Summary
Background
Liver abscess (LA) is an uncommon but potentially life-threatening disease with significant morbidity and mortality.
Methods
This review comprehensively describes epidemiology, pathogenesis, diagnosis, and treatment of LA, with a strong focus on antimicrobial treatment choices and the impact of multidrug-resistant pathogens.
Results
In industrialized areas, pyogenic liver abscess (PLA) accounts for over 80% of the cases, whereas Entamoeba histolyticais responsible for up to 10% of the cases, with a higher incidence in tropical areas. Highly virulent strains of Klebsiella pneumoniaehave emerged as a predominant cause of PLA in Asian countries and tend to spread to the USA, Australia, and European countries, therefore requiring special alertness. Most common symptoms of LA are fever, chills, and right upper quadrant abdominal pain, although a broad spectrum of non-specific symptoms may also occur.
Conclusion
Imaging studies (ultrasound, computed tomography scan) and microbiological findings play a crucial role in the diagnosis of LA. The treatment of choice for PLA is a multimodal approach combining broad-spectrum antibiotics and aspiration or drainage of larger abscess cavities. Amebic LA can be cured by metronidazole therapy without drainage.
Keywords: Liver abscess, Pyogenic liver abscess, Klebsiella pneumoniae, Multidrug-resistant pathogens, Amebic liver abscess, Entamoeba histolytica, Antibiotics, Aspiration, Drainage
Zusammenfassung
Hintergrund
Leberabszesse (LA) sind eine seltene, jedoch potenziell lebensbedrohliche Erkrankung mit relevanter Morbidität und Mortalität.
Methoden
Diese Übersicht beschreibt ausführlich Epidemiologie, Pathogenese, diagnostische Verfahren und Behandlung des LA. Der Schwerpunkt der Arbeit liegt dabei auf der Auswahl der geeigneten antibiotischen Therapie sowie des Managements bei Nachweis multiresistenter Erreger.
Ergebnisse
Eitrige/pyogene Leberabszesse (PLA) sind mit über 80% die häufigste Entität in industrialisierten Gebieten, während Entamoeba histolyticain ca. 10% der Fälle als Erreger identifiziert werden kann. In tropischen Gebieten ist die Inzidenz der Amöben-LA deutlich höher. Hochvirulente Stämme von Klebsiella pneumoniae haben sich in Asien zur Hauptursache der PLA entwickelt. Die zunehmende Verbreitung von K. pneumoniae auch in den USA, in Australien und in europäischen Ländern stellt eine Herausforderung für die Behandlung und Prävention von Sekundärkomplikationen dar. Typische Symptome bei LA sind Fieber, Schüttelfrost und rechtsseitiger Oberbauchschmerz, obgleich eine Vielzahl anderer, unspezifischer Symptome auftreten kann.
Schlussfolgerung
Bildgebung (Ultraschall, Computertomographie) sowie mikrobiologische Untersuchungen sind geeignete diagnostische Verfahren bei klinischem Verdacht auf einen LA. Das multimodale Management des PLA beinhaltet die Therapie mit einem Breitspektrum-Antibiotikum sowie die Aspiration oder Drainage größerer Abszesse. Amöben-LA können durch Metronidazol geheilt werden und benötigen keine Drainage.
Introduction
Liver abscess (LA) is an uncommon but potentially life-threatening disease with significant morbidity and mortality. LA have been described as far back as in ancient Greece in 400 B.C., when Hippocrates thought that prognosis of LA was related to the type of fluid in the lesion [1]. In 1890, Sir William Osler was the first to describe the presence of amebae in a patient's abscess and stools; however, it took until the early 20th century to conclude that Entamoeba histolytica was causally correlated to abscess formation in the liver [2]. In addition to pyogenic bacteria and E. histolytica, other pathogens, such as fungi and cytomegalovirus, can also cause LA, albeit rarely and especially in immunosuppressed patients.
In this review article, we comprehensively describe epidemiology, pathogenesis, diagnosis, and treatment of LA, with a strong focus on antimicrobial treatment choices and the impact of multidrug-resistant pathogens.
Epidemiology
In the first half of the last century and prior to the introduction of antibiotics, suppurative appendicitis was mainly responsible for the formation of pyogenic liver abscesses (PLA) [2,3]. In the last decades, however, hepatobiliary hitches have replaced portal pyemia and hematogenous spread as the most common cause of PLA [1,2,3,4]. The most relevant pathogens are Escherichia coli, Klebsiellaspp., and Enterococcusspp. Among anaerobic bacteria, Bacteroidesspp. and Fusobacteriumspp. predominate [1,2]. Aerobic, anaerobic, or microaerophilic streptococci are isolated in 25-30% of cultures from PLA. In a high proportion of cases polymicrobial bacterial infection is recorded, with a strong etiological shift towards Klebsiella pneumoniae [1,3,4] (table 1, 2).
Table 1.
Microbiological findings in 63 patients with PLA who attended a tertiary hospital in Hong Kong between 1991 and 2001 (modified from [3])
| Number of cultures, % |
||
|---|---|---|
| abscesses (n = 44) | blood (n = 46) | |
| Klebsiella | 26 (43) | 22 (35) |
| Escherichia coli | 12 (20) | 13 (20) |
| Streptococcus milleri | 7 (12) | 5 (8) |
| Anerobes | ||
| Bacteriodes fragilis | 6 (10) | 8 (13) |
| Peptostreptococcus | – | 2 (3) |
| Clostridium sordelii | – | 1 (1.5) |
| Fusobacterium | – | 1 (1.5) |
| Enterococcus | 3 (5) | 3 (5) |
| Miscellaneous Gram-negative organisms | 6 (10) | 8 (13) |
| Total isolates | 60 (100) | 63 (100) |
Table 2.
Number of bacterial isolates recovered per case in patients with PLA who attended two municipal hospitals in New York City over a 10-year period (modified from [1])
| Number of isolates | Cases, % |
|---|---|
| 0 | 31.2 |
| 1 | 44.2 |
| 2 | 15.6 |
| 3 | 6.5 |
| 4 | 2.6 |
In the USA, the incidence of PLA is 8-15/100,000 inhabitants/year, accounting for over 80% of LA cases [1]. Abscesses due to E. histolytica account for up to 10% of the cases, and are more common in tropical areas as well as in tourists and in immigrants from developing countries, while fungi and other infectious agents are responsible for less than 10%.
Before the 1980s, E. coliwas the most common bacterial pathogen that caused PLA. During the past three decades, however, highly virulent strains of K. pneumoniaehave emerged as a predominant cause in Asian countries, especially Taiwan [1,2,3,4]. The exact reason for this observation is not known, but it may be related to different host susceptibility to K. pneumoniae infection, expression of K. pneumoniae-specific virulence factors, higher K. pneumoniae carriage rates compared to the USA and Europe, and environmental factors [4]. For example, Chung et al. [5,6] noted that people of Korean ethnicity who had lived in countries other than Korea had a lower proportion of carrying serotype K1 of K. pneumoniaestrains than those who lived in Korea. These findings indicate a potential role of environmental factors in intestinal colonization by these strains. To a lesser extent, the shift towards K. pneumoniae has also been observed in the USA, in Europe, and in Australia [1,4,7,8,9,10].
Middle-to-older-aged patients are at higher risk of developing PLA [1,2]. The peak incidence is found in 50- to 65-year-old patients, whereas PLA in children are rare. Male dominance is observed in patients with PLA, and the male to female ratio is approximately 1.5-2.5 [1,2,3,4,11]. According to the literature, mortality is ranging from 5-26% [1,2,3,4,5,12,13,14,15]; however, owing to the improvement of diagnosis and treatment strategies, it has decreased in recent years and is now less than 10% [2,4].
Amebic LA was a relentlessly progressive and almost invariably fatal disease little more than a century ago, but since the introduction of effective medical treatment and rapid diagnosis, mortality rates have fallen to 1-3% [16].
Etiology
In contrast to other bacterial etiologies, PLA caused by K. pneumoniae are usually primary and cryptogenic. SinceK. pneumoniae strains may colonize the human gastrointestinal tract, colonization is commonly anticipated to precede invasion of the intestinal mucosa and portal venous flow or ascending biliary infection [4]. Some studies have suggested that translocation from the gastrointestinal tract is the most likely route by which K. pneumoniae causes formation of LA [4,11]. Fung et al. [14,15] demonstrated that gastrointestinal carriage is a strong predisposing factor, and Lin et al. [17] reported a fecal carriage rate of K. pneumoniae in healthy adults of 75% and a high prevalence rate of serotype K1/K2 isolates with the regulator of mucoid phenotype A (rmpA) plasmid gene among typical strains in Taiwan. In addition to intestinal colonization, cryptogenic invasive K. pneumoniae PLA are frequently associated with diabetes mellitus [4,11,12], but do not show any clear association with peritoneal sources of infections, such as hepatobiliary obstruction, pancreatitis, enterocolitis, or malignomas [2,4,11]. Diabetes mellitus correlates with a high incidence of serotype K1 in K. pneumoniae PLA [18,19]. Poor glycemic control plays an important role in impairing the neutrophil phagocytic function of patients with K1/K2-type K. pneumoniae PLA [4,11,18,19,20,21].
Amebic LA arise from hematogenous spread (probably via the portal circulation) of amebic trophozoites that have breached the colonic mucosa [16]. Only few individuals presenting with amebic LA have concurrent amebic colitis, but the majority of patients have no bowel symptoms, and stool microscopy is usually negative for E. histolyticatrophozoites and cysts.
Clinical Manifestations
Clinical presentations of patients with LA are not typical, and patients may present with vague constitutional symptoms. Most common are fever and chills, followed by abdominal pain restricted to the right upper quadrant, and hepatic tenderness [1,2,3,4]. Fever is a predominant symptom and has been reported in 90-95% of the cases [1,2,3,4] (table 3). A broad spectrum of non-specific symptoms like diarrhea, jaundice, right pleural effusion, anorexia, nausea, and vomiting may also occur [1,11,15].
Table 3.
Presenting features of 79 patients with PLA who attended two municipal hospitals in New York City over a 10-year period (modified from [1])
| Symptoms | Cases, % |
|---|---|
| Fever | 89.6 |
| Right upper quadrant pain | 72.2 |
| Chills | 69.0 |
| Nausea | 43.1 |
| Vomiting | 32.3 |
| Weight loss | 26.1 |
| Jaundice | 21.4 |
| Headache | 17.5 |
| Myalgias | 11.9 |
| Diarrhea | 10.7 |
Although spontaneous rupture of LA has been rarely reported, there is a higher incidence of abscess rupture in the group of K. pneumoniae PLA patients compared to other LA etiologies [11,12,20]. Suggested risk factors for spontaneous rupture in K. pneumoniae PLA are persistent hyperglycemic dysregulation in diabetic patients, large abscess size >5 cm, and thinned-wall and gas-forming abscess [4,7,11,12,13,15,19].
K. pneumoniae PLA is also associated with a higher likelihood of hematogenous spread and the potential for metastatic infection. The incidence rate of metastatic infection ranges from 10-45%, especially in patients with diabetes mellitus due to impaired host-defense mechanisms [4,5,11,12,19,20,21,22,23,24,25,26,27,28,29]. Furthermore, an abscess size of >5 cm has been described as a significant independent predictor [4,11,19,20,21,22]. Eyes, meninges, central nervous system (CNS), and lungs are the most common metastatic sites [7,21,22,23,24,25,26,27,28,29]. Endophthalmitis is probably the most serious septic complication of K. pneumoniae PLA, leading to subacute vision impairment. Affected patients usually do not recover their vision and may become entirely blind despite aggressive intravenous and intravitreal antibiotic treatment [4,11,23,25,26,29]. The mortality rate of K. pneumoniae PLA patients with evidence of metastatic infections is significantly higher than in patients without metastatic infections [4,11,22,26].
Clinical presentation of patients with amebic LA is similar to those with PLA [16]. Individuals can present with amebic LA months or even years after travel or residency in an endemic area, so a careful travel history is mandatory. The disease should be suspected in anyone with an appropriate exposure history and fever, right upper quadrant pain, and substantial hepatic tenderness. Cough may be present, and dullness and rales in the right lung base are not infrequent. Jaundice is rather unusual. Symptoms are usually acute (<10 days in duration) but can be chronic, with anorexia and weight loss as prominent features [16].
Laboratory Findings
Anemia, leukocytosis, high erythrocyte sedimentation rate, elevated C-reactive protein level, hypoalbuminemia, and hyperbilirubinemia, as well as elevated alanine aminotransferase (ALAT) and alkaline phosphatase (AP) levels are the most common laboratory findings [1,4,11]. None of the blood tests specifically help to diagnose LA; however, they can suggest a liver abnormality that leads to targeted imaging studies.
In patients with amebic LA, leukocytosis without eosinophilia, mild anemia, raised concentrations of AP, and a high erythrocyte sedimentation rate are the most common laboratory observations [16].
Imaging Findings
LA are mainly located in the right lobe of the liver [1,3,4,8,16,30]. A high proportion of LA manifests itself as a solitary abscess, but multiple bilateral abscesses are also possible. Chest radiographs may reveal right-sided pulmonary infiltrates with pleural effusion [1,3,4,11,30], and abdominal radiographs, which are rarely used but can be helpful in some cases, may show air-fluid levels or portal venous gas formation [3,8,11,30]. By now, ultrasound (US) and computed tomography (CT) are the two main diagnostic methods with a sensitivity of 96-100% [11,30].
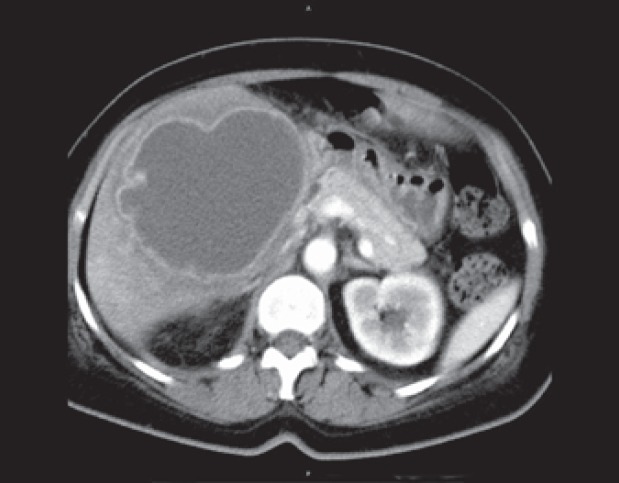
On CT imaging, LA are of lower attenuation than the surrounding normal liver parenchyma (fig. 1). The abscess wall usually shows a rim enhancement on contrast-enhanced CT. The appearance of LA at US imaging is typically hypoechoic, but may also range from hyperechoic to hypoechoic (fig. 2) [30]. Gas-forming LA showing aerobilia have been rarely reported in PLA patients in the past; however, with the etiologic shift to K. pneumoniaeas the primary causative agent, there is an increased risk of gas-producing PLA, especially in patients with diabetes mellitus failing glycemic control [4,11]. It is assumed that, under anaerobic conditions, K. pneumoniae strains being capable of facultative anaerobe metabolism can produce carbon dioxide by fermentation of glucose in tissue, especially under hyperglycemic conditions [4,11].
Fig. 1.
CT imaging of a large amebic LA in the right lobe of the liver.
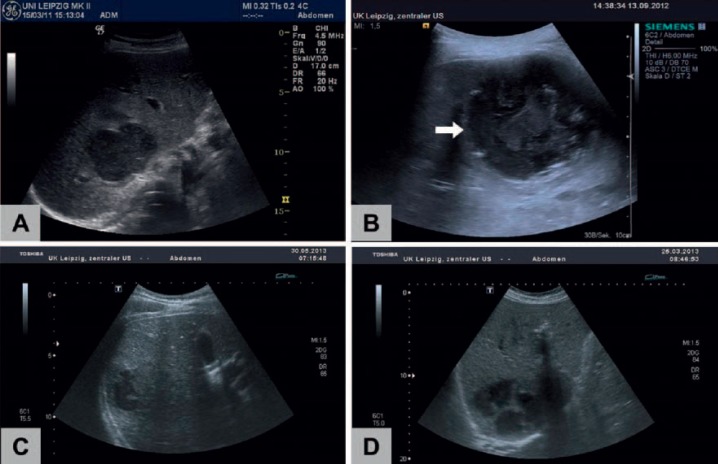
Fig. 2.
Ultrasound morphology of LA (B mode): PLA frequently show an inhomogeneous hypoechoic pattern (A) with a thickened, edematous wall (B, arrow). Blurred, irregular borders (C) and septa (D) can also be observed. Morphology cannot discriminate between pyogenic (A, B, D) and amebic (C) abscesses.
Diagnosis
Diagnosis of LA is mainly based on imaging studies, paired with microbiological findings. CT scans have been reported to be more sensitive than US in the diagnosis of LA [11,30]; however, from our own experience, both methods should be considered as equivalent. In terms of microbiological diagnosis, K. pneumoniaeisolates obtained from blood or LA puncture with the hypermucoviscous phenotype is suggestive of an invasive K. pneumoniaestrain, and the attending clinician should be notified as soon as possible by the microbiological laboratory [4]. Multiplex polymerase chain reaction (PCR) might be a useful rapid test for detection of the K. pneumoniaeserotype that causes LA [31].
Metastatic infections are a clinical challenge and are only diagnosed in one third of the cases on admission [4]. Lungs, CNS, and eyes are the most common metastatic sites [4,21,22,23,24,25,26,27,28,29]. Thus, in diabetic patients, especially in Asians or those of Asian origin, who present with K. pneumoniaebacteremia, endophthalmitis, meningitis, or other extrahepatic infections, a search for an occult LA is indicated [4].
The diagnosis of amebic LA relies on liver imaging and positive amebic serology. Diagnostic fluid aspiration from the lesion is therefore not necessary (fig. 3, fig. 4). US or CT scan are the tests of choice; both methods are very sensitive, but neither provides absolute specificity for amebic LA [16]. Formerly, such abscesses were described as solitary lesions in the right lobe of the liver, but modern imaging techniques such as high-resolution US or CT show a high frequency of multiple abscesses. Amebic serology is highly sensitive (>94%) and highly specific (>95%) for the diagnosis of amebic LA [16]. False-negative serological test results, however, can be obtained early in infection (within the first 7-10 days), but repeated tests will usually become positive. The successful use of an antigen detection enzyme-linked immunosorbent assay (ELISA) or PCR in patients with amebic LA suggests that these tests play an important part in the diagnosis of extraintestinal disease [16].
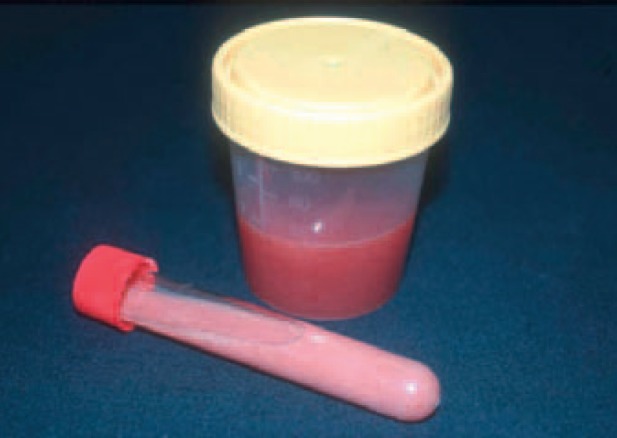
Fig. 3.
Creamy brownish aspiration fluid of a PLA, together with small traces of blood (A, B).
Fig. 4.
Pink-colored aspiration fluid from a large amebic LA in a 46-year-old patient returning from an 8-week backpacking trip to India (courtesy Prof. Stefan Schubert, Leipzig University Hospital, Germany).
Treatment
Antimicrobial Treatment of PLA
First, it is essential to exclude amebic LA as a differential diagnosis. When the diagnosis of PLA is suspected, there is a need to start broad-spectrum antibiotics immediately after collection of microbiological specimen (obtained from abscess puncture and blood cultures) to control ongoing bacteremia and its associated complications [4,11].
Recent studies have shown that most pathogens found in PLA are now resistant to ampicillin, but depending on the local resistance epidemiology, fluoroquinolones, third- and fourth-generation cephalosporins, piperacillin/tazobactam, aminoglycosides, and carbapenems remain effective treatment options [1,4,11,32,33,34]. Preceding antibiotic therapy with ampicillin/amoxicillin has been described as a risk factor for K. pneumoniae PLA manifestation in Asia due to selection pressure [32]. Taking into account resistance rates against fluoroquinolones of up to 30% in E. coli, K. pneumoniae, and other enterobacteriaceae, third-generation cephalosporins (ceftriaxone and cefotaxime) and piperacillin/tazobactam have backed up a dominant position in the antibiotic treatment of PLA [1,4,8,11,32,33]. Initial antibiotic regimens should therefore comprise either a third-generation cephalosporin and metronidazole or piperacillin/tazobactam, with the advantage of the latter variant that enterococcal infections are also partly covered.
When switching to de-escalation strategies, the selection of antimicrobials should be based on in vitro susceptibilities and clinical response. In most cases, targeted monotherapy with a beta-lactam tested active in vitro is sufficient. Combination therapy has been advocated for the treatment of bacteremic infections. There is current evidence that combination therapy with a beta-lactam and an aminoglycoside agent should be preferred in severely ill patients with Klebsiella spp. infections who experience hypotension [35]. Although PLA caused by ESBL(extended-spectrum beta-lactamase)-producing K. pneumoniaehave been reported in Taiwan, such strains still form a minority [4,11,32,33,34]. Carbapenems are the drug of choice for ESBL-producing E. coliandK. pneumoniae. Carbapenem use (primarily imipenem/cilastatin) has been found to be independently associated with lower mortality than other antibiotics [4]. The growing incidence of carbapenem-resistant K. pneumoniae, such as strains producing NDM-1 (New Delhi metallo-beta-lactamase-1) or KPC (K. pneumoniae carbapenemases), is of serious concern because of the few remaining treatment options (mainly tigecycline, colistin, and aminoglycosides) for these hyperresistant strains, which are associated with excess mortality [36,37,38,39].
The duration of therapy must be determined by response to treatment, as shown by repeated US of the abscess (fig. 5) and resolution of fever and leukocytosis [4]. The optimal duration of intravenous antimicrobial therapy, as well as the duration of subsequent oral therapy, remains unclear and does largely depend on the success rates of interventional source control. In studies from Taiwan, therapy generally consisted of 3 weeks of intravenous antibiotics followed by 1-2 months of oral therapy [4,11]. However, our own experience as well as studies performed in the USA indicate that shorter courses of antibiotics with targeted intravenous therapy for 2-3 weeks and consecutive oral therapy for 1-2 weeks are associated with extremely low mortality of less than 5% [1,8].
Fig. 5.
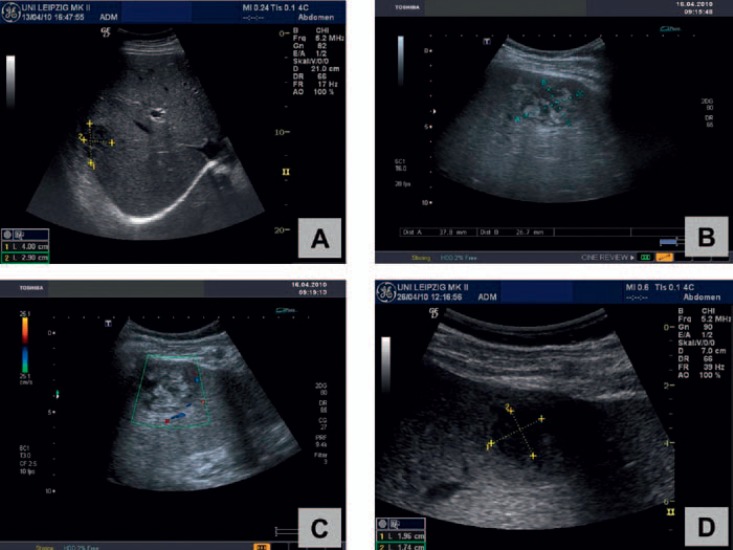
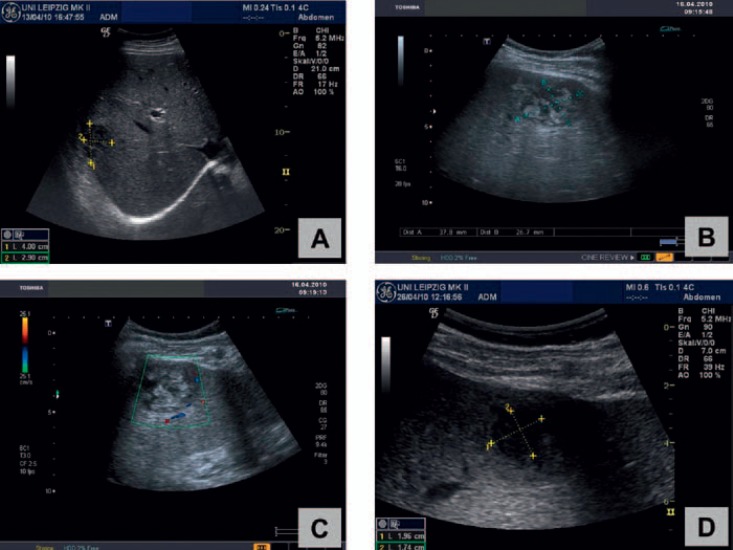
Repeated ultrasound imaging shows successful treatment of a PLA in the right lobe of the liver in a 54-year-old male patient: Under combined treatment with needle aspiration and antibiotics a ‘meltdown’ (A-D) of the abscess (initial size: 4.0 × 2.9 cm) could be demonstrated within 4 weeks.
Metastatic infections of the CNS and eyes in K. pneumoniae PLA are severe and difficult to treat. In the absence of ESBL-producing strains, third-generation cephalosporins are the drugs of choice for K. pneumoniaemeningitis in view of their better penetration into the cerebrospinal fluid (compared with first-generation and second-generation cephalosporins) [4]. Both cefotaxime and ceftriaxone are effective for the treatment of meningitis. Large doses are used for both intravenous cefotaxime (up to 2 g every 4 h) and ceftriaxone (2 g twice a day). 3 weeks of treatment has been recommended because of a high rate of relapse in individuals treated with shorter treatment courses [4]. Imipenem/cilastatin or meropenem should be given to patients instead of third-generation cephalosporins when ESBL-producing strains are suspected.
Both intravitreal and intravenous routes should be used for treatment of K. pneumoniaeendophthalmitis. Intravenous ceftazidime plus amikacin has been the most widely used combination [4]. Combination intravitreal treatment with cephalosporins (cefazolin and ceftazidime) and aminoglycosides (gentamicin and amikacin) have been applied successfully [4].
Furthermore, glycemic control in diabetic patients plays an essential role in the clinical features of K. pneumoniae PLA, especially in metastatic complications [4].
Interventional Therapy of PLA
Percutaneous drainage was widely used during the past three decades due to the obvious advantages of simplicity and avoidance of general anesthesia and laparotomy [1,4,8,11]. Approaching multiple PLA, percutaneous drainage may be associated with a higher failure rate. However, a recent retrospective study showed that treatment with percutaneous transhepatic drainage demonstrated similar effectiveness for patients with multiple abscesses but shorter hospitalization when compared with surgical drainage, which suggested that percutaneous aspiration or drainage should always be undertaken before surgery in terms of its lower morbidity and less cost [40].
Yu et al. [41] found that intermittent needle aspiration was probably as effective as continuous catheter drainage for the treatment of PLA. Due to the solid nature of K. pneumoniae PLA, procedure simplicity, patient comfort, and reduced costs, needle aspiration deserves to be considered as a first-line drainage approach in this disease entity, if not in all PLA. Patients with the following criteria have been suggested for percutaneous drainage: patients who continued to be febrile even after 48-72 h of adequate medical and aspiration treatment; LA > 6 cm in size; and clinical or US features suggesting impending perforation [11].
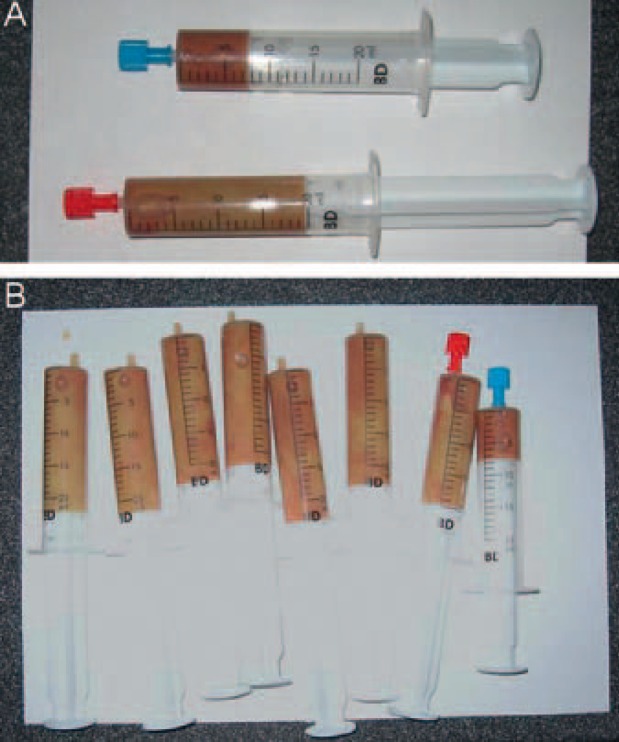
Common material for US-guided aspiration or drainage of LA is shown in figure 6.
Fig. 6.
Material for interventional therapy of LA: For drainage, a pigtail catheter (2) is placed into the abscess cavity using a guide needle (3). An adaptor (1) connects the catheter with a drainage bag (7). Alternatively, different fine needle types (4-6) can be used for aspiration of liquid abscess content.
Surgical Interventions in Patients with PLA
Surgical drainage has been reserved for patients who fail to respond to treatment with either aspiration or percutaneous drainage and antibiotics or who have concurrent intra-abdominal pathology which requires surgical management [1,4,41,42]. In addition to conventional laparotomy, laparoscopic drainage in association with systemic antibiotic therapy has been described as a safe and effective minimally invasive approach [2]. Thus, percutaneous and surgical techniques are not competing methods, but they have different indications, and surgery also represents an option for non-responders to percutaneous treatment [2,11,42].
Management of Amebic LA
Due to the excellent response to metronidazole, the management of amebic LA is unique. Abscesses occupying large areas of the liver can be cured without drainage, even by one dose of metronidazole [16]. Most patients show a response to treatment (reduced fever and abdominal pain) within 72-96 h. Individuals with amebic LA should also be treated with a luminal agent (diloxanide furoate or paromomycin) to eliminate intestinal colonization by E. histolytica.
Surgical drainage of uncomplicated amebic LA is generally unnecessary and should be avoided [16]. The role of percutaneous therapeutic aspiration guided by US or CT in the treatment of uncomplicated amebic LA remains controversial [16]. Clearly, most such abscesses can be cured by metronidazole treatment alone, and in a randomized controlled study no significant difference in either length of hospitalization or duration of time to becoming afebrile was seen between individuals treated with metronidazole alone and those treated with metronidazole as well as percutaneous therapeutic aspiration [16,43]. However, a prospective study in which large amebic LA underwent aspiration, while small abscesses were treated with drugs alone, showed benefits in mean hospital stay and the speed of clinical improvement in the aspiration group [16].
A reasonable policy might be to reserve aspiration for individuals in whom diagnosis is uncertain (where PLA or bacterial superinfection of the amebic LA is a concern), those who do not respond to metronidazole (persistent fever or abdominal pain after 4 days of treatment), individuals with large left lobe abscesses (because of the risk of rupture into the pericardium), and severely ill patients in whom an accelerated clinical course and large abscesses make rupture seem imminent [16]. Aspiration, percutaneous catheter drainage, or both improve outcomes in the treatment of amebic empyema after abscess rupture, and percutaneous catheter (or, if necessary, surgical) drainage could be lifesaving in the treatment of amebic pericarditis [16]. Amebic LA rupture into the peritoneum might also respond best to conservative treatment, medical management, and use of percutaneous catheter drainage if localized collections of fluid are noted [16,43].
Conclusion
LA remain an important and potentially life-threatening disease. However, the mortality rate in recent decades has been significantly reduced and is today less than 10% in PLA, in amebic LA even below 3%.
During the last three decades, highly virulent strains of K. pneumoniae (serotypes K1 and K2 with the rmpA plasmid gene) have emerged as a predominant cause of PLA in Asian countries, especially Taiwan, and this trend has been observed to a lesser extent also in the USA, in Europe, and in Australia. Therefore, corresponding changes in pathogen etiologies must be globally taken into account when setting diagnosis and treatment of PLA. Diabetes mellitus and serotype K1 infection are the most common risk factors for recurrent K. pneumoniae PLA. The prognosis for patients with endophthalmitis caused by K. pneumoniaeis still very poor, with more than 85% of the patients retaining a severe visual deficit.
The therapeutic success in PLA can be ensured best in a multimodal approach with inclusion of antibiotics, US- or CT-assisted aspiration or drainage, and surgical relief as a last option. According to our own experience, needle aspiration should be preferred, taking into account increased patient discomfort and substantial dislocation rates especially when dorsal or dorsolateral drainage is applied.
In consideration of current resistance rates reaching up to 90% against ampicillin and up to 30% against fluoroquinolones in most enterobacteriaceae, third-generation cephalosporins (combined with metronidazole) and piperacillin/tazobactam have obtained a dominant position in the antibiotic treatment of PLA. Fortunately, PLA caused by ESBL-producing K. pneumoniaestill form a minority; however, ESBL-producing strains are globally on the rise. Carbapenems are the drug of choice for ESBL-producing E. coliandK. pneumonia, and their use (primarily imipenem/cilastatin) has been found to be independently associated with lower mortality than other antibiotics.
Due to the excellent response to antiparasitic therapy with metronidazole, the management of amebic LA is different from PLA, with the result that even amebic LA occupying large areas of the liver can be cured without drainage.
Disclosure Statement
There was no funding. All authors indicate no potential conflicts of interest.
References
- 1.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 2.Romano G, Agrusa A, Frazzetta G, et al. Laporoscopic drainage of liver abscess: case report and review of the literature. G Chir. 2013;34:180–182. doi: 10.11138/gchir/2013.34.5.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong WM, Wong BC, Hui CK, et al. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year study period. J Gastroenterol Hepatol. 2002;17:1001–1007. doi: 10.1046/j.1440-1746.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- 4.Siu LK, Yeh KM, Lin JC, et al. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 5.Chung DR, Lee SS, Lee HR, et al. the Korean Study Group for Liver Abscess Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniaein Korea. J Infect. 2007;54:578–583. doi: 10.1016/j.jinf.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Chung DR, Lee H, Park MH, et al. Fecal carriage of serotype K1 Klebsiella pneumoniaeST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur J Clin Microbiol Infect Dis. 2012;31:481–486. doi: 10.1007/s10096-011-1334-7. [DOI] [PubMed] [Google Scholar]
- 7.Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniaeas a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100:322–331. doi: 10.1111/j.1572-0241.2005.40310.x. [DOI] [PubMed] [Google Scholar]
- 8.Pastagia M, Arumugam V. Klebsiella pneumoniaeliver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis. 2008;6:228–233. doi: 10.1016/j.tmaid.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Moore R, O'Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection. 2013;41:681–686. doi: 10.1007/s15010-013-0408-0. [DOI] [PubMed] [Google Scholar]
- 10.Chavada R, Ng J, Maley M, Descallar J. Emergence of Klebsiella pneumoniae liver abscesses in South-western Sydney. Infection. 2014;42:595–596. doi: 10.1007/s15010-014-0617-1. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Wang JY, Jiang W. An increasing prominent disease of Klebsiella pneumoniae liver abscess: etiology, diagnosis, and treatment. Gastroenterol Res Pract. 2013;2013:258514. doi: 10.1155/2013/258514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniaeand Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37:176–184. [PubMed] [Google Scholar]
- 13.Chen SC, Wu WY, Yeh CH, et al. Comparison of Escherichia coliand Klebsiella pneumoniaeliver abscesses. Am J Med Sci. 2007;334:97–105. doi: 10.1097/MAJ.0b013e31812f59c7. [DOI] [PubMed] [Google Scholar]
- 14.Fung CP, Siu LK. Virulence of Klebsiella pneumoniaeserotype K2 should not be underestimated in K pneumoniaeliver abscess. Clin Infect Dis. 2007;45:1530–1531. doi: 10.1086/523007. author reply 1532-1533. [DOI] [PubMed] [Google Scholar]
- 15.Fung CP, Lin YT, Lin JC, et al. Klebsiella pneumoniaein gastrointestinal tract and pyogenic liver abscess. Emerg Infect Dis. 2012;18:1322–1325. doi: 10.3201/eid1808.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley SL. Jr Amebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 17.Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniaecolonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol. 2012;12:13. doi: 10.1186/1471-2180-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu WL, Chan KS, Ko WC, Lee CC, Chuang YC. Lower prevalence of diabetes mellitus in patients with Klebsiella pneumoniaeprimary liver abscess caused by isolates of K1/K2 than with non-K1/K2 capsular serotypes. Clin Infect Dis. 2007;45:1529–1530. doi: 10.1086/523006. author reply 1532-1533. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Chung DR, Wie SH, et al. Risk factor analysis of invasive liver abscess caused by the K1 serotype Klebsiella pneumonia. Eur J Clin Microbiol Infect Dis. 2009;28:109–111. doi: 10.1007/s10096-008-0595-2. [DOI] [PubMed] [Google Scholar]
- 20.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. Comparison of prevalence of virulence factors for Klebsiella pneumoniaeliver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis. 2008;62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Yu WL, Hansen DS, Ko WC, et al. the International Klebsiella Study Group. Virulence characteristics of Klebsiellaand clinical manifestations of K pneumoniaebloodstream infections. Emerg Infect Dis. 2007;13:986–993. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SS, Chen YS, Tsai HC, et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis. 2008;47:642–650. doi: 10.1086/590932. [DOI] [PubMed] [Google Scholar]
- 23.Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniaeliver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913–1916. [PubMed] [Google Scholar]
- 24.Hu BS, Lau YJ, Shi ZY, Lin YH. Necrotizing fasciitis associated with Klebsiella pneumoniaeliver abscess. Clin Infect Dis. 1999;29:1360–1361. doi: 10.1086/313471. [DOI] [PubMed] [Google Scholar]
- 25.Casanova C, Lorente JA, Carrillo F, Perez-Rodriguez E, Nunez N. Klebsiella pneumoniaeliver abscess associated with septic endophthalmitis. Arch Intern Med. 1989;149:1467. [PubMed] [Google Scholar]
- 26.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniaegenotype K1:an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 27.Tang LM, Chen ST, Hsu WC, Chen CM. Klebsiella meningitis in Taiwan: an overview. Epidemiol Infect. 1997;119:135–142. doi: 10.1017/s0950268897007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang PW, Lin HD, Wang LM. Pyogenic liver abscess associated with septic pulmonary embolism. J Chin Med Assoc. 2008;71:442–447. doi: 10.1016/S1726-4901(08)70146-1. [DOI] [PubMed] [Google Scholar]
- 29.Yang CS, Tsai HY, Sung CS, Lin KH, Lee FL, Hsu WM. Endogenous Klebsiellaendophthalmitis associated with pyogenic liver abscess. Ophthalmology. 2007;114:876–880. doi: 10.1016/j.ophtha.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Lin AC, Yeh DY, Hsu YH, et al. Diagnosis of pyogenic liver abscess by abdominal ultrasonography in the emergency department. Emerg Med J. 2009;26:273–275. doi: 10.1136/emj.2007.049254. [DOI] [PubMed] [Google Scholar]
- 31.Turton JF, Baklan H, Siu LK, Kaufmann ME, Pitt TL. Evaluation of a multiplex PCR for detection of serotypes K1, K2 and K5 in Klebsiellaspp. and comparison of isolates within these serotypes. FEMS Microbiol Lett. 2008;284:247–252. doi: 10.1111/j.1574-6968.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 32.Lin YT, Liu CJ, Yeh YC, et al. Ampicillin and amoxicillin use and the risk of Klebsiella pneumonia liver abscess in Taiwan. J Infect Dis. 2013;208:211–217. doi: 10.1093/infdis/jit157. [DOI] [PubMed] [Google Scholar]
- 33.Lin JC, Siu LK, Fung CP, Yeh KM, Chang FY. Nosocomial liver abscess caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. J Clin Microbiol. 2007;45:266–269. doi: 10.1128/JCM.01413-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su SC, Siu LK, Ma L, et al. Community-acquired liver abscess caused by serotype K1 Klebsiella pneumoniaewith CTX-M-15-type extended-spectrum beta-lactamase. Antimicrob Agents Chemother. 2008;52:804–805. doi: 10.1128/AAC.01269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korvick JA, Bryan CS, Farber B, et al. Prospective observational study of Klebsiellabacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother. 1992;36:2639–2644. doi: 10.1128/aac.36.12.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 37.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lübbert C, Faucheux S, Becker-Rux D, et al. Rapid emergence of secondary resistance to gentamicin and colistin following selective digestive decontamination in patients with KPC-2-producing Klebsiella pneumoniae a single-centre experience. Int J Antimicrob Agents. 2013;42:565–570. doi: 10.1016/j.ijantimicag.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferraioli G, Garlaschelli A, Zanaboni D, et al. Percutaneous and surgical treatment of pyogenic liver abscesses: observation over a 21-year period in 148 patients. Dig Liver Dis. 2008;40:690–696. doi: 10.1016/j.dld.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Yu SC, Ho SS, Lau WY, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39:932–938. doi: 10.1002/hep.20133. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh HF, Chen TW, Yu CY, et al. Aggressive hepatic resection for patients with pyogenic liver abscess and APACHE II score ≥15. Am J Surg. 2008;196:346–350. doi: 10.1016/j.amjsurg.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 43.Allan RJ, Van Katz MD, Johnson MB, et al. Uncomplicated amebic liver abscess: prospective evaluation of percutaneous aspiration. Radiology. 1991;183:827–830. doi: 10.1148/radiology.183.3.1584941. [DOI] [PubMed] [Google Scholar]