Abstract
Background
Anemia is common in chronic kidney disease (CKD) and associated with poor outcomes. In cross-sectional studies, lower estimated glomerular filtration rate (eGFR) has been associated with increased risk for anemia. The aim of this study was to determine how hematocrit changes as eGFR declines and what factors impact this longitudinal association.
Methods
We followed 1094 African-Americans with hypertensive nephropathy who participated in the African-American Study of Kidney Disease and Hypertension. Mixed effects models were used to determine longitudinal change in hematocrit as a function of eGFR. Interaction terms were used to assess for differential effects of age, gender, baseline eGFR, baseline proteinuria, malnutrition and inflammation on eGFR-associated declines in hematocrit. In sensitivity analyses, models were run using iGFR (by renal clearance of I125 iothalamate) in place of eGFR.
Results
At baseline, mean hematocrit was 39% and 441 (40%) individuals had anemia. The longitudinal relationship between eGFR and hematocrit differed by baseline eGFR and was steeper when baseline eGFR was <45 mL/min/1.73 m2. For example, the absolute decline in hematocrit per 10 mL/min/1.73 m2 decline in longitudinal eGFR was −3.7, −1.3 and −0.5% for baseline eGFR values of 20, 40 and 60 mL/min/1.73 m2, respectively (P < 0.001 comparing the longitudinal association between baseline eGFR = 40 or 60 versus baseline eGFR = 20 mL/min/1.73 m2). Similarly, male sex, younger age (<65 years) and higher baseline proteinuria (protein-to-creatinine ratio >0.22) were associated with greater hematocrit declines per unit decrease in longitudinal eGFR compared with female sex, older age and low baseline proteinuria, respectively (P-interaction <0.05 for each comparison). The longitudinal eGFR–hematocrit association did not differ by body mass index, serum albumin or C-reactive protein.
Conclusions
Men, younger individuals and those with low baseline eGFR (<45 mL/min/1.73 m2) or baseline proteinuria are particularly at risk for eGFR-related declines in hematocrit.
Keywords: African-American Study of Kidney Disease and Hypertension (AASK), anemia, chronic kidney disease, hematocrit
INTRODUCTION
Anemia commonly develops in chronic kidney disease (CKD), presumably due to decreases in erythropoietin production and derangements in iron homeostasis that occur as kidney function declines [1, 2]. Defined by the Kidney Disease Outcomes and Quality Initiative (KDOQI) as hemoglobin levels of <13.5 g/dL (hematocrit <40.5%) for men and <12 g/dL (hematocrit <36%) for women, anemia has also been associated with poor clinical outcomes [3]. These outcomes include left ventricular hypertrophy, lower quality of life, cardiovascular hospitalization and all-cause mortality [4–6].
In prior studies, anemia was associated with reduced kidney function, as well as female gender, older age, African-American race, iron deficiency and chronic inflammation [2, 7, 8]. Using data from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994), Astor et al. demonstrated that mean hemoglobin levels were lower and prevalence of anemia was higher among individuals with estimated glomerular filtration rates (eGFRs) below 60 mL/min/1.73 m2 [2]. The Prevalence of Anemia in Early Renal Insufficiency (PAERI) study also reported greater odds of anemia with lower eGFR [7]. These findings, however, were cross-sectional and, therefore, provide limited insights about how anemia evolves in the context of CKD progression. Whether hematocrit declines linearly as eGFR declines and whether patterns of hematocrit change vary by age, gender, baseline proteinuria, malnutrition, inflammation or level of baseline eGFR remain to be elucidated.
Using up to 6.4 years of data from the trial phase of the African-American Study of Kidney Disease and Hypertension (AASK), we aimed to describe the longitudinal relationship between eGFR and hematocrit as well as its variation by baseline characteristics among African-American individuals with CKD attributed to hypertension.
MATERIALS AND METHODS
Study population
The study population consisted of 1094 participants who were enrolled in the AASK trial. Details of the study protocol have been reported elsewhere [9–12]. From February 1995 to September 1998, the 1094 African-American individuals, aged 18–70 years, with hypertensive CKD (iGFR 20–65 mL/min/1.73 m2 as determined by renal clearance of I125 iothalamate), were randomized to initial therapy with one of three blood pressure medications (ramipril, metoprolol or amlodipine in a randomization ratio of 2:2:1) and one of two blood pressure goals (mean arterial pressure ≤92 or 102–107 mmHg). Exclusion criteria included a history of diabetes, urine protein-to-creatinine ratio >2.5, malignant hypertension in the preceding 6 months, secondary hypertension, heart failure, severe systemic disease or a specific contraindication/need for any of the study drugs. The trial phase ended on 30 September 2001 [9, 10]. Institutional Review Boards from all participating institutions approved the trial protocol, and each participant provided written informed consent.
Outcome and predictors
The primary outcome in this report was hematocrit, treated as a continuous variable and obtained from annual complete blood counts processed locally. Hematocrit was used instead of hemoglobin because the latter was not entered into the AASK database; therefore, hemoglobin was unavailable for analysis [13]. Incident anemia was defined as a single hematocrit measurement of <40.5% for men or <36% for women, as specified by the 2006 KDOQI clinical practice guidelines for anemia in CKD [3].
The primary exposure of interest was eGFR, measured at annual visits and based on the following three-variable AASK prediction equation: eGFR = 329 × (serum creatinine)−1.096 × (age)−0.294 × (0.736 for women) [11, 14]. Lewis et al. derived this formula using data from AASK and found that it was nearly as accurate in estimating I125 iothalamate GFR as more complex equations like the Modification of Diet in Renal Disease (MDRD) formula [14]. Samples for serum creatinine were processed with an autoanalyzer at the AASK Central Biochemistry Laboratory in the Department of Laboratory Medicine at Cleveland Clinic [15]. Renal clearance of I125 iothalamate was also available for each individual at baseline and annually thereafter; in sensitivity analyses, similar analyses using iGFR were conducted [9, 14].
Additional covariates included age at randomization, gender, baseline eGFR, body mass index, baseline proteinuria, serum albumin and C-reactive protein. Baseline proteinuria was defined as a protein-to-creatinine ratio of >0.22 on 24-h urine collection [10]. Urine samples were processed at the AASK Central Biochemistry Laboratory, employing the pyrogallol red technique and modified Jaffe reaction for quantification of total urine protein and creatinine, respectively [13, 15]. Further details regarding the measurements of serum albumin and C-reactive protein in AASK have previously been described [16].
Statistical analyses
Baseline characteristics were described using the mean (standard deviation [SD]) or median (inter-quartile range [IQR]) for continuous variables and frequencies (%) for categorical variables. The cross-sectional association between eGFR and hematocrit at baseline was also displayed graphically using a two-way fractional–polynomial prediction plot.
Mixed effects models were constructed to relate longitudinal absolute change in hematocrit to longitudinal change in eGFR. In these models, baseline eGFR was characterized using a two-slope linear spline with knot at 45 mL/min/1.73 m2. This choice of 45 mL/min/1.73 m2 as the inflection point was made after considering alternative models with knots at 30, 40 and 50 mL/min/1.73 m2 as well as a linear model without a spline. Among these, the model with a knot at 45 mL/min/1.73 m2 had the lowest Akaike's information criteria, indicating the best fit to the data. Covariates included age at the time of randomization, gender, baseline proteinuria and randomized treatment groups.
To test whether the association between hematocrit and longitudinal change in eGFR varied by baseline characteristic, a product term of longitudinal change in eGFR and baseline characteristic was added to the model. Tested baseline characteristics included (i) eGFR (represented by two product terms, corresponding to the two separate splines), (ii) age, (iii) gender, (iv) proteinuria, (v) body mass index, (vi) serum albumin and (vii) C-reactive protein. In the latter three models, body mass index, serum albumin, and C-reactive protein were also included among the covariates, respectively.
All models also included interaction terms between follow-up time and randomized treatment groups to account for possible longitudinal changes in hematocrit associated with treatment assignment (independent of eGFR change) as well as random intercept and slope terms for hematocrit change over time to account for intra-individual correlations among repeated hematocrit measurements. A first-order auto-regressive model was used to allow for higher correlations between hematocrit values at neighboring visits than visits spaced apart in time.
In sensitivity analyses, hematocrit was log-transformed to assess relative change in hematocrit per unit change in eGFR [17]. In other analyses, we excluded those persons who received erythropoietin-stimulating agents (ESA) or iron supplements during the study. Finally, to assess the robustness of our findings, parallel analyses using iGFR in place of eGFR were also performed.
Data were analyzed using Stata statistical software (Version 12, 2011; College Station, TX). P values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics
Table 1 shows the baseline characteristics of the 1094 participants enrolled in the AASK trial and included in this analysis. Mean baseline hematocrit was 39.4% (SD 4.9%). At the start of the trial, 37 (3%) individuals had a hematocrit of <30% and 91 (8%) had a hematocrit of <33%, thresholds commonly used in clinical practice for initiating ESA therapy in the United States and Europe, respectively. When using sex-specific criteria as defined by KDOQI (hematocrit <40.5% for men and hematocrit <36% for women) [3], 441 (40%) individuals had anemia at baseline, with men and women having mean hematocrits of 41.1% (SD 4.6%) and 36.8% (SD 4.1%), respectively (P < 0.001). Median baseline eGFR was 48 mL/min/1.73 m2 (IQR 36–58 mL/min/1.73 m2), 42% of participants had an eGFR of <45 mL/min/1.73 m2 and one-third had a baseline urine protein-to-creatinine ratio of >0.22. Follow-up during the trial phase ranged from 3 to 6.4 years. The mean number of hematocrit measurements per participant was 4 (SD 2). Among individuals with a baseline hematocrit of ≥30%, 9% developed a hematocrit of <30% at least once during the follow-up; among those with a baseline hematocrit of ≥33%, 20% developed a hematocrit of <33% at least once during the follow-up.
Table 1.
Baseline characteristics of study participants by gender
| Males (n = 669) | Females (n = 425) | All (n = 1094) | |
|---|---|---|---|
| Mean age, years | 54.2 ± 10.8 | 55.2 ± 10.5 | 54.6 ± 10.7 |
| Current smoker | 201 (30%) | 120 (28%) | 321 (29%) |
| Mean blood pressure, mmHg | |||
| Systolic | 150 ± 24 | 150 ± 24 | 150 ± 24 |
| Diastolic | 96 ± 15 | 94 ± 13 | 96 ± 14 |
| Mean arterial blood pressure, mmHg | 114 ± 16 | 113 ± 15 | 114 ± 16 |
| Mean body mass index, kg/m2 | 30.1 ± 6.2 | 31.3 ± 7.1 | 30.6 ± 6.6 |
| Mean hematocrit, % | 41.1 ± 4.6 | 36.8 ± 4.1 | 39.4 ± 4.9 |
| Median eGFR, mL/min/1.73 m2 | 49 (38–59) | 45 (34–56) | 48 (36–58) |
| eGFR category, mL/min/1.73 m2 | |||
| ≥60 | 159 (24%) | 63 (15%) | 222 (20%) |
| 45–59 | 254 (38%) | 155 (36%) | 409 (37%) |
| 30–44 | 178 (26%) | 135 (32%) | 313 (29%) |
| <30 | 78 (12%) | 72 (17%) | 150 (14%) |
| Mean serum albumin, g/dL | 4.3 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 |
| Mean C-reactive protein, mg/dL | 0.7 ± 1.0 | 0.9 ± 1.0 | 0.8 ± 1.0 |
| Median urine protein-to-creatinine ratio | 0.08 (0.03–0.36) | 0.08 (0.04–0.37) | 0.08 (0.03–0.36) |
| Proteinuria >0.22 | 228 (34%) | 129 (30%) | 357 (33%) |
| Randomized blood pressure goal, mmHg | |||
| ≤92 | 334 (50%) | 206 (48%) | 540 (49%) |
| 102–107 | 335 (50%) | 219 (52%) | 554 (51%) |
| Randomized study drug | |||
| Ramipril | 267 (40%) | 169 (40%) | 436 (40%) |
| Metoprolol | 271 (40%) | 170 (40%) | 441 (40%) |
| Amlodipine | 131 (20%) | 86 (20%) | 217 (20%) |
Values presented as mean ± SD, median (IQR), or n (%).
eGFR, estimated glomerular filtration rate.
Cross-sectional association between baseline eGFR and hematocrit
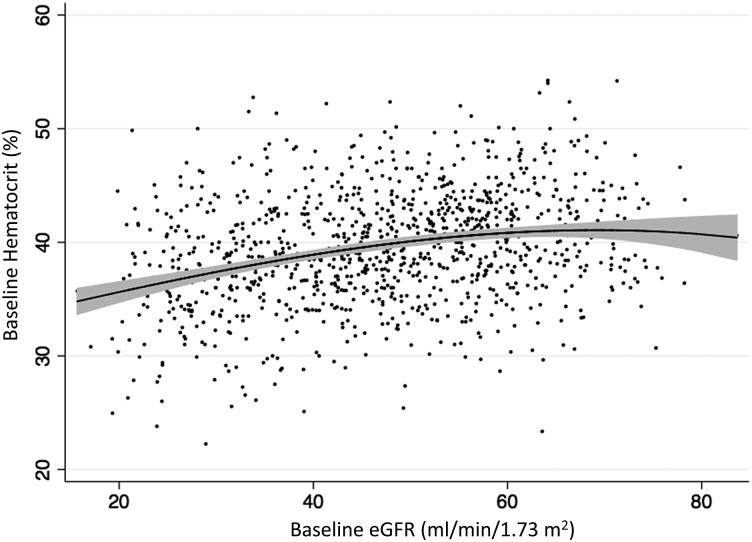
Figure 1 displays the distribution of baseline hematocrit by eGFR. As depicted in this fractional polynomial prediction plot, lower hematocrit was associated with lower eGFR levels. When assessed using the mixed effects model, baseline hematocrit and baseline eGFR were similarly associated: for every 10 mL/min/1.73 m2 lower eGFR at the start of the study, initial hematocrit was lower by 1.4% (absolute difference; 95% CI −1.8, −1.1; P < 0.001) among participants with baseline eGFR < 45 mL/min/1.73 m2 and 0.3% (absolute difference; 95% CI −0.6, −0.005; P = 0.046) among participants with baseline eGFR > 45 mL/min/1.73 m2. Older age at the time of randomization, female gender, and baseline proteinuria were also associated with lower baseline hematocrits (P < 0.05 for each comparison).
FIGURE 1:
Cross-sectional fractional polynomial prediction plot of hematocrit versus eGFR at baseline. Tinted bands indicate 95% pointwise confidence intervals.
Longitudinal association between eGFR and hematocrit
The results of the longitudinal analyses are presented in Table 2. After controlling for eGFR, hematocrit fell by an average of 1.4% per 5 years (absolute change; 95% CI −1.9, −0.8; P < 0.001). However, the relationship between longitudinal change in eGFR and longitudinal change in hematocrit varied by baseline eGFR, with a greater drop in hematocrit associated with eGFR decline in participants with lower baseline eGFR (P-interaction < 0.001 for baseline eGFR< 45 mL/min/1.73 m2 and P-interaction = 0.002 for baseline eGFR > 45 mL/min/1.73 m2). For example, an individual with a 10 mL/min/1.73 m2 decline in eGFR from a baseline eGFR of 20 mL/min/1.73 m2 would experience an absolute decline in hematocrit of 3.7%, whereas a similar decline in an individual with a baseline eGFR of 60 mL/min/1.73 m2 would result in an absolute decline in hematocrit of only 0.5% (P < 0.001). When hematocrit was log-transformed to reflect relative rather than absolute change, similar findings were obtained (data not shown). Finally, use of iron supplements and ESA was uncommon during the study, with only 12% of participants on iron and 2% on ESA. Repeated analyses excluding these individuals yielded similar conclusions (data not shown).
Table 2.
Results of the joint mixed-effects model relating longitudinal change in hematocrit to follow-up time and change in eGFR
| Predictor variables | Change in Hct (%) | 95% CI | P-value |
|---|---|---|---|
| Time, per 5 years of follow-up | −1.4 | (−1.9, −0.8) | <0.001 |
| Change in eGFR, per 10 mL/min/1.73 m2 decrease for baseline eGFR | |||
| 20 mL/min/1.73 m2 | −3.7 | (−4.2, −3.2) | <0.001 |
| 30 mL/min/1.73 m2 | −2.5 | (−2.8, −2.2) | <0.001 |
| 40 mL/min/1.73 m2 | −1.3 | (−1.5, −1.2) | <0.001 |
| 45 mL/min/1.73 m2 | −0.8 | (−1.0, −0.5) | <0.001 |
| 50 mL/min/1.73 m2 | −0.6 | (−0.8, −0.5) | <0.001 |
| 60 mL/min/1.73 m2 | −0.5 | (−0.6, −0.3) | <0.001 |
eGFR, estimated glomerular filtration rate; Hct, hematocrit; CI, confidence interval.
Data presented as absolute change in hematocrit for six sample baseline eGFR values.
In addition to the listed predictor variables, the joint model included additional terms to control for age at the time of randomization, gender, baseline proteinuria (urine protein-to-creatinine ratio >0.22), interaction terms between baseline eGFR (linear spline with a knot at 45 mL/min/1.73 m2) and time-varying eGFR, randomized drug assignment, randomized blood pressure target group, and interaction terms between treatment assignments and time.
Impact of other participant characteristics on the longitudinal association between eGFR and hematocrit
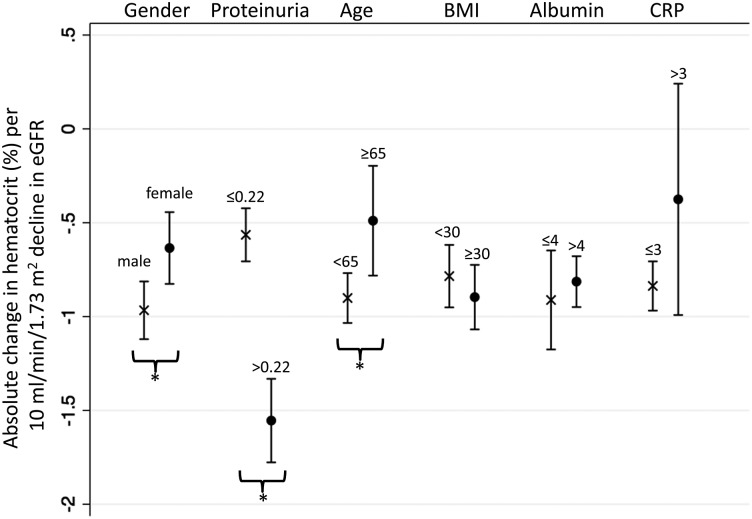
Men had greater absolute declines in hematocrit per 10 mL/min/1.73 m2 decline in longitudinal eGFR compared with women (−1.0 versus −0.6%, respectively; P-interaction = 0.01). Individuals with baseline proteinuria were also found to have greater absolute hematocrit declines compared with those without proteinuria (−1.6 versus −0.6% per 10 mL/min/1.73 m2 decline in eGFR; P-interaction < 0.001). Moreover, younger participants (<65 years) experienced steeper absolute declines in hematocrit compared with older participants (−0.9 versus −0.5% per 10 mL/min/1.73 m2 decline in eGFR; P-interaction = 0.01). In contrast, body mass index, serum albumin, and C-reactive protein levels did not affect the longitudinal relationship between eGFR and hematocrit (Figure 2).
FIGURE 2:
Longitudinal analyses of change in hematocrit per change in eGFR, by baseline participant characteristics. eGFR, estimated glomerular filtration rate; Proteinuria, urine protein-to-creatinine ratio; Age (years); BMI, body mass index (kg/m2); Albumin, serum albumin (g/dL); CRP, C-reactive protein (mg/dL). * denotes P-interaction <0.05.
Analyses using iGFR
Similar findings were obtained when iGFR was used in place of eGFR—persons with lower baseline iGFR values had greater absolute declines in hematocrit per unit decrease in longitudinal iGFR (Supplementary Table 1). Moreover, male gender, younger age and baseline proteinuria were each associated with steeper absolute declines in hematocrit as iGFR declined (Supplementary Figure 1).
Association between drug assignment and longitudinal hematocrit
Consistent with the trial design, we also conducted supplementary analyses examining the overall effect that each randomized drug had on hematocrit during the trial phase (Supplementary Table 2). Those randomized to ramipril did not experience greater rates of hematocrit decline compared with those assigned to the other drug groups (P > 0.05 for all comparisons).
DISCUSSION
In this study of 1094 participants with up to 6.4 years of follow-up data, we demonstrated that individuals with lower baseline eGFR had not only lower baseline hematocrit but also greater hematocrit declines associated with a given change in eGFR. We also observed steeper longitudinal associations between eGFR and hematocrit among men, younger individuals and persons with baseline proteinuria. These observations provide helpful information in identifying individuals with CKD at high risk for developing anemia.
Prior studies have suggested that hemoglobin declines over time [18, 19]. Others have reported that reduced kidney function is associated, at least cross-sectionally, with an increased risk for anemia [2, 7, 20]. Astor et al. reported that in NHANES III (1988–1994), the prevalence of anemia increased from 1% for eGFR 60–89 mL/min/1.73 m2 to 5% for eGFR 30–59 mL/min/1.73 m2 to 44% for eGFR 15–29 mL/min/1.73 m2. Of note, <1% of participants in NHANES III had an eGFR of < 30 mL/min/1.73 m2 [2]. In another cross-sectional study, Hsu et al. found that the threshold at which hematocrit became significantly lower occurred at creatinine clearances of 60 mL/min for men and 40 mL/min for women [20]. Our findings provide support that these associations also occur longitudinally. More importantly, we were able to quantify this association, demonstrating that longitudinal change in hematocrit per unit change in eGFR depends not only on an individual's baseline eGFR but also whether that value is above or below 45 mL/min/1.73 m2. These findings were confirmed in analyses using iGFR, thus providing support that the longitudinal association between eGFR and hematocrit observed in our primary analyses was not due to how GFR was estimated by the AASK trial equation [11, 14].
Several mechanisms have been proposed to explain how reduced kidney function leads to anemia. First, it has been well established that erythropoietin is an important stimulator of red blood cell production [21, 22]. Erythropoietin synthesis occurs primarily in the kidneys; as renal function declines, a relative deficiency of this hormone ensues [22, 23]. Second, patients with CKD commonly have elevated levels of hepcidin, a hormone that has been shown to decrease dietary iron absorption and macrophage iron recycling. Reduced renal clearance and chronic inflammation directly induce hepcidin transcription and may contribute to elevated hepcidin levels [24, 25]. Finally, poor nutrition from uremia can lead to dietary deficiencies in iron, vitamin B12 and folate, all of which are necessary for erythropoiesis [22].
The trend for a gender difference in the longitudinal association between hematocrit change and eGFR change was unanticipated. While it was not surprising that women had lower initial hematocrits, the explanation as to why men experienced greater declines in hematocrit as CKD progressed is less clear. Hsu et al. observed a similar cross-sectional pattern in their study, hypothesizing that larger body surface areas in men conferred higher demands for erythropoietin production and thus greater need for preserved renal function [20]. The AASK trial equation used in this study to estimate GFR accounted for body surface area. Analyses using iGFR yielded similar conclusions. Therefore, other mechanisms accounting for these gender differences likely exist. One potential explanation is that hormonal changes may occur differentially in men and women as CKD progresses. The presence of hypogonadism in renal disease has previously been established [26–28]. Carrero et al. reported that in CKD, men with a total testosterone of <10 nmol/L were 5.3 times more likely to develop anemia than men with sufficient testosterone levels [29]. Hence, the decreases in testosterone that occur as kidney function declines may account for this differential effect of gender on the eGFR–hematocrit relationship.
A given decline in eGFR was associated with a greater average decline in hematocrit in patients with baseline proteinuria. Estimated GFR likely fails to fully capture the risk associated with renal dysfunction. In a meta-analysis of 13 CKD cohorts, both lower eGFR and higher albuminuria were independently associated with increased risk for end-stage renal disease and mortality [30]. Similarly, we propose that baseline proteinuria may provide added prognostic information on a patient's risk for anemia. This is consistent with prior cross-sectional studies among patients with type 2 diabetes, in which macroalbuminuria was associated with lower hemoglobin levels and higher prevalence of anemia, independent of eGFR [31, 32]. We also found that younger individuals are more likely to experience steeper declines in hematocrit related to decreases in longitudinal eGFR. This is consistent with the lower relative risk of adverse events that has been observed in older versus younger individuals for a given eGFR [33].
Prior studies have suggested that the renin–angiotensin system may be important in regulating erythropoiesis [34]. In a study of 1513 individuals with diabetes and CKD, treatment with an angiotensin receptor blocker was associated with significant hemoglobin declines [35]. While ACE inhibitors also interfere with this pathway, our findings suggest that this class of medications does not lead to greater declines in hematocrit compared with other commonly used anti-hypertensive medications. One possible explanation is that the doses of ramipril (2.5–10 mg/day) in the AASK trial might not have been high enough to induce anemia [9]. Moreover, prior studies have suggested that beta-blockers can inhibit the release of renin [36–38]. This may have accounted for the lack of difference in longitudinal hematocrit decline observed between the ramipril and metoprolol groups. Lastly, our study population was limited to African-Americans with CKD. It is possible that the effects of ACE inhibition on anemia may be modified by race [9, 10].
Our study has limitations. First, we used hematocrit rather than hemoglobin, which was unavailable in our dataset. Most national guidelines utilize hemoglobin, as it is less influenced by plasma volume [2]. Moreover, most studies on the topic of anemia in CKD have preferentially used hemoglobin instead of hematocrit [2, 39–41]. Second, hematocrit was derived from complete blood counts that had been processed locally. However, presumably the same test was used at each laboratory. Third, we do not have information on specific causes of anemia, which could include decreased erythropoietin production, erythropoietin resistance, iron deficiency, reduced red blood cell survival and/or occult blood loss [22, 25, 42]. Fourth, it is difficult to tease apart the separate effects of reduced eGFR and proteinuria. Because baseline proteinuria was inversely correlated with baseline eGFR in the AASK trial, it is possible that the interactions of the longitudinal change in eGFR with baseline eGFR and with baseline proteinuria may reflect a common mechanism. Finally, our results may be less reliable at hematocrits <30% due to the small number of observations below this threshold.
Despite these limitations, our study also has several strengths. First, unlike previous studies, we analyzed data longitudinally, describing both differences in hematocrit at baseline and changes in hematocrit as CKD progressed. Second, duration of follow-up was long, with up to 6.4 years of data. Third, the cohort was large with participants from across the United States. Fourth, most studies on anemia in CKD have been derived from patient populations with varied racial backgrounds and CKD etiologies. Our study population focuses on African-Americans with hypertensive CKD, a group often underrepresented. Still, our findings may not be generalizable to other patient populations.
This study has several potential clinical implications. Current KDOQI guidelines recommend that hemoglobin testing be conducted at least once annually in all patients with CKD regardless of kidney disease severity or etiology [3]. Recently, the Kidney Disease: Improving Global Outcomes (KDIGO) work group recommended that individuals without anemia undergo hemoglobin testing at least once yearly for CKD stage 3 and twice yearly for CKD stages 4 and 5 not yet on dialysis, whereas those with known anemia should have their hemoglobin measured every 3 months regardless of CKD stage [43]. Our findings not only support this more individualized approach to anemia screening in CKD but also suggest that men and individuals with baseline proteinuria, aged <65 years or baseline eGFR < 45 mL/min/1.73 m2 may also benefit from closer monitoring. Additional studies are needed to determine whether these associations hold true in other CKD populations and whether race modifies the association between eGFR and anemia and its cardiovascular consequences.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
ACKNOWLEDGMENTS
We would like to thank participants of the AASK trial. T.K.C. was supported by NIH-NIDDK grant 5T32DK007732. M.M.E. is supported by NIH-NIDDK grant 1K23DK081317. B.C.A. was supported by NIH-NIDDK grant R21DK078218. The AASK trial and cohort were supported by institutional grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887 and DK 2818-02) and the following pharmaceutical companies: King Pharmaceuticals, Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia and Upjohn. Portions of this work have been presented at the 2012 Mid-Atlantic and National Young Investigator's Forums in Washington, DC, and the 2012 American Society of Nephrology Kidney Week in San Diego, CA.
CONFLICT OF INTEREST STATEMENT
T.K.C. previously owned stock in Pfizer Pharmaceuticals. The other authors have nothing to declare.
REFERENCES
- 1.Patel TV, Singh AK. Anemia in chronic kidney disease: new advances. Heart Fail Clin 2010; 6: 347–357 [DOI] [PubMed] [Google Scholar]
- 2.Astor BC, Muntner P, Levin A, et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2002; 162: 1401–1408 [DOI] [PubMed] [Google Scholar]
- 3.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis 2006; 47: S11–145 [DOI] [PubMed] [Google Scholar]
- 4.Thorp ML, Johnson ES, Yang X, et al. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology 2009; 14: 240–246 [DOI] [PubMed] [Google Scholar]
- 5.Kuwahara M, Iimori S, Kuyama T, et al. Effect of anemia on cardiac disorders in pre-dialysis patients immediately before starting hemodialysis. Clin Exp Nephrol 2011; 15: 121–125 [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein FO, Story K, Firanek C, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol 2009; 4: 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin 2004; 20: 1501–1510 [DOI] [PubMed] [Google Scholar]
- 8.Chonchol M, Lippi G, Montagnana M, et al. Association of inflammation with anaemia in patients with chronic kidney disease not requiring chronic dialysis. Nephrol Dial Transplant 2008; 23: 2879–2883 [DOI] [PubMed] [Google Scholar]
- 9.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288: 2421–2431 [DOI] [PubMed] [Google Scholar]
- 10.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363: 918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appel LJ, Middleton J, Miller ER, III, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol 2003; 14: S166–S172 [DOI] [PubMed] [Google Scholar]
- 12.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 2003; 14: S154–S165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sika M, Lewis J, Douglas J, et al. Baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Clinical Trial and Cohort Study. Am J Kidney Dis 2007; 50: 78–89, 89 e71 [DOI] [PubMed] [Google Scholar]
- 14.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis 2001; 38: 744–753 [DOI] [PubMed] [Google Scholar]
- 15.Toto RD, Greene T, Hebert LA, et al. Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: results from the African American Study of Kidney Disease and Hypertension (AASK) cohort. Am J Kidney Dis 2010; 56: 896–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras G, Hu B, Astor BC, et al. Malnutrition-inflammation modifies the relationship of cholesterol with cardiovascular disease. J Am Soc Nephrol 2010; 21: 2131–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CT, Greene T, Chertow GM, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging 2012; 5: 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Portoles J, Gorriz JL, Rubio E, et al. The development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney disease. BMC Nephrol 2013; 14: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas MC, Tsalamandris C, MacIsaac RJ, et al. The epidemiology of hemoglobin levels in patients with type 2 diabetes. Am J Kidney Dis 2006; 48: 537–545 [DOI] [PubMed] [Google Scholar]
- 20.Hsu CY, Bates DW, Kuperman GJ, et al. Relationship between hematocrit and renal function in men and women. Kidney Int 2001; 59: 725–731 [DOI] [PubMed] [Google Scholar]
- 21.Erslev A. Humoral regulation of red cell production. Blood 1953; 8: 349–357 [PubMed] [Google Scholar]
- 22.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012; 23: 1631–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson LO, Goldwasser E, Fried W, et al. Role of the kidney in erythropoiesis. Nature 1957; 179: 633–634 [DOI] [PubMed] [Google Scholar]
- 24.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306: 2090–2093 [DOI] [PubMed] [Google Scholar]
- 25.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis 2010; 55: 726–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albaaj F, Sivalingham M, Haynes P, et al. Prevalence of hypogonadism in male patients with renal failure. Postgrad Med J 2006; 82: 693–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iglesias P, Carrero JJ, Diez JJ. Gonadal dysfunction in men with chronic kidney disease: clinical features, prognostic implications and therapeutic options. J Nephrol 2012; 25: 31–42 [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz MI, Sonmez A, Qureshi AR, et al. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 1617–1625 [DOI] [PubMed] [Google Scholar]
- 29.Carrero JJ, Barany P, Yilmaz MI, et al. Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrol Dial Transplant 2012; 27: 709–715 [DOI] [PubMed] [Google Scholar]
- 30.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79: 1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasso FC, De Nicola L, Carbonara O, et al. Cardiovascular risk factors and disease management in type 2 diabetic patients with diabetic nephropathy. Diabetes Care 2006; 29: 498–503 [DOI] [PubMed] [Google Scholar]
- 32.Thomas MC, MacIsaac RJ, Tsalamandris C, et al. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care 2003; 26: 1164–1169 [DOI] [PubMed] [Google Scholar]
- 33.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 2012; 308: 2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato H, Ishida J, Imagawa S, et al. Enhanced erythropoiesis mediated by activation of the renin-angiotensin system via angiotensin II type 1a receptor. FASEB J 2005; 19: 2023–2025 [DOI] [PubMed] [Google Scholar]
- 35.Mohanram A, Zhang Z, Shahinfar S, et al. The effect of losartan on hemoglobin concentration and renal outcome in diabetic nephropathy of type 2 diabetes. Kidney Int 2008; 73: 630–636 [DOI] [PubMed] [Google Scholar]
- 36.Holmer S, Rinne B, Eckardt KU, et al. Role of renal nerves for the expression of renin in adult rat kidney. Am J Physiol 1994; 266: F738–F745 [DOI] [PubMed] [Google Scholar]
- 37.Holmer SR, Hengstenberg C, Mayer B, et al. Marked suppression of renin levels by beta-receptor blocker in patients treated with standard heart failure therapy: a potential mechanism of benefit from beta-blockade. J Intern Med 2001; 249: 167–172 [DOI] [PubMed] [Google Scholar]
- 38.Tkacs NC, Kim M, Denzon M, et al. Pharmacological evidence for involvement of the sympathetic nervous system in the increase in renin secretion produced by a low sodium diet in rats. Life Sci 1990; 47: 2317–2322 [DOI] [PubMed] [Google Scholar]
- 39.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 40.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 2010; 363: 1146–1155 [DOI] [PubMed] [Google Scholar]
- 41.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 42.Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis 2004; 44: 715–719 [PubMed] [Google Scholar]
- 43.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.