Abstract
Background
It is important to identify an easily defined subset of patients at increased risk of adverse clinical outcomes associated with mineral and bone disorder (MBD) biomarkers (parathyroid hormone, calcium and phosphate).
Methods
Observational cohort study of 26 221 prevalent hemodialysis patients in Davita clinics as of 31 August 2005 and followed up until 31 December 2006 (16 months). Predictors were 12 possible definitions of ‘clinically important’ MBD based on all 3 biomarkers, and 18 alternative definitions based on only 1 or 2 biomarkers. Events were death alone and a composite of cardiovascular hospitalization or death. Excess events were calculated based on a multivariate Cox model using 5224 patients in target for all MBD biomarkers and applied to 20 997 patients out of target for at least one biomarker. Excess events attributable to MBD were estimated by subtracting the multivariate model-derived predicted number from the actual number. Outcomes were the proportion of excess events attributable to MBD captured by each definition (threshold ≥70%) and the reduction in the population size considered to have clinically important MBD (threshold ≥30%). The excess fraction was excess events divided by actual events.
Results
Patients with more biochemical markers out of target tended to be younger, black and have longer times since starting dialysis. The excess fraction associated with MBD ranged from ∼10 to 26% depending on the clinical endpoint and definition. The only definition to meet the thresholds required at least two of the three MBD biomarkers to be out of target (high or low). It captured 82% of excess composite endpoints and 74% of excess deaths and reduced the at-risk population by 46%.
Conclusions
Patients with at least two of three MBD biomarkers out of target represent a subgroup of patients at elevated risk of adverse clinical events.
Keywords: cardiovascular disease, CKD-MBD, death, hemodialysis, risk prediction
INTRODUCTION
Mineral and bone disorder (MBD) is common in patients receiving dialysis and is characterized by abnormal bone mineralization and vascular calcification [1–5]. It is challenging to manage, both because clinicians need to consider multiple biochemical markers including parathyroid hormone (PTH), calcium (Ca) and phosphate (P), and because the available therapies have varying and often discordant effects on these markers [6]. Furthermore, effective management includes a substantial effort on the part of patients to be adherent with dietary restrictions, with thrice-daily doses of oral phosphate binders, and, for many patients, regular use of oral medication [7].
Despite these challenges, MBD is clinically important as evidenced by a variety of studies that document the associations of PTH, Ca and P with adverse clinical outcomes [8–12]. It has also been shown that having more biomarkers outside of recommended targets, as well as a longer duration of time out of target, is associated with increased risk of death in incident hemodialysis patients [9]. In fact, up to 20% of deaths in dialysis patients may be associated with poorly controlled MBD, illustrating the importance for clinicians to manage MBD effectively [9].
To facilitate standardized practice patterns, two expert panels have released MBD guidelines for the management of MBD: Kidney Disease Outcomes Quality Initiative (KDOQI) [4] and Kidney Disease Improving Global Outcomes (KDIGO) [5]. These guidelines are intended to guide physician decision making by setting independent target ranges for PTH, Ca and P. Borrowing from these classification systems, a recent investigation showed that patients can be grouped into more clinically relevant MBD ‘phenotypes’ defined as being below, within or above target for PTH, Ca and P simultaneously [13]. Although the resulting classification system provided useful insight into the variety of biomarker patterns and their associated outcomes, it did not yield a sufficiently simple framework for identifying patients at the highest risk of adverse clinical outcomes. Without such a framework, it is difficult for clinicians to readily identify patients in routine clinical practice who may benefit from more focused clinical attention on MBD. This is particularly important with the growing influence from emerging national quality standards [e.g. the ESRD Quality Incentive Program (QIP)] and evolving provider treatment protocols, which may conflict in their focus on the three MBD biomarkers.
Therefore, it is important to have a simple, but useful, framework for identifying patients at the highest risk of adverse clinical outcomes associated with MBD [14]. To accomplish this goal, the first step is to estimate the number of excess events attributable to MBD. Then, using this information, the second step is to compare simple definitions of ‘clinically important’ MBD and to select those decision rules that maximize the capture of patients who are at excess risk (akin to high sensitivity) while minimizing the capture of patients who are not (akin to high specificity).
MATERIALS AND METHODS
Data source and patients
This observational study used a combined dataset from DaVita, Inc. and the United States Renal Data System (USRDS) [15]. Additional detail on the database is provided in Supplementary data. The cohort consisted of a point prevalent dialysis population in August 2004 who survived for 12 months, were in the facility as of 31 August 2005 (baseline) and had received ≥6 dialysis sessions in August 2005 [13]. Patients with a parathyroidectomy within 12 months before baseline were excluded. Patients had to have at least one PTH, Ca and P result in the 4-month period before baseline (May through August 2005) and were followed for cardiovascular hospitalization and for death from 1 September 2005 until 31 December 2006.
MBD biomarkers and therapy
Average values for PTH, Ca and P over the 4 months before baseline were calculated. For PTH, patients were categorized as follows: Low = 0–149 pg/mL, Low Target = 150–299 pg/mL, High Target = 300–599 pg/mL and High = 600+ pg/mL for consistency with KDIGO and KDOQI recommendations. For Ca, patients were categorized into low, normal and high using the DaVita Laboratory normal reference range (8.4–10.2 mg/dL). For P, patients were categorized into low, target and high using the KDOQI target range of 3.5–5.5 mg/dL. Vitamin D, phosphate binder, and cinacalcet treatment were categorized monthly as yes or no if at least one dose was recorded during the month and were summarized over the same 4-month window to be consistent with the laboratory values. Calcium and non-calcium-containing phosphate binders could not be calculated as mutually exclusive subcategories.
Definitions of clinically important MBD
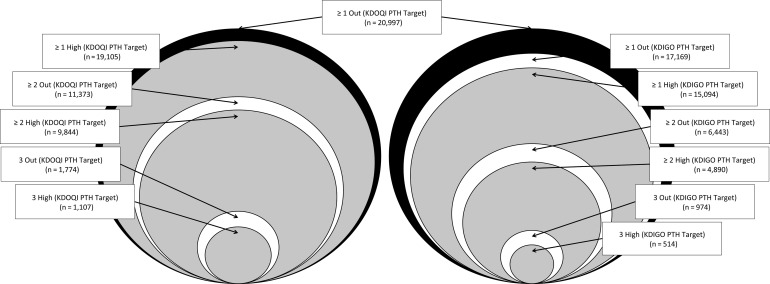
Patients with at least one biochemical marker out of target were categorized into one of three possible definitions of clinically important MBD based on their PTH, Ca and P values being above or below target: ≥1 Out of Target, ≥2 Out of Target and All 3 Out of Target. This set was defined using the KDOQI PTH target. Another analogous set of definitions of clinically important MBD was created that focused on being ‘above’ the target range, referred to as ≥1 High, ≥2 High and All 3 High. Patients were also classified according to an additional set of six definitions of clinically important MBD identical to those specified earlier, but replacing the KDOQI PTH target with the KDIGO target. These were the 12 primary definitions of clinically important MBD evaluated in this study (Figure 1).
FIGURE 1:
Graphical depiction of the 12 primary definitions. All definitions are subsets of the KDOQI definition, which is shown in both panels for reference. The area of each circle is proportional to the sample size of patients defined as having clinically important MBD by that definition. The KDIGO definitions are not fully nested within the KDOQI definitions when only high values are considered, due to the different PTH target ranges.
Because a single reference definition was used for all comparisons, the definitions restricted to values above target yielded a set of ‘leftover’ patients with at least one low value and no high values. For example, a patient with low target PTH, low Ca and target P would be classified as ≥1 Out of Target in the set of definitions focused on being out of target. However, in the set of definitions focused on patients who are above target, this patient could not be classified as being above target nor could the patient be included in the reference group without changing it. Therefore, such patients were grouped separately and referred to as the ‘Low Only’ group. Similarly, the use of the KDIGO PTH range leaves out a group of patients with both Ca and P in target who have a PTH between 300 and 599 pg/mL; these patients were referred to as the ‘High Target PTH Only’ group. Estimates for excess risk are provided for these two groups, in addition to the 12 primary definitions.
To permit comparisons to any other measure that might be conceived as a variation, an additional 18 ad hoc definitions were included that accounted for all individual and pairwise combinations of PTH, Ca and P (see Supplementary data). These were considered to be ad hoc definitions because they excluded at least one MBD-related biomarker.
Endpoints
The primary endpoint for the risk model was a composite endpoint based on the first occurrence of a cardiovascular hospitalization or death. All analyses were repeated for death alone. Details on the identification of endpoints are available in Supplementary data.
Predictive model of excess risk
We defined the number of patients with clinically important MBD as the number of excess cases of the composite event beyond what would be predicted by known risk factors in patients who were in target for all three MBD parameters. To identify excess events, we created a predictive model for each endpoint using the sample of patients who were in control for PTH, Ca and P (n = 5224), referred to hereafter as the ‘model development sample.’ The KDOQI PTH target was used for this group because it allowed for the most comprehensive definition of clinically important MBD. We used previously identified covariates from other similar studies to create multivariable Cox proportional hazards models of time to the composite endpoint and time to death [13, 16].
Because patients in the model development sample were in target for PTH, Ca and P at baseline, and because we excluded MBD biomarkers, the resulting model accounts for all risk factors except those related to MBD [17]. When applied to a population with MBD, the model underestimates the risk from MBD and ‘under-predicts’ the event probability for each patient. To predict the number of excess events captured with each definition of clinically important MBD, the individual event probabilities were summed across individuals in the groups formed according to our definitions and compared to the actual number of events. The difference was considered to be ‘excess’ events attributable to the model's inability to account for MBD. Predicting at the group level also avoided limitations in predicting individual-specific outcomes, which are highly variable, and using a separate development sample reduced the risk of over-fitting [18].
The predictive model results were used to compare the definitions of clinically important MBD against the results from the reference definition of ≥1 Out of Target using the KDOQI PTH range. Definitions were evaluated according to two opposing outcomes: the proportion of patients with clinically important MBD who were retained and the reduction in the size of the population considered to have clinically important MBD. We set arbitrary thresholds for choosing useful definitions as those that (i) captured ≥70% of the patients with clinically important MBD and (ii) reduced the ‘at-risk’ population by ≥30%.
The excess fraction was calculated as the number of excess events divided by the number of actual events. Confidence intervals for all study measures were based on a bootstrap using 1000 resamples. All analyses were performed in SAS (version 9.3).
RESULTS
The demographic and clinical characteristics of the 26 221 patients at baseline are summarized in Table 1. The results are presented both overall and by the number of biochemical markers out of target using the KDOQI PTH definition. The groups in Table 1 are mutually exclusive (i.e. they are not the groups defined by the 12 primary definitions, which are overlapping). There were more biochemical markers out of target in patients who were younger, black and had a longer time on dialysis (i.e. longer vintage). The overall crude event rates were 44.3% for the composite endpoint and 23.1% for death. The crude composite endpoint rate increased with increasing biomarkers out of target, while the crude death rate did not. Use of cinacalcet and non-calcium-containing phosphate binders increased as the number of laboratory values out of target increased.
Table 1.
Patient characteristics by the number of biochemical markers out of target
| Variables | Levels | None out of target (n = 5224) | 1 out of target (n = 9624) | 2 out of target (n = 9599) | 3 out of target (n = 1774) | All patients (n = 26 221) |
|---|---|---|---|---|---|---|
| Age group (%) | 0–29 years | 0.5 | 1.1 | 3.6 | 4.2 | 2.1 |
| 30–39 years | 2.6 | 4.5 | 8.9 | 9.9 | 6.1 | |
| 40–49 years | 7.5 | 11.0 | 16.6 | 18.3 | 12.8 | |
| 50–59 years | 16.1 | 20.3 | 24.5 | 25.1 | 21.4 | |
| 60–64 years | 11.6 | 12.7 | 12.3 | 11.8 | 12.3 | |
| 65–69 years | 13.2 | 13.1 | 11.5 | 10.0 | 12.3 | |
| 70–79 years | 29.5 | 22.9 | 15.6 | 14.4 | 21.0 | |
| ≥80 years | 18.9 | 14.3 | 7.0 | 6.4 | 12.0 | |
| Sex (%) | Men | 55.1 | 53.2 | 53.3 | 56.5 | 53.9 |
| Women | 44.9 | 46.8 | 46.7 | 43.5 | 46.1 | |
| Race (%) | White | 61.9 | 53.0 | 47.6 | 45.2 | 52.3 |
| African American | 29.3 | 37.8 | 43.0 | 47.9 | 38.7 | |
| Native American | 2.6 | 3.0 | 3.1 | 2.5 | 2.9 | |
| Asian | 4.6 | 4.8 | 4.8 | 3.3 | 4.7 | |
| Other/unknown | 1.5 | 1.4 | 1.4 | 1.2 | 1.4 | |
| Comorbid conditions (%) | ASHD | 42.5 | 38.2 | 33.6 | 31.6 | 36.9 |
| CHF | 44.6 | 42.7 | 41.9 | 39.0 | 42.5 | |
| COPD | 17.7 | 17.4 | 16.6 | 15.6 | 17.0 | |
| CVA | 17.5 | 16.7 | 13.6 | 14.4 | 15.6 | |
| Diabetes | 62.1 | 59.6 | 54.5 | 47.1 | 57.3 | |
| Vintage (%) | 12–24 months | 30.2 | 25.0 | 20.4 | 15.5 | 23.7 |
| 25–48 months | 36.2 | 33.9 | 31.3 | 25.1 | 32.8 | |
| ≥49 months | 33.6 | 41.1 | 48.4 | 59.4 | 43.5 | |
| Albumin, g/dL | Mean (SD) | 3.85 (0.34) | 3.86 (0.35) | 3.90 (0.34) | 3.90 (0.36) | 3.87 (0.35) |
| Hemoglobin, g/dL | Mean (SD) | 12.36 (0.85) | 12.34 (0.91) | 12.37 (0.94) | 12.41 (0.96) | 12.36 (0.91) |
| Kt/V | Mean (SD) | 1.71 (0.25) | 1.68 (0.25) | 1.63 (0.24) | 1.60 (0.23) | 1.66 (0.25) |
| PTH, pg/mL | Mean (SD) | 228 (40.8) | 341 (243) | 589 (486) | 702 (652) | 434 (403) |
| Calcium, mg/dL | Mean (SD) | 9.56 (0.38) | 9.59 (0.47) | 9.59 (0.61) | 9.83 (1.13) | 9.60 (0.58) |
| Phosphate, mg/dL | Mean (SD) | 4.64 (0.51) | 5.09 (0.96) | 6.29 (1.26) | 6.60 (1.21) | 5.54 (1.26) |
| Event rate (%) | Death | 24.3 | 23.9 | 21.5 | 24.2 | 23.1 |
| Composite | 43.0 | 43.6 | 45.1 | 47.6 | 44.3 | |
| Any MBD-directed therapy (%) | Any vitamin D | 85 | 83 | 85 | 79 | 84 |
| Any phosphate binder | 90 | 92 | 95 | 95 | 93 | |
| Any non-calcium-containing phosphate binder | 52 | 62 | 74 | 75 | 65 | |
| Any calcium-containing phosphate binder | 55 | 53 | 52 | 52 | 53 | |
| Any cinacalcet | 11 | 21 | 35 | 37 | 25 |
Only selected comorbidities are shown, including ASHD, atherosclerotic heart disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident. Groups included in this table are mutually exclusive and are used to construct definitions of ≥1 Out of Target, ≥2 Out of Target and ≥3 Out of Target. Because information about MBD-directed therapy is summarized on a monthly basis as yes/no, calcium and non-calcium-containing phosphate binder categories are not mutually exclusive.
There was a clear relationship between a greater number of biochemical markers out of target and a higher excess fraction associated with MBD (see Tables 2 and 3). Across the endpoints and definitions, the excess fraction estimates ranged between 9.7 and 25.9%. In general, the excess fraction associated with MBD was higher for death than for the composite endpoint. Also, the KDIGO-based PTH target generally, but not always, yielded higher excess fraction estimates than the KDOQI PTH target.
Table 2.
Actual events, excess events and excess risk of the composite endpoint
| MBD definition | N | Composite events (cardiovascular hospitalization or death) |
|||
|---|---|---|---|---|---|
| Actual | Predicted | Excess | Excess fraction (%) | ||
| Model development sample | |||||
| All in Target | 5224 | 2248 | 2248 | 0 | 0 |
| KDOQI PTH Target | |||||
| ≥1 Out* | 20 997 | 9368 | 8455 | 913 (605–1266) | 9.7 (6.5–13.4) |
| ≥2 Out | 11 373 | 5174 | 4423 | 751 (531–974) | 14.5 (10.4–18.6) |
| 3 Out | 1774 | 845 | 681 | 164 (113–215) | 19.4 (13.7–24.7) |
| ≥1 High | 19 105 | 8489 | 7581 | 908 (610–1246) | 10.7 (7.3–14.5) |
| ≥2 High | 9844 | 4461 | 3755 | 706 (508–913) | 15.8 (11.6–20.2) |
| 3 High | 1107 | 551 | 441 | 110 (73–147) | 20.0 (14.0–25.4) |
| KDIGO PTH Target | |||||
| ≥1 Out | 17 169 | 7712 | 6871 | 841 (577–1139) | 10.9 (7.5–14.7) |
| ≥2 Out | 6443 | 2971 | 2510 | 461 (327–609) | 15.5 (11.1–20.3) |
| 3 Out | 974 | 453 | 366 | 87 (53–120) | 19.2 (12.3–25.6) |
| ≥1 High | 15 094 | 6750 | 5916 | 834 (582–1119) | 12.4 (8.7–16.4) |
| ≥2 High | 4890 | 2258 | 1851 | 407 (291–529) | 18.0 (13.0–23.0) |
| 3 High | 514 | 242 | 197 | 45 (21–69) | 18.7 (9.6–26.5) |
*KDOQI PTH Target with ≥1 Out is the reference definition for comparing all other definitions. The All in Target population was used as the model development sample for the risk estimation. The excess fraction was calculated as the number of excess events divided by the number of actual events. Values in parentheses reflect the middle 95% of the bootstrap distribution.
Table 3.
Actual events, excess events and excess risk of death
| MBD definition | N | Deaths |
|||
|---|---|---|---|---|---|
| Actual | Predicted | Excess events | Excess fraction (%) | ||
| Model development sample | |||||
| All in Target | 5224 | 1271 | 1271 | 0 | 0 |
| KDOQI PTH Target | |||||
| ≥1 Out | 20 997 | 4799 | 4284 | 515 (280–771) | 10.7 (5.9–13.2) |
| ≥2 Out | 11 373 | 2497 | 2118 | 379 (228–528) | 15.2 (9.2–21.0) |
| 3 Out | 1774 | 430 | 330 | 100 (60–140) | 23.2 (14.5–30.7) |
| ≥1 High | 19 105 | 4242 | 3755 | 487 (259–729) | 11.5 (6.2–16.9) |
| ≥2 High | 9844 | 2082 | 1743 | 339 (200–474) | 16.3 (9.7–22.3) |
| 3 High | 1107 | 281 | 217 | 64 (35–94) | 22.7 (13.2–30.7) |
| KDIGO PTH Target | |||||
| ≥1 Out | 17 169 | 3901 | 3453 | 448 (249–666) | 11.5 (6.5–16.8) |
| ≥2 Out | 6443 | 1490 | 1203 | 287 (185–388) | 19.2 (12.7–25.5) |
| 3 Out | 974 | 236 | 175 | 61 (34–89) | 25.8 (15.7–34.2) |
| ≥1 High | 15 094 | 3296 | 2879 | 417 (242–607) | 12.6 (7.4–18.3) |
| ≥2 High | 4890 | 1084 | 842 | 242 (158–327) | 22.3 (15.0–29.2) |
| 3 High | 514 | 126 | 93 | 33 (14–52) | 25.9 (13.1–36.5) |
KDOQI PTH Target with ≥1 Out is the reference definition for comparing all other definitions. The All in Target population was used as the model development sample for the risk estimation. The excess fraction was calculated as the number of excess events divided by the number of actual events. Values in parentheses reflect the middle 95% of the bootstrap distribution.
Patients in the Low Only group (at least one low biochemical marker and none high; n = 1894) had 12 excess composite events for a composite event excess fraction of 1.5% (95% CI 0–5.9%). These patients also had 31 excess deaths for a death excess fraction of 5.2% (95% CI 0–13.1%). Patients in the High Target PTH Only group (PTH of 300–599 pg/mL and both Ca and P in target; n = 3828) had 71 excess composite events for a composite event excess fraction of 4.5% (95% CI 0–9.4%). They had 68 excess deaths for a death excess fraction of 8.2% (95% CI 0.1–15.7%).
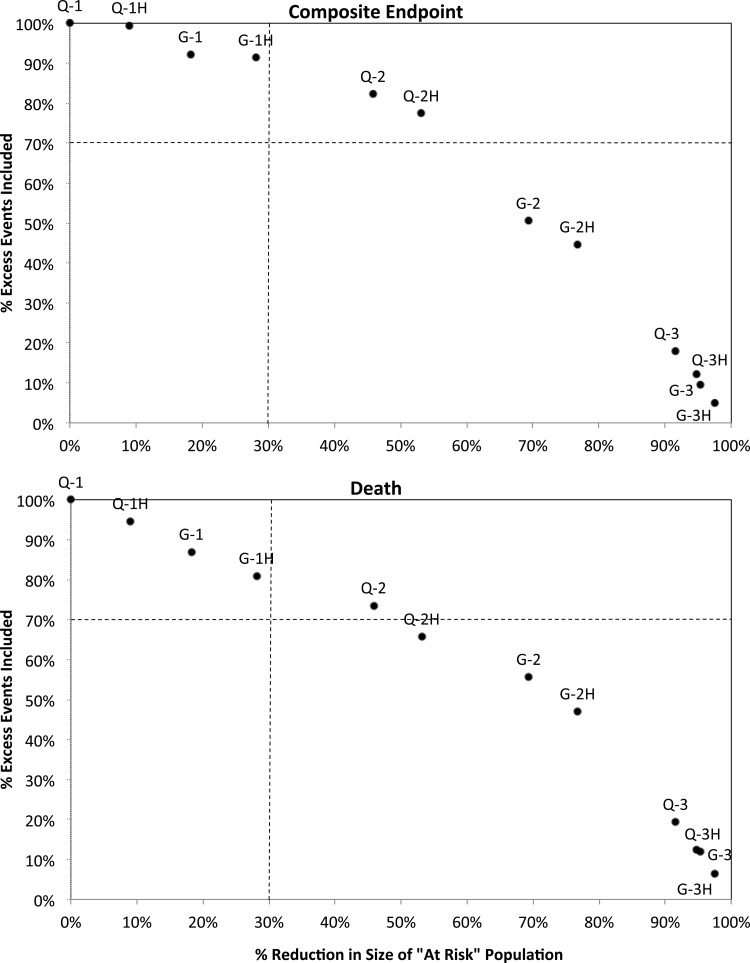
There was a consistent inverse relationship between the reduction in the MBD population size and the proportion of excess events captured by each definition of clinically important MBD. The only definition that included all three biomarkers and met our threshold of retaining at least 70% of the excess cases for both the composite endpoint and for death and reduced the ‘at-risk’ population size by at least 30% was one that included patients with any two biomarkers out of target (Figure 2, point Q-2). It captured 82% of excess composite endpoints and 74% of excess deaths and reduced the population by 46%.
FIGURE 2:
Reduction in the ‘at-risk’ MBD population versus the proportion of excess events captured for 12 primary MBD definitions. MBD definitions using the KDOQI PTH target begin with ‘Q-’, and those using the KDIGO PTH target begin with ‘G-’. This is followed by either the number of biochemical markers out of target (1, 2 or 3) or the number high (1H, 2H or 3H).
Of the four definitions of clinically important MBD that focused on at least two biochemical markers (out of target or high), only one other was close to our threshold. It included patients with at least two biomarkers above target according to the KDOQI PTH range, identified 77% of excess composite events and 66% of excess deaths, and reduced the population by 53% and (Figure 2, point Q-2H).
The four definitions that focused on at least one biomarker (out of target or high) tended to capture most of the excess composite events and deaths but did not reduce the at-risk population sufficiently to meet our threshold. The closest was the definition using at least one biomarker above target according to the KDIGO PTH range. This definition of clinically important MBD identified 91% of excess composite events and 81% of excess deaths but reduced the population by only 28% (Figure 2, point G-1H).
None of the four definitions of clinically important MBD that focused on all three biomarkers (out of target or high) captured more than 19% of the excess events; however, all reduced the population substantially by 92% or more.
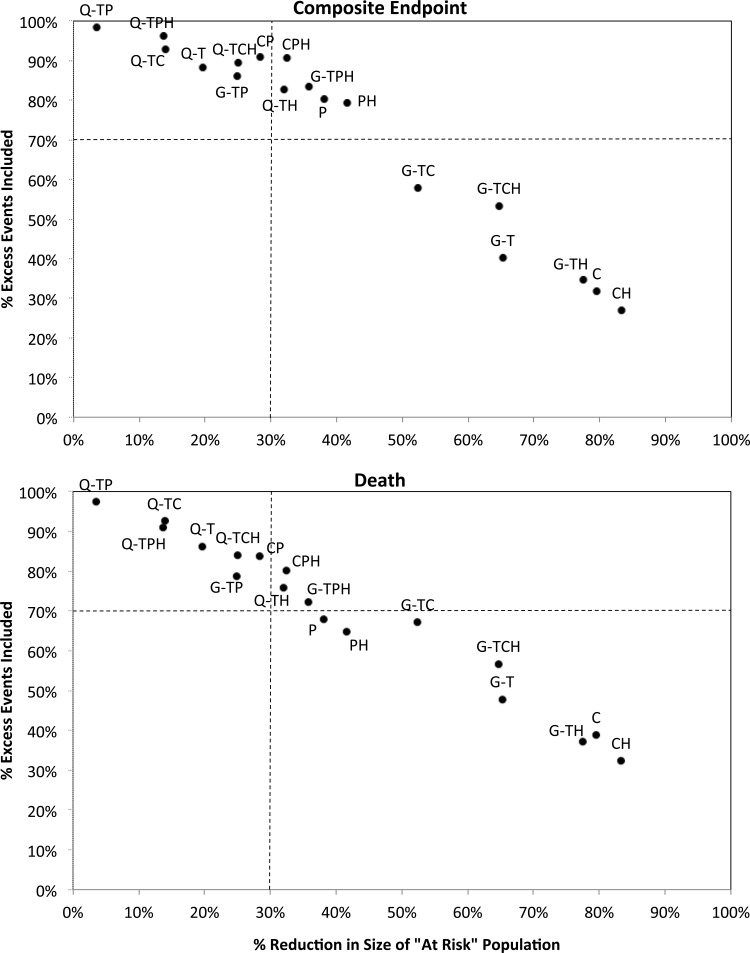
Hypercalcemia (regardless of PTH or Ca levels) captured only 27% of excess composite events and 32% of excess deaths but reduced the population by 83% (Figure 3, point CH). Other ad hoc results are discussed in Supplementary data.
FIGURE 3:
Reduction in the ‘at-risk’ MBD population versus the proportion of excess events captured for 18 ad hoc MBD definitions. MBD definitions using the KDOQI PTH target begin with ‘Q-’, and those of KDIGO begin with ‘G-’ unless PTH was not included in the definition. Out of target markers for ad hoc definitions are indicated as follows: PTH = ‘T’, calcium = ‘C’ and phosphate = ‘P’. If out of target was only considered as above target, this is indicated with an ‘H’ at the end.
DISCUSSION
In this investigation, we used data on over 26 000 hemodialysis patients to evaluate definitions of clinically important MBD. Our goal was to identify definitions that effectively balanced two competing objectives: (i) maximizing the capture of excess clinical events potentially attributable to MBD and (ii) reducing the number of patients considered to have clinically important MBD in order to help clinicians focus clinical attention on the highest risk patients. Our results suggest that a definition of at least two biomarkers out of target using the KDOQI PTH range best balances these objectives. This definition could be considered for use in identifying patients undergoing hemodialysis for whom more focused clinical attention may be warranted due to a high excess risk of adverse clinical events.
The definitions we evaluated are based on the existing KDOQI and KDIGO MBD guidelines. However, because the evidence base (and therefore the guidelines) is limited regarding the effect of simultaneous biochemical control, the guidelines are limited in their ability to help clinicians identify real-world patients at elevated risk of adverse clinical events. For example, patients who are below target for one or more biomarkers have just a very slight excess risk of the composite endpoint. The fact that these patients are at a higher excess risk of death than of the composite endpoint suggests that their risk is mediated through different pathways than cardiovascular disease. If so, few of these patients may be likely to experience cardiovascular benefit from traditional approaches to MBD management. In contrast, patients with elevated levels of two or more biomarkers appear to account for a large proportion of excess events related to MBD and may be more appropriate candidates for focused management with respect to MBD.
There are many reasons why providers and provider organizations might choose one definition over another. Our data show that there are a variety of useful definitions that could be implemented with varying trade-offs. However, to the extent providers choose to implement definitions that leave out specific patient groups or measures (e.g. any of our ad hoc definitions), they must be careful to avoid inadvertently providing incentives that adversely affect patients. For example, it may be appealing to focus on hypercalcemia, or all patients with either hypercalcemia or hyperphosphatemia because they are readily measured. This could result in PTH elevations or reductions depending on the approaches used to modulate Ca and/or P. Our data suggest that such an approach would ignore a sizable proportion of patients with clinically meaningful MBD. As the nephrology community works diligently to improve patient outcomes, the effects of such inadvertent incentives should be considered.
As a case in point, reimbursement for dialysis services in the United States now incorporates a component based on the achievement of guideline-based clinical targets (i.e. QIP), and these guidelines are intended to include MBD metrics [19]. Currently, the collection of regular Ca and P measures is a QIP target, with the goal of, as early as 2016, incorporating hypercalcemia (Ca > 10.2 mg/dL) as a QIP measure [20, 21]. Many studies have shown that physicians respond to both clinical and financial incentives; therefore, QIP measures that are comprehensive for MBD may be better for aligning financial incentives with patient outcomes [22, 23].
The goal of this study was to define a subset of MBD patients who might benefit from focused clinical attention by using all three biomarkers simultaneously, consistent with guidelines and anticipated QIP measures. Knowing how to identify such patients is the first step toward improving outcomes. This is particularly important when financial incentives might not fully align with clinical incentives, a situation that might occur as the QIP program evolves in its ability to measure laboratory values consistently.
There are a number of limitations to our analyses. First, our study is based on a counter-factual scenario created by comparing a predicted event count to an actual event count. To the extent that other strong confounders are missing in the data and are more likely to occur in MBD patients, they could affect the estimates of excess events. Our ‘unexposed’ group is likely to have been exposed to MBD, either before or after baseline. Therefore, our risk model is likely to include some influence from PTH, Ca and P, a situation that is likely to have reduced our counts of excess events. There are also many potential variations in analytical methods that others might prefer, including specific interactions, time horizons, calendar years of data, outcome definitions and target ranges. Although we tested multiple definitions and outcome measures, it would be useful to replicate these findings in different datasets, perhaps with different specifications, to test the robustness of the findings.
The patients in this study were based on a prevalent population and were required to survive for 12 months from the time of initial observation. This was done for two reasons: to allow us to compare different look-back periods for the measurement of mean PTH, Ca and P (data not shown) [13], and to increase the homogeneity of the sample for our prediction model. Therefore, these results are more relevant for patients with a mix of times since the start of dialysis for whom management of MBD is an ongoing concern. Also, we should note that PTH, Ca and P were treated as categorical variables. Patients who fall within one group but have laboratory values near the cutoff values that define their group may not have the same risk as the rest of their group.
Our measure of excess risk is conservative in that it estimates excess events at the 16-month time horizon of the study. It does not account for events that might have been delayed but that still occurred by the end of the study (or were otherwise altered in time). In addition, our goal of finding a definition that retained at least 70% of excess events and reduced the number of patients considered ‘at risk’ by 30% is arbitrary. Others may favor higher or lower thresholds. We could not easily measure true sensitivity and specificity or a net reclassification index, because we estimated excess events at the level of the subgroups in our analyses. However, our results are intended to align with these concepts, and the trade-off between excess events captured and the size of the ‘at-risk’ population is intended to align with the idea of an receiver-operating characteristic curve. The reason for analyzing the data in this way was to avoid individual predictions that are notoriously challenging [24]. In essence, we elected to estimate the number of events accurately at the expense of determining which specific patients would have an event.
The question of whether our results are externally valid is important. To help maximize its generalizability, we selected risk factors based on published risk factors from other studies. To avoid over-fitting our predictive model, we built it using a subset of the data (patients within target for PTH, Ca and P); then, we applied it to the remaining MBD patients [18]. This approach yielded results comparable with a previous investigation estimating the attributable risk of MBD [9]. In the previous investigation, the attributable risk of death using KDOQI targets ranged from 12 to 28%, whereas in our study the estimates were slightly lower and ranged from 11 to 23%. Part of this difference may be attributable to less over-fitting in our models compared with the earlier study. Given the differences the population and the statistical models used, this level of concordance is very encouraging.
Patients within target for PTH, Ca and P (on whom we built the predictive model) may have been different from the rest of the population in ways that were not captured with our adjusted predictive model. The most obvious difference may be related to MBD-directed treatments, since these patients were within all target ranges and the other patients were not. Based on simple analyses of treatments, the use of vitamin D and phosphate binders was similar across the patient subgroups defined by the number of parameters out of target. However, it appears that patients in target for all three risk factors tended to use less cinacalcet and less non-calcium-containing binders than patients in the other MBD subgroups. This may suggest that our reference group was easier to bring into target with respect to PTH, Ca and/or P. The greater use of cinacalcet and non-calcium-containing binders in the subgroup with all three out of target also suggests that these patients may be harder to bring into target.
Along these lines, it is important to discuss the effect of other therapies in reaching target. Implicit in our goal was to identify a laboratory-based assessment tool and to ignore the role of treatment. It is possible that there are effects of medications beyond their direct effect on PTH, Ca and P. For example, excessive doses of calcium might be useful in bringing patients into target, but they may not reduce the risk of cardiovascular disease. Since the population in target in our study appeared to use more calcium-containing phosphate binders, if this binder use increases cardiovascular risk, our study may under-estimate the benefit bringing patients into target with non-calcium-containing binders. However, we should also be clear that this is hypothesis-generating speculation for future studies to consider, perhaps by using marginal structural models to incorporate time-dependent confounders affected by prior treatment. Our study was not designed to evaluate the role of therapy in modifying risk.
This study focuses exclusively on cardiovascular events and mortality as important outcomes related to MBD. However, it is well recognized that there are other important clinical outcomes related to MBD including fracture and parathyroidectomy. Although these are less common outcomes, additional studies focused on these outcomes might be informative. One final thought is that it would be preferable if one could rule out more patients who are not really at excess risk. For example, because the excess fraction is generally <25%, it might be useful to reduce the population size by at least 60%, rather than 30%, while still capturing at least 70% of excess events. None of the definitions we used achieved such a goal in this dataset. This likely requires additional understanding in the group with a single biochemical marker out of target. Perhaps there is a way to evaluate their temporal patterns of PTH, Ca and P to make finer distinctions in these patients.
In conclusion, patients with at least two biomarkers out of target represent a subgroup of MBD patients at clinically important risk of cardiovascular hospitalization and death. Additional focus on these patients may provide a way to improve outcomes in patients with MBD. Additional investigation is important for elucidating the appropriate interventions in these patient subgroups.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
G.A.B. serves as advisor and consultant for Amgen, Inc. and as a medical director for Davita. M.D.D. and M.H. are employees of Outcomes Insights, Inc., which receives research and consulting funding from Amgen, Inc. B.D.B., T.P.D. and K.A.L. work in the Center for Observational Research at Amgen, Inc. and are stockholders of Amgen, Inc. This research was funded by Amgen, Inc. The Amgen study protocol created prior to initiating research specified that the results would be published regardless of outcome.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the work of the NIH and the USRDS Coordinating Center in developing and maintaining the USRDS and of DaVita for enabling the merging of the datasets. However, the opinions of the authors do not reflect those of DaVita, the NIH or the USRDS. The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
REFERENCES
- 1.Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18: 875–885 [DOI] [PubMed] [Google Scholar]
- 2.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69: 1945–1953 [DOI] [PubMed] [Google Scholar]
- 3.Moe SM, Drueke T, Lameire N, et al. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis 2007; 14: 3–12 [DOI] [PubMed] [Google Scholar]
- 4.NKF. KDOQI Guidelines for CKD Care. Available from URL: http://www.kidney.org/professionals/kdoqi/guidelines_commentaries.cfm 2013 [Google Scholar]
- 5.NKF. KDIGO: Kidney Disease—Improving Global Outcomes Clinical Practice Guidelines. Available from URL: http://kdigo.org/home/guidelines/ 2013 [Google Scholar]
- 6.Martin KJ, Gonzalez EA. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: What is normal, when to start, and how to treat? Clin J Am Soc Nephrol 2011; 6: 440–446 [DOI] [PubMed] [Google Scholar]
- 7.Bover J, Farre N, Andres E, et al. Update on the treatment of chronic kidney disease-mineral and bone disorder. J Ren Care 2009; 35(Suppl 1): 19–27 [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 9.Danese MD, Belozeroff V, Smirnakis K, et al. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 1423–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int 2006; 70: 351–357 [DOI] [PubMed] [Google Scholar]
- 11.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530 [DOI] [PubMed] [Google Scholar]
- 12.Block GA. Therapeutic interventions for chronic kidney disease-mineral and bone disorders: focus on mortality. Curr Opin Nephrol Hypertens 2011; 20: 376–381 [DOI] [PubMed] [Google Scholar]
- 13.Block GA, Kilpatrick RD, Lowe KA, et al. CKD-mineral and bone disorder and risk of death and cardiovascular hospitalization in patients on hemodialysis. Clin J Am Soc Nephrol 2013; 8: 2132–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cozzolino M, Urena-Torres P, Vervloet MG, et al. Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol Dial Transplant 2014; 68: 429–436 [DOI] [PubMed] [Google Scholar]
- 15.USRDS. Annual Data Report 2012
- 16.Liu J, Huang Z, Gilbertson DT, et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 2010; 77: 141–151 [DOI] [PubMed] [Google Scholar]
- 17.Glynn RJ, Gagne JJ, Schneeweiss S. Role of disease risk scores in comparative effectiveness research with emerging therapies. Pharmacoepidemiol Drug Saf 2012; 21(S2): 138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation and Updating. New York, NY: Springer, 2010 [Google Scholar]
- 19.2013. Medicare Program: End-Stage Renal Disease Prospective Payment System, Quality Incentive Program, and Durable Medical Equipment, Prosthetics, Orthotics, and Supplies: Department of Health and Human Services; Centers for Medicare & Medicaid Services.
- 20.CMS. ESRD QIP Payment Year 2015 Program Details. Available from URL: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/QIP-Details-PY15.pdf [Google Scholar]
- 21.CMS. ESRD QIP Payment Year 2016 Program Details. Available from URL: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/QIP-Details-PY16.pdf [Google Scholar]
- 22.Brunelli SM, Monda KL, Burkart JM, et al. Early trends from the Study to Evaluate the Prospective Payment System Impact on Small Dialysis Organizations (STEPPS). Am J Kidney Dis 2013; 61: 947–956 [DOI] [PubMed] [Google Scholar]
- 23.Powe NR, Griffiths RI, Anderson GF, et al. Medicare payment policy and recombinant erythropoietin prescribing for dialysis patients. Am J Kidney Dis 1993; 22: 557–567 [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Eijkemans MJ, Boersma E, et al. Applicability of clinical prediction models in acute myocardial infarction: a comparison of traditional and empirical Bayes adjustment methods. Am Heart J 2005; 150: 920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.