
| QoL, EQ-5D (0 to 1)b | 0.74 (0.16) | 0.67 (0.25) | ||||
A higher score is worse.
A higher score is better. ACR = American College of Rheumatology. ADL = Activities of daily living. KOOS = Knee steoarthritis Outcome Score. NRS = numerical rating scale. QoL = Quality of life. SD = standard deviation.
Of the patients in the paracetamol group, 65.3% (n = 32) had knee symptoms lasting 3 months compared with 48.1% (n = 25) of the patients in the diclofenac group. Patients in the diclofenac group had slightly higher knee pain severity (mean of 5.4 versus a mean of 5.1 in the paracetamol group) and somewhat more often had indication of knee OA on radiology (K&L score of ≥2; 36.2% versus 30.8% in the paracetamol group).
Of the 104 patients, 33 (diclofenac: n = 16; paracetamol: n = 17) were included via the medical record searches. No significant differences between incident and prevalent patients were found for knee pain and function at baseline. Not surprisingly, however, prevalent cases had a longer duration of symptoms than incident cases.
Primary outcomes
Daily knee pain severity
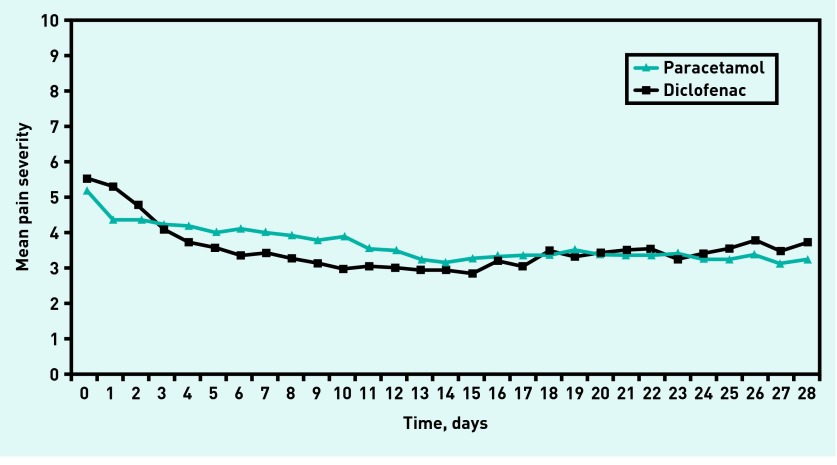
The course of the daily knee pain severity over the first 4 weeks is presented in Figure 2. Over a period of 2-weeks follow-up, no significant difference in the course of daily knee pain severity was found between the paracetamol and diclofenac groups; adjusted estimated difference of 0.5 (95% CI = −0.2 to 1.3). Also, over a period of 4-weeks follow-up no significant difference was found between both groups; adjusted estimated difference of −0.2 (95% CI = −1.0 to 0.7) (Table 2).
Figure 2.
Mean daily measured knee pain severity of the paracetamol and diclofenac group over a period of 4-weeks follow-up. NRS = Numeric Rating Scale.
Table 2.
Primary outcomes: daily knee pain severity measured with a diary and knee pain and function measured with the Knee Injury and Osteoarthritis Outcome Score
| Paracetamol (n= 52) Mean (SD) | Diclofenac (n= 52) Mean (SD) | Mean differencesa (95% CI) | Effect size | |
|---|---|---|---|---|
| Course of daily knee pain during: | ||||
| 2 weeks | 3.5 (2.1) | 3.0 (2.1) | 0.5 (−0.2 to 1.3) | 0.35 |
| 4 weeks | 3.3 (2.1) | 3.5 (2.1) | −0.2 (−1.0 to 0.7) | −0.03 |
|
| ||||
| KOOS painb | ||||
| 3 weeks | 38.3 (19.5) | 39.6 (20.7) | −1.6 (−9.5 to 6.3) | 0.04 |
| 6 weeks | 35.0 (19.4) | 40.2 (22.1) | −5.6 (−13.7 to 2.5) | −0.16 |
| 9 weeks | 34.8 (18.8) | 38.5 (20.7) | −3.5 (−11.3 to 4.4) | −0.03 |
| 12 weeks | 34.8 (19.4) | 37.4 (21.0) | −2.8 (−10.7 to 5.1) | 0.01 |
|
| ||||
| KOOS functionb | ||||
| 3 weeks | 33.9 (19.3) | 34.2 (21.7) | −0.6 (−8.7 to 7.6) | 0.07 |
| 6 weeks | 31.9 (19.9) | 33.1 (20.6) | −1.8 (−9.7 to 6.2) | 0.04 |
| 9 weeks | 28.3 (19.7) | 33.1 (20.3) | −4.6 (−12.6 to 3.3) | −0.10 |
| 12 weeks | 28.4 (19.5) | 31.4 (20.2) | −2.7 (−10.6 to 5.0) | −0.02 |
Results of the generalised estimating equations analyses.
A higher score is more pain/worse function.
KOOS= Knee Injury and Osteoarthritis Outcome Score.
KOOS pain and function
The KOOS knee pain and KOOS function scores assessed over a period of 12-weeks follow-up are presented in Table 2. Over a period of 12-weeks follow-up no significant or clinically relevant significant differences were found between groups. Over the 12-weeks follow-up the adjusted estimated difference for KOOS knee pain was −2.8 (95% CI = −10.7 to 5.1) on a 0–100 scale. For KOOS function this was −2.7 (95% CI = −10.6 to 5.0) over the 12-weeks follow-up.
Secondary outcomes
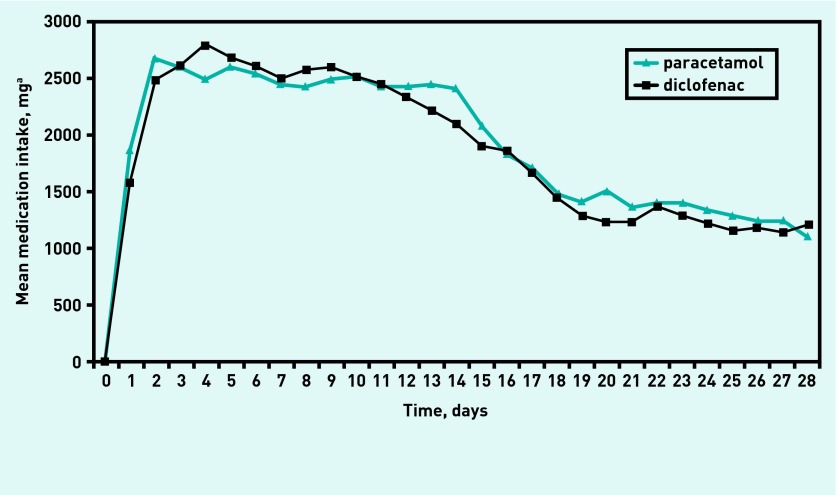
No statistically significant or clinical relevant significant differences were found between paracetamol and diclofenac use regarding severity of knee pain averaged over the last week and quality of life over a period of 12-weeks follow-up (Table 3). Regarding compliance with the medication, over a period of 2-weeks follow-up, 44 patients (85%) were compliant with diclofenac treatment compared with 45 patients (87%) in the paracetamol group (Figure 3).
Table 3.
Secondary outcomes measured over a period of 12-weeks follow-up
| Paracetamol (n= 52) Mean (SD) | Diclofenac (n= 52) Mean (SD) | Mean differences (95% CI)a | Effect size | |
|---|---|---|---|---|
| Knee pain severityb | ||||
| 3 weeks | 3.4 (2.5) | 3.4 (2.4) | −0.1 (−1.1 to 0.9) | 0.09 |
| 6 weeks | 3.0 (2.4) | 3.4 (2.6) | −0.4 (−1.4 to 0.6) | −0.03 |
| 9 weeks | 2.9 (2.2) | 3.1 (2.4) | −0.1 (−1.1 to 0.8) | 0.12 |
| 12 weeks | 2.8 (2.3) | 2.9 (2.4) | −0.2 (−1.1 to 0.7) | 0.07 |
|
| ||||
| Quality of lifec | ||||
| 3 weeks | 0.8 (0.2) | 0.8 (0.2) | 0.02 (−0.1 to 0.1) | 0.09 |
| 6 weeks | 0.8 (0.1) | 0.8 (0.2) | 0.1 (0.01 to 0.1) | 0.41 |
| 9 weeks | 0.8 (0.1) | 0.8 (0.2) | 0.1 (0.01 to 0.1) | 0.40 |
| 12 weeks | 0.8 (0.1) | 0.8 (0.2) | 0.0 (−0.05 to 0.1) | 0.09 |
Results of the generalised estimating equations analyses.
A higher score is more pain (knee pain severity averaged over last week measured with 0–10 numeric rating scale).
A higher score is better quality of life (measured with EQ-5D).
Figure 3.
Mean medication intake of the paracetamol and diclofenac group over a period of 4-weeks follow-up. aThe daily dose of diclofenac was converted (for example, total use of 150 mg of diclofenac per day was converted to 3000 mg).
Adverse events
Table 4 presents the number of self-reported adverse events at 3-weeks follow-up, as reported in the 3-weekly questionnaire. Patients in the diclofenac group more often reported gastrointestinal (36.5% versus 13.5%), respiratory (34.6% versus 15.4%), skin (26.9% versus 11.5%), and/or psychiatric (38.5% versus 28.8%) reactions.
Table 4.
Adverse events reported by patients at 3-weeks follow-up
| Paracetamol (n= 52) | Diclofenac (n= 52) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Patients reporting one or more adverse events | 24 | 46.2 | 33 | 63.5 |
| Psychiatric | 15 | 28.8 | 20 | 38.5 |
| Respiratory, thoracic, and connective tissue | 8 | 15.4 | 18 | 34.6 |
| Gastrointestinal | 7 | 13.5 | 19 | 36.5 |
| Nervous system | 13 | 25 | 14 | 26.9 |
| Skin and subcutaneous tissue | 6 | 11.5 | 14 | 26.9 |
| General | 6 | 11.5 | 10 | 19.2 |
| Cardiovascular | 5 | 9.6 | 8 | 15.4 |
| Immune system | 0 | 0 | 2 | 3.8 |
| Musculoskeletal and connective tissue | 0 | 0 | 1 | 1.9 |
| Organ of vestibular system | 0 | 0 | 1 | 1.9 |
Post-hoc adjusted analyses
The results of the post-hoc adjusted GEE analyses on the primary outcomes showed no significant differences in the course of daily knee pain severity between the paracetamol and diclofenac groups over a period of 2- and 4-weeks follow-up: 0.7 (95% CI = −0.03 to 1.4) and −0.1 (95% CI = −0.8 to 0.7), respectively. Also, no significant differences between paracetamol and diclofenac in KOOS pain and KOOS function over a period of 12-weeks follow-up were found: 0.2 (95% CI = −6.5 to 6.9) and −0.3 (95% CI = −7.0 to 6.5), respectively.
DISCUSSION
Summary
No significant differences were found in daily knee pain severity over a period of 2- and 4-weeks follow-up between patients taking paracetamol and those taking diclofenac. Also, over a period of 12-weeks follow-up no significant differences were found regarding KOOS pain and KOOS function between patients in both groups. Patients in the diclofenac group reported more adverse events.
Strengths and limitations
A limitation of the trial was the open-labelled design in which patients and GPs were aware of the treatment assignments. This may influence patients’ response to the outcome(s) and introduce bias. For example, a patient’s preference for the medication to which he/she was assigned to could lead to better outcomes (detection bias) regardless of any treatment efficacy. This is also the case the other way round. Also, performance bias could have occurred as the treating GP was aware of the assigned medication.24
The pragmatic open-labelled design made the generalisability (external validity) high, however; the results reflect daily practice better than other studies that used flare designs, wash-out periods, and blinding of patients. Most patients included in the present study had not used pain medication for their knee symptoms during the previous 3 months. And those who had used medication prior to the study (diclofenac group: n = 11; paracetamol group: n = 17) did not use it in the same dosage as prescribed in the trial. Other studies often use a wash-out period,11,14 or even need a flare of symptoms after a wash-out period25 prior to randomisation. Use of a flare design might result in higher treatment effects,26 and this might reduce generalisability of the results in daily practice. Although placebo-controlled trials are important as proof of principle, pragmatic trials (open label) are also needed to assess the effectiveness of treatments in daily practice.
Another strength was the measurement of daily knee pain severity and daily medication intake in a diary. This allowed patients’ daily fluctuations in pain severity and medication use to be followed.
The study by Perrot et al27 recommended daily measures in clinical trials because average pain severity over the past week, past 14 days, or past month are strongly influenced by pain intensity on the day of assessment. The current study’s daily knee pain measurements (Appendix 1) show significant differences between groups at days 6–10 in favour of the diclofenac group. The results from the post-hoc adjusted GEE analyses at days 6–10 ranged from 0.8 to 0.9 on the 0–10 scale.
A limitation of this study was the low level of enrolment of eligible patients. The original sample size calculation required 154 patients to be included. However, even after extending the planned inclusion period of 18 months by an additional 12 months, there were only 104 eligible patients.
Comparison with existing literature
Until now, only one previous study was performed solely in primary care.11 Boureau et al.11 performed a multi-centre, double-blinded study assessing pain intensity over 14 days between ibuprofen and paracetamol use in 222 patients who visited their GP pain because of hip and/or knee OA. They found that ibuprofen was more effective than paracetamol (effect size: 0.5) over 14 days. In the current study no significant differences were found in the course of average daily knee pain severity during the first 2 weeks follow-up. However, based on the 95% confidence intervals of the daily knee pain severity (Table 2) over 2-weeks follow-up, which includes 1.0, a statistically and clinically relevant significant difference cannot be excluded. Also for the KOOS pain and KOOS function a clinically relevant significant difference cannot be excluded (10 points on the KOOS) based on the 95% confidence intervals over a period of 12-weeks follow-up (Table 2). Compared with patients in the study of Boureau et al,11 the patient population in the current study was slightly younger, included more males, and patients had less severe knee pain at baseline.
Implications for practice
No significant differences in knee pain severity and knee function over a period of 2, 4, and 12-weeks follow-up were found between patients taking diclofenac or paracetamol in this study. These findings support the currently available clinical guidelines recommending paracetamol as the first choice pain medication for knee OA.
Appendix 1. Daily knee pain severity scores measured with a diary and results of post-hoc adjusted GEE analyses
| Paracetamol (n= 52) Mean (SD) | Diclofenac (n= 52) Mean (SD) | Mean differences (95% CI) | |
|---|---|---|---|
| Knee pain severity on: | |||
| Day 1 | 4.3 (2.0) | 5.2 (2.0) | −0.3 (−1.1 to 0.5) |
| Day 2 | 4.3 (2.0) | 4.7 (2.2) | 0.04 (−0.7 to 0.8) |
| Day 3 | 4.1 (2.1) | 4.0 (2.3) | 0.4 (−0.4 to 1.2) |
| Day 4 | 4.1 (2.0) | 3.7 (2.2) | 0.5 (−0.2 to 1.3) |
| Day 5 | 4.0 (2.0) | 3.5 (2.3) | 0.7 (−0.1 to 1.4) |
| Day 6 | 4.1 (1.8) | 3.3 (2.3) | 0.8 (0.1 to 1.5) |
| Day 7 | 4.0 (2.0) | 3.4 (2.2) | 0.9 (0.2 to 1.7) |
| Day 8 | 3.9 (1.9) | 3.2 (2.1) | 0.9 (0.1 to 1.6) |
| Day 9 | 3.7 (2.0) | 3.1 (2.2) | 0.8 (0.1 to 1.5) |
| Day 10 | 3.8 (2.2) | 2.9 (2.0) | 0.8 (0.04 to 1.5) |
| Day 11 | 3.5 (2.2) | 3.0 (2.0) | 0.7 (−0.03 to 1.4) |
| Day 12 | 3.5 (2.0) | 3.0 (2.2) | 0.6 (−0.1 to 1.4) |
| Day 13 | 3.2 (2.0) | 2.9 (2.2) | 0.6 (−0.2 to 1.3) |
| Day 14 | 3.1 (2.1) | 2.9 (2.2) | 0.5 (−0.3 to 1.3) |
| Day 15 | 3.2 (2.1) | 2.8 (2.1) | 0.4 (−0.3 to 1.2) |
| Day 16 | 3.3 (2.2) | 3.2 (2.3) | 0.4 (−0.4 to 1.2) |
| Day 17 | 3.3 (2.2) | 3.0 (2.2) | 0.3 (−0.5 to 1.1) |
| Day 18 | 3.4 (2.3) | 3.5 (2.2) | 0.3 (−0.5 to 1.0) |
| Day 19 | 3.5 (2.1) | 3.3 (2.3) | 0.2 (−0.6 to 1.0) |
| Day 20 | 3.3 (2.1) | 3.4 (2.2) | 0.1 (−0.7 to 1.0) |
| Day 21 | 3.3 (2.3) | 3.5 (2.4) | 0.1 (−0.8 to 0.9) |
| Day 22 | 3.3 (2.3) | 3.5 (2.3) | 0.03 (−0.8 to 0.9) |
| Day 23 | 3.4 (2.4) | 3.2 (2.3) | 0.003 (−0.8 to 0.8) |
| Day 24 | 3.3 (2.1) | 3.4 (2.1) | −0.03 (−0.8 to 0.7) |
| Day 25 | 3.2 (2.0) | 3.5 (2.0) | −0.1 (−0.8 to 0.7) |
| Day 26 | 3.4 (1.9) | 3.7 (2.0) | −0.1 (−0.9 to 0.7) |
| Day 27 | 3.1 (1.9) | 3.4 (1.9) | −0.1 (−0.9 to 0.7) |
| Day 28 | 3.2 (2.4) | 3.7 (2.1) | −0.1 (−0.9 to 0.7) |
Bold numbers are statistically significant (P value ≤0.05). GEE = generalised estimating equations.
Funding
This study was supported by the NutsOhra Foundation in Amsterdam, the Netherlands, and partly funded by a programme grant of the Dutch Arthritis Foundation for its centre of excellence ‘Osteoarthritis in primary care’. Trial registration Dutch Trial Registration NTR1485 (www.trialregister.nl).
Ethical approval
The medical ethics committee of the Erasmus MC University Medical Center Rotterdam, the Netherlands approved the study.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58(2):150–167. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 3.Belo JN, Bierma-Zeinstra SMA, Raaijmakers AJ, van der Wissel F. Dutch general practice guideline ‘Non-traumatic knee disorders in adults’. [In Dutch] Huisarts en Wetenschap. 2008;51(5):229–240. [Google Scholar]
- 4.Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62(12):1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15(9):981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Smink AJ, van den Ende CH, Vliet Vlieland TP, et al. “Beating osteoARThritis”: development of a stepped care strategy to optimize utilization and timing of non-surgical treatment modalities for patients with hip or knee osteoarthritis. Clin Rheumatol. 2011;30(12):1623–1629. doi: 10.1007/s10067-011-1835-x. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F, Zhao S, Lane N. Preference for nonsteroidal antiinflammatory drugs over acetaminophen by rheumatic disease patients: a survey of 1,799 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Arthritis Rheum. 2000;43(2):378–385. doi: 10.1002/1529-0131(200002)43:2<378::AID-ANR18>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Ausiello JC, Stafford RS. Trends in medication use for osteoarthritis treatment. J Rheumatol. 2002;29(5):999–1005. [PubMed] [Google Scholar]
- 9.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(4):476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Barozzi N, Tett SE. Perceived barriers to paracetamol (acetaminophen) prescribing, especially following rofecoxib withdrawal from the market. Clin Rheumatol. 2009;28(5):509–519. doi: 10.1007/s10067-008-1077-8. [DOI] [PubMed] [Google Scholar]
- 11.Boureau F, Schneid H, Zeghari N, et al. The IPSO study: ibuprofen, paracetamol study in osteoarthritis. A randomised comparative clinical study comparing the efficacy and safety of ibuprofen and paracetamol analgesic treatment of osteoarthritis of the knee or hip. Ann Rheum Dis. 2004;63(9):1028–1034. doi: 10.1136/ard.2003.011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Case JP, Baliunas AJ, Block JA. Lack of efficacy of acetaminophen in treating symptomatic knee osteoarthritis: a randomized, double-blind, placebo-controlled comparison trial with diclofenac sodium. Arch Intern Med. 2003;163(2):169–178. doi: 10.1001/archinte.163.2.169. [DOI] [PubMed] [Google Scholar]
- 13.Yelland MJ, Nikles CJ, McNairn N, et al. Celecoxib compared with sustained-release paracetamol for osteoarthritis: a series of n-of-1 trials. Rheumatology (Oxford) 2007;46(1):135–140. doi: 10.1093/rheumatology/kel195. [DOI] [PubMed] [Google Scholar]
- 14.Batlle-Gualda E, Román Ivorra J, Martín-Mola E, et al. Aceclofenac vs paracetamol in the management of symptomatic osteoarthritis of the knee: a double-blind 6-week randomized controlled trial. Osteoarthritis Cartilage. 2007;15(8):900–908. doi: 10.1016/j.joca.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Lequesne M, Fannius J, Reginster JY, et al. Floctafenin versus acetaminophen for pain control in patients with osteoarthritis in the lower limbs. Franco-Belgian Task Force. Rev Rhum Engl Ed. 1997;64(5):327–333. [PubMed] [Google Scholar]
- 16.Verkleij SP, Luijsterburg PA, Koes BW, et al. Effectiveness of diclofenac versus acetaminophen in primary care patients with knee osteoarthritis: [NTR1485], DIPA-trial: design of a randomized clinical trial. BMC Musculoskelet Disord. 2010;11:7. doi: 10.1186/1471-2474-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heintjes EM, Berger MY, Koes BW, Bierma-Zeinstra SM. Knee disorders in primary care: design and patient selection of the HONEUR knee cohort. BMC Musculoskelet Disord. 2005;6:45. doi: 10.1186/1471-2474-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 19.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE., Jr . Regression modeling strategies. New York, NY: Springer-Verlag New York Inc; 2010. [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 24.Boutron I, Moher D, Altman DG, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 25.Schnitzer TJ, Tesser JR, Cooper KM, Altman RD. A 4-week randomized study of acetaminophen extended-release vs rofecoxib in knee osteoarthritis. Osteoarthritis Cartilage. 2009;17(1):1–7. doi: 10.1016/j.joca.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Trijau S, Avouac J, Escalas C, et al. Influence of flare design on symptomatic efficacy of non-steroidal anti-inflammatory drugs in osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage. 2010;18(8):1012–1018. doi: 10.1016/j.joca.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Perrot S, Rozenberg S, Moyse D, et al. Comparison of daily, weekly or monthly pain assessments in hip and knee osteoarthritis. A 29-day prospective study. Joint Bone Spine. 2011;78(5):510–515. doi: 10.1016/j.jbspin.2010.11.009. [DOI] [PubMed] [Google Scholar]