Summary
Background
Clostridium difficile infections (CDI) are increasingly important in patients with antibiotic treatments, ranging from mild, self-limiting to severe, life-threatening disease. Currently, diagnostic algorithms and treatment guidelines are being adapted to novel tests and therapeutic options for recurrent CDI.
Methods
A systematic literature search using the terms ‘Clostridium difficile’ and ‘treatment’ was carried out. Current guidelines are being discussed from a clinical point of view.
Results
State-of-the-art diagnostics for C. difficile diagnosis rely on the patient's history, clinical symptoms, and laboratory examination of stool. Recommendations are in favour of glutamate dehydrogenase (GDH) screening tests and confirmatory detection of C. difficile toxin genes (polymerase chain reaction (PCR)). Therapeutic strategies depend on disease severity (mild vs. severe) and endorse metronidazole and vancomycin as well as fidaxomycin for recurrent disease. In very severe cases, surgical therapy is recommended. For relapsing diseases, faecal transfer is considered as a therapeutic option if available.
Conclusion
Current guidelines have been adapted to new pathways in diagnosing CDI and have included statements on novel therapeutic options such as fidaxomycin and faecal transplant for recurrent disease. Depending on the severity of the disease, standard therapy with either metronidazole or vancomycin is recommended.
Keywords: Clostridium difficile, Diagnostics, Guidelines, Therapy
Zusammenfassung
Hintergrund
Infektionen mit Clostridium difficile (CDI) erlangen bei Patienten zunehmend an Bedeutung während und nach einer antibiotischen Behandlung. Das Spektrum reicht von leichten, selbstlimitierenden bis hin zu schweren, lebensbedrohlichen Verläufen. Derzeit werden Diagnosealgorithmen und Behandlungsrichtlinien an neue Tests und Therapiemöglichkeiten angepasst.
Methoden
Eine systematische Literatursuche mit den Begriffen ‘Clostridium difficile’ und ‘Behandlung’ wurde durchgeführt, und die aktuellen Leitlinien werden aus einer klinischen Perspektive beleuchtet.
Ergebnisse
Im Mittelpunkt der Diagnosestellung einer CDI stehen weiterhin Anamnese, klinische Beurteilung und Laboruntersuchungen des Stuhls. Aktuell wird ein Screening auf Glutamatdehydrogenase (GDH) mit Bestätigungsnachweisverfahren mittels PCR (Polymerase-Kettenreaktion) der Toxingene von C. difficile empfohlen. Therapeutische Strategien hängen von der Schwere der Erkrankung ab (mild bis schwer) und stützen sich auf Metronidazol und Vancomycin sowie Fidaxomycin bei Rezidiven. In sehr schweren Fällen wird die chirurgische Therapie empfohlen. Für rezidivierende Erkrankungen steht vielerorts mit der fäkalen Bakteriotherapie zudem eine vielversprechende Option zur Verfügung.
Schlussfolgerung
Die aktuellen Leitlinien zur CDI berücksichtigen neue Wege in der Diagnostik und beurteilen neue therapeutische Optionen wie Fidaxomycin und die fäkale Bakteriotherapie für rezidivierende Erkrankungen. Abhängig von der Schwere der Erkrankung besteht die Standardtherapie weiterhin in der Gabe von Metronidazol oder Vancomycin.
Introduction
Clostridium difficileis an anaerobic Gram-positive bacterium that can cause antibiotic-associated diarrhoea. Normal gut flora resists colonisation and overgrowth with C. difficile[1]. The use of antibiotics alters the intestinal microbiome and allows proliferation of C. difficile. Colonization occurs by the faecal-oral route. Hospitalized patients are the primary targets of C. difficile infection (CDI) although C. difficile is present as a colonizer in 2-3% of healthy adults and in as many as 70% of healthy infants [2]. CDI is associated with antibiotic treatments and may occur in every clinical setting. Current data suggest that 20% of individuals who are hospitalized become colonized with C. difficile during hospitalization, and about 30% of these patients develop diarrhoea [3]. CDI commonly manifests as mild-to-moderate diarrhoeal disease with abdominal cramping. In severe cases CDI can present with acute abdomen as well as fulminant, life-threatening colitis. The diagnosis of C. difficile colitis should be suspected in any patient with diarrhoea who has received antibiotics within the previous 3 months, who has been recently hospitalized, and/or who has an occurrence of diarrhoea 48 h or more after hospitalization [4]. However, recent studies have shown that C. difficile can be a cause of diarrhoea in community dwellers without previous hospitalization or antibiotic exposure [5]. C. difficile produces heat-resistant spores that can persist in the environment for several months, thus providing the basis for nosocomial outbreaks even after extensive cleaning measures.
Pathophysiology of Clostridium difficile Infection
Pathogenic strains of C. difficile produce multiple distinct toxins [6]. The best characterized toxins are toxin A, which is an enterotoxin, and toxin B, a cytotoxin. Both are high molecular weight proteins (308 kDa and 270 kDa) capable of binding to specific receptors on intestinal mucosal cells. Receptor-bound toxins gain intracellular entry by catalysing a specific alteration (inactivation) of Rho proteins (small glutamyl transpeptidase(GTP)-binding proteins) that assist in actin polymerization, cytoskeletal architecture, and cell movement. Both toxin A and toxin B appear to play a role in the pathogenesis of C. difficile colitis in humans, mainly by induction of apoptosis in target mucosal cells. Toxin damage of the colonic mucosa leads to an accumulation of fibrin, mucin, and dead cells, finally forming a layer of debris in the colon (pseudomembrane). Subsequent inflammatory activation adds to the direct toxin-associated damage resulting in mild diarrhoeal disease up to extensive intestinal wall damage with septic shock and death.
Clinical Presentation of Clostridium difficile Infection
Several societies of gastroenterologists as well as infectious disease specialists have recently published updates of CDI management guidelines [7,8,9]. The most recent guidance document has been released by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in 2014 [8]. An up-to-date definition of an episode of CDI comprises a clinical picture (table 1) compatible with CDI, microbiological evidence of free toxins, and the presence of C. difficile in stool without reasonable evidence of another cause of diarrhoea or pseudomembranous colitis (diagnosed by endoscopy, after colectomy, or on autopsy).
Table 1.
Patient characteristics correlating with disease severity when associated with CDI (adapted from [8])
| Category | Signals/Symptoms |
|---|---|
| Physical examination | fever, rigors, hemodynamic instability including signs of shock, respiratory failure with need for mechanical ventilation, peritonism, ileus |
| Laboratory tests | leukocytosis > 15 Gpt/l, left shift with >20% neutrophils, rise in serum creatinine >1.5 × baseline, lactate > 5 mmol/l, albumin < 30 g/l |
| Endoscopy | presence of pseudomembranes |
| Imaging | colonic distension > 6 cm in transverse colon/toxic megacolon, colonic wall thickening, pericolonic fat stranding, ascites due to CDI |
Severity of Disease
Severe CDI is defined as an episode of CDI with one or more specific signs and symptoms of severe colitis or a complicated course of disease with systemic toxin effects, shock, or even death. Severe CDI requires intensive care unit (ICU) treatment and/or colectomy [10]. On the contrary, the course can be very mild and self-limiting, especially once the causing antibiotic is ceased. The challenge lies in the prediction of severe disease and in the awareness for risk factors of an unfavourable outcome as shown in table 2[11].
Table 2.
Prognostic markers that can be used to determine severe CDI
| Characteristics | Degree of recommendation | Quality of evidence | Reference |
|---|---|---|---|
| Age (>65 years) | A | IIr | [12] |
| Marked leukocytosis (leukocyte count > 15 Gpt/l) | A | IIrht | [12] |
| Decreased blood albumin (<30 g/l) | A | IIr | [12] |
| Rise in serum creatinine level (>133 μmol/l or >1.5 times the premorbid level) | A | IIht | [12] |
| Comorbidity (severe underlying disease and/or immunodeficiency) | B | IIht | [13] |
Diagnosis of Clostridium difficile Infection
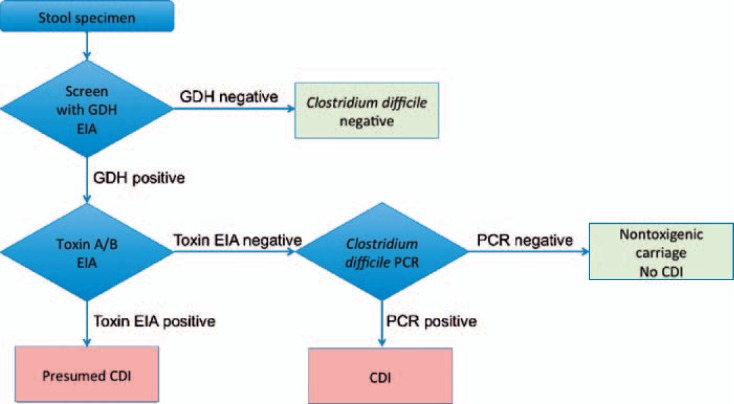
As mentioned above, the diagnosis of CDI is based on a combination of signs and symptoms and confirmed by microbiological evidence of C. difficile and toxin-producing C. difficile in stools, given the absence of another cause. The presence of pseudomembranes on colonoscopy or histopathology can help to establish the diagnosis of CDI; however, it cannot rule out CDI. Laboratory workup uses several tests for the detection of C. difficile and its toxins. Diagnostic tests for CDI include the cell culture-based cytotoxicity test, which has been formerly regarded as the gold standard test in terms of sensitivity and specificity, but is very laborious and has been abandoned for commercial reasons. Further available tests are glutamate dehydrogenase (GDH) and toxin A and/or B enzyme immune assays (EIAs). Toxigenic culture of C. difficile may be performed in specialized laboratories and more often nucleic amplification tests (NAAT) targeting either 16S rRNA genes, toxin genes, and GDH genes are available to the physicians in care of CDI patients. ESCMID recommendations are clearly in favour of a two- or three-stage algorithm combining a sensitive screening test with a more specific confirmatory test to diagnose CDI (fig. 1) [8]. Suspected stools should first be screened with an EIA detecting GDH. Like bacterial culture, tests that detect GDH do not distinguish toxin-producing from non-toxigenic C. difficile isolates [12]. The rationale for the approach is indeed that GDH is produced in significantly higher quantities than the C. difficile toxin and should yield a more sensitive assay than solid-phase toxin A/B EIAs. The commercial GDH tests offer a turnaround time of 15-45 min, compatible with laboratory routine needs. The greatest utility of stool GDH assays appears to be a screen to rule out negative specimens and to select specimens for further testing. Published sensitivity of GDH tests is about 90% with a specificity of 93% [13]. Alternatively, EIAs detecting toxins A and/or B (sensitivity ranging from 44 to 99% and specificity from 75 to 100%) or NAAT detecting toxin B gene (tcdB) may be used as screening tests. Samples with a negative test result can be reported as negative, while samples with a positive first test result should be retested with a method to detect free toxins. The multi-step procedure to ensure presence of free toxins in the patient's faeces should allow discrimination of CDI from asymptomatic colonization. Two commonly recommended methods in the laboratory diagnosis of CDI are the use of a GDH screening confirmed by NAAT to detect toxigenic strains. Diarrhoea is defined as loose stools, i.e. taking the shape of the receptacle or corresponding to the Bristol stool chart types 5-7, plus a stool frequency of three bowel movements in 24 h or fewer consecutive hours (definition by World Health Organisation; www.who.int/topics/diarrhoea). Testing of formed stool and/or retesting of faeces of patients with formerly confirmed CDI is not recommended due to the possibility of false positive test results in formed stools and asymptomatic carrier stage after an episode of CDI.
Fig. 1.
Diagnostic algorithm for the laboratory diagnosis of CDI.
Rational Concepts in the Therapy of Clostridium difficile Infection
Once the diagnosis of a CDI is made, it is necessary to apply appropriate infection control measures to prevent further spread within the ward or hospital. To prevent spreading of Clostridium spores, hands need to be washed, patients are to be isolated, and the use of gloves and protective clothing by the staff is necessary in addition to regular hand hygiene after patient contact [14]. In all patients with CDI it is mandatory to consider stopping the causing antibiotic therapy. This may be sufficient as the only treatment in a patient with little symptoms. Patients need adequate replacement and monitoring of fluids and electrolytes. Antimotility medications should be avoided.
Nowadays, all recommendations for antibiotic therapy are based on differentiation between mild-to-moderate or severe disease [8]. The American guidelines further differentiate a severe and complicated course [7,9]. For the clinician to successfully treat CDI it is most important to screen for risk factors of severe disease and parameters associated with an unfavourable outcome. There are many clinical and laboratory variables that correlate with severity of CDI as shown in table 1[8]. The most important prognostic markers for the development of severe disease (table 2) seem to be age > 65 years, leukocytosis (>15 Gpt/l), decreased serum albumin (<3 g/l), rise in serum creatinine (>133 µmol/l or >1.5 times of the premorbid level) and underlying co-morbidities [15,16,17].
In a patient with strong suspicion of CDI empiric treatment is encouraged by European and American guidelines. We recommend this approach merely for patients with severe disease or risk factors for an unfavourable outcome. Again discontinuation of unnecessary antibiotic treatment is mandatory.
Mild-to-Moderate CDI
In cases of mild-to-moderate or non-severe CDI, the ESCMID guideline recommends for CDI clearly induced by antibiotics after stopping the inducing antibiotic to observe clinical response and to begin antibiotic treatment with metronidazole if clinical deterioration occurs [8]. The most commonly used and studied antibiotics to treat CDI are metronidazole and vancomycin. Metronidazole 500 mg orally three times a day for 10 days is the recommended initial antibiotic treatment in mild-to-moderate disease as it has been shown to be effective in inducing a clinical response and no statistically significant difference compared to vancomycin [18]. In 335 patients, symptomatic cure rates were 71% for metronidazole and 79% in patients treated with vancomycin. Other studies show the inferiority of metronidazole compared to vancomycin and a faster symptomatic response to a treatment with vancomycin [19]. When treating patients with oral or intravenous metronidazole, one should be aware of its possible side effects. Most patients state gastrointestinal complaints and a metallic taste; however, peripheral and optic neuropathy can occur in long-term treatment [20,21]. Besides higher treatment costs of oral vancomycin, there is concern of inducing vancomycin-resistant enterococci, although this is merely a theoretical concern and clinical evidence is lacking [22]. Changes in antibiotic resistance have been reported for both antibiotics in specific ribotypes causing CDI. This emerges merely from in vitro studies, and so far there are no reports that treatment failure could be linked to antimicrobial resistance to metronidazole or vancomycin [23].
Although not recommended for the first episode of mild-to-moderate CDI, fidaxomycin has proven its efficacy in the treatment of these patients with cure rates of up to 88% [24,25]. 200 mg are administered orally twice daily. The advantage of this drug is the lower risk of recurrence. Therefore, it is being discussed primarily for recurrent CDI but could also be considered as first-line therapy in patients with a high risk of recurrence (table 3) [26].
Table 3.
Summary of treatment recommendations according to disease severity (adapted from [9])
| Severity | Criteria | Treatment | Comment |
|---|---|---|---|
| Mild-to-moderate disease | diarrhoea, no signs or symptoms of severe disease | metronidazole 500 mg p.o. 3×/day for 10 days | if no improvement in 5–7 days switch to vancomycin 4 × 125 mg p.o. |
| Severe disease |
|
vancomycin 125 mg p.o. 4×/day for 10 days | other authors consider age < 65 years and a rise in creatinine >1.5 × baseline as equal risk factors for severe disease |
| Severe and complicated disease |
|
vancomycin 500 mg p.o. 4×/day and metronidazole 500 mg i.v. 3×/day and vancomycin per rectum (500 mg vancomycin in 500 ml Nalco 0.9%) 2–4×/day | consider surgical consultation |
Although not included in current guidelines, it is worth mentioning that oral teicoplanin, a glycopeptide not available in the USA, has equal clinical cure rates as vancomycin and a licensed indication for the treatment of CDI (teicoplanin p.o. 100 mg two times per day) [27]. Additionally, the American guideline recommends reviewing the response to therapy in a patient with mild-to-moderate CDI and switching to vancomycin if the clinical symptoms do not improve after 5-7 days [7]. In patients intolerant or allergic to metronidazole or in pregnant or breast-feeding women, Surawicz et al. [9]] recommend vancomycin in standard dosing. To this day, there is insufficient evidence to recommend the use of probiotics, toxin-binding resins and polymers, or monoclonal antibodies [8].
Severe CDI and Severe and Complicated CDI
Patients with severe disease should be treated with oral vancomycin 125 mg four times daily for 10 days. Since the definition of severity varies among treatment studies and in other trials, treatment response is not specified for the severity of CDI. This decision is based merely on predictors and clinical signs associated with a severe course of disease. Single studies show the inferiority of metronidazole over vancomycin, with clinical cure rates of 72.7 versus 81.1% [28] and 97 vs. 76% for severe disease [29]. Fidaxomycin has been shown to be non-inferior to vancomycin although severely ill patients were excluded from these trials [24,25]. The use of very high doses of vancomycin was recommended for severe and complicated disease in the Infectious Diseases Society of America (IDSA) guideline [7]. However, there is no evidence that these higher doses change the clinical course considering duration of diarrhoea, rate of recurrence, or microbiological cure [30.] As shown in table 3, the American guidelines discuss a third group of patients with severe and complicated disease. In these patients with fulminant, life-threatening disease, signs of systemic toxicity, peritonitis, or toxic colonic dilation, it is crucial to consider surgical intervention. It remains a major challenge to predict the clinical course and response to medical treatment in patients with complicated CDI. This is further complicated by high mortality rates of emergency operations for CDI ranging from 19 to 71% [31]. It has become evident that early colectomy can lower mortality and improve survival [32]. The more negative prognostic signs (shock with need for vasopressors, lactate > 5 mmol/l, mental status changes, end organ failure, need for intubation and ventilation) a patient has, the earlier surgical treatment should be considered. The established operative management of severe, complicated CDI is subtotal colectomy with end ileostomy. Although not supported by the ESCMID guideline, Surawicz et al. [9] as well as the IDSA [7] recommend treatment with vancomycin orally in a higher dose of 500 mg four times daily and rectally (500 mg in 500 ml normal saline) up to four times a day in combination with metronidazole intravenously (500 mg three times a day) as the treatment of choice in patients with complicated CDI. Especially in patients with ileus or toxic colonic dilation and inability to tolerate oral intake, adjunctive use of rectal vancomycin and intravenous metronidazole has been shown to be effective [33,34].
Recurrent CDI
The risk of recurrence of CDI after an initial episode is reported to be 10-20% within 8 weeks and further increases with every other episode up to 40-65% [35]. It is considered similar for the treatment with metronidazole and vancomycin. Fewer secondary recurrences are reported after treatment with fidaxomycin for patients with mild-to-moderate disease [36]. The first recurrence can be treated with the same regimens used for the initial episode, depending on the severity of disease. Especially in patients with risk of further recurrence (table 4), treatment with fidaxomycin should be considered.
Table 4.
Prognostic markers that can be used to predict risk of recurrent CDI (adapted from [8])
|
For the second relapse of CDI, metronidazole is no longer recommended based upon concerns regarding its side effects, especially neuropathy. In this situation fidaxomycin (200 mg twice daily for 10 days) or vancomycin (125 mg four times daily for 10 days) followed by either a pulsed or tapered strategy is recommended. Up to now, there is no single strategy that can be recommended; however, McFarland et al. [35] could reduce the frequency of relapse to 14.3% by a pulsed regime up and to 31% by using a tapered strategy [37]. The pulsed strategy proposed requires a standard vancomycin course over 10 days followed by 125 mg vancomycin every 2-3 days for 10 doses. Of the multiple strategies used for tapering vancomycin, the IDSA guidelines 2010 recommended stepping down to 125 mg twice daily for a week after the regular 10 days of vancomycin, followed by 125 mg once daily for a week which is then followed by pulse of 125 mg every 2-3 days for 2-8 weeks [7].
For multiple recurrences, the European as well as the American guideline on CDI recommend that faecal microbiota transplant (FMT) should be considered for these patients. FMT means the instillation of faecal bacteria from a healthy person into a sick recipient in order to cure a certain disease. During the past few years, studies have investigated this treatment for recurrent CDI by endoscopically administering the faeces in the duodenum, in the ileocolon, or by enema. Cure rates reported are approximately 92% in a systematic review of 317 patients [38]. Long-term follow-up of FMT is limited and more research is needed to determine the optimal route of administration [39].
Disclosure Statement
Both authors declare no conflict of interest.
References
- 1.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14:82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Carroll KC, Bartlett JG. Biology of Clostridium difficile implications for epidemiology and diagnosis. Ann Rev Microbiol. 2011;65:501–521. doi: 10.1146/annurev-micro-090110-102824. [DOI] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011;8:17–26. doi: 10.1038/nrgastro.2010.190. [DOI] [PubMed] [Google Scholar]
- 4.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Ad Hoc Clostridium difficile Surveillance Working Group Recommendations for surveillance of Clostridium difficileassociated disease. Infect Control Hosp Epidemiol. 2007;28:140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 5.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, Harmsen WS, Zinsmeister AR. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rineh A, Kelso MJ, Vatansever F, Tegos GP, Hamblin MR. Clostridium difficile infection: molecular pathogenesis and novel therapeutics. Expert Rev Anti Infect Ther. 2014;12:131–150. doi: 10.1586/14787210.2014.866515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH Society for Healthcare Epidemiology of America; Infectious Diseases Society of America Clinical practice guidelines for Clostridium difficile infection in adults. 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 8.Debast SB, Bauer MP, Kuijper EJ Committee European Society of Clinical Microbiology and Infectious Diseases. update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 9.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 10.Sailhamer EA, Carson K, Chang Y, Zacharias N, Spaniolas K, Tabbara M, Alam HB, DeMoya MA, Velmahos GC. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144:433–439. doi: 10.1001/archsurg.2009.51. discussion 439-440. [DOI] [PubMed] [Google Scholar]
- 11.Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008;196:384–388. doi: 10.1016/j.amjsurg.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Barbut F, Lalande V, Daprey G, Cohen P, Marle N, Burghoffer B, Petit JC. Usefulness of simultaneous detection of toxin A and glutamate dehydrogenase for the diagnosis of Clostridium difficileassociated diseases. Eur J Clin Microbiol Infect Dis. 2000;19:481–484. doi: 10.1007/s100960000297. [DOI] [PubMed] [Google Scholar]
- 13.Eastwood K, Else P, Charlett A, Wilcox M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol. 2009;47:3211–3217. doi: 10.1128/JCM.01082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vonberg RP, Kuijper EJ, Wilcox MH, Barbut F, Tüll P, Gastmeier P European C difficileInfection Control Group; European Centre for Disease Prevention and Control (ECDC) van den Broek PJ, Colville A, Coignard B, Daha T, Debast S, Duerden BI, van den Hof S, van der Kooi T, Maarleveld HJ, Nagy E, Notermans DW, O'Driscoll J, Patel B, Stone S, Wiuff C. Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect. 2008;14(suppl 5):2–20. doi: 10.1111/j.1469-0691.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- 15.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficileassociated disease. Emerg Infect Dis. 2009;15:415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lungulescu OA, Cao W, Gatskevich E, Tlhabano L, Stratidis JG. CSI: a severity index for Clostridium difficile infection at the time of admission. J Hosp Infect. 2011;79:151–154. doi: 10.1016/j.jhin.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Abou Chakra CN, Pepin J, Valiquette L. Prediction tools for unfavourable outcomes in Clostridium difficile infection: a systematic review. PloS One. 2012;7:e30258. doi: 10.1371/journal.pone.0030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drekonja DM, Butler M, MacDonald R, Bliss D, Filice GA, Rector TS, Wilt TJ. Comparative effectiveness of Clostridium difficile treatments: a systematic review. Ann Intern Med. 2011;155:839–847. doi: 10.7326/0003-4819-155-12-201112200-00007. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox MH, Howe R. Diarrhoea caused by Clostridium difficile response time for treatment with metronidazole and vancomycin. J Antimicrob Chemother. 1995;36:673–679. doi: 10.1093/jac/36.4.673. [DOI] [PubMed] [Google Scholar]
- 20.Lofmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50(suppl 1):S16–23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 21.McGrath NM, Kent-Smith B, Sharp DM. Reversible optic neuropathy due to metronidazole. Clin Experiment Ophthalmol. 2007;35:585–586. doi: 10.1111/j.1442-9071.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller M, Bernard L, Thompson M, Grima D, Pepin J. Lack of increased colonization with vancomycin-resistant enterococci during preferential use of vancomycin for treatment during an outbreak of healthcare-associated Clostridium difficile infection. Infect Control Hosp Epidemiol. 2010;31:710–715. doi: 10.1086/653613. [DOI] [PubMed] [Google Scholar]
- 23.Baines SD, O'Connor R, Freeman J, Fawley WN, Harmanus C, Mastrantonio P, Kuijper EJ, Wilcox MH. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother. 2008;62:1046–1052. doi: 10.1093/jac/dkn313. [DOI] [PubMed] [Google Scholar]
- 24.Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, Sears P, Gorbach S OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–289. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 25.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. OPT-80-003 Clinical Study Group Fidaxomicin versus vancomycin for Clostridium difficile infection. New Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 26.Hu MY, Katchar K, Kyne L, Maroo S, Tummala S, Dreisbach V, Xu H, Leffler DA, Kelly CP. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136:1206–1214. doi: 10.1053/j.gastro.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 27.de Lalla F, Nicolin R, Rinaldi E, Scarpellini P, Rigoli R, Manfrin V, Tramarin A. Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficileassociated diarrhea. Antimicrob Agents Chemother. 1992;36:2192–2196. doi: 10.1128/aac.36.10.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan-Taber S, Fitts D, Gelone SP, Broom C, Davidson DM. Polymer Alternative for CDI Treatment (PACT) investigators Vancomycin, metronidazole, or tolevamer for Clostridium difficileinfection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345–354. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 29.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficileassociated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 30.Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med. 1989;86:15–19. doi: 10.1016/0002-9343(89)90223-4. [DOI] [PubMed] [Google Scholar]
- 31.Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P. West Midlands Research Collaborative Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. 2012;99:1501–1513. doi: 10.1002/bjs.8868. [DOI] [PubMed] [Google Scholar]
- 32.Koss K, Clark MA, Sanders DS, Morton D, Keighley MR, Goh J. The outcome of surgery in fulminant Clostridium difficile colitis. Colorectal Dis. 2006;8:149–154. doi: 10.1111/j.1463-1318.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 33.Longo WE, Mazuski JE, Virgo KS, Lee P, Bahadursingh AN, Johnson FE. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum. 2004;47:1620–1626. doi: 10.1007/s10350-004-0672-2. [DOI] [PubMed] [Google Scholar]
- 34.Olson MM, Shanholtzer CJ, Lee JT, Jr, Gerding DN. Ten years of prospective Clostridium difficileassociated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991. Infect Control Hosp Epidemiol. 1994;15:371–381. doi: 10.1086/646934. [DOI] [PubMed] [Google Scholar]
- 35.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 36.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154–161. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tedesco FJ, Gordon D, Fortson WC. Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am J Gastroenterol. 1985;80:867–868. [PubMed] [Google Scholar]
- 38.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 39.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]