Summary
Background
The increasing prevalence of obese patients will lead to a more frequent use of bariatric procedures in the future. Compared to conservative medical therapy, bariatric procedures achieve greater weight loss and superior control of comorbidities, resulting in improved overall mortality.
Methods
A search for current literature regarding mechanisms, indications, and outcomes of bariatric surgery was performed.
Results
In order to care for patients after bariatric surgery properly, it is important to understand its mechanisms of action and effects on gastrointestinal physiology. Recent investigations indicate that the beneficial effects of bariatric procedures are much more complex than simply limiting food intake or an associated malabsorption. Changes in gastrointestinal hormone secretion, energy expenditure, intestinal bacterial colonization, bile acid metabolism, and epigenetic modifications resulting in altered gene expression are likely responsible for the majority of the beneficial effects of bariatric surgery. Malabsorptive bariatric procedures divert the flow of bile and pancreatic enzymes from food and therefore limit the digestion and absorption of nutrients, resulting in reduced calorie intake and subsequent weight loss. Essential micronutrients such as vitamins and trace elements are also absorbed to a lesser extent, potentially leading to severe side effects.
Conclusion
To prevent malnutrition, dietary supplementation and regular control of micronutrient levels are mandatory for patients undergoing malabsorptive bariatric procedures, in whom the fat-soluble vitamins A and D are commonly deficient.
Keywords: Bariatric surgery, Metabolic surgery, Biliopancreatic diversion, BPD, Bbiliopancreatic diversion with duodenal switch, BPD-DS, Malabsorption, Gastric bypass
Zusammenfassung
Hintergrund
Die Zunahme an adipösen Patienten wird in Zukunft zu mehr bariatrischen Operationen führen. Diese Operationen sind effizient, um den erwünschten Gewichtsverlust und eine der konservativen medizinischen Therapie überlegenen Kontrolle von Komorbiditäten zu erreichen.
Methoden
Eine Recherche zur aktuellen Literatur bezüglich der Wirkmechanismen, Indikationen und Resultate bariatrischer Operationen wurde durchgeführt.
Ergebnisse
Um Patienten nach bariatrischen Eingriffen gut behandeln zu können, sollten die Wirkmechanismen und die Auswirkungen auf die Physiologie des Magen-Darm-Trakts verstanden werden. Neuere Untersuchungen zeigen, dass bariatrische Operationen nicht nur über eine Restriktion der Nahrungszufuhr oder Malabsorption wirken, sondern zu komplexen Veränderungen der Sekretion gastrointestinaler Hormone, der Energieverbrennung, der bakteriellen Kolonisation des Darms, des Gallensäurestoffwechsels und epigenetischen Veränderungen, welche die Genexpression modulieren, führen. Diese Veränderungen sind wahrscheinlich hauptverantwortlich für die positiven Effekte bariatrischer Eingriffe. Malabsorptive bariatrische Operationen separieren die Galle sowie die Pankreasenzyme von der Nahrung und limitieren damit die Verdauung und Resorption von Nährstoffen. Durch die reduzierte Kalorienaufnahme wird der gewünschte Gewichtsverlust erreicht. Durch die Diversion des Verdauungsprozesses werden auch Vitamine und Spurenelemente ungenügend resorbiert, woraus eine potenziell gefährliche Mangelernährung resultieren kann.
Schlussfolgerung
Um eine Unterernährung zu verhindern, ist es wichtig, dass Patienten nach malabsorptiven bariatrischen Eingriffen lebenslang Nahrungsergänzungsmittel zu sich nehmen und regelmäßige Kontrollen des Ernährungszustands durchführen, da vor allem die fettlöslichen Vitamine A und D sehr häufig unzureichend vorhanden sind.
Introduction
The percentage of individuals who are either overweight or obese is increasing in Germany as well as worldwide. In 2012, more than 53% of women and men in Germany were overweight, and 2.8% of women and 1.2% of men were severely obese, with a BMI > 40 kg/m2[1]. It is a well-established fact that bariatric surgery is the most effective and cost-efficient means of achieving weight loss for both mild and severely obese patients [2,3,4]. Most importantly, however, bariatric surgery and its associated weight loss results in a strong improvement of obesity-associated comorbid conditions and reduces overall mortality. For example, diabetes mellitus type 2 may be better controlled, and rates of cardiovascular events, including myocardial infarction and stroke, decrease following bariatric surgery [5,6,7]. Hence, in upcoming years, bariatric surgery will likely become the treatment of choice for many obese patients with or without comorbid conditions. It is important for clinicians to be familiar with changes in gastrointestinal physiology that may be attributed to bariatric surgery, as well as with the potential problems that may occur in these patients. Furthermore, the recent literature has demonstrated that bariatric procedures exert their beneficial effects through a variety of mechanisms, and that the mere restriction of food intake or malabsorption is not as important as initially thought [8,9,10]. Bariatric surgery results in changes to multiple organ systems, such as the secretion of gastrointestinal hormones, energy expenditure, intestinal bacterial colonization, and bile acid metabolism. Epigenetic changes resulting in modified gene expression have also been demonstrated after bariatric surgery. These mechanisms, while less obvious, seem to be major contributors to the beneficial effects of such procedures [11,12,13,14,15,16,17,18]. The purpose of this review is to provide an overview of bariatric procedures with a focus on malabsorptive procedures, their potential long-term complications, and recommendations for follow-up.
Indications for Bariatric Surgery
A worldwide consensus on which patients are suitable candidates for bariatric surgery has been reached. The German S3 guidelines, the guidelines of the American Society for Metabolic and Bariatric Surgery, and the British National Institute for Health and Care Excellence guidelines all recommend bariatric surgery for patients with a BMI > 40 kg/m2, or for patients with a BMI > 35 kg/m2 and at least one comorbid condition such as diabetes mellitus type 2, arterial hypertension, nonalcoholic fatty liver disease, sleep apnea, lipid abnormalities, osteoarthritis, or heart disease. Many obese patients suffer from one or more of these diseases and are therefore candidates for bariatric surgery. We and others have shown that patients with poorly controlled diabetes can become insulin-free after laparoscopic Roux-en-Y gastric bypass (RYGB) [19,20,21]. Based on these promising results, it can be expected that extended indications for bariatric surgery may be established in the future.
Physiology of Digestion and Nutrient Absorption
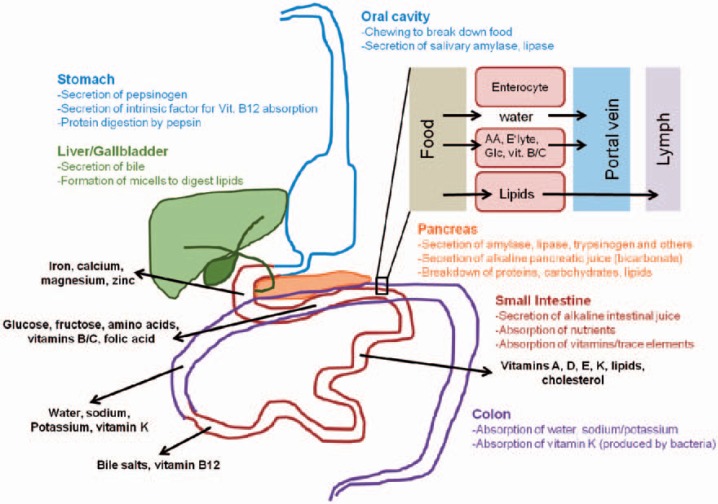
In order to understand the principles of bariatric surgery, the physiology of digestion and nutrient absorption must be understood (fig. 1) [22,23]. Digestion is the process by which food is broken up into parts that are small enough to be absorbed by the intestinal lining. The majority of nutrients are actively transported into enterocytes (absorption), while water, lipids, and other substances are taken up passively. The major steps of digestion are chewing and the secretion of amylase by the salivary glands, which starts the digestion of carbohydrates. This step is followed by the secretion of hydrochloric acid and pepsinogen in the stomach, which is in turn split into active pepsin. Pepsin and hydrochloric acid start the process of digesting protein into polypeptides. The chime is then transported to the duodenum, where the pancreatic enzymes and bile are secreted. With the help of additional enzymes expressed on the enterocytes, the enzymes of the pancreas digest polypeptides into amino acids and carbohydrates into monosaccharides. Lipids are degraded by the pancreatic lipase into fatty acids and glycerol with the help of bile. Medium chain fatty acids, which can be absorbed without the help of lipase or bile, are an important exception [24]. These nutrients are then absorbed through the enterocytes by various transport mechanisms and transported to the liver by the portal or lymph system. Under normal conditions, over 75% of sugars, proteins, and lipids are absorbed within 70 cm of small bowel [25]. Furthermore, the gut can compensate if some parts are resected and shortened; a length of >240 cm of small bowel is sufficient to prevent any malabsorption syndrome [26]. Micronutrients such as vitamins, minerals, and trace elements are absorbed by specific transporters distributed over the entirety of the small intestine. The majority of these (iron, calcium, zinc, and selenium in particular), however, are absorbed in the duodenum and upper jejunum. Procedures that bypass the duodenum and upper jejunum, such as the RYGB, may result in deficiencies of these trace metals. Vitamin B12 is bound by the intrinsic factor secreted by the stomach, and the vitamin B12 and intrinsic factor complex is absorbed in the terminal ileum by specific transporters. Therefore, any bariatric procedure involving a gastric bypass or resection may result in vitamin B12 deficiency. The absorption of lipid-soluble vitamins such as A, D, E, and K depends on bile and lipase, which is diverted from food in malabsorptive procedures, resulting in potentially severe deficiencies. Thus, the order and effectiveness of digestion and nutrient absorption is affected by malabsorptive bariatric procedures, leading to a high risk of malnutrition. The nutritional status of bariatric patients must be closely monitored before and after surgery as the majority of obese patients also exhibit preexisting, clinically significant vitamin and trace metal deficiencies [27,28,29]. Due to their nature, malabsorptive bariatric procedures induce deficiencies, especially of fat-soluble vitamins, as well as caloric and protein malnutrition, which are caused by impaired fat digestion and food absorption limitations [30,31,32]. In contrast, the RYGB is prone to trace metal deficiencies because it bypasses the duodenum, where trace metals are absorbed [33]. Patients thus require high-dose vitamin and trace metal supplementation after malabsorptive procedures, while those who have had other bariatric procedures can be supplemented with over-the-counter vitamin preparations [34]. In either case it is pivotal to monitor vitamin and trace metal levels in bariatric patients.
Fig. 1.
Overview of digestion and absorption (AA = amino acids; E'lyte = electrolytes; Glc = glucose; vit. B/C = vitamins of the B complex except B12 and C).
Overview of Bariatric Procedures
Bariatric procedures have previously been divided into three groups: restrictive, malabsorptive, and a combination of both [2,35,36]. Restrictive operations limit gastric volume and thereby reduce food intake. Malabsorptive procedures divert digestive liquids such as bile and pancreatic enzymes and shorten the length of bowel that participates in food absorption. Combined procedures have both restrictive and malabsorptive components. Recent research, however, demonstrates that the essential effects of bariatric procedures, especially of the RYGB and gastric sleeve, cannot be explained based on the restriction of food intake alone. These data suggest that bariatric procedures produce multiple effects, such as changes in gastrointestinal hormone secretion, energy expenditure, intestinal bacterial colonization, bile acid metabolism, and epigenetic changes modifying gene expression [11,12,13,14,15,16,17,18,37,38,39]. It appears that especially the effects of bariatric surgery on comorbid conditions, such as diabetes, are mediated by the aforementioned mechanisms [9,10]. It is noteworthy that malabsorptive procedures are rarely performed in Germany. According to the German Bariatric Surgery Registry, 14 malabsorptive procedures were performed in 2012, while 2,733 RYGB, 2,553 sleeve gastrectomies, and 259 gastric bands were performed during the same period [40].
Restrictive Bariatric Procedures
The laparoscopic adjustable gastric band (LAGB) is the only purely restrictive procedure. The gastric band procedure involves the creation of a small stomal pouch which leads to rapid satiety and reduced food intake. The circumference of the band can be adjusted through a percutaneously accessible port which allows the surgeon to adapt the level of restriction according to the desired weight loss. Though initially thought to be a restrictive procedure, the gastric sleeve mainly acts through changes in intestinal hormone secretion and energy expenditure and has hardly any effect on food intake [10]. The gastric sleeve is created by resecting the greater curvature of the stomach. This procedure was originally developed as a step to initiate weight loss in super-obese patients until a malabsorptive procedure could be performed with reduced mortality [41,42]. The surprising success of the gastric sleeve alone in achieving the desired weight loss has established it as one of the most common bariatric procedures today [43,44,45]. Due to its purely restrictive nature, the LAGB also has the lowest success rate, with <50% of excess weight loss (EWL) after 2 years, while the gastric sleeve achieves up to 70% EWL after 3 years [7,46].
Malabsorptive Bariatric Procedures
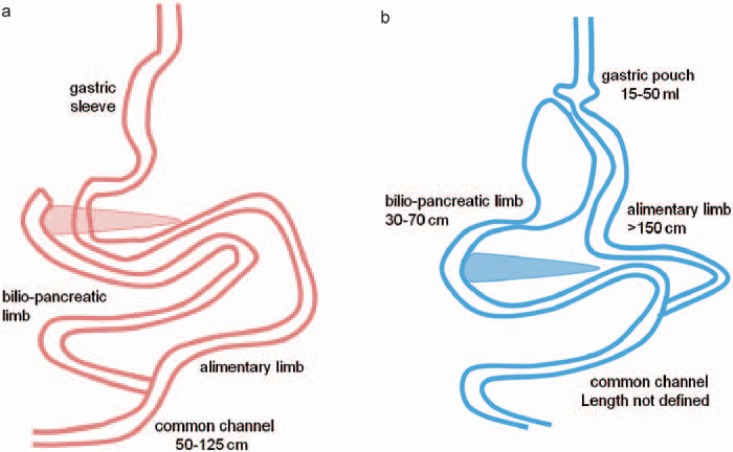
The classic malabsorptive procedures are the biliopancreatic diversion (BPD, according to Scopinaro) and the biliopancreatic diversion with duodenal switch (BPD-DS). Scopinaro described the BPD as a diversion of the bile and pancreatic juice (via the biliopancreatic limb) from food (in the alimentary limb), combined with a subtotal gastrectomy [47]. The BPD-DS (fig. 2) was described by Marceau and features a sleeve gastrectomy with pyloric preservation and reconstruction, plus an ileoduodenostomy [48]. The degree of malabsorption for BPD/BPD-DS varies according to the length of the common channel (50-125 cm), in which the digestion and absorption occur. The shorter the common channel, the more effective the weight loss [49,50,51]. However, side effects such as diarrhea and severe vitamin A and D deficiencies also increase as the length of the common channel decreases. Sufficient gastric volume, ideally including the pylorus (which allows for a dosed release of food into the small bowel), and a sufficient alimentary limb length (>200 cm) are crucial for protein malnutrition prevention [52,53]. Vage et al. [49] demonstrated that, in order to achieve the desired weight loss while minimizing malnutrition, an alimentary limb of 40% and a common channel of 10% of the total small bowel length are required. This combination yields the best results regarding both weight loss and vitamin D level preservation [49]. A common channel length of >100 cm, however, appears to be insufficient for achieving the desired weight loss [50]. The BPD/BPD-DS has a high success rate, with 70% EWL after 2 years [7,31]. However, this procedure has by far the highest long-term complication rate and results in caloric, protein, and micronutrient deficiencies (table 1) [32,33,54]. Hence, BPD/BPD-DS may not be considered a first-choice bariatric procedure, although it does play a role as a redo procedure in patients who fail to achieve their desired weight loss after RYGB or gastric sleeve [55].
Fig. 2.
Overview of a biliopancreatic diversion with duodenal switch and b distal Roux-en-Y gastric bypass.
Table 1.
Short- and long-term outcomes after bariatric surgery
| BPD/BPD-DS | RYGB | |
|---|---|---|
| Complications | ||
| Mortality, % | 1.1–1.2 | 0.3–0.5 |
| Reoperation rate, % | 11.5 | 7.2 |
| Weight loss/comorbid conditions | ||
| Excess weight loss after 2 years, % | 70 | 62–70 |
| Improved glycemic control, % | 83–88 | 76–91 |
| Improved hypercholesterolemia, % | 68–100 | 44–91 |
| Arterial hypertension, % | 70–92 | 63–87 |
| Improved sleep apnea, % | 79–87 | 49–95 |
| Long-term malnutrition (>1 year) | ||
| Severe nutritional deficiency, % | 4.1 | 2.1 |
| Parenteral nutrition required, % | 2.7 | 0.3 |
| Protein malnutrition, % | 0–18 | 0–1.4 |
| Iron deficiency, % | 0–44 | 5.9–50 |
| Vitamin B12 deficiency, % | 22 | 8–37 |
| Vitamin D deficiency, %a | 17–63 | 51 |
Combined Bariatric Procedures
As the most commonly used bariatric procedure today, the laparoscopic RYGB combines both concepts of bariatric surgery, although it mainly acts restrictively. Similar to the gastric sleeve, the main effect of the RYGB is to induce changes in intestinal hormone secretion, energy expenditure, intestinal bacterial colonization, bile acid metabolism, and epigenetic changes [9,11,12,13,15,16,17,18]. This operation was developed in the 1970s and initially used a loop gastrojejunostomy, until the Roux-en-Y reconstruction was used, to address a high rate of bile reflux [56]. The RYGB now combines a small gastric pouch (15-50 ml) with a gastrojejunostomy that bypasses the duodenum and early jejunum. The gastric outlet is narrow (1-2 cm), which delays gastric emptying. The biliopancreatic limb is 30-70 cm in length, while the alimentary limb in the traditional RYGB is typically 75-150 cm long. A modified version of the RYGB, the so-called ‘long limb’ or ‘distal RYGB’, uses a longer alimentary limb, i.e. >150 cm (fig. 2). The longer the alimentary limb, the greater is the risk that a malabsorptive component will result. It is noteworthy that the length of the common channel in the RYGB procedure is not routinely measured and mainly depends on the length of the patientss' small bowel. Odstrcil et al. [57] demonstrated that malabsorption contributes only minimally to weight loss after traditional RYGB, with a reduction of only 180 kcal/day due to malabsorption, but 1,410 kcal/day due to reduced food intake. The long-limb RYGB increases the malabsorptive effect to 452 kcal/day [57]. Caloric or protein malnutrition rarely occurs after RYGB, but calcium, selenium, zinc, iron, and vitamin D levels may become deficient due to the duodenal bypass. Although there is no general consensus, over-the-counter multivitamin supplementation is usually recommended [34,58]. The EWL after 2 years is 60-70% [7,46]. Additionally, it appears that the RYGB has the greatest impact on comorbid conditions such as diabetes, with 31% complete and 30% partial diabetes remission rates >5 years after surgery [59].
Perioperative Management, Complication Rate and Mortality
Bariatric procedures are safe (table 1), with a perioperative mortality of 0.5% for RYGB and about 1.1% for BPD/BPD-DS in experienced centers. These rates hold true even for high-risk patients with a BMI > 50 kg/m2[7,60,61,62]. BPD/BPD-DS has a longer operative time, longer hospital stay, and a higher overall complication rate. Complications such as surgical site, organ space, and systemic infections are infrequent but more common among patients undergoing BPD/BPD-DS procedures [61,62]. Anastomotic complications differ between RYGB and BPD/BPD-DS: Marginal ulcers and anastomotic strictures are more common in RYGB (1.2 vs. 0.3% and 3.3 vs. 1.9%, respectively) whereas anastomotic leakage is more common in BPD/BPD-DS (1.6 vs. 0.8%). Overall, approximately 15% of RYGB and 25% of BPD/BPD-DS patients suffer from complications [7,62].
Short- and Long-Term Outcomes
The main goals of bariatric surgery aim at inducing and maintaining EWL as well as improving comorbid conditions. Bariatric procedures are much more efficient in controlling comorbid conditions than medical therapy [20,63]. A comparison of long-term outcomes is shown in table 1. Malabsorptive procedures such as BPD/BPD-DS achieve the highest EWL, followed by RYGB [7,31,60,62,64,65]. The impact on diabetes remission, dyslipidemia normalization, arterial hypertension, and sleep apnea is comparable [7,31,62]. The BPD-DS maintains a higher EWL (75%) after 10 years [64]. In contrast, the RYGB appears to achieve superior control of comorbid conditions, with 31% complete and 30% partial diabetes remission rates >5 years after surgery. These results are likely related to changes in the secretion of intestinal hormones and energy expenditure [12,59]. Despite having similar effects on comorbid conditions, the BPD/BPD-DS has a significantly higher risk for malnutrition of both macro- and micronutrients than the RYGB [30,31,32,66]. Patients require increased protein intake (about 30% additionally) and high-dose vitamin (especially of the lipid-soluble vitamins A, D, E, and K) as well as trace element supplementation after BPD/BPD-DS, while patients after RYGB receive over-the-counter multivitamin supplementation [30,34]. Table 2 shows a proposed daily dietary supplementation for both procedures, although there is no general consensus on an ideal dietary supplementation after bariatric surgery. We recommend a basic multivitamin after RYGB and gastric sleeve, with additional supplementation determined according to laboratory values. Iron deficiency with anemia is common among women in general and likely worsens after bariatric procedures; therefore, intravenous substitution may be required [34]. Special attention should be paid to vitamin B1 levels if a patient vomits frequently after bariatric surgery, and should be supplemented accordingly [33]. It is noteworthy that deficiencies in the lipid-soluble vitamins tend to occur 1 year postoperatively, whereas deficiencies in water-soluble vitamins and trace elements occur earlier [32,33]. All patients should receive at least annual follow-up visits after bariatric surgery during which the surgeon should look for signs of vitamin deficiencies, including anemia, hair loss, skin or nail problems, and abnormal laboratory results.
Table 2.
Proposed dietary supplementation after bariatric procedures
| Recommended daily dietary supplementation | BPD/BPD-DS [30, 32] | RYGB |
|---|---|---|
| Multivitamins | 2 × 1 per day | 2 × 1 per day |
| Vitamin A | 2 × 25,000 per day | none |
| Vitamin D | 2 × 25,000 IU per day | according to laboratory values |
| Vitamin B12 | 1 mg i.m. every 3 months | according to laboratory values |
| Calcium | 2 × 1,000 mg | according to laboratory values |
| Iron | 2 × 100 mg per day | women: 2 × 100 mg per day men: according to laboratory values |
| Additional protein intake | 100 g per day | none |
BPD = Biliopancreatic diversion according to Scopinaro; BPD-DS = biliopancreatic diversion with duodenal switch; RYGB = Roux-en-Y gastric bypass.
Conclusion
Bariatric procedures are safe and effective in both inducing weight loss and controlling comorbid conditions among obese patients. Such procedures yield better mortality rates and long-term outcomes than medical therapy alone. Malabsorptive procedures have a stronger effect on weight loss, although patients undergoing them are also at a higher risk for significant malnutrition. The RYGB and gastric sleeve achieve similar results with minimal risk for malnutrition or vitamin deficiency. Lifetime high-dose proteins and vitamin supplementation, as well as controlling the nutritional status of all patients after malabsorptive bariatric surgery, are mandatory.
Disclosure Statement
The authors do not have any conflicts of interest to disclose.
References
- 1.Mensink GB, Schienkiewitz A, Haftenberger M, Lampert T, Ziese T, Scheidt-Nave C. Overweight and obesity in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1) (article in German) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:786–794. doi: 10.1007/s00103-012-1656-3. [DOI] [PubMed] [Google Scholar]
- 2.Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009. p. CD003641. [DOI] [PubMed]
- 3.O'Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, Strauss B, Marks S, Schachter L, Chapman L, Anderson M. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006;144:625–633. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Neovius M, Narbro K, Keating C, Peltonen M, Sjoholm K, Agren G, Sjostrom L, Carlsson L. Health care use during 20 years following bariatric surgery. JAMA. 2012;308:1132–1141. doi: 10.1001/2012.jama.11792. [DOI] [PubMed] [Google Scholar]
- 5.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 6.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 8.Kohli R, Stefater MA, Inge TH. Molecular insights from bariatric surgery. Rev Endocr Metab Disord. 2011;12:211–217. doi: 10.1007/s11154-011-9172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pournaras DJ, Osborne A, Hawkins SC, Vincent RP, Mahon D, Ewings P, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252:966–971. doi: 10.1097/SLA.0b013e3181efc49a. [DOI] [PubMed] [Google Scholar]
- 10.Scott WR, Batterham RL. Roux-en-y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301:R15–27. doi: 10.1152/ajpregu.00038.2011. [DOI] [PubMed] [Google Scholar]
- 11.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werling M, Olbers T, Fandriks L, Bueter M, Lonroth H, Stenlof K, le Roux CW. Increased postprandial energy expenditure may explain superior long term weight loss after Roux-en-Y gastric bypass compared to vertical banded gastroplasty. PLoS One. 2013;8:e60280. doi: 10.1371/journal.pone.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, Sharkey KA, Lutz TA, le Roux CW. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013. p. 5178141. [DOI] [PMC free article] [PubMed]
- 15.Aron-Wisnewsky J, Dore J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9:590–598. doi: 10.1038/nrgastro.2012.161. [DOI] [PubMed] [Google Scholar]
- 16.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, Welbourn R, le Roux CW. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 18.Barres R, Kirchner H, Rasmussen M, Yan J, Kantor FR, Krook A, Naslund E, Zierath JR. Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep. 2013;3:1020–1027. doi: 10.1016/j.celrep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Muller-Stich BP, Fischer L, Kenngott HG, Gondan M, Senft J, Clemens G, Nickel F, Fleming T, Nawroth PP, Buchler MW. Gastric bypass leads to improvement of diabetic neuropathy independent of glucose normalization-results of a prospective cohort study (DiaSurg 1 study) Ann Surg. 2013;258:760–765. doi: 10.1097/SLA.0b013e3182a618b2. discussion 765-766. [DOI] [PubMed] [Google Scholar]
- 20.Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ, Thomas AJ, Leslie DB, Chong K, Jeffery RW, Ahmed L, Vella A, Chuang LM, Bessler M, Sarr MG, Swain JM, Laqua P, Jensen MD, Bantle JP. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–2249. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon JB, Hur KY, Lee WJ, Kim MJ, Chong K, Chen SC, Straznicky NE, Zimmet P. Gastric bypass in type 2 diabetes with BMI < 30: weight and weight loss have a major influence on outcomes. Diab Med. 2013;30:e127–134. doi: 10.1111/dme.12107. [DOI] [PubMed] [Google Scholar]
- 22.Whitney EN, Rolfes SR. Understanding Nutrition. ed 12. Belmont, CA: Wadsworth, Cengage Learning; 2011. [Google Scholar]
- 23.Heizer WD. Normal and abnormal intestinal absorption by humans. Environ Health Perspect. 1979;33:101–106. doi: 10.1289/ehp.7933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linscheer WG, Patterson JF, Moore EW, Clermont RJ, Robins SJ, Chalmers TC. Medium and long chain fat absorption in patients with cirrhosis. J Clin Invest. 1966;45:1317–1325. doi: 10.1172/JCI105438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson C. Studies of gastrointestinal interactions. VII. Characteristics of the absorption pattern of sugar, fat and protein from composite meals in man. A quantitative study. Scand J Gastroenterol. 1975;10:33–42. [PubMed] [Google Scholar]
- 26.Booth CC. The metabolic effects of intestinal resection in man. Postgrad Med J. 1961;37:725–739. doi: 10.1136/pgmj.37.434.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. 2008;18:870–876. doi: 10.1007/s11695-007-9349-y. [DOI] [PubMed] [Google Scholar]
- 28.Ernst B, Thurnheer M, Schmid SM, Schultes B. Evidence for the necessity to systematically assess micronutrient status prior to bariatric surgery. Obes Surg. 2009;19:66–73. doi: 10.1007/s11695-008-9545-4. [DOI] [PubMed] [Google Scholar]
- 29.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25:1150–1156. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. 2006;331:219–225. doi: 10.1097/00000441-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Sovik TT, Aasheim ET, Taha O, Engstrom M, Fagerland MW, Bjorkman S, Kristinsson J, Birkeland KI, Mala T, Olbers T. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Ann Intern Med. 2011;155:281–291. doi: 10.7326/0003-4819-155-5-201109060-00005. [DOI] [PubMed] [Google Scholar]
- 32.Aasheim ET, Bjorkman S, Sovik TT, Engstrom M, Hanvold SE, Mala T, Olbers T, Bohmer T. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90:15–22. doi: 10.3945/ajcn.2009.27583. [DOI] [PubMed] [Google Scholar]
- 33.Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S. Nutritional deficiencies following bariatric surgery: what have we learned? Obes Surg. 2005;15:145–154. doi: 10.1381/0960892053268264. [DOI] [PubMed] [Google Scholar]
- 34.Schweitzer DH, Posthuma EF. Prevention of vitamin and mineral deficiencies after bariatric surgery: evidence and algorithms. Obes Surg. 2008;18:1485–1488. doi: 10.1007/s11695-008-9489-8. [DOI] [PubMed] [Google Scholar]
- 35.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132:2253–2271. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 36.Van Hee RH. Biliopancreatic diversion in the surgical treatment of morbid obesity. World J Surg. 2004;28:435–444. doi: 10.1007/s00268-004-7364-x. [DOI] [PubMed] [Google Scholar]
- 37.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 38.Dixon AF, Dixon JB, O'Brien PE. Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J Clin Endocrinol Metab. 2005;90:813–819. doi: 10.1210/jc.2004-1546. [DOI] [PubMed] [Google Scholar]
- 39.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 40.Stroh CW, Weiner R, Benedix F, Horbach T, Birk D, Luderer D, Ludwig K, Meyer G, Wilhelm B, Wolff S, Knoll C, Manger T. Adipositas- und metabolische Chirurgie in Deutschland 2012-Ergebnisse der Qualitätssicherungsstudie zur operativen Therapie der Adipositas (GBSR) Zentralbl Chir. 2014;139:e1–e5. doi: 10.1055/s-0033-1360227. [DOI] [PubMed] [Google Scholar]
- 41.Regan JP, Inabnet WB, Gagner M, Pomp A. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg. 2003;13:861–864. doi: 10.1381/096089203322618669. [DOI] [PubMed] [Google Scholar]
- 42.Milone L, Strong V, Gagner M. Laparoscopic sleeve gastrectomy is superior to endoscopic intragastric balloon as a first stage procedure for super-obese patients (BMI ≥ 50) Obes Surg. 2005;15:612–617. doi: 10.1381/0960892053923833. [DOI] [PubMed] [Google Scholar]
- 43.Mognol P, Chosidow D, Marmuse JP. Laparoscopic sleeve gastrectomy as an initial bariatric operation for high-risk patients: initial results in 10 patients. Obes Surg. 2005;15:1030–1033. doi: 10.1381/0960892054621242. [DOI] [PubMed] [Google Scholar]
- 44.Daskalakis M, Weiner RA. Sleeve gastrectomy as a single-stage bariatric operation: indications and limitations. Obes Facts. 2009;2(suppl 1):8–10. doi: 10.1159/000198239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mognol P, Chosidow D, Marmuse JP. Laparoscopic sleeve gastrectomy (LSG): review of a new bariatric procedure and initial results. Surg Technol Int. 2006;15:47–52. [PubMed] [Google Scholar]
- 46.Fischer L, Hildebrandt C, Bruckner T, Kenngott H, Linke GR, Gehrig T, Buchler MW, Muller-Stich BP. Excessive weight loss after sleeve gastrectomy: a systematic review. Obes Surg. 2012;22:721–731. doi: 10.1007/s11695-012-0616-1. [DOI] [PubMed] [Google Scholar]
- 47.Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66:618–620. doi: 10.1002/bjs.1800660906. [DOI] [PubMed] [Google Scholar]
- 48.Marceau P, Biron S, Bourque RA, Potvin M, Hould FS, Simard S. Biliopancreatic diversion with a new type of gastrectomy. Obes Surg. 1993;3:29–35. doi: 10.1381/096089293765559728. [DOI] [PubMed] [Google Scholar]
- 49.Vage V, Gasdal R, Laukeland C, Sletteskog N, Behme J, Berstad A, Andersen JR. The biliopancreatic diversion with a duodenal switch (BPDDS): how is it optimally performed? Obes Surg. 2011;21:1864–1869. doi: 10.1007/s11695-011-0496-9. [DOI] [PubMed] [Google Scholar]
- 50.McConnell DB, O'Rourke RW, Deveney CW. Common channel length predicts outcomes of biliopancreatic diversion alone and with the duodenal switch surgery. Am J Surg. 2005;189:536–540. doi: 10.1016/j.amjsurg.2005.01.023. discussion 540. [DOI] [PubMed] [Google Scholar]
- 51.Hamoui N, Anthone GJ, Kaufman HS, Crookes PF. Maintenance of weight loss in patients with body mass index >60 kg/m2 importance of length of small bowel bypassed. Surg Obes Relat Dis. 2008;4:404–406. doi: 10.1016/j.soard.2007.08.020. discussion 406-407. [DOI] [PubMed] [Google Scholar]
- 52.Schauer PR, Schirmer BD, Brethauer SA. Minimally Invasive Bariatric Surgery. New York, NY: Springer; 2007. [Google Scholar]
- 53.Scopinaro N, Adami GF, Marinari GM, Gianetta E, Traverso E, Friedman D, Camerini G, Baschieri G, Simonelli A. Biliopancreatic diversion. World J Surg. 1998;22:936–946. doi: 10.1007/s002689900497. [DOI] [PubMed] [Google Scholar]
- 54.Gracia JA, Martinez M, Elia M, Aguilella V, Royo P, Jimenez A, Bielsa MA, Arribas D. Obesity surgery results depending on technique performed: long-term outcome. Obes Surg. 2009;19:432–438. doi: 10.1007/s11695-008-9762-x. [DOI] [PubMed] [Google Scholar]
- 55.Elnahas A, Graybiel K, Farrokhyar F, Gmora S, Anvari M, Hong D. Revisional surgery after failed laparoscopic adjustable gastric banding: a systematic review. Surg Endosc. 2013;27:740–745. doi: 10.1007/s00464-012-2510-2. [DOI] [PubMed] [Google Scholar]
- 56.Griffen WO, Jr, Young VL, Stevenson CC. A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg. 1977;186:500–509. doi: 10.1097/00000658-197710000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odstrcil EA, Martinez JG, Santa Ana CA, Xue B, Schneider RE, Steffer KJ, Porter JL, Asplin J, Kuhn JA, Fordtran JS. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92:704–713. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 58.Stroh C, Benedix F, Meyer F, Manger T. Nutrient deficiencies after bariatric surgery-systematic literature review and suggestions for diagnostics and treatment (article in German) Zentralbl Chir. 2013. DOI: 10.1055/s-0032-1328594. [DOI] [PubMed]
- 59.Brethauer SA, Aminian A, Romero-Talamas H, Batayyah E, Mackey J, Kennedy L, Kashyap SR, Kirwan JP, Rogula T, Kroh M, Chand B, Schauer PR. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628–636. doi: 10.1097/SLA.0b013e3182a5034b. discussion 636-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prachand VN, Davee RT, Alverdy JC. Duodenal switch provides superior weight loss in the super-obese (BMI ≥ 50 kg/m2) compared with gastric bypass. Ann Surg. 2006;244:611–619. doi: 10.1097/01.sla.0000239086.30518.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sovik TT, Taha O, Aasheim ET, Engstrom M, Kristinsson J, Bjorkman S, Schou CF, Lonroth H, Mala T, Olbers T. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. Br J Surg. 2010;97:160–166. doi: 10.1002/bjs.6802. [DOI] [PubMed] [Google Scholar]
- 62.Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg. 2012;147:847–854. doi: 10.1001/archsurg.2012.1654. [DOI] [PubMed] [Google Scholar]
- 63.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hess DS, Hess DW, Oakley RS. The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg. 2005;15:408–416. doi: 10.1381/0960892053576695. [DOI] [PubMed] [Google Scholar]
- 65.Laurenius A, Taha O, Maleckas A, Lonroth H, Olbers T. Laparoscopic biliopancreatic diversion/duodenal switch or laparoscopic Roux-en-Y gastric bypass for super-obesity-weight loss versus side effects. Surg Obes Relat Dis. 2010;6:408–414. doi: 10.1016/j.soard.2010.03.293. [DOI] [PubMed] [Google Scholar]
- 66.Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26:1031–1037. doi: 10.1016/j.nut.2009.12.003. [DOI] [PubMed] [Google Scholar]