Summary
Background
Since the beginning of the new millennium gender medicine has become more and more relevant. The goal has been to unveil differences in presentation, treatment response, and prognosis of men and women with regard to various diseases.
Methods
This study encompassed 1,061 patients who underwent surgery for rectal cancer at the Department of Surgery, University Medical Center Schleswig-Holstein Campus Lübeck, Germany, between January 1990 and December 2011. Prospectively documented demographic, clinical, pathological, and follow-up data were obtained. Analysis encompassed the comparison of clinical, histopathological, and oncological parameters with regard to the subcohorts of male and female patients.
Results
No statistically significant differences could be found for clinical and histopathological parameters, location of tumor, resection with or without anastomosis, palliative or curative treatment, conversion rates, duration of surgery, and long-term survival. For the entire cohort, gender-related statistically significant differences in complications encompassed anastomotic leakage, burst abdomen, pneumonia, and urinary tract complications all of which occurred more often in men.
Conclusion
Data obtained in this study suggest that there are no gender-related differences in the oncologic surgical treatment of patients with rectal carcinoma. However, male sex seems to be a risk factor for increased early postoperative morbidity.
KeyWords: Gender, Rectal neoplasms, Complications, Laparoscopy
Zusammenfassung
Hintergrund
Seit dem neuen Jahrtausend rücken geschlechtsspezifische Untersuchungen zu verschiedenen Erkrankungen zunehmend in den Fokus. Ziel ist die Erfassung von Unterschieden bezüglich Symptomen, Therapien, Therapieansprechraten und Prognosen bei Männern und Frauen.
Methoden
Diese Studie schließt 1061 Patienten mit operativer Therapie beim Rektumkarzinom in der Klinik für Allgemeine Chirurgie, Universitätsklinik Schleswig-Holstein Campus Lübeck, Deutschland, zwischen Januar 1990 und Dezember 2011 ein. Prospektiv wurden Daten zu demographischen, klinischen, pathologischen und Langzeitüberlebensraten erfasst. Die Untersuchung schließt klinische, onkologische, histopathologische und Morbiditätsdaten unter besonderer Berücksichtigung des Geschlechts ein.
Ergebnisse
Für klinische und histopathologische Parameter, Tumorlokalisationen, Resektionen mit oder ohne Anastomose, palliative oder kurative Therapien, Konversionsraten, Schnitt-Naht-Zeiten und Langzeitüberleben konnte kein geschlechtsabhängiger signifikanter Unterschied erfasst werden. Für das Gesamtkollektiv wurden geschlechtsspezifische signifikante Unterschiede zu den Parametern Anastomoseninsuffizienz, Platzbauch, Pneumonie und Harnwegskomplikationen erfasst, die allumfänglich bei Männern gehäuft auftraten.
Schlussfolgerung
Die in dieser Studie erhobenen Daten lassen den Schluss zu, dass keine geschlechtsspezifischen Unterschiede bezüglich der onkologisch-chirurgischen Behandlung beim Rektumkarzinom vorliegen. Männliches Geschlecht scheint jedoch ein Risikofaktor für eine erhöhte früh postoperative Morbidität zu sein.
Background
Twenty to thirty years ago the American National Institutes of Health (NIH) and the Food and Drug Administration (FDA) recognized that most diagnostic and therapeutic strategies and operation procedures had been devised for men [1]. The aspect of gender-related differences of both appearance and prognosis of diseases was first focused on in female endocrinology and reproduction medicine. Later on, differences of gender had become apparent in common diseases like diabetes or cardiovascular diseases [2]. Studies showed that prevention of myocardial infarction by intake of low-dose acetylsalicylic acid was inadequate and that myocardial infarction causes a different spectrum of symptoms in women than in men [3,4]. Several clinical and experimental investigations have revealed sex-related differences in infectious diseases and sepsis. Female gender appears to be protective under such conditions, whereas male gender seems to imply a diminished immune response [5].
Since the beginning of the new millennium gender medicine has become increasingly relevant. The goal has been to unveil differences in presentation, treatment response, and prognosis of men and women concerning various diseases. In 2001, the first center for gender medicine was founded in New York, USA, followed by the Swedish Karolinska Institute in 2002 as well as by a center in Berlin, Germany, in the following year. For several years now, the scientific journal Gender Medicine has been published regularly. The first textbook of gender medicine, titled ‘Sex and Gender Aspects in Clinical Medicine’, does not contain a single chapter regarding gender-related differences in surgery [6]. Furthermore, only few studies have focused on this issue. In cardiac surgery, women seem to require more transfusions in bypass surgery, and the increased risk of mortality and morbidity is related to an increase of transfusions [7]. Several differences between men and women were revealed in a study analyzing the perioperative and long-term outcome after mitral valve procedures [8]. The influence of gender in endoprosthetic surgery has been addressed in an analysis including more than 35,000 patients. Herein, the authors demonstrated gender as an independent risk factor for revision surgery in women [9].
Colorectal cancer (CRC) constitutes the third most common cause of death from malignant disease in Europe and North America. The risk of developing CRC is higher in men than in women and is influenced by both environmental and genetic factors [10]. The incidence of CRC per 100,000 inhabitants amounts to 19.4 in men and 15.3 in women [11]. In Germany, the incidence has increased continuously until 2006, with a higher rate in men (34%) than in women (26%) [12]. Mortality has declined throughout the same period, albeit this was more pronounced in women than in men.
Data on clinical and oncologic outcome after surgery for rectal cancer with regard to gender are limited. On the one hand, some studies suggest that anastomotic leakage occurs more frequently in male than in female patients after anterior resection [13,14]. Also, male sex may imply a higher risk for in-hospital mortality [15]. On the other hand, overall morbidity and the number of reoperations have been described to be significantly higher in women than in men [16]. Furthermore, there seems to be an advantage of the female sex with regard to survival following surgery for CRC. However, the available data is conflicting [16,17,18].
The aim of this study was to identify possible gender-related differences regarding clinical and histopathological features as well as oncologic outcome in patients with rectal cancer undergoing surgery.
Patients and Methods
This study encompassed 1,061 patients who underwent surgery for rectal cancer at the Department of Surgery, University Medical Center Schleswig-Holstein Campus Lübeck, Germany, between January 1990 and December 2011. Prospectively documented demographic, clinical, pathological, and follow-up data were obtained after patientss' informed consent and in accordance with the approval of the local Ethical Committee (#07-124). Patients diagnosed with synchronous colon cancer had been excluded in advance. All patients were treated according to best surgical practice, including total mesorectal excision (TME) with high ligation of vessels in rectal cancer of the middle and lower third. Partial mesorectal excision with high ligation of vessels was performed for tumors in the upper rectum. Individual surgical approaches were performed in palliative or emergency situations and in consensus with the local tumor board panel. In accordance with guideline recommendations, neoadjuvant treatment had been offered when indicated.
Analysis encompassed the comparison of clinical, histopathological, and oncological parameters with regard to the subcohorts of male and female patients. In addition, for some parameters possible differences between laparoscopic and open surgery were analyzed, taking gender into consideration.
Continuous variables were expressed as mean or median ± standard deviation and range. Categorical variables were expressed as percent. The Kaplan-Meier curves for female versus male were calculated and assessed for significance by the log-rank test. The 5-year survival rates were estimated using the Kaplan-Meier method. The 95% confidence intervals and the p values were based on an asymptotic approach by using the standard normal distribution.
Student's t test or chi-squared test was performed to compare gender-related differences. All results with p < 0.05 were considered significant. All calculations were performed using SPSS software (IBM SPSS Statistics®; Chicago, IL, USA).
Results
A total of 1,061 patients were identified from our database. Of these, 599 (56.5%) were male and 462 (43.5%) were female. Clinical and histopathological data is listed in table 1. None of the parameters differed significantly between the male and female subcohorts. There was a notable difference in the rate of patients with a Karnofsky index of less than 100 which, however, was still not significant. Both groups were documented to have tumor-related symptoms very frequently with 92.0 and 93.1%, respectively. Symptoms also included subclinical symptoms such as anemia, vague abdominal discomfort, and increased discharge of mucus.
Table 1.
Clinical and histopathological parameters of patients with rectal tumor for the entire cohort as well as for the male and female subcohorts
| Variable | Total (n = 1,061) | Male (n = 599) | Female (n = 462) | p value |
|---|---|---|---|---|
| Median age, years | 67.0 (23–97) | 65.0 (23–94) | 70.0 (31–97) | n.s. |
| Karnofsky < 100% | 458 (43.7%)a | 224 (37.7%)b | 234 (51.5%)c | n.s. |
| Elevated CEA | 244 (41.4%)d | 143 (42.7%)e | 101 (39.6%)f | n.s. |
| Symptomatic | 970 (91.4%) | 551 (92.0%) | 433 (93.1%) | n.s. |
| Neoadjuvant therapy R-status | 166 (15.6%) | 111 (18.5%) | 55 (11.9%) | n.s. |
| R0 | 795 (74.9%) | 454 (75.8%) | 341 (73.8%) | n.s. |
| R1 | 22 (2.1%) | 6 (1.0%) | 16 (3.5%) | n.s. |
| R2 | 244 (23.0%) | 139 (23.2%) | 105 (22.7%) | n.s. |
| Mean LN yield | 15.2 (± 6.0) | 15.0 (± 5.9) | 15.5 (± 6.3) | n.s. |
| UICC | n.s. | |||
| UICC 0 | 17 (1.6%) | 8 (13%) | 9 (1.9%) | |
| UICC I | 246 (23.2%) | 151 (25.2%) | 95 (20.6%) | |
| UICC II | 208 (19.6%) | 116 (19.4%) | 92 (19.9%) | |
| UICC III | 252 (23.8%) | 141 (23.5%) | 111 (24.0%) | |
| UICC IV | 216 (20.4%) | 128 (21.4%) | 88 (19.0%) | |
| UICC xg | 122 (11.5%) | 55 (9.2%) | 67 (14.5%) | |
| T-category | n.s. | |||
| T0 | 56 (5.3) | 29 (4.8%) | 27 (5.8%) | |
| Tis | 17 (1.6%) | 8 (1.3%) | 9 (1.9%) | |
| T1 | 97 (9.1%) | 59 (9.8%) | 38 (8.2%) | |
| T2 | 245 (23.1%) | 143 (23.9%) | 102 (22.1%) | |
| T3 | 461 (43.4%) | 268 (44.7%) | 193 (41.8%) | |
| T4 | 163 (15.4%) | 55 (9.1%) | 57 (13.4%) | |
| Tx | 22 (2.1%) | 37 (6.2%) | 36 (7.8%) | |
| N-category | n.s. | |||
| N0 | 541 (51.0%) | 312 (52.1%) | 229 (49.6%) | |
| N1 | 191 (18.0%) | 111 (18.5%) | 80 (17.3%) | |
| N2 | 199 (18.8%) | 105 (17.5%) | 77 (16.7% | |
| N xg | 147 (13.9%) | 71 (11.9%) | 76 (16.4%) | |
| M-category | n.s. | |||
| M0 | 829 (78.1%) | 465 (77.6%) | 364 (78.8%) | |
| M1 | 216 (20.4%) | 128 (21.4%) | 88 (18.1%) | |
| M xg | 16 (1.5%) | 6 (1.0%) | 10 (2.2%) | |
| Grading | n.s. | |||
| G1 | 19 (1.8%) | 8 (1.3%) | 11 (2.4%) | |
| G2 | 712 (67.1%) | 403 (67.3%) | 310 (67.1%) | |
| G3 | 329 (31.0%) | 187 (31.2%) | 141 (30.5%) | |
| G4 | 1 (0.1%) | 1 (0.2%) | 0 |
LN = Lymph node; n.s. = not significant, CEA = carcinoembryonic antigen;
UICC = Union internationale contre le cancer.
Of n = 1,048 available.
Of n = 594 available.
Of n = 454 available
Of n = 590 available.
Of n = 334 available.
Of n = 255 available.
Not assessable.
The tumor was located in the upper rectum in 293 (27.6%) patients, in the mid rectum in 337 (31.8%) patients, and in the lower third in 431 (40.6%) patients. There were no significant differences regarding the location when analyzing the female and male cohorts separately (table 2).
Table 2.
Location of the rectal tumor, surgical treatment, post-surgery mortality, and length of hospital stay (LOS) for the entire cohort as well as for the male and female subcohorts; data include elective and emergency cases
| Variable | Total (n = 1,061) | Male (n = 599) | Female (n = 462) | p value |
|---|---|---|---|---|
| Tumor location | n.s. | |||
| Upper third, n | 293 (27.6%) | 151 (25.2%) | 142 (30.7%) | |
| Mid third, n | 337 (31.8%) | 195 (32.7%) | 142 (30.7%) | |
| Lower third, n | 431 (40.6%) | 253 (42.2%) | 178 (38.5%) | |
| Surgical treatment | n.s. | |||
| Resection with anastomosis, n | 661 (62.3%) | 380 (63.4%) | 281 (60.8%) | |
| Resection without anastomosis, n | 264 (24.9%) | 154 (25.7%) | 110 (23.8%) | |
| Other, n | 136 (12.8%) | 65 (10.9%) | 71 (15.4%) | |
| 30-day mortality, n | 61 (5.7%) | 29 (4.8%) | 32 (6.9%) | n.s. |
| LOS, days | 15 (1–125) | 15 (1–123) | 15 (1–125) | n.s. |
n.s. = Not significant.
The majority of patients (n = 661, 62.3%) underwent resection with anastomosis. 264 (24.9%) patients underwent resective surgery without anastomosis including extirpation. Other modes of surgical treatment were performed in 136 (12.8%) patients. Table 2 shows the distribution of surgical treatments with regard to male and female patients. There were no statistically significant differences between the two subcohorts.
For the entire cohort, palliative surgery was performed in 266 patients (25%). In the female subcohort, 121 patients (45.5%) underwent palliative surgery, and in the male subcohort, palliative surgery was performed in 145 patients (54.5%). This difference was statistically not significant. Open surgery was performed in 716 (67.5%) patients. Within the female subcohort, 320 patients (69.3%) underwent open surgery, while 396 patients (66.1%) within the male subcohort received open surgery. Laparoscopic surgery was performed in 345 (32.5%) patients. In the female subcohort, 142 patients (30.7%) underwent laparoscopic surgery, and 203 (33.8%) of the male patients were operated on laparoscopically. Conversion rates did not differ significantly with regard to gender, with 8 (1.7%) and 15 (2.5%) patients in the female and male subcohort, respectively.
The median duration of surgery was 220 min (range 35-540 min), with 225 min (range 35-525 min) for the male and 210 min (range 35-540 min) for the female patients, without being statistically significant.
Complications occurred in 330 (31.1%) patients and were statistically not significantly different between the female and male subcohort with 130 (28.1%) and 200 (33.4%) patients, respectively. Male patients more often developed anastomotic leakage than women (n = 64 (16.8%) vs. n = 6 (9.3%); p = 0.005). This difference was slightly pronounced in the laparoscopic subcohort with 6 (6.7%) female patients and 21 (16.8%) male patients being affected (p = 0.0027) but was not significant for the open surgery subcohort. For the entire cohort, gender-related statistically significant differences in complications encompassed burst abdomen, pneumonia, and urinary tract complications all of which occurred more often in men (table 3). When analyzing the open and laparoscopic subcohort, statistical significance for these complications was only found within the group of patients undergoing open surgery (table 3).
Table 3.
Complications after surgery for rectal cancer with regard to gender as well as open and laparoscopic surgery. Significant p values in italics. Numbers and percent relate to individual complications, i.e., one patient may have had one or more complications
| Variable, n | Total |
Open surgery |
Laparoscopic surgery |
||||||
|---|---|---|---|---|---|---|---|---|---|
| female (n = 462) | male (n = 599) | p value | female (n = 320) | male (n = 396) | p value | female (n = 142) | male (n = 203) | p value | |
| Total | 130 (28.1%) | 200 (33.4%) | n.s. | 92 (28.8%) | 138 (34.8%) | n.s. | 38 (26.8%) | 62 (30.5%) | n.s. |
| Hemorrhage | 21 (4.5%) | 24 (4.0%) | n.s. | 16 (5.0%) | 14 (3.5%) | n.s. | 5 (3.5%) | 10 (4.9%) | n.s. |
| Wound healing deficit | 27 (5.8%) | 31 (5.2%) | n.s. | 22 (6.9%) | 23 (5.8%) | n.s. | 5 (3.5%) | 8 (3.9%) | n.s. |
| Burst abdomen | 2 (0.4%) | 16 (2.7%) | 0.005 | 1 (0.3%) | 13 (3.3%) | 0.004 | 1 (0.7%) | 3 (1.5%) | n.s. |
| Ileus/sub-ileus | 6 (1.3%) | 15 (2.5%) | n.s. | 2 (0.6%) | 6 (1.5%) | n.s. | 4 (2.8%) | 9 (4.4%) | n.s. |
| Anastomotic leakage | 43 (9.3%) | 101 (16.8%) | 0.005 | 33 (10.5%) | 67 (16.9%) | n.s. | 10 (6.7%) | 34 (16.8%) | 0.027 |
| Abscess | 9 (1.9%) | 9 (1.5%) | n.s. | 7 (2.2%) | 5 (1.3%) | n.s. | 2 (1.4%) | 4 (2.0%) | n.s. |
| Peritonitis | 7 (1.5%) | 18 (3.0%) | n.s. | 5 (1.6%) | 9 (2.3%) | n.s. | 2 (1.4%) | 9 (4.4%) | n.s. |
| Sepsis | 10 (2.2%) | 16 (2.7%) | n.s. | 6 (1.9%) | 9 (2.3%) | n.s. | 4 (2.8%) | 7 (3.4%) | n.s. |
| Other local complications | 18 (3.9%) | 23 (3.8%) | n.s. | 14 (4.4%) | 10 (2.5%) | n.s. | 4 (2.8%) | 13 (6.4%) | n.s. |
| Hepatic | 2 (0.4%) | 2 (0.2%) | n.s. | 1 (0.3%) | 1 (0.3%) | n.s. | 1 (0.7%) | 0 (0.0%) | n.s. |
| Cardiac | 16 (3.4%) | 24 (4.0%) | n.s. | 12 (3.7%) | 18 (4.5%) | n.s. | 4 (2.8%) | 6 (3.0%) | n.s. |
| Embolism | 1 (0.2%) | 3 (0.3%) | n.s. | 1 (0.3%) | 2 (0.5%) | n.s. | 0 (0.0%) | 0 (0.0%) | n.s. |
| Neurological/psychiatric | 8 (1.7%) | 6 (1.0%) | n.s. | 7 (2.2%) | 5 (1.3%) | n.s. | 1 (0.7%) | 1 (0.5%) | n.s. |
| Pneumonia | 9 (1.9%) | 31 (5.2%) | 0.006 | 6 (1.9%) | 20 (5.1%) | 0.024 | 3 (2.1%) | 11 (5.4%) | n.s. |
| Pulmonary other | 17 (3.7%) | 26 (4.3%) | n.s. | 8 (2.5%) | 20 (5.1%) | n.s. | 9 (6.3%) | 6 (3.0%) | n.s. |
| Nephric | 8 (1.7%) | 20 (3.3%) | n.s. | 6 (1.9%) | 12 (3.0%) | n.s. | 2 (1.4%) | 8 (3.9%) | n.s. |
| Urinary tract | 10 (2.2%) | 27 (4.5%) | 0.039 | 8 (2.5%) | 22 (5.6%) | 0.042 | 2 (1.4%) | 5 (2.5%) | n.s. |
| Other | 37 (8.0%) | 34 (5.7%) | n.s. | 31 (9.8%) | 24 (6.1%) | n.s. | 7 (4.8%) | 15 (7.3%) | n.s. |
n.s. = Not significant.
In 153 (14.4%) patients, reintervention, i.e. surgically and radiologically, endoscopically, or by other means, was necessary, with 57 (12.3%) being female and 96 (16.0%) male patients. The difference was statistically not significant. There were 32 (6.9%) female patients and 29 (4.8%) male patients who died within the first 30 days after surgery.
Median length of hospital stay (LOS) for the entire cohort was 15 days (range 1-125 days) with the same median of 15 days for the female (range 1-125 days) and the male (range 1-123 days) patients (table 2). Within the laparoscopic subcohort, median LOS for the female and male patients was 13 days (range 4-57 and 5-104 days, respectively). For the open subcohort, the median for both female and male patients was 16 days (range 1-125 and 1-123 days, respectively).
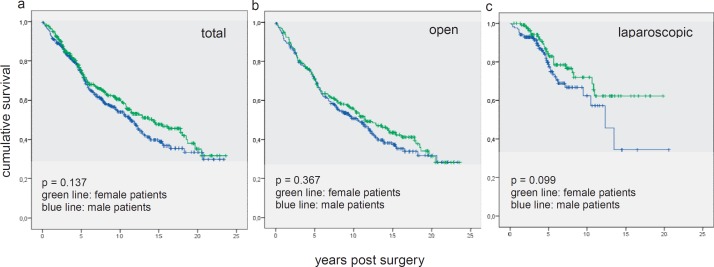
There were no significant differences in survival between the female and the male subcohort (fig. 1a). The 10-year survival rate was 60% for the female patients and 54% for the male patients (p = 0.137). When analyzing the subcohorts of laparoscopically and openly operated patients, again there were no statistically significant differences in survival, with 10-year survival rates of 55 and 51% for the female and male patients, respectively, in the open group (p = 0.367) (fig. 1b). For the laparoscopic group, the 10-year survival rates were 72% for the female and 71% for the male patients (p = 0.099) (fig. 1c).
Fig. 1.
Kaplan-Meier curves for female versus male long-term survival show a not statistically significant advantage for women (a all patients, b open resection, c laparoscopic resection).
Discussion
Worldwide, approximately 1.2 million new patients are diagnosed with CRC per year, with almost 600,000 dying of this malignancy [11,19,20]. Male sex has been identified to be an independent risk factor to develop CRC and, accordingly, the incidence in the male population exceeds the incidence in the female population [11]. Especially for carcinomas of the rectum, the male sex is affected more frequently with a reported incidence rate ratio of 1.63 [21]. In recent years, studies comparing the outcome for the two sexes have reported improved survival of women with CRC [17,22,23].
There may be gender-specific differences in CRC biology. A genome-wide methylation analysis as well as the analysis of specific cancer-related genes demonstrated differential methylation based on colon location, individual age, and gender negatively associated with male sex [24]. Another study described a significantly higher mutation rate in the v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) proto-oncogene in males which is an important event in the methylator pathway of CRC development [25].
In the present study, we did not analyze localization of the CRC with regard to gender but merely focused on a cohort with a carcinoma of the rectum. Screening programs for CRC have been introduced in order to detect (pre-)malignant lesions at an early stage, thereby diminishing the CRC-related mortality [26]. Although there seems to be an adequate awareness of CRC screening programs in general, only a minority of both men and women actually undergo screening investigations. Gender-related differences have been described with regard to perception and psychological barriers towards CRC screening, with men tending to suppress negative views while strongly procrastinating the task of completing screening and with women being withheld by their own reservations about CRC screening-associated distress [26,27].
In our cohort, symptoms occurred very frequently (>90%) in both male and female patients. However, symptoms according to our definition, besides those being clinically evident, also encompassed only mildly apparent symptoms such as anemia or increased anal mucus discharge. We did not analyze the time interval between first onset of symptoms and CRC diagnosis. Concerning this aspect, studies described longer intervals in female than in male patients [28,29].
In a study by Offner et al. [30], male gender was identified as an independent risk factor for the development of severe infection in surgical patients. One of the reasons why women are less likely to have perioperative complications could be explained by the fact that they seem to have a better posttraumatic immune response following major abdominal surgical procedures [5,31]. This may be of relevance for the short- and long-term outcome after colorectal surgery, and studies have addressed the use of sex steroids and antagonists as a therapeutic approach in order to improve perioperative immune dysfunction [5]. Male sex hormones appear to depress immune function whereas female sex steroids lead to an increased humoral immune response [32,33,34].
Much in line with the reported results, we found significantly more infectious pulmonary and urinary complications in men than in women. The differences in these complications were pronounced in the subcohort of patients who underwent open surgery. Although hypothetical, one could argue that the observed difference in anastomotic leakage was related to differences in immune response and thus to wound and anastomotic healing. However, other studies regularly reported higher rates of anastomotic leakage in male patients in comparison to females [13,14].
A recent study reporting on 25,413 patients with CRC showed that male sex was associated with synchronous CRC [35]. Of the 25,413 patients, 884 (3.5%) had synchronous colorectal tumors. Patients with synchronous colorectal carcinoma were older and more often of male sex compared with patients with solitary colorectal carcinoma. In ≥35% of the cases, an extended surgical procedure was conducted. In our study, both cohorts were limited to solitary rectal carcinoma.
In our study, the overall rate of anastomotic leakage differed significantly between men and women. Although a differentiation into grade A, B, and C according to the International Study Group of Rectal Cancer [36] is not reproducible based on our prospective register data, this effect could also be seen in both subgroups (open and laparoscopic resection). Male gender seems to be a risk factor for the development of anastomotic insufficiency after rectal resection. Technical difficulties in a narrow male pelvis are very well known in laparoscopic and open rectal resections. Variation in pelvic diameters, the obstetric conjugate and interspinous distance, may influence the quality of TME, for example. Short distances are associated with bad samples and worse oncologic outcome. There is some evidence that interspinous distance was a predicting factor of a positive circumferential resection margin [37]. In contrast, a multivariate analysis showed that a deep and narrow pelvis with long sacral length, shallow sacral angle, narrow intertuberous diameter, and large tumor size were significantly associated with longer pelvic dissection time but had no influence on postoperative outcome [38].
To which extent different anatomical factors in men and women may lead to a higher rate of anastomotic insufficiency has to be studied.
Wichmann et al. [31] found that women had a significantly longer overall and disease-free survival after a curative resection for CRC when compared to men (57.8 vs. 52.0 months and 51.6 vs. 46.0 months, respectively). This effect was most pronounced above the age of 50 years. Interestingly, this gender difference was only seen in patients diagnosed with rectal cancer and not in those with colon cancer. However, in the present study, there was no significant gender difference in cancer-specific survival in any age category, although there was a tendency of improved long-term survival in women.
Data obtained in this study suggest that there are no gender-related differences in the oncologic surgical treatment of patients with rectal carcinoma. Male sex, however, seems to be a risk factor for early postoperative increased morbidity.
Disclosure Statement
The authors disclose any sponsorship or funding arrangements relating to this publication. The authors declare no conflict of interests.
Acknowledgement
The authors would like to thank Mrs. Claudia Killaitis and Dr. Elisabeth Övermann for administration of the prospective colorectal database in our Department of Surgery.
References
- 1.Regitz-Zagrosek V. Oertelt-Prigione S, Regitz-Zagrosek V, editors. Why do we need Gender Medicine? Sex and Gender Aspects in Clinical Medicine. London, Springer. 2012:1. [Google Scholar]
- 2.Legato MJ. Cardiovascular disease in women: what's different? What's new? What's unresolved? Ann N Y Acad Sci. 1994;736:147–157. doi: 10.1111/j.1749-6632.1994.tb12827.x. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 4.McSweeney JC, Cody M, O'Sullivan P, Elberson K, Moser DK, Garvin BJ. Women's early warning symptoms of acute myocardial infarction. Circulation. 2003;108:2619–2623. doi: 10.1161/01.CIR.0000097116.29625.7C. [DOI] [PubMed] [Google Scholar]
- 5.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. 2014;5:12–19. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oertelt-Prigione S, Regitz-Zagrosek V. (eds) Sex and Gender Aspects in Clinical Medicine. London: Springer; 2012. [Google Scholar]
- 7.Williams ML, Trivedi JR, Doughtie C, Slaughter MS. Is female sex an independent risk factor for perioperative transfusion in coronary artery bypass graft surgery? J Am Coll Surg. 2011;212:362–366. doi: 10.1016/j.jamcollsurg.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Kendel F, Dunkel A, Muller-Tasch T, et al. Gender differences in health-related quality of life after coronary bypass surgery: results from a 1-year follow-up in propensity-matched men and women. Psychosom Med. 2011;73:280–285. doi: 10.1097/PSY.0b013e3182114d35. [DOI] [PubMed] [Google Scholar]
- 9.Inacio MC, Ake CF, Paxton EW, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173:435–441. doi: 10.1001/jamainternmed.2013.3271. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 11.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. 1. [DOI] [PubMed] [Google Scholar]
- 12.Onkopedia. www.dgho-onkopedia.de/de/onkopedia/leitlinien/rektumkarzinom.
- 13.Reilly F, Burke JP, Appelmans E, Manzoor T, Deasy J, McNamara DA. Incidence, risks and outcome of radiological leak following early contrast enema after anterior resection. Int J Colorectal Dis. 2014;29:453–458. doi: 10.1007/s00384-013-1820-8. [DOI] [PubMed] [Google Scholar]
- 14.Kang CY, Halabi WJ, Chaudhry OO, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013;148:65–71. doi: 10.1001/2013.jamasurg.2. [DOI] [PubMed] [Google Scholar]
- 15.Masoomi H, Kang CY, Chen A, et al. Predictive factors of in-hospital mortality in colon and rectal surgery. J Am Coll Surg. 2012;215:255–261. doi: 10.1016/j.jamcollsurg.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 16.van Leeuwen BL, Pahlman L, Gunnarsson U, Sjovall A, Martling A. The effect of age and gender on outcome after treatment for colon carcinoma. A population-based study in the Uppsala and Stockholm region. Crit Rev Oncol Hematol. 2008;67:229–236. doi: 10.1016/j.critrevonc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 17.McArdle CS, McMillan DC, Hole DJ. Male gender adversely affects survival following surgery for colorectal cancer. Br J Surg. 2003;90:711–715. doi: 10.1002/bjs.4098. [DOI] [PubMed] [Google Scholar]
- 18.Wichmann MW, Muller C, Hornung HM, Lau-Werner U, Schildberg FW. Gender differences in long-term survival of patients with colorectal cancer. Br J Surg. 2001;88:1092–1098. doi: 10.1046/j.0007-1323.2001.01819.x. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 20.O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 21.Murphy G, Devesa SS, Cross AJ, Inskip PD, Mc-Glynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668–1675. doi: 10.1002/ijc.25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratto C, Sofo L, Ippoliti M, Merico M, Doglietto GB, Crucitti F. Prognostic factors in colorectal cancer. Literature review for clinical application. Dis Colon Rectum. 1998;41:1033–1049. doi: 10.1007/BF02237397. [DOI] [PubMed] [Google Scholar]
- 23.Iversen LH, Norgaard M, Jepsen P, et al. Trends in colorectal cancer survival in northern Denmark: 1985-2004. Colorectal Dis. 2007;9:210–217. doi: 10.1111/j.1463-1318.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaz AM, Wong CJ, Dzieciatkowski S, Luo Y, Schoen RE, Grady WM. Patterns of DNA methylation in the normal colon vary by anatomical location, gender, and age. Epigenetics. 2014;9:492–502. doi: 10.4161/epi.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clancy C, Burke JP, Kalady MF, Coffey JC. BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e711–718. doi: 10.1111/codi.12427. [DOI] [PubMed] [Google Scholar]
- 26.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 27.Wong RK, Wong ML, Chan YH, Feng Z, Wai CT, Yeoh KG. Gender differences in predictors of colorectal cancer screening uptake: a national cross sectional study based on the health belief model. BMC Public Health. 2013;13:677. doi: 10.1186/1471-2458-13-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esteva M, Leiva A, Ramos M, et al. Factors related with symptom duration until diagnosis and treatment of symptomatic colorectal cancer. BMC Cancer. 2013;13:87. doi: 10.1186/1471-2407-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen AF, Olesen F, Hansen RP, Zachariae R, Vedsted P. Social support, gender and patient delay. Br J Cancer. 2011;104:1249–1255. doi: 10.1038/bjc.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–938. doi: 10.1001/archsurg.134.9.935. discussion 938-940. [DOI] [PubMed] [Google Scholar]
- 31.Wichmann MW, Muller C, Meyer G, et al. Different immune responses to abdominal surgery in men and women. Langenbecks Arch Surg. 2003;387:397–401. doi: 10.1007/s00423-002-0346-2. [DOI] [PubMed] [Google Scholar]
- 32.Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6:366–372. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 34.Panchanathan R, Shen H, Bupp MG, Gould KA, Choubey D. Female and male sex hormones differentially regulate expression of Ifi202, an interferon-inducible lupus susceptibility gene within the Nba2 interval. J Immunol. 2009;183:7031–7038. doi: 10.4049/jimmunol.0802665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Leersum NJ, Aalbers AG, Snijders HS, et al. Synchronous colorectal carcinoma: a risk factor in colorectal cancer surgery. Dis Colon Rectum. 2014;57:460–466. doi: 10.1097/DCR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 36.Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Baik SH, Kim NK, Lee KY, Sohn SK, Cho CH, Kim MJ, Kim H, Shinn RK. Factors influencing pathologic results after total mesorectal excision for rectal cancer: analysis of consecutive 100 cases. Ann Surg Oncol. 2008;15:721–728. doi: 10.1245/s10434-007-9706-z. [DOI] [PubMed] [Google Scholar]
- 38.Kim JY, Kim YW, Kim NK, Hur H, Lee K, Min BS, Cho HJ. Pelvic anatomy as a factor in laparoscopic rectal surgery: a prospective study. Surg Laparosc Endosc Percutan Tech. 2011;21:334–339. doi: 10.1097/SLE.0b013e31822b0dcb. [DOI] [PubMed] [Google Scholar]