Abstract
Gastrin-releasing peptide (GRP) receptors are over-expressed in various human tumor including breast and prostate which can be targeted with bombesin for diagnosis and targeted therapy. High abdominal accumulation and the poor in vivo stability of radiolabeled bombesin analogues may represent a limitation for diagnostic imaging and targeted therapy. In this study a new bombesin derivative was labeled with 99mTc via HYNIC and tricine as a coligand and investigated further. The peptide HYNIC conjugate was synthesized on a solid phase using Fmoc strategy. Labeling with 99mTc was performed at 100 °C for 10 min and radiochemical analysis involved ITLC and HPLC methods. The stability of radiopeptide was checked in the presence of human serum at 37 °C up to 24 h. Internalization was studied with the human GRP receptor cell line PC-3. The Biodistribution was studied in mice. Labeling yield of >98 % was obtained to correspond a specific activity of ~80.9 GBq/μmol. Radioconjugate internalization into PC-3 cells was high and specific (15.6 ± 1.9 % at 4 h). A high and specific uptake in GRP-receptor-positive organs such as mouse tumor and pancreas (2.11 ± 0.18 and 1.78 ± 0.09 % ID/g after 1 h respectively) was also determined.
Keywords: GRP, Bombesin, Tumor, 99mTc, Radiopeptide
Introduction
In recent years, small radiolabeled receptor binding peptides exhibit a great potential for receptor-imaging and tumor targeting because of their easy preparation, easy radiolabeling, rapid clearance from blood, non-target tissues, low toxicity, low immunogenicity, high affinity and specificity for receptors [1–3]. Peptide receptors are over-expressed in various human tumor cells which can be targeted with peptide-based radiopharmaceuticals for diagnosis and targeted therapy [1–4]. Bombesin (BB), a tetradecapeptide analogue of human gastrin-releasing peptide (GRP) is one of the most promising peptides which show high affinity for GRP receptor. Over-expression of GRP receptors in several malignant tumors, particularly prostate, lung, breast and colon cancers which make these receptors promising molecular targets for radiolabeled BB derivatives [2–8].
High abdominal accumulation and the poor in vivo stability of radiolabeled BB analogues may represent a limitation for diagnostic imaging and targeted therapy [9–12]. A variety of techniques are available concerning the design and development of new BB derivatives that include introduction/substitution of specific amino acids, chelating group and more important spacer chain[8, 11, 13]. All these agents have influence on radioactivity accumulation in the abdominal region and tumor/normal organ rations [11, 13].
Among the BB derivatives labeled with various radionuclides, BB (7–14) analogues seem to be one of the most BB peptides for development and design of new BB derivatives. It has been shown that the C-terminal region (Gln7-Trp8-Ala9-Val10-Gly11-His12-leu13-Met14-NH2) of BB is necessary for retaining receptor binding affinity and its biological activity [6, 8, 14–18]. Labeling of BB analogues with 99mTc has been performed either directly or mainly indirectly via bifunctional chelator agents such as MAG3 (mercaptoacetyltriglycine), N2S2. DTPA (diethlenetriaminepentaacetic acid) and HYNIC (2-hydrazinonicotinamide) with or without the presence of a linker or spacer function [5, 6, 8, 11, 15, 16, 19–21]. The HYNIC-biomolecules including antibodies and peptides require coligands such as tricine and ethylenediamine diacetic acid (EDDA) for completing the coordination sphere of the technetium (V) core with allowing easy modification of the hydrophilicity and pharmacokinetics [6, 8]. It has been demonstrated in recent studies that 99mTc-radiolabeling yield of HYNIC-BB derivatives using tricine as co-ligand and tricine/EDDA the exchange labeling is high [22–25]. In recent reports, utilizing various types of spacer chains has demonstrated that uncharged hydroxyl amino acids and spacer length in addition reduced kidney uptake resulted in significantly better tumor-to-tissue ration [2, 11]. Probably proteolysis of BB derivatives in plasma occur between His12-Leu13 and the slightly modified of BB derivatives have influence on stability and receptor binding affinity [11, 13, 26].
We also recently reported the preparation and evaluation of new 99mTc labeled bombesin derivatives via HYNIC as a bifunctional chelating agent and tricine as co-ligand and tricine/EDDA exchange labeling [22–24]. To extend our previous study and enhance in vivo stability together with improve tumor targeting and pharmacokinetics characteristics, we chose a BB (7–14) and (d-Tyr)2 as a spacer to improve excretion pattern via kidney and modified d-Phe13 versus Leu13 to decrease proteolysis in plasma and increase biological activity.
Here we present data on the synthesis of [HYNIC-d-Tyr5-d-Tyr6-d-Phe13] BB (5–14), describe optimum conditions for radiolabeling with 99mTc using tricine as coligand and in vitro/in vivo study of the radiolabeled peptide for targeting GRP receptor-positive tumors.
Experimental
Materials and methods
Rink amide 4-methylbenzhydrylamine (MBHA) resin and all Fmoc-protected amino acids were obtained from NovaBiochem. The prochelator HYNIC-Boc was synthesized according to Abrams et al. [27]. Other reagents were purchased from Fluka, and used without further purification. The reactive side chains of the amino acids were masked with one of the following groups: Trp, t-butoxycarbonyl; His, trityl; Tyr, t-butyl. The cell culture medium was Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % fetal bovine serum (FBS), amino acids, vitamins and penicillin/streptomycin from Gibco. Sodium pertechnetate (Na 99mTcO4) obtained from commercial 99Mo/99mTc generator (Radioisotope Division, AEOI). Analytical reverse phase high performance liquid chromatography (RP-HPLC) was performed on a JASCO 880-PU intelligent pump HPLC system equipped with a multiwavelength detector and a flow-through Raytest–Gabi γ-detector. CC 250/4.6 Nucleosil 120-5 C18 column from Teknokroma was used for analytical HPLC, and a VP 250/10 Nucleosil 100-5 C18 column was used for semipreparative HPLC. The gradient systems consisted of 0.1 % trifluoroacetic acid/water (Solvent A) and acetonitrile (Solvent B). For analytic HPLC, Gradient I was used: 0 min 95 % A (5 % B), 5 min 95 % A (5 % B), 30 min 0 % A (100 % B), 33 min 0 % A (100 % B), 35 min 95 % A (5 % B), flow = 1 ml/min, λ = 280 nm. For semipreparative HPLC Gradient II was used: 0 min 80 % A (20 % B), 2 min 80 % A (20 % B), 17 min 50 % A (50 % B), 19 min 0 %A (100 % B), 21 min 0 % A (100 % B), 25 min 80 % A (20 % B) flow = 2 ml/min, λ = 280 nm. Mass spectrum was recorded on a HP 1100 series LC/MSD. Quantitative gamma counting was performed on an ORTEC Model 4001 M γ-system well counter.
Synthesis
The peptide-chelator conjugate was synthesized by standard Fmoc solid phase synthesis on Rink Amide MBHA resin with substitution, 0.69 mmol/g. Coupling of each amino acid was performed in the presence of 3 mol excess of Fmoc-amino acid, 3 mol excess of N-hydroxybenzotriazole, 3 mol excess of Diisopropylcarbodiimide and 5 mol excess of diisopropylethylamine (DIPEA) in dimethylformamide (DMF). Coupling success was checked by the established 2,4,6-trinitrobenzenesulfonicacid test. Cleavage of the Fmoc group was achieved by repetitive treatment with 20 % piperidine in DMF. Coupling of HYNIC to peptide was performed in the presence of 1.2 mol excess of HYNIC-BOC 2.5 mol excess of (2-(7-Aza-1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate), 5 mol excess of DIPEA in DMF. The peptide HYNIC conjugate was removed from the resin and amino acid side chains were deprotected by treatment with a cocktail of trifluoro acetic acid, triisopropylsilane and water (95:2.5:2.5). After removing the organic solvents in vacuo, the crude product was precipitated with cold diethyl ether. The crude peptide HYNIC conjugate was dissolved in water and purified by semi-preparative (Gradient II) RP-HPLC, next the purified product was characterized by LC/MSD and analytic HPLC.
Radiolabeling with 99mTc
Radiolabeling of peptide HYNIC conjugate was performed by adding 20 μg (13.9 nmol) of the stock solution new HYNIC-BB derivative (1 mmol/l in water) and 20 mg (112 μmol) of tricine co-ligand in 0.5 mL of water. 40 μg SnCl2 (20 μl of 2 mg/ml SnCl2, 2H2O in nitrogen-purged 0.1 M HCl) were added to this solution. Finally, 370–1,110 MBq of 99mTcO4 − in 0.5 mL saline was added to the solution and incubated for 10 min at 100 °C.After cooling down to room temperature, the reaction mixture was analyzed.
Radiochemical analysis
After cooling up to room temperature, the radiolabeling yield of the labeled peptide was determined by analytical RP-HPLC (Gradient I) and ITLC on silica gel 60 (Merck) using different mobile phases: 2-butanone for free 99mTcO4 (Rf = 1), 0.1 M sodium citrate (pH 5) to determine the non-peptide bound 99mTc coligand with 99mTcO4 (Rf = 1) and methanol/1 M ammonium acetate 1/1 for 99mTc colloid (Rf = 0). The radioactivity was quantified by cutting the strip (1.5 × 10 cm2) into 1 cm pieces and counting in a well type gamma counter.
Human serum stability
To 1 mL of freshly prepared human serum, we added 100 μl (14.8–29.6 MBq) radiolabeled BB derivative and mixture was incubated at 37 °C. 100 μl aliquots was removed and treated with 200 μl of ethanol [11] at different times up to 24 h. Sample was centrifuged at 3,000 rpm for 15 min to precipitate serum proteins. The supernatant was filtered through a 0.20 μm pore filter and analyzed with RP-HPLC Gradient I to determine radiochemical stability.
Cell culture
The human androgen-independent prostate carcinoma cell line PC-3 was obtained from National Cell Bank of Iran (NCBI) affiliated to Pasteur Institute of Iran. The cells were grown in DMEM supplemented with 10 % (v/v) fetal bovine serum (FBS), 1 % l-glutamine (2 mM),1 % penicillin(100 IU/ml)/streptomycin(100 μg/ml) and 1 % amphotericin B(0.25 μg/ml). The cells were incubated at 37 °C in a humidified atmosphere containing 5 % CO2 and were subcultured weekly detaching with trypsin/EDTA solution (0.25 %).
Internalization assay
Medium was removed from the 6-well plates contain PC-3 cells with density of 1 million cells per well and cells were washed once with 2 ml of internalization medium (DMEM with 1 % FBS). Furthermore, 1.5 ml internalization medium was added to each well, and the plates were incubated at 37 °C for about 1 h. Afterwards, about 150 kBq (2.5 pmol total peptide mass per well) was added to the medium, and the cells were incubated at 37 °C for various time periods. To determine nonspecific internalization, we incubated cells with the radioligand in the presence of 150 μl, 1 μmol/l Bombesin. The cellular uptake was stopped at appropriate time periods (30 min, 1 h, 2 h and 4 h) by removing medium from the cells and washing twice with 1 ml of ice-cold phosphate-buffered saline (PBS). An acid wash for 10 min with a glycine buffer (pH 2.8) on ice was also performed twice. This step was to distinguish between membrane-bound (acid releasable) and internalized (acid resistant) radioligand. Finally, the cells were treated with 1 N NaOH. The culture medium, the receptor-bound and internalized fractions were measured radiometrically in a gamma counter.
Biodistribution
Animal experiments were performed in compliance with the regulations of our institution and with generally accepted guidelines governing such a work. A suspension of human PC-3 cells (1 × 107) in PBS buffer was subcutaneously injected in the right flank of each nude mouse. Seven to 10 days after inoculation, the tumors were inducted and then an activity of 20 MBq (0.35 nmol) of 99mTc-Bombesin was injected via the femoral vein. In order to determine the non-specific uptake of the radiopeptides, in receptor-positive organs, a group of 3 animals were injected with 100 μg cold peptide in 50 μl saline as a co-injection with the radiopeptides (blocked animals). After 1, 4 and 24 h, the mice in groups of 3 animals were scarified, organs of interest were collected, weighed and radioactivity was measured in a gamma counter. The percentage of the injected dose per gram (% ID/g) was calculated for each tissue.
Statistical analyses
The calculations of means and standard deviations for internalization and biodistribution were performed on Microsoft Excel. Student’s t test was used to determine statistical significance. Differences at the 95 % confidence level (P < 0.05) were considered significant.
Results
Synthesis
[HYNIC-d-Tyr5-d-Tyr6-d-Phe13] BB (5–14) (Fig. 1) was synthesized by Fmoc strategy supplying an overall yield of nearly 47 %. The composition and structural identity of HYNIC peptide was verified by LC-MSD (Table 1). The chemical purity was >99 % as confirmed by RP-HPLC method.
Fig. 1.
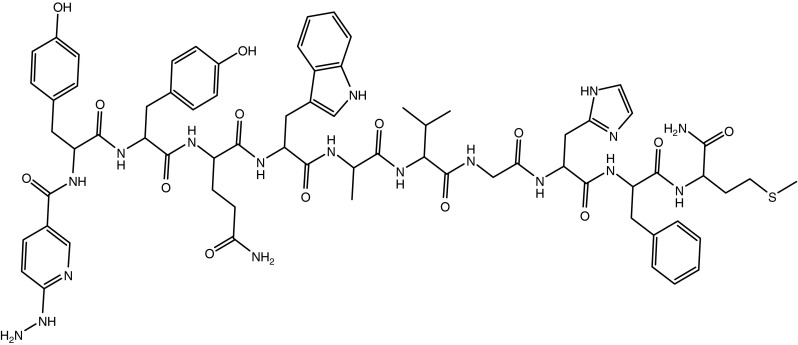
Structure of [HYNIC-d-Tyr5-d-Tyr6-d-Phe13] BB (5–14)
Table 1.
Data for [HYNIC0, d-Tyr6, d-Trp8]-BZ [6–14] NH2 from LC/MSD analysis
| Compound | Calculated mass (g) | Observed mass (g) |
|---|---|---|
| [HYNIC]-peptide | 1434.61 | 1435.09 [M+H]+;100 % |
Radiolabling
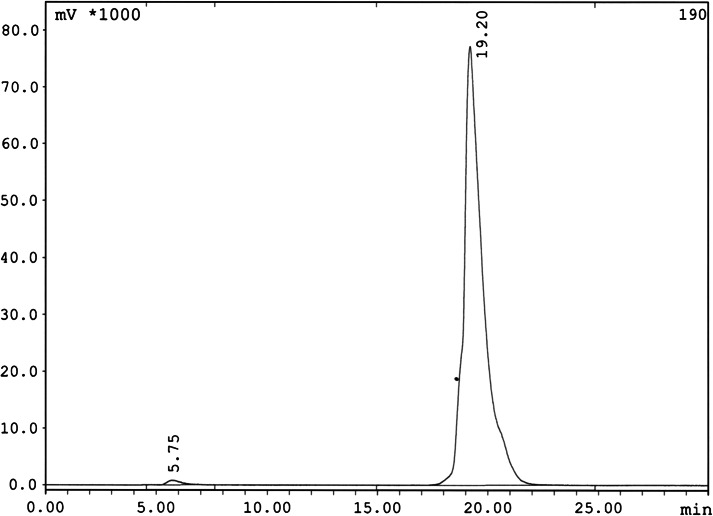
The radiochemical yield of [99mTc/tricine/HYNIC0, d-Tyr5-d-Tyr6-d-Phe13] Bombesin (5–14) NH2, was higher than 98 % by HPLC and also ITLC at a specific activity of 81 GBq/μmol. The HPLC elution time (gradient I) were 19.20 min for 99mTc-peptide and 5.72 min for 99mTcO4 (Fig. 2).
Fig. 2.
RP-HPLC profile of [99mTc/tricine/HYNIC0, d-Tyr5- d-Tyr6-d-Phe13] Bombesin (5–14)
Internalization assay and stability
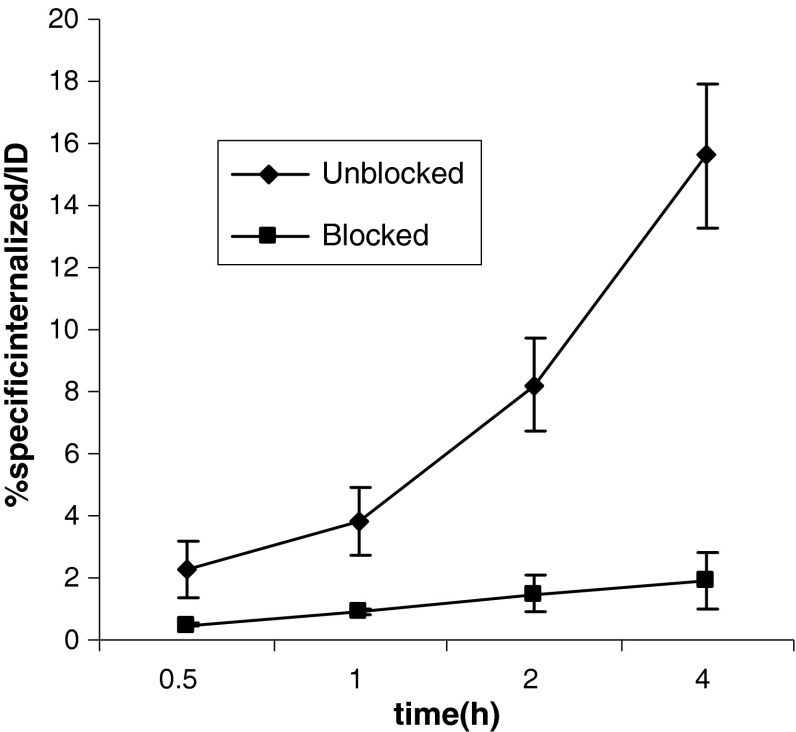
The result of the in vitro assay of the radioligand into PC-3 cell showed fast receptor-specific internalization (5.3 ± 1.1 % at 1 h and 15.6 ± 2.3 % at 4 h). As it shows the significant differences of uptake between blocked and unblocked cells in various time periods are very noticeable (P < 0.05) (Fig. 3). After 24 h in human serum, the radiochemical purity remained >90 %.
Fig. 3.
Time course internalization of [99mTc/tricine/HYNIC0, d-Tyr5-d-Tyr6-d-Phe13] bombesin (5–14) in unblocked and blocked PC-3 cells. Result of three independent experiments with triplicates in each experiment
Animal biodistribution studies
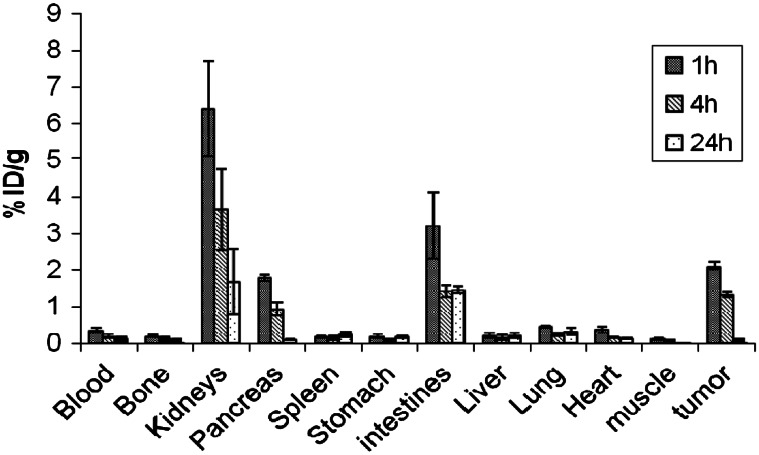
Figure 4 shows the results of biodistribution studies. Radiopeptide exhibited a rapid clearance from the blood with 0.19 ± 0.06 % after 4 h. There was also fast clearance from the GRP receptor-negative tissues with predominantly renal excretion. New 99mTc labeled BB derivative showed a high uptake of radioactivity in the PC-3 tumor and in the GRP receptor-positive organs such as the pancreas. By blocking the receptor through prior injection of cold peptide, the uptake in tumor and pancreas is diminished and this confirms the specificity of radioconjugate. Reduction uptake percentages were 82 % (1.32 vs. 0.24 % ID/g, P < 0.05) and 76 % (0.93 vs. 0.22 % ID/g, P < 0.05) respectively (Table 2). On the other hand, the uptake reduction in non-targeted tissues due to the blocking dose was not significantly.
Fig. 4.
Biodistribution findings in mice (% injected dose per gram organ ± SD, n = 3)
Table 2.
Biodistribution in mice (% injected dose per gram organ ± SD, n = 3) 4 h after administration [99mTc/tricine/HYNIC0, d-Tyr5-d-Tyr6-d-Phe13] Bombesin (5–14)
| Organ | Unblocked | Block |
|---|---|---|
| Blood | 0.19 ± 0.06 | 0.21 ± 0.08 |
| Bone | 0.11 ± 0.05 | 0.14 ± 0.02 |
| Kidneys | 3.65 ± 1.12 | 3.85 ± 1.7 |
| Pancreas | 0.93 ± 0.17 | 0. 22 ± 0.12 |
| Spleen | 0.14 ± 0.06 | 0.17 ± 0.09 |
| Stomach | 0. 13 ± 0.03 | 0.11 ± 0.03 |
| Intestines | 0.96 ± 0.44 | 1.2 ± 0.21 |
| Liver | 0.15 ± 0.08 | 0.17 ± 0.04 |
| Lung | 0.23 ± 0.04 | 0.28 ± 0.07 |
| Heart | 0.15 ± 0.02 | 0.16 ± 0.02 |
| Muscle | 0.06 ± 0.02 | 0.07 ± 0.03 |
| PC-3 tumor | 1.32 ± 0.08 | 0.24 ± 0.05 |
Discussion
Peptide sequences influence on tumor uptake, in vivo stability, pharmacokinetic characteristics, binding affinity for the receptors and the coordination of 99mTc by HYNIC peptide conjugate [11, 13, 25, 28]. If 99mTc-HYNIC peptide becomes more stable, then it may result in improved tumor targeting and body retention. On the other hand, a change in configuration would be likely to reduce the performance of the radiopharmaceutical in vivo. HYNIC makes labeling with 99mTc in high specific activity possible followed by using various coligands, which permit control of the hydrophilicity and pharmacokinetics of the labeled peptide [6, 22–25, 28]. High specific activity achieves with low concentration of the HYNIC peptide conjugate. One of the most widely used coligands is tricine. Tricine gives the best radiolabeling efficiency but it has been reported that tricine as a coligand, 99mTc-complex was not stable, particularly in dilute solutions, due to different bonding modalities of the hydrazine moiety of the HYNIC and the tricine coligand [6, 25, 28]. As we have previously shown that bombesin derivative [HYNIC-d-Tyr6-d-Trp8] BB (6–14) is as potential targeted tumor imaging agent [22, 23]. Therefore we extended our pervious study with a new radiolabeled bombesin derivative with sequences bombesin (7–14), D-Phe13 versus leu13 modification and (d-Tyr)2 as spacer to improve excretion pattern via kidney, improve binding affinity and to decrease enzymatic metabolism. In this study we used HYNIC peptide with tricine as a coligand in amounts of 20 μg and 20 mg in final volume of labeled solution respectively. We obtained high radiochemical yield (>98 %) with very low amount of 99mTc-pertechnetate (<0.5 %), 99mTc-radiocolloid (<1 %) and 99mTc-coligand (<0.3 %). In RP-HPLC analysis, we observed a single major peak without any impurities due to isomeric forms of the new 99mTc-HYNIC-conjugates. In comparison to those reports regarding 99mTc-tricine-HYNIC complex instability [28, 29], our new labeled peptide conjugate was stable up to a 24 h post labeling period in the room temperature. These high labeling yield and stability may be due to optimization of condition in amount of materials, Peptide sequence and also in our labeling method.
Radiotracer showed internalization profile with increased value from 0.5 h (2.3 ± 0.9 %) to 4 h (15.6 ± 1.9 %) incubation time. The efflux rate of radiopeptide from PC-3 tumor cell after 4 h showed an acceptable intercellular trapping. Pervious works of BB derivatives also demonstrate internalization and receptor mediated trapping of labeled compounds [4, 21]. Compare with our pervious compound [99mTc-tricine-HYNIC0-d-Tyr6-d-Trp8] BB (6–14) [22, 23], this new derivative showed higher rate of internalization after 4 h in PC-3 (15.6 ± 1.9 vs. 10.7 ± 1.2 %).It could be due to d-Phe13 versus leu13 modification and replacement of (d-Tyr)2 as spacer instead of d-Tyr6. As liolios et al. [11] reported that the incorporation of positively and negatively charged the amino acid spacer decrease the binding affinity of the BB analogue for GRP receptor. In the present study, it seems that uncharged the amino acid composed from tyrosine increases binding affinity of new BB analogue.
The results of biodistribution showed fast blood clearance of radiopeptide with <0.19 % ID/g and rapid excretion performed mainly by renal pathway at 4 h. In addition, in vivo studies exhibited low abdominal accumulation and significant and specific uptake of radioconjugate in tumor and pancreas. The highest non-specific uptake was found in kidneys. A significant uptake of radioactivity was observed in the pancreas which expresses GRP receptors. The specificity of radioconjugate was confirmed by blocking the receptor through prior injection of cold peptide.
Garayoa et al. [30] reported that uncharged hydroxyl linker as serine improved the biodistribution and resulting in increase in the tumor uptake. Compare with our pervious study [22, 23] the uptake of radiopeptide in tumor and pancreas increased (2.11 vs. 1.12 % ID/g, P < 0.05 and 1.78 vs. 1.04 % ID/g, P < 0.05 at 1 h) respectively. These results indicate that the charge in linker might be a key factor for determining the pharmacological characteristics of BB derivatives. As the most of BB derivatives exhibit high abdominal accumulation which may represent a problem in their clinical for diagnostic imaging and targeted therapy [12]. Our compound with modified lipophilicity has a good improvement in renal excretion, significant and specific tumor uptake.
Conclusions
In general, this study performed for new [HYNIC-d-Tyr5-d-Tyr6-d-Phe13] BB (5–14) derivative showed that hydrophilicity, charge of spacer and sequence of peptide influenced on the bidistribution and the affinity. In this study, labeling of [HYNIC-d-Tyr5-d-Tyr6-d-Phe13] BB (5–14) with 99mTc was completed within a very short time by using tricine coligand in high specific activity (~80.9 GBq/μmol) which makes it an ideal conjugate for usage in clinical nuclear medicine laboratories. Furthermore, this labeled peptide conjugate demonstrated excellent radiochemical stability even up to 24 h post labeling. Our new radiopeptide had a specific cell binding and internalization followed by a good stability in human serum at 37 °C for at least 24 h and no significant impurities were detected by HPLC. The prepared radiopeptide showed a high accumulation of radioactivity in tumor and pancreas as a positive GRP receptors targeted tissue followed by excretion via the kidneys. These promising Characteristics make our new designed labeled peptide conjugate as a very suitable candidate for diagnostic imaging of GRP receptor-positive tumors.
Acknowledgments
The authors thank Mr. Soleman Faraji for reviewing the English grammar.
References
- 1.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocor Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 2.Smith CJ, Volkert WA, Hoffman TJ. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl Med Biol. 2005;32:733–740. doi: 10.1016/j.nucmedbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Okarvi SM. Peptide-based radiopharmaceuticals: future tools for diagnostic imaging of cancers and other diseases. Med Res Rev. 2004;24:357–397. doi: 10.1002/med.20002. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Chen J, Waldherr C, Hinni K, Waser B, Reubi JC, Maecke HR. Synthesis and evaluation of bombesin derivatives on the basis of pan-bombesin peptides labeled with indium-111, lutetium-177, and yttrium-90 for targeting bombesin receptor expressing tumors. Cancer Res. 2004;64:6707–6715. doi: 10.1158/0008-5472.CAN-03-3845. [DOI] [PubMed] [Google Scholar]
- 5.Smith CJ, Volkert WA, Hoffman TJ. Gastrin releasing peptide (GRP) receptor targeted radiopharmaceuticals: a concise update. Nucl Med Biol. 2003;30:861–868. doi: 10.1016/S0969-8051(03)00116-1. [DOI] [PubMed] [Google Scholar]
- 6.Faintuch BL, Santos RLSR, Souza ALFM, Hoffman TJ, Greeley M, Smith CJ. 99 mTc-HYNIC-bombesin (7–14) NH2: radiochemical evaluation with co-ligands EDDA (EDDA = ethylenediamine-N,N′-diacetic acid), tricine, and nicotinic acid. Synth React Inorg Met Org Nano Met Chem. 2005;35:43–51. doi: 10.1081/SIM-200047545. [DOI] [Google Scholar]
- 7.Breeman WA, de jong M, Erion JL, Bugaj JE, Srinivasan A, Bernard BF, et al. Preclinical comparison of in-labeled DTPA—or DOTA—bombesin analogs for receptor targeted scintigraphy and radionuclide therapy. J Nucl Med. 2002;43:1650–1656. [PubMed] [Google Scholar]
- 8.Faintuch BL, Teodoro R, Duatti A, Muramoto E, Faintuch S, Smith CJ. Radiolabeled bombesin analogs for prostate cancer diagnosis: preclinical studies. Nucl Med Biol. 2008;35:401–411. doi: 10.1016/j.nucmedbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Smith CJ, Gali H, Sieckman GL, Higginbotham C, Volkert WA, Hoffman TJ. Radiochemical investigations of 99mTc-N3B-X-BBN[7–14]NH2: an in vitro/in vivo structure-activity relationship study where X = 0-,3-,5-,8-, and 11-carbon tethering moieties. Bioconjug Chem. 2003;14:93–102. doi: 10.1021/bc020034r. [DOI] [PubMed] [Google Scholar]
- 10.Däpp S, Garayoa EG, Maes V, Brans L, Tourwé DA, Müller C, Schibli R. PEGylation of 99mTc-labeled bombesin analogues improves their pharmacokinetic properties. Nucl Med Biol. 2011;38:997–1009. doi: 10.1016/j.nucmedbio.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Liolios CC, Fragogeorgi EA, Zikos C, Loudos G, Xanthopoulos S, Bouziotis P, Paravatou-Petsotas M, livaniou E, Varvarigou AD, Sivolapenko GB. Structural modifications of 99mTc-labelled bombesin-like peptides for optimizing pharmacokinetics in prostate tumor targeting. Int J Pharmaceutics. 2012;430:1–17. doi: 10.1016/j.ijpharm.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Pujatti PB, Santos JS, Couto RM, Melero LTUH, Suzuki MF, Soares CRJ, Grallert SR, Mengatti J, De Araújo EB. Novel series of 177Lu-labeled bombesin derivatives with amino acidic spacers for selective targeting of human PC-3 prostate tumor cells. Q J Nucl Med Mol Imaging. 2011;55:310–323. [PubMed] [Google Scholar]
- 13.Okarvi SM, Jammaz IA. Preparation and evaluation of bombesin peptide derivatives as potential tumor imaging agents: effects of structure and composition of amino acid sequence on in vitro and in vivo characteristics. Nucl Med Biol. 2012;39:795–804. doi: 10.1016/j.nucmedbio.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman TJ, Quinn TP, Volkert WA. Radiometallated receptor-avid peptide conjugates for specific in vivo targeting of cancer cells. Nucl Med Biol. 2001;28:527–539. doi: 10.1016/S0969-8051(01)00209-8. [DOI] [PubMed] [Google Scholar]
- 15.Smith CJ, Sieckman GL, Owen NK, Hayes DL, Mazuru DG, Kannan R, Volkert WA, Hoffman TJ. Radiochemical investigations of gastrin-releasing peptide receptor-specific [99mTc(X)(CO)3-Dpr-Ser-Ser-Ser-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2] in PC-3, tumor-bearing, rodent models: syntheses, radiolabeling, and invitro/in vivo studies where Dpr = 2,3-diaminopropionic acid and X = H2O or P(CH2OH)3. Cancer Res. 2003;63:4082–4088. [PubMed] [Google Scholar]
- 16.Smith CJ, Gali H, Sieckman GL, Hayes DL, Owen NK, Mazuru DG, Volkert WA, Hoffman TJ. Radiochemical investigations of P177Lu-DOTA-8-Aoc-BBN[7-14] NH2: an in vitro/in vivo assessment of the targeting ability of this new radiopharmaceutical for PC-3 human prostate cancer cells. Nucl Med Biol. 2003;30:101–109. doi: 10.1016/S0969-8051(02)00391-8. [DOI] [PubMed] [Google Scholar]
- 17.Alves S, Paulo A, Correia JD, Santos I, Gano L, Veerendra BCJ, Sieckman GL, Hoffman TJ, Rold TL, Figueroa SD, Retzloff L, McGrate J, Prasanphanich A, Smith CJ. Pyrazolyl conjugates of bombesin: a new tridentate ligand framework for the stabilization of fac-[M(CO)3]+moiety. Nucl Med Biol. 2006;33:625–634. doi: 10.1016/j.nucmedbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Yang YS, Zhang X, Xiong Z, Chen X. Comparative in vitro and in vivo evaluation of two 64Cu-labelled bombesin analogs in a mouse model of human prostate adenocarcinoma. Nucl Med Biol. 2006;33:371–380. doi: 10.1016/j.nucmedbio.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Van de Wiele C, DumontF Broecke RV, Oosterlink W, Cocguyt V, serreyn R, peers S, Thornback J, Slegers G, Dierckx RA. Technetium-99m RP527, a GRP analogue for visulisation of GRP receptor-expressing malignancies: a feasibility study. Eur J Nucl med. 2000;27:1694–1699. doi: 10.1007/s002590000355. [DOI] [PubMed] [Google Scholar]
- 20.Van de Wiele C, Dumont F, Van Belle S, Slegeres G, Peers SH, Dierckx RA. Is there a role for agonist gastrin-relasing peptide receptor radioligands in tumor imaging? Nucl Med Commun. 2001;22:5–15. doi: 10.1097/00006231-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 21.La Bella R, Garcia-Garayoa E, Langer M, Blauenstein P, Beck-Sickinger AG, Schubiger PA. In vitro and in vivo evaluation of a 99mTc(I)-labeled bombesin analogue for imaging of gastrin releasing peptide receptor-positive tumors. Nucl Med Biol. 2002;29:553–560. doi: 10.1016/S0969-8051(02)00314-1. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghzadeh N, Gandomkar M, Najafi R, Shafiei M, Sadat Ebrahimi SE, Shafiee A, Larijani B. Preparation and evaluation of a new 99mTc labeled bombesin derivative for tumor imaging. J Radioanal Nucl Chem. 2010;283:181–187. doi: 10.1007/s10967-009-0138-z. [DOI] [Google Scholar]
- 23.Sadeghzadeh N, Gandomkar M, Shafiei M, Mazidi M, Goudarzi M, Mirfallah SH, Sadat Ebrahimi SE. Synthesis and evaluation of a new radiolabeled bombesin analogue for diagnosis of GRP receptor expressing tumors. Iran J Nucl Med. 2009;17:18–26. [Google Scholar]
- 24.Shirmardi SP, Gandomkar M, Mazidi M, Shafiei M, Ghannadi Maragheh M. Synthesis and evaluation of a new bombesin analog labeled with 99mTc as a GRP receptor imaging agent. J Radioanal Nucl Chem. 2011;288:327–335. doi: 10.1007/s10967-011-0985-2. [DOI] [Google Scholar]
- 25.Lambrecht FY, Durkan K, Bayrak E. Labeling bombesin-like peptide with 99mTc via hydrazinonicotinamide: description of optimized radiolabeling conditions. J Radioanal Nucl Chem. 2010;284:539–545. doi: 10.1007/s10967-010-0530-8. [DOI] [Google Scholar]
- 26.Garayoa GE, Ruegg D, Blauenstein PZ, wimpfer M, Khan IA, Maes V, Blanc A, Beck-Sickinger A, Tourwe AG, Schubiger PA. Chemical and biological characterization of new Re(CO)3/99mTc(CO)3 bombesin analogues. Nucl Med Biol. 2007;34:17–28. doi: 10.1016/j.nucmedbio.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Abrams MJ, Juweid M, tenKate CI, Schwartz DA, Hauser MM, Gaul FE, Fuccello AJ, Rubin RH, Strauss W, Fischman AJ. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J Nucl Med. 1990;31:2022–2028. [PubMed] [Google Scholar]
- 28.King R, Surfraz MBU, Finucane C, Biagini SCG, Blower PJ, Mather SJ. 99mTc-HYNIC-gastrin peptides: assisted coordination of 99mTc by amino acid side chains results in improved performance both in vitro and in vivo. J Nucl Med. 2009;50:591–598. doi: 10.2967/jnumed.108.058289. [DOI] [PubMed] [Google Scholar]
- 29.Edwards DS, Liu S, Harris AR, Looby RJ, Ziegler MC, Heminway SJ, Barrett JA, Carroll TR. New and versatile ternary ligand system for technetium radiopharmaceuticals: water soluble phophines and tricine as coligands in labeling a hydrazinonicotinamide-modified cyclic glycoprotein Iib/IIIa recptor antagonist with 99mTc. Bioconjug Chem. 1997;8:146–154. doi: 10.1021/bc970002h. [DOI] [PubMed] [Google Scholar]
- 30.García Garayoa E, Schweinsberg C, Maes V, Brans L, Bläuenstein P, Tourwe DA, Schibli R, Schubiger PA. Influence of the molecular charge on the biodistribution of bombesin analogues labeled with the [99mTc(CO)3]-core. Bioconjug Chem. 2008;19:2409–2416. doi: 10.1021/bc800262m. [DOI] [PubMed] [Google Scholar]