Summary
Background
Due to the adenoma-carcinoma sequence, complete removal of colorectal polyps is essential.
Method
This article analyzes the role of surgery in the removal of colorectal adenoma.
Results
Nowadays, most adenomas are removed properly by endoscopic methods. Also in the resection of giant polyps and recurrent adenoma endoscopic data is convincing. Therefore, surgical resection of colorectal adenomas is required in the case of endoscopic inaccessibility. Reasons for this may be the location of the polyp, incomplete endoscopic resection, or suspected malignancy. Endoscopic or limited surgical resection of malignant adenomas is acceptable only if ‘low-risk’ criteria are fulfilled. Otherwise oncologic radical resection is recommended. In general, radical resection is also necessary in the case of polyps that are not suitable for endoscopic removal, because here the rate of colorectal carcinoma is high.
Conclusion
If a surgical approach is necessary, minimally invasive surgery in the hands of an experienced laparoscopic surgeon is a suitable option. Adenomas in the lower two thirds of the rectum are suitable for transanal full-thickness resection. This is done by conventional resection or transanal endoscopic microsurgery. The histopathological preparation of these specimens provides diagnostic and therapeutic benefits, particularly compared to piecemeal resection of early carcinoma.
Keywords: Colorectal adenoma, Conventional resection, Laparoscopic resection, ‘Low-risk’ criteria, Transanal resection
Zusammenfassung
Hintergrund
Die Adenom-Karzinom-Sequenz erfordert die komplette Entfernung kolorektaler Adenome.
Methode
In der vorliegenden Arbeit wird die Rolle der Chirurgie bei der Entfernung kolorektaler Adenome analysiert.
Ergebnisse
Die meisten Adenome lassen sich einwandfrei endoskopisch entfernen. Die Daten der Endoskopie sind auch bei Vorliegen sehr großer Adenome und bei Adenomrezidiven überzeugend. Deshalb kommen chirurgische Eingriffe erst nach Scheitern endoskopischer Maßnahmen zum Zuge. Gründe hierfür können die Polypenlokalisation, eine inkomplette endoskopische Abtragung oder der Malignomverdacht sein. Eine endoskopische oder chirurgisch limitierte Resektion ist beim Frühkarzinom onkologisch nur zulässig, wenn «Low-Risk»-Kriterien erfüllt sind. Ansonsten erfolgt die onkologische Standardresektion. Diese ist in der Regel auch beim nicht abtragbaren Polypen zu empfehlen, da sich hinter derartigen Befunden häufig bereits Karzinome verbergen.
Schlussfolgerung
Bei entsprechender Expertise können Eingriffe laparoskopisch erfolgen. Adenome in den beiden unteren Rektumdritteln eignen sich für chirurgische transanale Vollwandresektionen; diese können konventionell oder mittels transanaler endoskopischer Mikrochirurgie erfolgen. Die histopathologische Aufarbeitung dieser Resektate bietet Vorteile in diagnostischer und therapeutischer Hinsicht, insbesondere gegenüber einer Piecemeal-Resektion beim Frühkarzinom.
Introduction
Endoscopic resection of colorectal adenomas has been an established procedure for many years. Surgical resection is a standard therapeutic procedure in the treatment of colorectal cancer. Because of technical advances in endoscopy, surgery in itself has become less important in the therapy of colorectal adenomas. Endoscopy and surgery are complementary procedures in the treatment of benign lesions. Colon adenomas that are not sufficiently removable with endoscopic techniques due to size, location, or suspected malignancy represent surgical indications. In rectal adenomas, transanal surgery is a competitive alternative to endoscopic procedures.
The need for therapeutic intervention in the case of colorectal adenoma is based on the adenoma-carcinoma sequence: Intraepithelial neoplasia proceed from ‘low-grade’ to ‘highgrade’ neoplasia to invasive colorectal cancer over a period of years. However, statistical analysis shows that in the end only few adenomas develop into cancer. The comparison of adenoma prevalence and carcinoma incidence indicates a yearly transformation rate of about 0.25%. Adenomas can also remain stable in size or even regress completely. Most adenomas never develop into malignant lesions. The potential for malignant transformation correlates with size, grade of dysplasia, and growth pattern. In this regard, special attention must be paid to flat and depressed adenomas and serrated adenomas with a higher risk of malignant transformation [1]. As long as the individual progression is unknown, total removal of the neoplastic lesion is essential. A clear risk reduction for development of colorectal carcinoma by consistent removal of polyps was already shown in the National Polyp Study in 1993 [2].
The removal of adenomas ranges from endoscopic mucosal resection (EMR) to endoscopic submucosal dissection (ESD) to surgical resection. Surgical resection is performed either by laparotomy, laparoscopy, or transanal procedures. There are also reports of intersections between endoscopy and surgery in ‘rendezvous maneuvers’: laparoscopic resection under endoscopic guidance, or endoscopic resection under laparoscopic guidance. The advantage of ESD and surgical resection over EMR is the high rate of en bloc and R0 resections resulting in low recurrence rates even in large adenomas. As a result, the pathologist is enabled to evaluate the circumferential as well as the deep resection margins. On the other hand, endoscopic snare resection is the least invasive procedure. The intention of this article was to analyze the role of surgery among the competing methods in this field.
Indications
Aware of all treatment options, a calculation regarding chance of success, invasiveness of the therapeutic procedure, and possible complications has to be made. In the case of suspected malignancy with deep submucosal invasion (non-lifting sign) (fig. 1), chromoendoscopic findings such as pit pattern type V according to Kudo, endomorphologic findings such as type 3 according to the NICE classification for narrow band imaging (NBI), or proven malignancy, oncologic surgical resection is the preferred option. In pedunculated lesions, endoscopic resection should be preferred regardless of morphologic findings in the lesion head. Developments over the last years have brought about new endoscopic techniques as well as laparoscopic procedures that are advantageous for our patients. In order to choose the best procedure, one should use all relevant diagnostics to find the best individualized option without regard for one's own specialization. Ideally, these decisions are made on the basis of an interdisciplinary board or discussion in which the surgeon and the gastroenterologist act as partners. In this setting, the therapy of colorectal adenomas is understood as a prime example of an interdisciplinary approach in gastrointestinal medicine and surgery with special regards for efficacy and the safety of the patient.
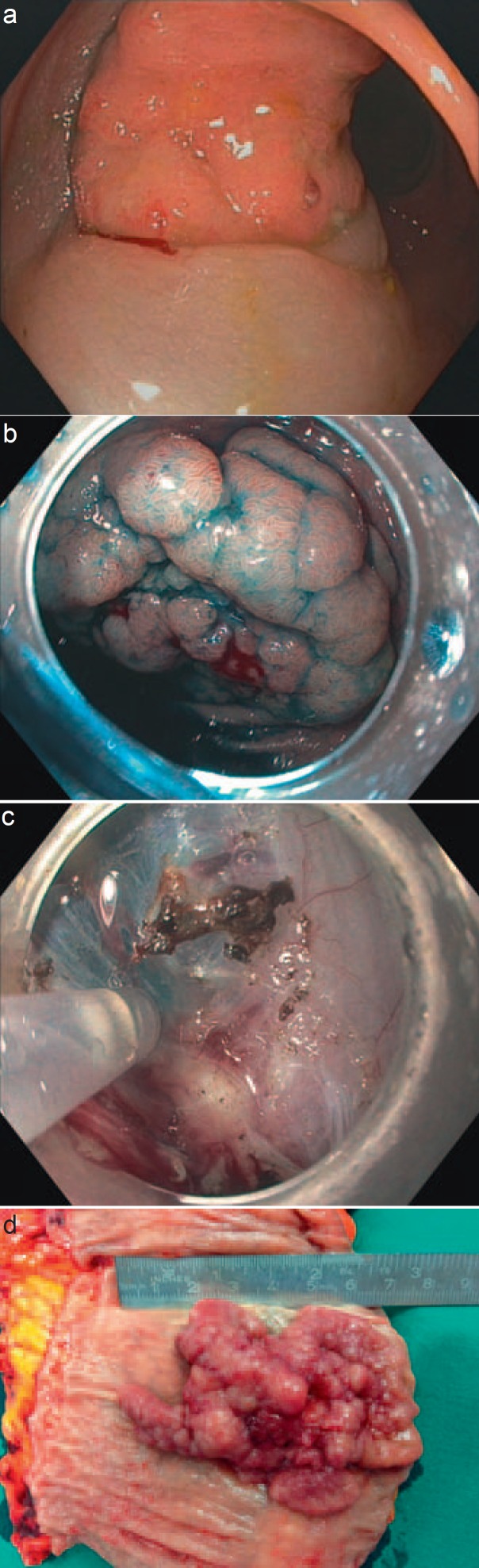
Fig. 1.
‘Non-lifting sign’ indicating invasion of deep layers of the colon wall and ulceration suspicious for colorectal carcinoma. a‘Non-lifting sign’: the whole polyp without lifting (pT2 N0 carcinoma); bbenign aspect of the polyp depicted in c; c‘non-lifting sign’ during ESD: upper part of the adenoma shows lifting while lower part is fixed to the deep layers of the colon wall (pT3 N1 carcinoma); dsurgical specimen of a polyp with endoscopic aspect of malignancy due to ulceration in the center (pT1 (SM3), N1a (1/46), L0, V0, G2, R0).
Hochdörffer et al. [3] reported results and outcomes of EMR in ‘giant’ colorectal lesions. ‘Giant’ was defined as polyps larger than 3 cm in diameter. In 165 patients, 167 polyps were evaluated. In 31 cases, complete resection was not possible, in another 15 cases the lesion was not suitable for endoscopic removal. For this reason, the rate of failure of endoscopic therapy in this cohort of selected patients was 27.6%. Although polyps appearing malignant macroscopically were excluded in this study, colorectal carcinoma was found in 10%. In 121 polyps, complete resection was achieved (73.6% piecemeal resections). After a median follow-up of 72.2 months, in 99 patients a local recurrence rate of 26.3% (n = 26) was found. 19 of these patients were again treated endoscopically. 6 patients with local recurrence (23.1%) were operated on, and in 4 cases an invasive carcinoma was found. Other groups reported series of EMR of large polyps (larger than 3 and 4 cm, respectively), with local recurrence rates of 12, 17, and 4% [4,5,6]. Of these, 66, 89, and 99% could again be treated endoscopically. Overall, the success rate of endoscopic removal of recurrent adenomas seems to be significant.
According to a meta-analysis by Puli et al. [7], the rate of curative endoscopic resections in ‘high-volume hospitals’ is higher and piecemeal resections are less common. This shows a correlation between expertise and endoscopic results. Accordingly, recurrence rates and rates of surgical resection vary. Some authors do not report any need for surgical resection, and surgical resection rates in large polyps range from 0% [4] to 25% [3]; however, the definition of ‘large’ is inconsistent.
According to Japanese papers, ESD shows high rates of en bloc and R0 resections. Therefore, the rates of local recurrence are low (about 2%) [8,9,10,11]. European data on this topic is rare; however, Probst et al. [12] could demonstrate that at least in the distal colorectum ESD is a feasible option even in Europe. The authors showed that results depended on a learning curve, but ended up with high en bloc resection rates of 96.2% and R0 resection rates of 84.5%.
These results give endoscopy a leading role even in the therapy of extensive findings. Limitations of endoscopy are specific anatomical locations (fig. 2), particular polyp shapes, and suspicion of malignancy. Under these conditions, or if endoscopic resection is incomplete, surgical resection is required. In any case, complete colonoscopy is necessary to detect synchronous lesions and to remove them before surgery is done. A biopsy should be taken to differentiate between benign and malignant lesions. Given the high rate of malignancy of large lesions, colorectal carcinoma should be proven preoperatively in order to perform an adequate oncologic resection. In colonic adenomas, preoperative endoscopic ink labelling is helpful to make intraoperative detection easier.
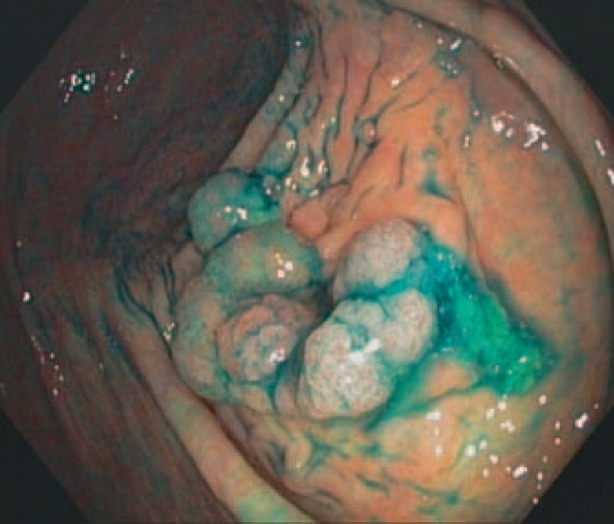
Fig. 2.
Adenoma protrusion from the ostium appendicis vermiformis.
Procedures
Laparoscopy/Laparotomy
Minimally invasive laparoscopic resections are oncologically comparable to open surgical resections in colorectal cancer, but laparoscopic treatment offers some advantages to the patient. The implementation of laparoscopy had a wide influence on the therapy of colorectal adenomas and colorectal cancer, yet minimally invasive surgery must not be misunderstood as a competitor of endoscopy. Surgical colonic resection still is a more invasive method and therefore regarded as a complementary therapeutic procedure. Usually it is complementary to endoscopy and therefore appropriate when endoscopic resection is impossible or incomplete, or when malignancy is suspected. Recent data show a high rate of colorectal carcinomas (17.7%) in polyps not suitable for endoscopic procedures, and therefore recommend in this situation radical oncologic resection as best treatment [13]. Jang et al. [14] demonstrated a similar rate of malignancy in this group (16.1%), but for a subpopulation of 98 patients noted the presence of dysplasia upon preoperative biopsy in 34.7% of cases. This result was based on any type of dysplasia, and no distinction was drawn between different grades of dysplasia. Therefore, the authors also recommend oncologic resection in dysplastic adenomas.
A German prospective multicenter observational study [15] analyzed the results of 525 patients with endoscopically unresectable colorectal polyps. Mainly colorectal standard resections were performed (24% anterior resections, 32.2% sigmoid resections, 1.3% left-sided hemicolectomies, 16.8% right-sided hemicolectomies, 14.1% ileocecal resections); only in 61 patients limited resections as wedge, full-thickness, or segmental resection were done. All patients had laparoscopic resections. Conversion to laparotomy became necessary in 3.2%. Malignancy was found in 18.1%. 14.8% of these colorectal cancers already exhibited lymph node metastases. A subgroup analysis of the postoperatively diagnosed colorectal carcinomas showed that only in 65.3% a radical oncologic resection took place. Postoperative complications were found in 20.8% (1.5% bleeding requiring surgery, 3.8% postoperative ileus, 2.1% anastomotic leakage followed by conservative treatment, 1.5% anastomotic leakage requiring surgery, 3.0% hematoma/abscess). The perioperative mortality rate was 0.9%. Complications requiring surgical revision occurred in 4.8%. Rates of local recurrences were not investigated. Pokala et al. [16], Lo and Law [17], and Lai et al. [18] found similar results in smaller collectives of 51, 45, and 78 patients with rates of colorectal cancer of 20, 35.6, and 44.3%, respectively (partially including preoperatively diagnosed malignant adenomas). Hauenschild et al. [19] had a slightly higher conversion rate of 6.9%, but less postoperative complications (9.3%).
The frequency of particularly severe complications is acceptable. Most authors favor the laparoscopic procedure. Several studies demonstrate the superiority of laparoscopy over laparotomy, especially in terms of the postoperative short-term outcome [16,20,21]. In difficult locations, especially in the transverse colon, the laparoscopic skills of the surgeon are crucial so that an open resection may be recommended. In our clinic, we tend to prefer open resection with a limited transverse incision in the right abdomen if a highly suspicious tumor is located in the right colon. When indicated, the operation is performed as a hybrid intervention where laparoscopic mobilization of the right colon leads to a shorter incision (laparoscopically assisted) [22].
Rendezvous Maneuvers
Rendezvous maneuvers are regarded by some authors as another application for laparoscopy. In inaccessible polyps of the colon, laparoscopic mobilization of the colon might improve exposure for the endoscopist. At the same time, laparoscopy allows surveillance of the endoscopic resection. In the case of perforation of the colon, the repair is managed laparoscopically at the same time [23,24,25]. The largest study about laparoscopically monitored colonoscopic polyectomy was performed by Franklin et al. [23] with 110 patients. Here, the rate of cancer was also high (10%). Because of malignancy and persistent endoscopic unresectability, an overall 19% of patients were in need of surgical resection. Other combinations were reported by Wilhelm et al. [26], namely endoscopically assisted wedge resections, endoscopically assisted transluminal resections, and endoscopically assisted segmental resections. Without a subgroup analysis, they reported a conversion rate of 5%, a perioperative mortality rate of 0.9%, a local recurrence rate of 0.9%, and a reoperation rate of 11%. The reason for reoperation in 9 out of 16 patients was the need for radical oncologic resection after colorectal cancer was found. Winter et al. [27] presented similar data with a conversion rate of 5%, a periprocedural complication rate of 5%, an anastomotic leakage rate of 3%, and a colorectal cancer rate of 13%. Rendezvous maneuvers require a high level of experience on both sides (surgeon and endoscopist). Another disadvantage is the expansion of the colon during colonoscopy, which can result in a worsening of the laparoscopic view.
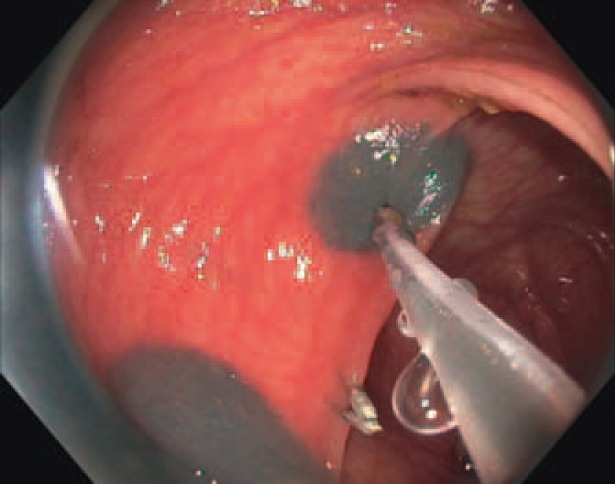
Preoperative ink labelling (4 quadrants of the colonic diameter) results in clear intraoperative visualization of the region of interest. We rarely operate with clip labels, since their detection during laparoscopy without the sense of touch may be complex and often requires X-ray for localization. This, in our opinion, interferes with an efficient operative procedure. Splitting of endoscopic localization and surgical resection by preoperative labelling can reduce the time and manpower needed in the operating theatre (fig. 3, 4). Based on our experience, we cannot confirm reports about complicated courses after ink labelling [22]. According to the current German guideline for colorectal carcinoma (S3-Leitlinie Kolorektales Karzinom), labelling with ink or clips is obligatory [28].
Fig. 3.
Endoscopic ink (and clip) labelling in each circumferential quadrant (all endoscopic images with kind permission of Dr. A. Probst, Medical Clinic III, Klinikum Augsburg, Germany).
Fig. 4.
Laparoscopic view of labelled colon.
Transanal Procedures
Kneist et al. [29] reported on 552 patients with rectal adenoma or carcinoma over a time period of 27 years. The size of the reported lesions was <2 cm in 25% of cases. 513 patients underwent transanal endoscopic microsurgery (TEM), whereas in 39 patients local excisions were performed using a Parks retractor. Specimen retrieval resulted in 331 full-thickness resections, 106 partial resections of the rectal wall, 66 mucosectomies, and in 49 cases in a combination of these procedures. Local excision was performed when Mason clinical stage was I, transanal ultrasound was without evidence of infiltration of the muscularis propria, and histological examination was without evidence of undifferentiated carcinoma. Although there was no limitation to the size of the adenomas, the pathological results revealed 67% adenomas, 29.5% carcinomas, and 2.4% carcinoid tumors. Despite preoperative biopsy, only 41% of the carcinomas were diagnosed before complete excision.
In view of the fact that more than 50% of EMR of large polyps result in piecemeal resections and that there is a high rate of cancer in rectal lesions, transanal surgery plays an important role in this field. Endoscopic piecemeal resections in early rectal carcinomas complicate the pathological examination and increase the rate of local recurrence. The completeness of resection is difficult to determine with respect to circumferential and deep resection margins. Furthermore, the depth of submucosal invasion remains unclear in contrast to a surgical full-thickness specimen. Therefore, in a ‘low-risk’ situation, transanal R0 resection preserves the option of an oncologically adequate limited resection. Herein, transanal surgery shows clear advantages compared to endoscopic piecemeal resections. Especially in the supraanal region, where transanal full-thickness resections can include also mesorectal fat and lymph nodes, further advantages arise for the patient: In early cancer, deep rectal resection with coloanal anastomosis and even abdominoperineal resection can be avoided [22]. In the case of a low-risk situation with invasion of the superficial layers (<1,000 µm) of the submucosa, the risk of lymph node metastasis is about 6% [30,31]. In this situation, a risk stratification has to be made; the risks of surgery must be compared to the risks of lymph node metastasis.
Finally, regarding the treatment of rectal neoplasms, it is of particular importance to consider the circumstances and invasiveness of competing methods. Advantages of the endoscopic techniques are that there is no need for general or spinal anesthesia and that there is good preservation of the anal sphincter which is not exposed to any dilatation damage. On the other hand, tumors that are close to the anal verge are accessible only through colonoscopic inversion so that endoscopic resection becomes more complicated and time-consuming. In contrast, polyps of the lower rectum are easily accessible for transanal resection under short anesthesia. A full-thickness specimen can be extracted using magnifying glasses and a headlight on a day-case or outpatient basis, whilst ESD takes a mean procedural time of more than 120 min and a corresponding length of time to sedate the patient. The required effort in men and surveillance compare to the surgical procedure. In our opinion, transanal resection of lesions in the lower parts of the rectum achieve optimal results. As long as ESD is not a standard therapy in Europe, its role is not clearly defined. It could probably be a competing method in the upper third of the rectum (above 12 cm) and the distal colon. At the moment, the current German guideline for colorectal carcinoma (S3-Leitlinie Kolorektales Karzinom) [28] regards ESD as being in a state of scientific evaluation.
An unsolved problem for surgeons and endoscopists are giant adenomas in the supraanal parts of the rectum that spread circularly over 5-10 cm in longitudinal direction in the rectal wall. Their extent and texture interfere with any type of limited procedure, which in turn results in piecemeal resections or multiple resections. Here, an interdisciplinary discussion considering the patient's interests is essential [22].
Conclusion
Colonic adenomas that are unsuitable for endoscopic resection are clear indications for surgery. Due to the adenoma-carcinoma sequence, total removal of all polyps is necessary. Colorectal carcinoma is common, especially in polyps that are not suitable for endoscopic resection. Therefore, radical oncologic resection is reasonable at least in dysplastic polyps. When a limited resection is performed, an intraoperative frozen section of the specimen should be examined to rule out colorectal cancer. Malignant adenomas that do not fulfill ‘low-risk’ criteria (R0 resection of pT1 tumors, maximal submucosal invasion of 1,000 µm, G1/2, L0, V0) require surgery. Due to the high risk of lymph node metastasis, radical oncologic resection is recommended. There is a high prevalence of malignancy in adenomas in the lower two thirds of the rectum. These should be transanally resected. This is a safe and fast procedure generating a full-thickness en bloc specimen that allows valid histological evaluation. Especially the depth of invasion into the submucosal layers can be clearly determined, and with that the existence of ‘low-risk’ or ‘high-risk’ criteria.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Risio M. The natural history of adenomas. Best Pract Res Clin Gastroenterol. 2010;24:271–280. doi: 10.1016/j.bpg.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF, Ackroyd F, Shike M, Kurtz RC, Hornsby-Lewis L, Gerdes H, Stewart ET. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. New Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Hochdörffer R, Eickhoff A, Apel D, Eickoff JC, Hartmann D, Jakobs R, Riemann JF. Endoscopic resection of ‘giant’ colorectal lesions: long-term outcome and safety. Z Gastroenterol. 2010;48:741–747. doi: 10.1055/s-0028-1109971. [DOI] [PubMed] [Google Scholar]
- 4.Ah Soune P, Ménard C, Saleh E, Desjeux A, Grimaud JC, Barthet M. Large endoscopic mucosal resection for colorectal tumors exceeding 4 cm. World J Gastroenterol. 2010;16:588–595. doi: 10.3748/wjg.v16.i5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seitz U, Bohnacker S, Seewald S, Thonke F, Soehendra N. Long-term results of endoscopic removal of large colorectal adenomas. Endoscopy. 2003;35:41–44. doi: 10.1055/s-2003-41535. [DOI] [PubMed] [Google Scholar]
- 6.Doniec JM, Löhnert MS, Schniewind B, Bokelmann F, Kremer B, Grimm H. Endoscopic removal of large colorectal polyps: prevention of unnecessary surgery? Dis Col Rectum. 2003;46:340–348. doi: 10.1007/s10350-004-6553-x. [DOI] [PubMed] [Google Scholar]
- 7.Puli SR, Kakugawa Y, Gotoda T, Antillon D, Saito Y, Antillon MR. Meta-analysis and systematic review of colorectal endoscopic mucosal resection. World J Gastroenterol. 2009;15:4273–4277. doi: 10.3748/wjg.15.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D. A prospective multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Saito Y, Sakamoto T, Fukunga S, Nakajima T, Kiriyama S, Matsuda T. Endoscopic submucosal dissection (ESD) for colorectal tumors. Dig Endosc. 2009;21(suppl 1):S7–12. doi: 10.1111/j.1443-1661.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 10.Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, Minatsuki C, Yamamichi N, Koike K. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42:723–729. doi: 10.1055/s-0030-1255675. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida N, Naito Y, Sakai K, Sumida Y, Kanemasa K, Inoue K, Morimoto Y, Konishi H, Wakabayashi N, Kokura S, Yagi N, Yanagisawa A, Yoshikawa T. Outcome of endoscopic submucosal dissection for colorectal tumors in elderly people. Int J Colorectal Dis. 2010;25:455–461. doi: 10.1007/s00384-009-0841-9. [DOI] [PubMed] [Google Scholar]
- 12.Probst A, Golger D, Anthuber M, Märkl B, Messmann H. Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy. 2012;44:660–667. doi: 10.1055/s-0032-1309403. [DOI] [PubMed] [Google Scholar]
- 13.Bertelson NL, Kalkbrenner KA, Merchea A, Dozois EJ, Landmann RG, De Petris G, Young-Fadok TM, Etzioni DA. Colectomy for endoscopically unresectable polyps: how often is it cancer? Dis Col Rectum. 2012;55:1111–1116. doi: 10.1097/DCR.0b013e3182695115. [DOI] [PubMed] [Google Scholar]
- 14.Jang JH, Balik E, Kirchoff D, Tromp W, Kumar A, Grieco M, Feingold DL, Cekic V, Njoh L, Whelan RL. Oncologic colorectal resection, not advanced endoscopic polypectomy, is the best treatment for large dysplastic adenomas. J Gastrointest Surg. 2012;16:165–172. doi: 10.1007/s11605-011-1746-9. [DOI] [PubMed] [Google Scholar]
- 15.Benedix F, Köckerling F, Lippert H, Scheidbach H. Laparoscopic resection for endoscopically unresectable colorectal polyps: analysis of 525 patients. Surg Endosc. 2008;22:2576–2582. doi: 10.1007/s00464-008-0059-x. [DOI] [PubMed] [Google Scholar]
- 16.Pokala N, Delaney CP, Kiran RP, Brady K, Senagore AJ. Outcome of laparoscopic colectomy for polyps not suitable for endoscopic resection. Surg Endosc. 2007;21:400–403. doi: 10.1007/s00464-006-9069-8. [DOI] [PubMed] [Google Scholar]
- 17.Lo SH, Law WL. Laparoscopic colorectal resection for polyps not suitable for coloscopic removal. Surg Endosc. 2005;19:1252–1255. doi: 10.1007/s00464-004-2220-4. [DOI] [PubMed] [Google Scholar]
- 18.Lai JH, NG KH, Ooi BS, Ho KS, Lim JF, Tang CL, Eu KW. Laparoscopic resection for colorectal polyps: a single institution experience. ANZ J Surg. 2011;81:275–280. doi: 10.1111/j.1445-2197.2010.05580.x. [DOI] [PubMed] [Google Scholar]
- 19.Hauenschild L, Bader FG, Laubert T, Czymek R, Hildebrand P, Roblick UJ, Bruch HP, Mirow L. Laparoscopic colorectal resection for benign polyps not suitable for endoscopic polypectomy. Int J Colorectal Dis. 2009;24:755–759. doi: 10.1007/s00384-009-0688-0. [DOI] [PubMed] [Google Scholar]
- 20.Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;(3):CD003145. doi: 10.1002/14651858.CD003145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy AM. Colon cancer laparoscopic or open resection study group (COLOR) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomized trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 22.Rüth S, Spatz J, Anthuber M. Colorectal adenoma: pro conventional/laparoscopic resection. Chirurg. 2011;82:520–525. doi: 10.1007/s00104-010-2062-8. [DOI] [PubMed] [Google Scholar]
- 23.Franklin ME, Jr, Leyva-Alvizo A, Abrego-Medina D, Glass JL, Trevino J, Arellano PP, Portillo G. Laparoscopically monitored colonoscopic polypectomy: an established form of endoluminal therapy for colorectal polyps. Surg Endoc. 2007;21:1650–1653. doi: 10.1007/s00464-007-9237-5. [DOI] [PubMed] [Google Scholar]
- 24.Prohm P, Weber J, Bönner C. Laparoscpic-assisted coloscopic polypectomy. Dis Col Rectum. 2001;44:746–748. doi: 10.1007/BF02234579. [DOI] [PubMed] [Google Scholar]
- 25.Smedh K, Skullman S, Kald A, Anderberg B, Nyström P. Laparoscopic bowel mobilization combined with intraoperative colonoscopic polypectomy in patients with an inaccessible polyp of the colon. Surg Endosc. 1997;11:643–644. doi: 10.1007/s004649900411. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm D, von Delius S, Weber L, Meining A, Schneider A, Friess H, Schmid RM, Frimberger E, Feussner H. Combined laparoscopic-endoscopic resections of colorectal polyps: 10-year experience and follow-up. Surg Endosc. 2009;23:688–693. doi: 10.1007/s00464-008-0282-5. [DOI] [PubMed] [Google Scholar]
- 27.Winter H, Lang RA, Spelsberg FW, Jauch KW, Hüttl TP. Laparoscopic colonoscopic rendezvous procedures for the treatment of polyps and early stage carcinomas of the colon. Int J Colorectal Dis. 2007;22:1377–1381. doi: 10.1007/s00384-007-0345-4. [DOI] [PubMed] [Google Scholar]
- 28.Leitlinienprogramm Onkologie. Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF: 2013. S3-Leitlinie Kolorektales Karzinom. [Google Scholar]
- 29.Kneist W, Terzic A, Burghardt J, Heintz A, Junginger T. Selection of patients with rectal tumors for local excision based on preoperative diagnosis. Results of a consecutive evaluation study of 552 patients. Chirurg. 2004;75:168–175. doi: 10.1007/s00104-003-0746-z. [DOI] [PubMed] [Google Scholar]
- 30.Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K, Yoshimura K, Bekku S. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–394. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Choi PW, Yu CS, Jang SJ, Jung SH, Kim HC, Kim JC. Risk factors for lymph node metastasis in sub-mucosal invasive colorectal cancer. World J Surg. 2008;32:2089–2094. doi: 10.1007/s00268-008-9628-3. [DOI] [PubMed] [Google Scholar]