Abstract
Background:
The aim of the present study was to evaluate the efficiency of two irrigating techniques - static and dynamic (passive ultrasonic instrumentation) irrigation in the elimination of bacterial biofilm.
Materials and Methods:
Forty extracted human permanent maxillary central incisors teeth with straight roots and single canals, were randomly allocated to two groups for static irrigation and passive ultrasonic irrigation (PUI). The root canal irrigant used was 2.5 % sodium hypochlorite. The root canals were prepared to tip sizes (20, 40) and tapers (0.04, 0.08). Using system GT instruments (Dentsply Malliefer, Switerzland). The teeth were split longitudinally into two, stained collagen was applied to the canal surfaces and the tooth reassembled for static and PUI. Digital images of the canal surface were taken before and after irrigation with 9, 18, 27 and 37 mL solution. The digital images were analyzed using ImageJ software (National Institute of Health, USA) to quantify residual canal coverage by the stained collagen. The data were analyzed using linear regression models and subjected to statistical analysis.
Results:
The mean percentage of canal surface with residual collagen increased with the coronal level of canal, decrease in apical size and taper of canal preparation and decrease in the volume of the irrigant. There was less residual collagen after PUI compared with static irrigation. The canal surface facing the open side port of the needle had less residual collagen after irrigation than the opposing surface.
Conclusion:
The stained collagen biomolecular film could not be removed completely either by passive ultrasonic instrumentation or static irrigation. The PUI was found to be more effective in the removal of collagen, especially in the apical part of the root canal.
Keywords: Biofilm, Collagen, sodium hypochlorite, irrigation, ultrasonic
Introduction
The primary objective of root canal treatment is debridement to achieve thorough disinfection and obturation of the canal space. It includes the mechanical cleansing by instruments and the use of irrigating solutions. Development of a periapical lesion signifies the presence of bacteria in the root canal system, in particular in its apical portion where they can exist in a biofilm and pose a challenge to treatment.1,2 An important role of root canal irrigation is to remove the residual bacterial biofilm from the inaccessible, un-instrumented surfaces.3
Biofilms can be defined as polysaccharide matrix enclosed bacterial populations adherent to each other and/or to surfaces or interfaces.4
Irrigants acts as a flush to remove organic and inorganic debris that would otherwise be left in the canal after mechanical instrumentation.5 Root canal irrigants should have a broad antimicrobial spectrum and high efficacy against anaerobic and facultative microganisms organized in biofilms.
The exact mechanism by which a biofilm is removed from the root canal surface is only a speculation and the exact means is not known. It was further speculated that the removal of a bacterial biofilm would be influenced by a number of factors and their interactions, including canal dimensions, properties of an irrigant, irrigant regimen and the properties of the biofilm.6
Irrigation devices play an important role in the delivery of the irrigant solution to the end point of canal preparation in removing intra-canal debris. The modes of irrigation include static irrigation as well as dynamic irrigation (active and passive ultrasonic irrigation [PUI]). Two types of ultrasonic irrigation has been described in the literature: One where irrigation is combined with simultaneous ultrasonic instrumentation (ultrasonic instrumentation) and another related to the non cutting action of the ultrasonically activated file called as passive ultrasonic instrumentation.7
The aim of the present study was to evaluate the efficiency of two irrigating techniques - static and dynamic (passive ultrasonic instrumentation) irrigation in the elimination of bacterial biofilm. The objectives of this study were (1) To use an ex vivo split tooth model to simulate the bacterial biofilm (2) To use the above biofilm model to assess the influence of various factors like canal taper, volume of the irrigant, mode of irrigation, open port of the needle upon the removal of bio-molecular film at various levels of root canal.
Materials and Methods
Preparation of ex vivo biofilm model
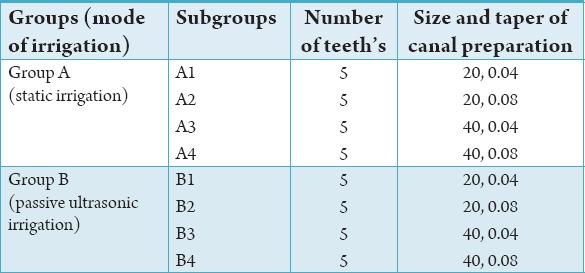
Forty extracted human permanent maxillary central incisors teeth with straight roots and single canals, were collected and stored in 4% formal saline. The forty teeth were divided into two groups each with twenty samples for static irrigation and ultrasonic irrigation. The root canal irrigant used was 2.5% sodium hypochlorite. The root canals were prepared to tip sizes (20, 40) and tapers (0.04, 0.08).
After access preparation was done, the tooth length was determined by placing a size 10 stainless steel K file (Mani Inc., Japan) in the canal and extending it until visible at the apical foramen. All the teeth were decoronated to give a standardized working length of 17.5 mm; the working length was set at 0.5 mm short of the apical foramen. The canals were prepared to tip size 20, 40 with tapers 0.04 and 0.08 using System GT instruments (Dentsply Malliefer, Switerzland) in a 70:1 controlled-torque, low speed rotary handpiece at 300 rpm according to subgroup designation (Table 1) following manufacturers protocol. During instrumentation, each tooth was irrigated with 50 mL of 2.5% sodium hypochlorite (Ammdent, India), delivered with a 3 mL Monoject Luer lock syringe with a 25-guage needle (Dentsply Rinn, USA).
Table 1.
Allocation of tooth samples for 2.5% sodium hypochlorite.
Each tooth was partly embedded in silicone putty (Aquasil Putty Impression Material, Dentsply USA) to create a set matrix which allowed reassembly of the tooth for irrigation tests after splitting. The tooth was then grooved on the buccal and palatal aspects along its entire length using a diamond disc (Axis Dental Corporation, USA) and placed longitudinally on another silicone matrix, to allow cushioning when split into two halves using an osteome and mallet. The two halves of the canal were randomly assigned as side A or B.
Four layers of organic collagen solution (in 0.6% acetic acid; Type I rat tail collagen, First Link Ltd., Birmingham, UK) mixed with Chinese Calligraphic Ink (Pebeo Fine Arts, France), in a ratio of 5:1, were applied to the canal surfaces using a small brush and allowed to gel into a three-dimensional matrix by evaporation of the acetic acid solvent at room temperature for 48 h.
Each split half of the tooth was equally divided into coronal, middle and apical sections and marked with a sharp pencil. The split tooth was reassembled for irrigation tests and disassembled for examination. Digital images were taken before and after irrigation with 9, 18, 27 and 36 mL of Sodium Hypochlorite using a digital camera (Sony DSC, Sony Corporation, USA).
Irrigation experiments
Irrigation experiments were done for static irrigation and ultrasonic irrigation using sodium hypochlorite.
From a sample of 40 teeth, 20 teeth (A1-A4) were irrigated with 2.5% sodium hypochlorite, delivered with a Monoject endodontic 3 mL syringe through a Luer lock 25 guage max-I-prob needle (Dentsply Rinn) at a rate of 6 ml/min. The irrigating needle was inserted 2 mm short of the working length and a total of 36 ml of solution was delivered in twelve 3 ml boluses (each 3 ml bolus delivered over 30 s with sufficient duration to allow syringe recharging and canal surface evaluation after 9, 18, 27 and 36 ml). The open side port of the needle faced the canal side designated “A”, in a fixed orientation.
For the rest 20 teeth (B1-B4), PUI was done. The protocol for irrigation was the same as for Static Irrigation with the addition of an ultrasonically activated file tip of size 15 K-file performed after introducing the first 3 ml of the irrigant, followed by delivery of the next 6 ml in two 3 ml boli.
Image analysis
The digital images were analyzed using ImageJ software (National Institute of Health, USA) to quantify residual canal coverage by the stained collagen. In total, 960 images (24 images per tooth) were taken and digitized. The means and standard deviation of the percentage of canal surface coverage with residual collagen after irrigation were calculated for each corono-apical section on each side of the canal. The generalized estimating equation (GEE) approach (STATA 9; STATA Corporation: College Station, TX, USA, 2005) was used to investigate the influence of the potential factors (Corono-apical level of canal, Canal dimension, Volume of irrigant, Mode of irrigation and Orientation of irrigation needle port) on the efficacy of irrigation using percentage of canal coverage with residual collagen as the independent variable. The clustering effect of the four repeated measurements taken on the same canal surface was accounted for in the GEE linear regression model.
For static irrigation of 2.5% sodium hypochlorite, two tabular columns were done. One for facing the needle and another opposite to the side of needle (Graphs 1 and 2). For PUI of 2.5% sodium hypochlorite, two tabular columns were done. One for facing the needle and another opposite to the side of needle (Graphs 3 and 4). The values were tabulated and subjected to statistical analysis.
Graph 1.
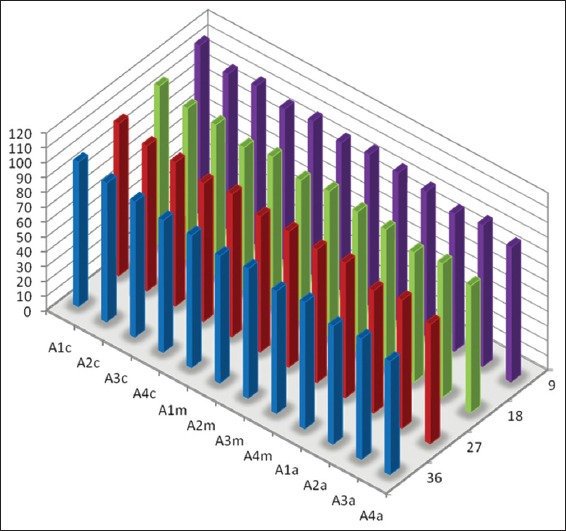
Mean percentage of canal surface coverage with residual collagen on the surface Facing the side port of needle after static irrigation (A: Samples from static irrigation group, 1-4 denote different canal dimensions [1= size 20/0.04; 2 = size 20/0.08; 3 = size 40/0.04 and 4 = size 40/0.08], c: Coronal third of canal, m: Middle third of canal and a: Apical third of canal).
Graph 2.
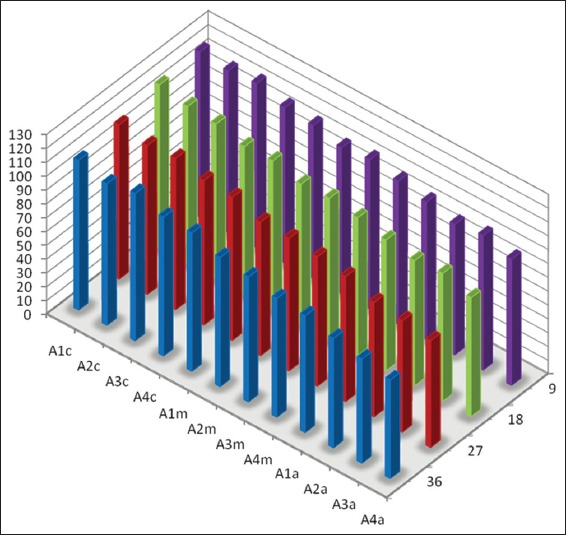
Mean percentage of canal surface coverage with residual collagen on the surface Opposite to the side port of needle after static irrigation (A: Samples from static irrigation group, 1-4 denote different canal dimensions [1 = size 20/0.04; 2 = size 20/0.08; 3 = size 40/0.04 and 4 = size 40/0.08], c: Coronal third of canal, m: Middle third of canal and a: Apical third of canal).
Graph 3.
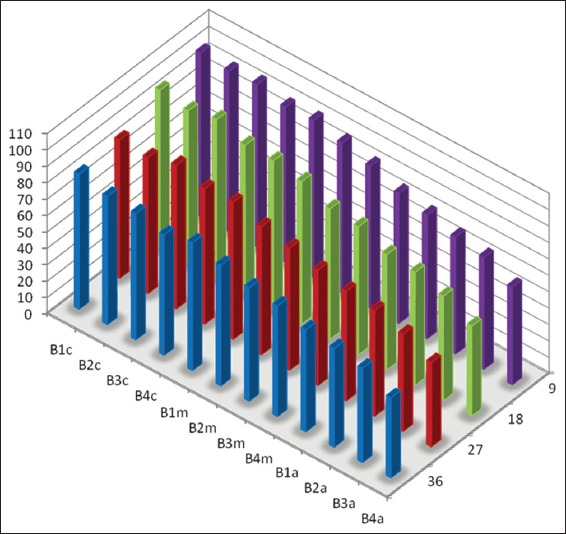
Mean percentage of canal surface coverage with residual collagen on the surface facing the side port of needle after passive ultrasonic irrigation (B: Samples from the PUI group, 1-4 denote different canal dimensions [1 = size 20/0.04; 2 = size 20/0.08; 3 = size 40/0.04 and 4 = size 40/0.08], c: Coronal third of canal, m: Middle third of canal and a: Apical third of canal).
Graph 4.
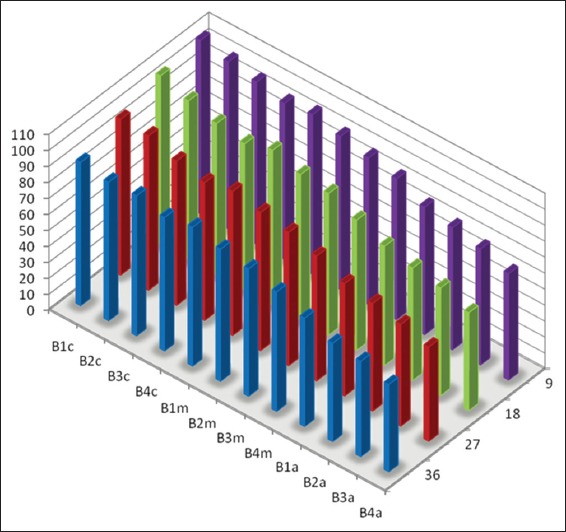
Mean percentage of canal surface coverage with residual collagen on the surface Opposite to the side port of needle after passive ultrasonic irrigation (B: Samples from the PUI group, 1-4 denote different canal dimensions [1 = size 20/0.04; 2 = size 20/0.08; 3 = size 40/0.04 and 4 = size 40/0.08), c: Coronal third of canal, m: Middle third of canal and a: Apical third of canal).
Results
The mean percentage of canal surface coverage with residual collagen after static and PUI are summarized graphically.
The mean percentage of canal surface with residual collagen increased with: (a) Coronal level of canal. (b) Decrease in apical size and taper of canal preparation (c) Decrease in the volume of the irrigant.
There was less residual collagen after PUI compared with static irrigation. The canal surface facing the open side port of the needle had less residual collagen after irrigation than the opposing surface.
Sodium hypochlorite
The efficacy of irrigation measured as “percentage of canal coverage with residual collagen” was significantly influenced by all the five explanatory variables. In descending order of effect, the relative ranking was: (i) corono-apical level of canal, (ii) volume of irrigation, (iii) canal dimension,(iv) mode of irrigation and (v) orientation of the needle port.
Discussion
It is generally accepted that one of the main causes of periapical disease is the bacterial infection of the root canal system. In cases of asymptomatic, persistent post treatment periapical radiolucencies, clinicians should consider the necessity of removing the extra-radicular factors like a biofilm.
Few bacteria can attach to Type I collagen in dentine through expression of surface adhesions and biofilms. In the clinical setting, biofilm removal from the instrumented part of the canal may be facilitated by the mechanical contact of the instruments. In the non-instrumented part of the canal, the biofilm is likely to be removed as a function of progressive chemical dissolution, along with a limited amount of fluid agitation as the fluid environment is likely to be viscous dominant. In view of such difficulties, this study used a split tooth model simulating a bacterial biofilm along the canal walls and comparing the efficacy of two irrigating techniques-static and dynamic (PUI) irrigation in the elimination of bacterial biofilm. This model was in accordance with the similar studies done by Huang et al.6 It was further speculated that the removal of the bacterial biofilm would be influenced by a number of factors and their interactions, including canal dimensions, properties of irrigant, irrigation regimen and the properties of the biofilm.
The aim of the present study was to evaluate the efficiency of two irrigating techniques - Static and dynamic (passive ultrasonic instrumentation) irrigation in the elimination of a bacterial biofilm by the physical and chemical action of irrigation. The test model used in the present study was designed to quantify the relative importance of factors like canal taper, volume of the irrigant, mode of irrigation, open port of the needle upon the removal of bio-molecular film at various levels of root canal.
In the present study to prepare the split tooth study model, human maxillary central incisors with single root and straight canals were chosen. Further in this study design, Type 1 rat tail collagen was used as a surface adherent test substrate, exhibiting the hydrodynamic properties of a biofilm because: they are visible when applied in root surface. had good adhesion to the root dentin and can be removed by physical abrasion or fluid turbulence (flushing action of irrigants). The film gradually dissolved and disintegrated from the surface by the irrigation and the removal helped by the physical abrasion or fluid turbulence IV among the available materials, it was the most cost economic.
The selected collagen, which was neither dried nor frozen, retained its native long molecule state, without denaturation in 0.6% acetic acid solution. On application, it returns to a nonacidic state. The collagen then gels into a three-dimensional matrix after evaporation of the acidic solution, a state that is reversible on addition of acetic acid (Type I rat tail collagen).
Type I rat tail collagen was transparent and hence eventually have to be stained. The criteria for stain selection were: it should contrast with dentine. It should aggregate with the collagen so as not to be decolourized by either sodium hypochlorite. It should not spread to the dentine collagen. When set, it should not flake or peel off. The Chinese Calligraphic Ink (Peebo, France) met all the requirements and retained the black colour after exposure to an equal volume of 2.5% NaOCl for 1 day.
The removal characteristics of the stained rat tail collagen layer on the canal surfaces were tested using sodium hypochlorite, and water. It was found that water did not remove the layer but the Sodium Hypochlorite did, leaving a clean surface without evidence of ink spread into dentinal tubules at or beyond the site of initial application. Four layers of the stained collagen gave good contrast and a predictable thickness of coating (5-15 um), a dimension within the range of bacterial film thickness.8
A Max-i-Probe 25-guage needle (Dentsply, USA) was used in this study. The Max-i-Probe has a closed-ended, side vented channel, which delivers the irrigant laterally.
Salzgeber et al.9 showed that increasing the volume of an irrigant significantly reduced the bacterial load, regardless of the irrigation needle depth. Huang et al.6 have used 4 different volumes of sodium hypochlorite and found that the larger volume of 36 mL significantly removed more collagen material from the tooth surface when compared with smaller volumes. Several other studies10,11 also showed the influence of volume on the removal of collagen. Thus in the present study different volume regimens were chosen (9, 18, 27 and 36 mL) in accordance with these studies.
Narrow canals compromise the efficacy of irrigation and may need to be enlarged and the taper increased to allow for effective irrigation. Salzgeber et al.9 advocated the need for the canals to be enlarged to a 40-file, so that the maximum irrigants is in direct contact with the apical debris. In the present study, the teeth samples were enlarged to apical preparation sizes 20 and 40 in accordance with the above literature. In the present study, canals were prepared to 20 and 40 tip size and a taper of 0.04 and 0.08.
Review of the literature have shown that the tip of the needle have been placed from 1 mm to 10 mm short of the apical end of the canal. In this present study, to standardize the depth of the needle penetration for all the samples, the tip was maintained at 2 mm short of the working length. Moreover this was the deepest penetration without the needle tip binding against the canals prepared to apical size 20 with a 0.04 taper.
It is desirable to control the irrigant delivery by rate and pressure throughout a study.12,13 The average rate of irrigant delivery in this study was set at 6 mL/min with a gauge 25 needle as it seemed clinically optimal and allowed the operator to maintain a constant force during extrusion.11
This study design employed a special imaging method (ImageJ, NIH, USA) to quantify residual canal coverage by the stained collagen which renders three-dimensional irregularities on the root canal surface into two-dimensional surfaces on the images. The GEE approach (STATA 9, USA) was used to investigate the influence of potential factors (coronal-apical level, canal dimension, volume of irrigant, mode of irrigation and orientation of irrigation needle port) on the efficacy of irrigation using “percentage of canal coverage with residual collagen” as the independent variable. The data was analyses using linear regression models.
The tooth split model used in this study simulated a bacterial biofilm. This ex vivo model was used to test the influence of several factors like (a) volume of irrigation, (b) canal dimension and taper, (c) mode of irrigation, (d) corono-apical level of canal and (e) canal surface, using 2.5% solution of sodium hypochlorite in the removal of bacterial biofilm.
Volume of irrigant
The volume of irrigant had a role in the removal of collagen. In static irrigation, the volumes of irrigation in the 9, 18, 27 and 36 ml did remove significant amount of collagen. For PUI, more amount of collagen was removed with all the 4 volumes. This can be probably due to the higher velocity and volume of irrigant flow created in the canal. Also the mechanical flushing action of the irrigant (NaOCl) would have been facilitated by the PUI.
Dimension of canal preparation
In this study, teeth samples are prepared to apical preparation sizes 20 and 40 with 0.04 and 0.08 taper. For static irrigation, the canal dimensions at 0.04 taper removed collagen less in the apical region, when compared with the 0.08 taper. For PUI the collagen was removed more with 0.08 taper, when compared with 0.04 taper. Overall, collagen was removed more with PUI than with static irrigation, especially in the apical region and with 0.08 taper. More the taper and larger the canal dimension, more will be the removal of the collagen and this can be attributed to the better apical flushing by the irrigant.
Corono-apical level of canal
In this study all tooth samples were divided into coronal, middle and apical sections. The collagen removal was more in the apical section when compared with the middle and coronal sections. The efficacy of irrigation was thus marked apically because of the greater fluid exchange/interaction that could have occurred in the apical area. This was more pronounced in the PUI.
Canal surface
The root canal surface facing the open side port of the needle appeared more cleaner than the opposite side, reinforcing the notion of the flushing effect of the irrigant in addition to the chemical effect. The collagen was removed more for the PUI technique in the facing side of the needle than with the static irrigation.
Mode of irrigation
The ability of irrigant to penetrate into areas not instrumented by mechanical instrumentation is critical for debridement and disinfection of the root canal system. Previous studies have shown that PUI resulted in significantly cleaner canals than hand filing alone. Hence in this study, the modes of irrigation compared were static and PUI.
PUI removed more amount of collagen than Static Irrigation in the present study. Several reasons can be attributed to this factor.
Flushing action of NaOCl facilitated by the PUI.
Much higher velocity and the volume of the irrigant volume.
Oscillations of the ultrasonic activated file.14
PUI combines acoustic waves with the chemical action of the irrigant and generates microstreaming. This microstreaming moves the solution against the root canal surfaces, enhancing mechanical cleansing of the canal walls and bacterial destruction.
Conclusion
Within the limitations of this study, it can be concluded that a bacterial biofilm, adherent to the root canal surface at the apical third of the root canal may be efficiently removed by:
Sufficient enlargement of the canal by increasing the canal taper.
Agitation of an irrigant, with an passive ultrasonically activated file.
Irrigation of the canal with a side vent needle like Max-I-Probe.
Large volumes of irrigant imparting lavaging effect.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Ramachandran Nair PN. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987;13(1):29–39. doi: 10.1016/S0099-2399(87)80089-4. [DOI] [PubMed] [Google Scholar]
- 2.Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(2):231–52. doi: 10.1016/j.tripleo.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Svensater G, Bergenholtz G. Biofilms in endodontic infections. Endod Top. 2004;9:27–36. [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35(6):791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Huang TY, Gulabivala K, Ng YL. A bio-molecular film ex-vivo model to evaluate the influence of canal dimensions and irrigation variables on the efficacy of irrigation. Int Endod J. 2008;41(1):60–71. doi: 10.1111/j.1365-2591.2007.01317.x. [DOI] [PubMed] [Google Scholar]
- 7.van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40(6):415–26. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 8.Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J Endod. 2002;28(10):689–93. doi: 10.1097/00004770-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Salzgeber RM, Brilliant JD. An in vivo evaluation of the penetration of an irrigating solution in root canals. J Endod. 1977;3:394–8. doi: 10.1016/S0099-2399(77)80172-6. [DOI] [PubMed] [Google Scholar]
- 10.Baker NA, Eleazer PD, Averbach RE, Seltzer S. Scanning electron microscopic study of the efficacy of various irrigating solutions. J Endod. 1975;1(4):127–35. doi: 10.1016/S0099-2399(75)80097-5. [DOI] [PubMed] [Google Scholar]
- 11.Moser JB, Heuer MA. Forces and efficacy in endodontic irrigation systems. Oral Surg Oral Med Oral Pathol. 1982;53(4):425–8. doi: 10.1016/0030-4220(82)90446-7. [DOI] [PubMed] [Google Scholar]
- 12.Ram Z. Effectiveness of root canal irrigation. Oral Surg. 1977;2:306. doi: 10.1016/0030-4220(77)90285-7. [DOI] [PubMed] [Google Scholar]
- 13.Chow TW. Mechanical effectiveness of root canal irrigation. Int Endod J. 1983;9(11):475–9. doi: 10.1016/S0099-2399(83)80162-9. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad M, Pitt Ford TR, Crum LA. Ultrasonic debridement of root canals: an insight into the mechanisms involved. J Endod. 1987;13(3):93–101. doi: 10.1016/S0099-2399(87)80173-5. [DOI] [PubMed] [Google Scholar]


