Abstract
Background
Galactose-deficient IgA1 (Gd-IgA1) is a critical effector molecule in the pathogenesis of IgA nephropathy (IgAN). Although many researchers have measured serum levels of Gd-IgA1 using snail helix aspersa agglutinin (HAA) lectin-based assay, the lectin-dependent assay has some serious problems in robustness. In this study, we aimed to establish a more robust and stable enzyme-linked immunosorbent assay (ELISA) method that uses a specific monoclonal antibody to recognize a hinge region in human Gd-IgA1 (Gd-IgA1 ELISA).
Methods
Rats were immunized with human Gd-IgA1 hinge region peptide to obtain Gd-IgA1-specific monoclonal antibody KM55. Gd-IgA1 ELISA for specifically detecting serum Gd-IgA1 was consequently constructed. Serum Gd-IgA1 concentrations in human subjects were measured using KM55 ELISA assay. To further confirm specificity of the Gd-IgA1-specific antibody, KM55 was also applied for immunofluorescence staining of glomerular Gd-IgA1 in paraffin-embedded sections of renal biopsy specimens.
Results
Measurement of serum levels of Gd-IgA1 in human subjects by Gd-IgA1 ELISA revealed increased serum Gd-IgA1 level in patients with IgAN compared with patients with other renal diseases or non-renal diseases. Importantly, the results obtained from Gd-IgA1 ELISA positively correlated with those from the HAA lectin-based assay (R = 0.75). Immunofluorescence staining of renal biopsy specimens with KM55 detected glomerular co-localization of Gd-IgA1 and IgA.
Conclusion
This novel lectin-independent method with KM55 for measuring serum levels of Gd-IgA1 can pave the way for more convincing diagnosis and activity assessment of IgAN, and can expedite clinical research to better understand this difficult disease.
Keywords: ELISA, galactose-deficient IgA1, helix aspersa agglutinin (HAA), IgA nephropathy, immunofluorescence, monoclonal antibody
INTRODUCTION
IgA nephropathy (IgAN) is one of the most frequently diagnosed primary glomerulonephritides worldwide, especially in Asian countries, including Japan [1]. Most cases of IgAN are discovered incidentally by urinalysis and diagnosed by renal biopsy [1, 2]. However, because renal biopsy has its accompanying procedural risks and limitation of insurance coverage, development of non-invasive diagnostic methods that employ disease-specific pathogens or biomarkers is required for clinical purposes. Non-invasive diagnosis of IgAN before the onset or at the early stage of disease progression is desired for specific treatment. Galactose-deficient IgA1 (Gd-IgA1) has been identified as one of the most convincing key mediators in the pathogenesis of IgAN, although the underlying molecular mechanisms are still under investigation [3–7].
HAA lectin-based assay, which can detect Gd-IgA1 in human serum samples, has played an indispensable role in this research, leading to several important findings because of its specific recognition of N-acetyl galactosamine (GalNAc) residues [3, 7, 8]. In general, lectins are capable of binding to various types of glycan of glycoproteins and they are widely distributed in mycobacteria, plants and animals. With regard to O-linked glycans, lectins that are purified from snail helix aspersa agglutinin (HAA), snail helix pomatia agglutinin or plant vicia villosa are known to possess specific affinity to GalNAc [9, 10]. Several studies on serum Gd-IgA1 measurement using HAA lectin-based assay have shown that circulating levels of Gd-IgA1 are significantly higher in IgAN patients than in non-renal disease controls [4, 6, 9]. Moreover, serum Gd-IgA1 levels in IgAN are associated with a risk of progression to end-stage renal disease [6]. HAA lectin has also been applied for Gd-IgA1 detection in supernatant of cultured cells, such as primary and immortalized B cells from human subjects [4, 11, 12]. Thus, HAA lectin-based assay has been a useful tool for clinical and basic research for years, and it is expected to be more widely used in future studies regarding the pathology, diagnosis and treatment of IgAN [13–15]. However, HAA lectin-based assay has several limitations. One of these is that its bioactivity and stability depend on the product lot of HAA lectin. Therefore, a more robust assay for detecting circulating Gd-IgA1 is strongly desired.
The aims of the study were the following: (i) to obtain and characterize a novel and unique monoclonal antibody against Gd-IgA1; and (ii) to apply it for a robust enzyme-linked immunosorbent assay (ELISA) system to detect serum Gd-IgA1.
MATERIALS AND METHODS
Animals
Sprague-Dawley rats (4 weeks of age, female) were purchased from Japan SLC, Inc. (Shizuoka, Japan), and maintained in specific pathogen-free conditions according to the institutional guidelines of Kyowa Hakko Kirin Co., Ltd.
Generation of Gd-IgA1
Gd-IgA1 was enzymatically generated from human plasma IgA1. Commercially available human plasma IgA1 (BioPur AG, Switzerland) was incubated with β-galactosidase from bovine testes (ProZyme, CA) and neuraminidase (Nacalai tesque, Kyoto, Japan) for 3 h at 37°C in sodium acetate buffer (pH5.0).
Acquisition of anti-Gd-IgA1 monoclonal antibody
Gd-IgA1-specific antibody, which is named as KM55, was obtained as described below. As the antigen, human IgA1 hinge region peptide (amino acid sequence: H-C223PST*PPT*PS*PS*TPPT*PSPS240-NH2) with five GalNAc residues added on specific serine/threonine residues (asterisks) was synthesized (Sigma-Aldrich Japan, Tokyo, Japan). After four times of KLH-conjugated antigen peptide administration to immunize SD rats, candidate hybridomas were established from splenocytes. Hybridomas that produce Gd-IgA1-specific monoclonal antibodies were selected by binding ELISA with the antigen peptide and the enzymatically generated Gd-IgA1.
Gd-IgA1 ELISA
A sandwich ELISA for Gd-IgA1 was constructed using KM55. KM55 was immobilized at 7.5 µg/mL on 96-well ELISA plates (NUNC MaxiSorp; Thermo Fisher Scientific, MA) for 18 h at room temperature. This was followed by blocking with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) for 2 h at room temperature. Serum samples were diluted in proportions of 1:50 with sodium acetate buffer (pH5.0) and desialylated by treatment with neuraminidase for 3 h at 37°C. After washing five times with PBS containing 0.05% Tween-20 (PBST), desialylated serum samples diluted with fetal bovine serum (1:5 dilution) were incubated on KM55-coated plates for 2 h at room temperature. Plates were washed and incubated in 1:1000 dilution with HRP-conjugated mouse anti-human IgA1 α1 chain-specific monoclonal antibody (SouthernBiotech, AL) for 2 h at room temperature. After washing, plates were eventually colored by SIGMAFAST O-phenylenediamine (OPD) solution (Sigma-Aldrich Japan) and stopped by 1 mol/L sulfuric acid (Wako, Osaka, Japan). The levels of each serum Gd-IgA1 were extrapolated by referring to a standard curve (4-parameter logistic curve fitting) of optical density (OD) (492 nm) and expressed as units. Enzymatically generated Gd-IgA1 from human plasma IgA1 was serially diluted (1.37–1000 ng/mL) and used as standards. In this study, 1 unit was defined as 1 µg/mL of enzymatically generated Gd-IgA1.
In the present study, serum samples were desialylated by treatment with neuraminidase, similar to the serum sample preparation in HAA lectin-based assay. Neuraminidase treatment has been conventionally performed because of the following reasons: (i) the existence of sialic acids on GalNAc might affect the recognition of serum Gd-IgA1 by HAA lectin and (ii) the pattern and number of sialic acids on the hinge region of IgA1 possibly vary among individuals. Unlike HAA lectin-based assay, neuraminidase treatment of serum samples was performed before adding to KM55 pre-coated plates to avoid detachment of KM55 due to the acidic condition.
HAA lectin-based assay
HAA lectin-based assay was performed according to previous reports [4]. Briefly, serum samples of 100 ng of IgA were incubated on plates where anti-IgA α1 chain-specific polyclonal antibodies (Jackson ImmunoResearch Laboratories, PA) were immobilized (2.5 µg/mL) and blocked with PBS containing 1% BSA and 0.05% Tween-20. In-well desialylation treatment with neuraminidase (10 mU/mL, Roche diagnostics, Basel, Switzerland) was perfomed for 3 h at 37°C. After washing with PBST, plates were incubated with biotinylated HAA lectin (1:500 dilution; Sigma-Aldrich Japan) for 2 h at 37°C. Captured Gd-IgA1 was detected by ExtraAvidin-Peroxidase (1:2000 dilution; Sigma-Aldrich Japan) and OPD solution (Sigma-Aldrich Japan) with hydrogen peroxide. Results were expressed as units, calculated by multiplying OD value (492 nm) with serum IgA concentration (μg/mL).
Measurement of serum Gd-IgA1
IgAN was diagnosed by IgA deposition with mesangial proliferation in the glomeruli of renal biopsy specimens from donors with positive findings on urinalysis. One hundred and fifty-two serum samples of IgAN, 20 control samples of other renal diseases (Supplementary data, Table S1) and 71 control samples of non-renal diseases without any positive findings on urinalysis were subjected to separate measurements of Gd-IgA1 levels by Gd-IgA1 ELISA and HAA lectin-based assay. Blood samples were collected at Juntendo University Hospital with the informed consent of each donor. All protocols were reviewed and approved by the Research Ethics Review Committees of Juntendo University Hospital and Kyowa Hakko Kirin Co., Ltd.
Competition assay
An assay to examine competitive binding activity between KM55 and HAA lectin to the enzymatically generated Gd-IgA1 was performed using HAA lectin-based assay method. HAA lectin was pre-mixed and incubated with serial concentration of KM55 for 30 min at room temperature. The mixture was added to plates in place of single HAA lectin. Inhibitory effect was calculated as a relative percentage of the OD value of the KM55-free sample.
Immunofluorescence staining
Renal biopsy specimens were obtained at Juntendo University Hospital with the informed consent from patients and approval of the Research Ethics Review Committees of Juntendo University Hospital and Kyowa Hakko Kirin Co., Ltd. Paraffin-embedded sections of 3-μm thickness were prepared for staining. After deparaffinization by series of xylene/ethanol and rehydration, antigen retrieval with subtilisin A (Sigma-Aldrich Japan) was performed at room temperature for 2 h. Then, samples were rinsed with distilled water and blocked with Protein Block (Dako Japan, Tokyo, Japan) at room temperature for 30 min, followed by incubation with KM55 (100 µg/mL) at 37°C for 60 min. After several washes by PBS and TBS containing 0.05% tween-20 (TBST), Alexa Fluor 555-conjugated goat anti-rat IgG antibody (1:1000 diluted; Life Technologies, CA) was reacted at 37°C for 30 min. Samples were washed with PBS/TBST and incubated with FITC-conjugated polyclonal rabbit anti-human IgA antibody (100 µg/mL; Dako Japan) at 37°C for 30 min. After washing with PBS/TBST, slides were sealed in Fluoromount (Diagnostic BioSystems, CA). Fluorescence was observed using BIOREVO Fluorescence Microscopy BZ-9000 (KEYENCE, Osaka, Japan).
Statistical analysis
Statistical analyses were performed with SAS release 9.2 (SAS Institute, Inc., Cary, NC). Differences among multiple disease groups were assessed by ANOVA, followed by Bonferroni correction for multiple comparisons. Relationship between measurements from two different methods was evaluated by simple linear regression analysis. Data are expressed as mean ± SD. P-values of <0.05 were considered to be statistically significant.
RESULTS
GalNAc-specific monoclonal antibody KM55 recognizes Gd-IgA1
To obtain Gd-IgA1-specific monoclonal antibodies, a hybridoma method using human IgA1 hinge region peptide as antigen was employed. Because the potential existence of galactose-deficient O-glycans has been suggested in at least five sites (230Ser, 232Ser, 225Thr, 228Thr and 236Thr) on the IgA1 hinge region in IgAN patients [15–17], we synthesized a human IgA1 hinge region peptide by adding GalNAc to these five amino acid sites. Immunization of rats with this peptide resulted in several hybridoma clones.
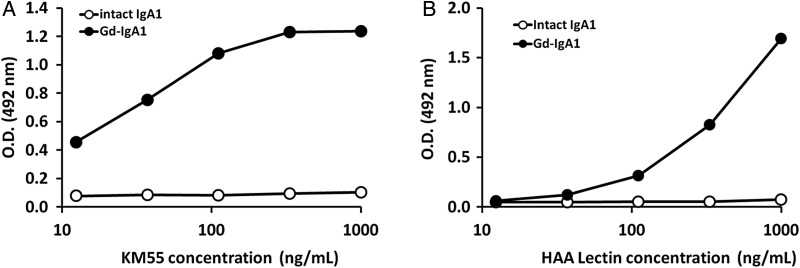
In the subsequent binding assays using enzymatically generated human Gd-IgA1 and antigen peptides, KM55, a monoclonal rat IgG2b antibody, was selected. On a plate, we immobilized the enzymatically produced Gd-IgA1 with β-galactosidase and neuraminidase or plasma-derived glycan-intact human IgA1; then we added KM55. We observed that KM55 bound to Gd-IgA1 in a dose-dependent manner, whereas it did not bind to glycan-intact human IgA1 even at high doses (Figure 1A), indicating that KM55 recognizes Gd-IgA1 but not glycan-intact IgA1. A comparable binding property in terms of dose dependency and specificity was observed when HAA lectin was added instead of KM55 (Figure 1B). In these binding assays, the amounts of both immobilized Gd-IgA1 and glycan-intact IgA1 were confirmed using anti-IgA1 antibody (data not shown). In addition, KM55 was evaluated in a BIAcore analysis to have Ka value of 1.68 × 104 (M−1s−1), KD value of 1.24 × 10−3 (s−1) and KD value of 7.4 × 10−8 (M) to Gd-IgA1. Because KM55 specifically recognized Gd-IgA1 but not glycan-intact IgA1, it was suggested that KM55 can be applied in lectin-independent ELISA of pathogenic Gd-IgA1.
FIGURE 1:
Specific binding of KM55 to Gd-IgA1. The horizontal axis represents concentration of KM55 or HAA lectin and the vertical axis represents OD. (A) KM55 specifically recognized enzymatically generated Gd-IgA1 from human plasma IgA1 (closed circles), but did not recognize glycan-intact human plasma IgA1 (open circles); (B) HAA lectin exhibited its specificity to the enzymatically generated Gd-IgA1 (closed circles); however, it did not recognize glycan-intact IgA1 (closed circles) as well as KM55.
Lectin-independent Gd-IgA1 ELISA revealed increased levels of serum Gd-IgA1 in patients with IgAN
KM55 enabled us to successfully establish a sandwich ELISA assay (Gd-IgA1 ELISA), wherein serum Gd-IgA1 is captured by an immobilized KM55 and consequently detected by horseradish peroxidase (HRP)-conjugated anti-human IgA polyclonal antibodies. A typical standard curve of enzymatically generated human Gd-IgA1 determination by Gd-IgA1 ELISA is shown in Supplementary data, Figure S1.
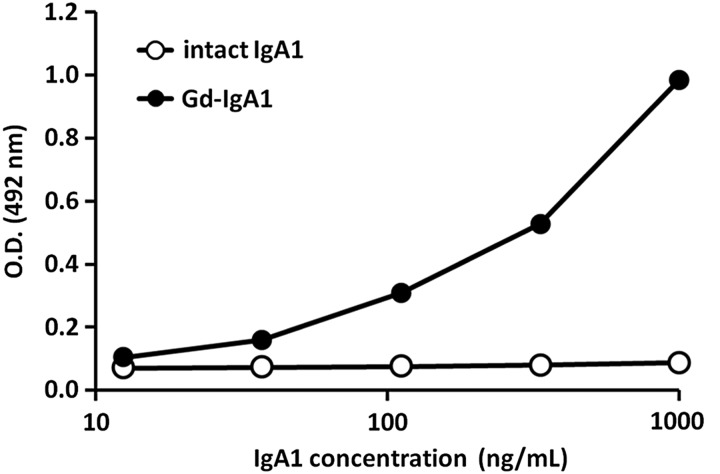
Specificity of Gd-IgA1 ELISA to Gd-IgA1 was examined using glycan-intact human IgA1 (Figure 2). Gd-IgA1 ELISA recognized enzymatically generated Gd-IgA1 in a dose-dependent manner. The absorbance of glycan-intact IgA1 from human plasma remained constant even at high concentrations. The inter-assay coefficients of variation (CV) were evaluated by five separate measurements of three independent human serum samples. The intra-assay CVs were evaluated by 20 measurements of 3 independent human serum samples on a single plate. The inter-assay and intra-assay CVs were 3.2–7.4% and 2.8–5.1%, respectively (Table 1A and B).
FIGURE 2:
Establishing Gd-IgA1 ELISA as a reliable and specific method to detect for Gd-IgA1. The horizontal axis represents concentration of Gd-IgA1 or intact IgA1 and the vertical axis represents OD. Enzymatically generated Gd-IgA1 (closed circles) was specifically recognized, whereas glycan-intact IgA1 from human plasma (open circles) was not recognized in the Gd-IgA1 ELISA (R = 0.99).
Table 1.
Characteristics of Gd-IgA1 ELISA
| A. Inter-assay | |||
|---|---|---|---|
| Measurement value (units) | SD value (units) | CV value (%) | n |
| 3.5 | 1.0 | 7.4 | 5 |
| 6.6 | 0.8 | 3.2 | 5 |
| 15.6 | 2.8 | 4.6 | 5 |
| B. Intra-assay | |||
| 3.5 | 0.4 | 2.8 | 20 |
| 6.6 | 1.4 | 5.1 | 20 |
| 15.6 | 2.0 | 3.1 | 20 |
Inter-assay (A) and intra-assay (B) coefficient of variation (CV) are shown as the reproducibility of measurements.
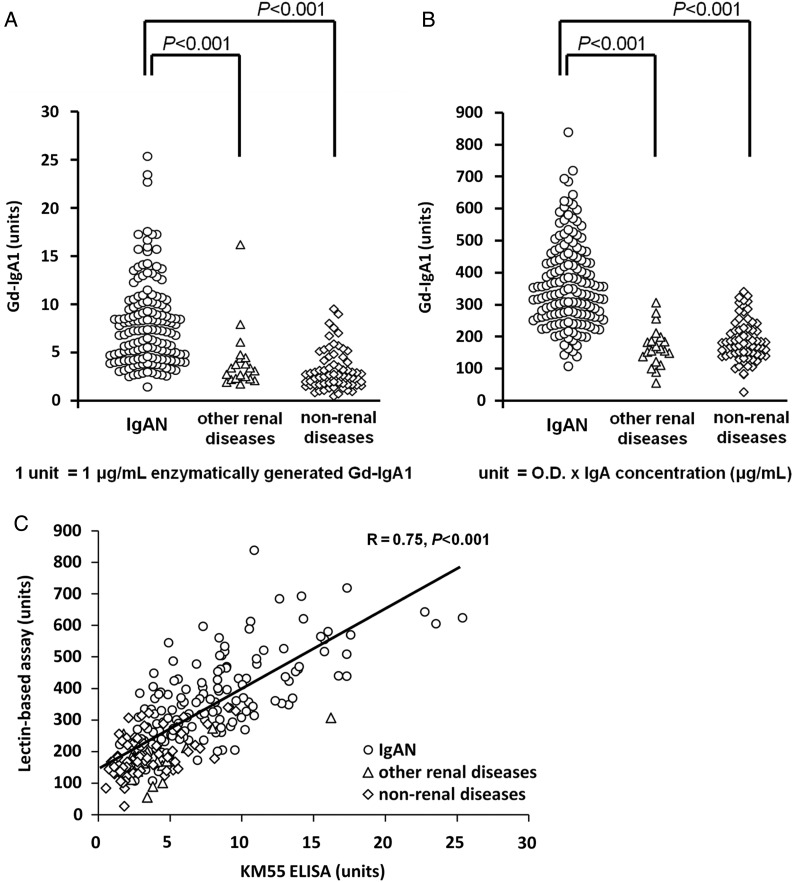
Using the GalNAc-specific monoclonal antibody KM55, we examined the serum levels of Gd-IgA1 in patients with IgAN, other renal diseases (Supplementary data, Table S1) and non-renal diseases. After extrapolating the OD value of serum samples from the standard curve of enzymatically generated Gd-IgA1, the final values of serum Gd-IgA1 level were expressed in units. In this study, 1 unit was defined as 1 μg/mL of standard Gd-IgA1. In our cohorts, the serum level of Gd-IgA1 in patients with IgAN ranged from 1.5 to 25.4 units, whereas it ranged from 1.8 to 16.2 units in other renal diseases and 0.5 to 9.6 units in non-renal diseases (Figure 3A). Among IgAN patients (N = 152), 27.6% had serum level of Gd-IgA1 above 9.6 units, the maximum value measured in the cohort with other renal diseases; the remaining 72.4% had serum level of Gd-IgA1 within the expected normal range (<9.6 units). Statistical analysis revealed that patients with IgAN (N = 152) had significantly higher average Gd-IgA1 levels (8.0 ± 4.4 units) compared with patients with other renal diseases (4.1 ± 3.2 units, N = 20, P < 0.001 versus IgAN) and with patients with non-renal diseases (3.2 ± 2.1 units, N = 70, P < 0.001 versus IgAN).
FIGURE 3:
Serum Gd-IgA1 levels measured by Gd-IgA1 ELISA and HAA lectin-based assay. (A) Serum Gd-IgA1 levels in patients with IgAN, other renal diseases and non-renal diseases were determined by Gd-IgA1 ELISA; (B) The same samples were measured for serum Gd-IgA1 levels by HAA lectin-based assay; (C) Relationship between Gd-IgA1 ELISA and HAA lectin-based assay was analyzed. Results from patients with IgAN (circles), other renal diseases (triangles) and non-renal diseases (rhombi) were plotted. The values obtained by these two independent methods exhibited positive correlation (R = 0.75). P-values of <0.05 were considered to be statistically significant.
To confirm whether Gd-IgA1 ELISA possesses similar reactivity to serum Gd-IgA1 as the conventional HAA lectin-based method, correlation between Gd-IgA1 ELISA and HAA lectin-based assay was examined using the same samples. Consistent with previous reports [4, 6], serum Gd-IgA1 level detected by HAA lectin-based assay in our cohorts was also significantly higher in IgAN patients (361.0 ± 134.8 units, N = 152), compared with that in other renal diseases (168.4 ± 62.0 units, N = 20, P < 0.001 versus IgAN) and non-renal diseases (187.3 ± 65.6 units, N = 70, P < 0.001 versus IgAN) (Figure 3B). Figure 3C shows the positive correlation between serum Gd-IgA1 levels separately determined by these two methods (R = 0.75, P < 0.001).
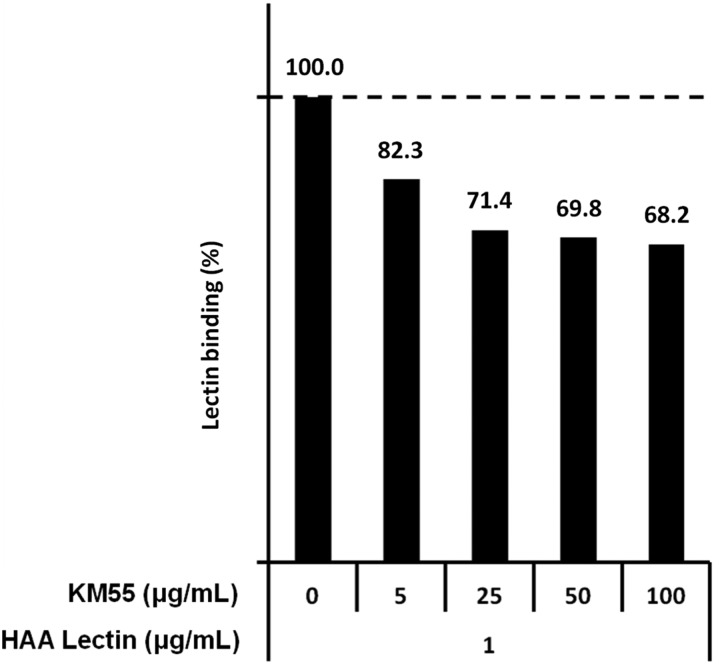
Next, we examined the competitive activity of KM55 against HAA lectin in an HAA lectin-based assay. We found that KM55 competed with HAA lectin in binding to Gd-IgA1 in a dose-dependent manner (Figure 4), suggesting an overlap of the target regions of KM55 and HAA lectin in Gd-IgA1. These results strongly indicate that KM55 is also capable of detecting serum Gd-IgA1 molecules, similar to HAA lectin.
FIGURE 4:
Competition analysis between KM55 and HAA lectin with regard to binding to Gd-IgA1. KM55 partially inhibited the binding of HAA lectin to the enzymatically generated Gd-IgA1 in a dose-dependent manner.
Finally, to further confirm whether KM55 recognizes pathogenic Gd-IgA1 in patients with IgAN, an immunohistochemical analysis was performed on renal biopsy specimens of IgAN. Immunofluorescence with KM55 revealed diffuse and global glomerular staining of Gd-IgA1 in the renal cortex, a pattern that is similar to that of IgA. Moreover, double staining of Gd-IgA1 and IgA showed that Gd-IgA1 was localized predominantly in the mesangial region; however, the localization of IgA was similar to that of Gd-IgA1 but was more broadly observed (Figure 5A–C). Immunofluorescence with KM55 exhibited no non-specific glomerular staining in minimal change nephrotic syndrome (data not shown).
FIGURE 5:
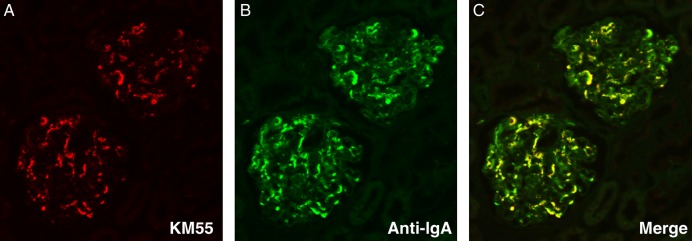
Immunofluorescent staining for glomerular deposition of Gd-IgA1 and IgA in renal biopsy specimens from a patient with IgAN. (A) KM55 recognized pathogenic Gd-IgA1 which was predominantly localized in the mesangial region; (B) IgA deposition was more broadly observed; (C) A merged image exhibited co-localization of Gd-IgA1 and IgA.
DISCUSSION
The present study demonstrated that the novel lectin-independent ELISA assay using an anti-Gd-IgA1 monoclonal antibody KM55 was a reliable method to detect serum Gd-IgA1, suggesting that Gd-IgA1 ELISA may overcome the several limitations of the conventional HAA lectin-based method.
HAA lectin can be isolated from a natural source, i.e. snail HAA, and supplied by manufactures as a highly purified protein by affinity chromatography. HAA lectin has a unique characteristic of recognizing GalNAc on Gd-IgA1. However, this bioactivity, which depends on the source of the lectin, has burdened researchers in this field for many years because of the difficulty in acquiring HAA lectins that are suitable for serum Gd-IgA1 detection.
On the other hand, Gd-IgA1 ELISA makes use of an anti-Gd-IgA1 monoclonal antibody that can be steadily obtained from hybridoma cells. According to our competition assays, KM55 partially inhibited HAA lectin from binding to enzymatically generated Gd-IgA1; we assumed that KM55 and HAA lectin partially share a binding site on the hinge region of a circulating Gd-IgA1 molecule. Consequently, we found a positive correlation between Gd-IgA1 ELISA and conventional HAA lectin-based assay in the measurement of serum Gd-IgA1 level. Thus, the new Gd-IgA1 ELISA for Gd-IgA1 determination in IgAN is a reliable assay to physicians in clinical practice and researchers [13].
Because Gd-IgA1 ELISA used a series of enzymatically generated Gd-IgA1 as the standard, the calculated Gd-IgA1 levels do not always equate to the levels of physiological Gd-IgA1. Therefore, we adopted a system of extrapolating physiological Gd-IgA1 levels and expressing these in unit in this study. Referring to the standard curve of enzymatically generated Gd-IgA1, serum Gd-IgA1 levels was ranged between 1 and 30 µg/mL. This enables us to estimate the ratio of serum Gd-IgA1 to total IgA.
Immunofluorescence staining with KM55 on renal biopsy specimens clearly demonstrated specific glomerular localization of Gd-IgA1, supporting that KM55 recognizes pathogenic Gd-IgA1 in patients with IgAN [18, 19]. Specificity of KM55 to glomerular Gd-IgA1 in patients with IgAN was confirmed by diminished staining of KM55, similar to a blackout image of glomeruli, upon simultaneous addition of a large amount of recombinant Gd-IgA1 but not recombinant glycan-intact IgA1 (data not shown). It was noteworthy that the deposition of Gd-IgA1 was found in every patient with IgAN, however, as expected, although the staining pattern and magnitude varied among patients. Because Gd-IgA1 ELISA showed elevation of serum Gd-IgA1 levels in patients with IgAN, these findings in immunofluorescence provide additional support for the hypothesis that circulating Gd-IgA1 is one of the potent inducers [5] and real-time biomarkers for IgAN [13, 20].
Several basic and clinical issues remain to be addressed for better understandings of nephritogenic Gd-IgA1 in IgAN. First, the relationship between serum Gd-IgA1 levels and their pathological activity in IgAN needs to be further evaluated by focusing on disease history and therapies received by individual patients. In particular, majority of IgAN patients with serum Gd-IgA1 levels within an expected normal range should be further examined and evaluated. Second, whether serum Gd-IgA1 level reflects clinical efficacy, such as remission and recurrence, in patients with IgAN who continuously receive therapies is of interest [2, 13]. Elucidating such relationship will be helpful in the improvement of therapeutic modalities for IgAN [2, 13]. Third, serum levels of Gd-IgA1 measured by Gd-IgA1 ELISA have the potential to be one of the key and real-time biomarkers for the diagnosis and indication of therapy in IgAN [20]. However, the existence of immune complexes containing Gd-IgA1 should also be assessed [5, 11, 21]. Fourth, the next important challenge is to investigate how KM55-positive renal Gd-IgA1 affects every glomerular lesion, such as mesangial proliferation and extracellular matrix expansion [21, 22]. Finally, the analysis of the specific binding site of KM55 on Gd-IgA1 hinge region needs to be considered as well.
In conclusion, we established a novel lectin-independent Gd-IgA1 ELISA that can detect serum Gd-IgA1 in patients with IgAN. This robust assay could help us understand the pathogenesis of IgAN with regard to glycan aberration.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared. This study was supported by a research grant from Kyowa Hakko Kirin Co., Ltd. based on the collaboration research between Juntendo University and Kyowa Hakko Kirin Co., Ltd.
(See related article by Coppo. A new monoclonal antibody for detecting degalactosylated IgA1 as serum biomarker of IgA nephropathy. Nephrol Dial Transplant 2015; 30: 1234–1236.)
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Tatsuya Ono, Kentaro Maki, Yuki Tanbo, Masayuki Maiguma, Terumi Shibata, Takako Ikegami and Tomomi Ikeda for their fruitful scientific advice and excellent research assistance. This study was supported, in part, by the JSPS KAKENHI Grant Number 22790802.
REFERENCES
- 1.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011; 26: 414–430 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Matsuzaki K, Suzuki H et al. Proposal of remission criteria for IgA nephropathy. Clin Exp Nephrol 2014; 18: 481–486 [DOI] [PubMed] [Google Scholar]
- 3.Moore JS, Kulhavy R, Tomana M et al. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol 2007; 44: 2598–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki H, Moldoveanu Z, Hall S et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 2008; 118: 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki H, Kiryluk K, Novak J et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol 2011; 22: 1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthoux F, Suzuki H, Thibaudin L et al. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 2012; 23: 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moldoveanu Z, Wyatt RJ, Lee JY et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 2007; 71: 1148–1154 [DOI] [PubMed] [Google Scholar]
- 8.Gomes MM, Suzuki H, Brooks MT et al. Recognition of galactose-deficient O-glycans in the hinge region of IgA1 by N-acetylgalactosamine-specific snail lectins: a comparative binding study. Biochemistry 2010; 49: 5671–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomana M, Matousovic K, Julian BA et al. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int 1997; 52: 509–516 [DOI] [PubMed] [Google Scholar]
- 10.Laitinen L, Juusela H, Virtanen I. Binding of the blood group-reactive lectins to human adult kidney specimens. Anat Rec 1990; 226: 10–17 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Fan R, Zhang Z et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity . J Clin Invest 2009; 119: 1668–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Raska M, Yamada K et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem 2014; 289: 5330–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki Y, Matsuzaki K, Suzuki H et al. Serum levels of galactose-deficient immunoglobulin (Ig) A1 and related immune complex are associated with disease activity of IgA nephropathy. Clin Exp Nephrol 2014; 18: 770–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanagawa H, Suzuki H, Suzuki Y et al. A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One 2014; 23: e98081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renfrow MB, Cooper HJ, Tomana M et al. Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation Fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem 2005; 280: 19136–19145 [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Wall SB, Suzuki H et al. Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics 2010; 9: 2545–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K, Smith AD, Poulsen K et al. Naturally occurring structural isomers in serum IgA1 O-glycosylation. J Proteome Res 2012; 11: 692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen AC, Bailey EM, Brenchley PE et al. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int 2001; 60: 969–973 [DOI] [PubMed] [Google Scholar]
- 19.Hiki Y, Odani H, Takahashi M et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 2001; 59: 1077–1085 [DOI] [PubMed] [Google Scholar]
- 20.Nakata J, Suzuki Y, Suzuki H et al. Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One 2014; 9: e89707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak J, Raskova Kafkova L, Suzuki H et al. IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured human mesangial cells. Nephrol Dial Transplant 2011; 26: 3451–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coppo R, Fonsato V, Balegno S et al. Aberrantly glycosylated IgA1 induces mesangial cells to produce platelet-activating factor that mediates nephrin loss in cultured podocytes. Kidney Int 2010; 77: 417–427 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.