Abstract
The short half-life of 212Bi and 213Bi limits the application of these radionuclides in α radionuclide therapy. The labeling of biomolecules with 212Pb (mother nuclide of 212Bi) instead of 212Bi or 213Bi has the advantage of obtaining a conjugate with a half-life of 10.6 h, compared with of 60 min for 212Bi or 46 min for 213Bi. Previous attempts to prepare a potential in vivo generator with 212Pb complexed by the DOTA chelator failed, because about 36 % of Bi was reported to escape as a result of the radioactive decay
Keywords: In vivo generator, 212Pb, 212Bi, Polyaminipolycarboxylate ligands
Introduction
In the field of targeted radiotherapy, the selection of radionuclide depends on the type of the treated disease. Solid tumors are generally treated with high and medium energy β −-emitters such as 90Y, 188Re and 131I, because their β −-particles have a tissue range of several millimeters. The effective tissue range of β −-particles is not optimal for treatment of tumors forming small clusters of cells and for treatment of single cancer cells and micrometastases. Treatment of these neoplastic diseases could be more effective with α-emitters, which combine short range with high linear energy transfer, combination that results in the relatively high biological effect and cytotoxicity [1]. Owing to this, α-particles are able to make lethal double strand breaks in DNA. When the double stranded DNA molecules breaks, there is very little chance to repair such damage. Humm and Cobb [2] reported that to attain single cell kill probability of 99.99 % tens of thousands of β-decays at the cell membrane are required, whereas in the case of α-emitters only few α-decays at the cell membrane are sufficient to kill malignant cells. Due to high radiotoxicity of α-particles, high degree of accuracy is required to deliver the radiation to the target cells without targeting normal cells. From the medical point of view, α-particles can be used either for treatment of cancer micro-metastasis, or to destroy tumor margins after surgical resection. Another potential application is in treating cancers such as lymphoma and leukemia, which are present as free-floating tumor cells in the circulation system [3]. Till now, only few clinical studies with 213Bi and 211At labeled peptides and monoclonal antibodies have demonstrated the potential of alpha particle emitting isotopes in radionuclide therapy [4, 5].
There are only few α-particle emitting radionuclides, which have properties suitable for developing therapeutic radiopharmaceuticals: generator-obtained 212Bi (t 1/2 = 60 min), 213Bi (t 1/2 = 46 min), 226Th (t 1/2 = 30 min), 225Ac (t 1/2 = 10 days), 227Th (t 1/2 = 18.7 days), as well as the cyclotron-produced 211At (t 1/2 = 7.2 h). The available α-emitters have serious shortcomings, because in the case of 225Ac and 227Th the designed ligand must form chemically stable complexes with both parent and decay radionuclides. 225Ac decays directly to 221Fr (alkali metal), which has a half-life of 4.9 min and escapes from 225Ac-radiobioconjugate. Similar situation appears in the case of 227Th, where the decay product, the gaseous 219Rn, easily liberates itself from 227Th-radioconjugate. Application of 211At is limited, because astatine as the heaviest halogen forms weak bond with a carbon atom in the biomolecule. Therefore, 211At-bioconjugates are unstable under physiological conditions.
In the case of 212Bi, 213Bi and 226Th short half-life often limits the application of these nuclides to situations when the tumor cells are rapidly accessible to the targeting agent. However, the short half-life of 212Bi could be effectively lengthened by chelation of the parent 212Pb radionuclide (t 1/2 = 10.6 h) to a biomolecule [6]. In comparison with direct use of 212Bi, radiopharmaceuticals based on 212Pb would have much broader applicability, because the half-life of 212Pb corresponds better with the pharmacokinetics of various biomolecules. Moreover, the 212Pb–212Bi in vivo generator delivers the dose per unit of administered activity ten times greater than that in the case of 212Bi alone or of the 213Bi α-emitter [7]. Thus, the required activity of the radiopharmaceutical preparation would be greatly reduced, and making this way generation and administration of the α-emitting radiopharmaceutical much easier.
It is very important that 212Bi formed in the β
−-decay of 212Pb remains bound to the carrier. This is because free bismuth localizes in the kidneys, prohibiting this way the use of structures that are not effective in stabilizing 212Bi in vivo [8]. In theory, the decay of 212Pb should not generate a problem with retention of 212Bi. The calculated recoil energy of the Bi nucleus is only about 0.5 eV. This is not sufficient to break a chemical bond, which requires about 10 eV. However, over 30 % of the γ-rays emitted when 212Pb decays are internally converted during the decay time. The resulting cascade of conversion electrons brings 212Bi to highly ionized states such as Bi5+ and Bi7+, hence the energy required to neutralize the charge is sufficient to break chemical bonds [9]. The potential use of 212Pb as an in vivo generator has been studied in earlier works [8, 10, 11]. Previous attempts to prepare a potential in vivo generator with 212Pb complexed by the DOTA chelator [11] failed, because about 36 % of Bi was reported to escape as a result of the radioactive decay
In this paper we report the formation and stability studies of 212Pb complexes with various polydentate ligands exhibited faster than DOTA kinetics of complex formation.
Experimental
Lead-212
The 1 MBq of 212Pb (t 1/2 = 60 min) was obtained from 232U as one of the decay products. Separation of 212Pb from 232U and other decay products was performed in a two-step procedure. In the first step, 224Ra was eluted by 0.1 M HNO3 from HDEHP-Teflon column loaded with 232U. In the second step 212Pb was separated from 224Ra on cation exchange resin Dowex 50 × 8 by elution with 1.0 M HCl. The effluent was acidified with HNO3, evaporated and the residue was dissolved in 0.01 M HNO3.
Measurements
The radioactivity was measured by γ-spectrometer using the HPGe detector (Canberra) with associated electronics (resolution 2.09 keV for 1,332 keV 60Co line, efficiency ca. 30 %), coupled to the multichannel analyzer TUKAN (The Andrzej Soltan Institute for Nuclear Studies, Świerk, Poland).
Ligands
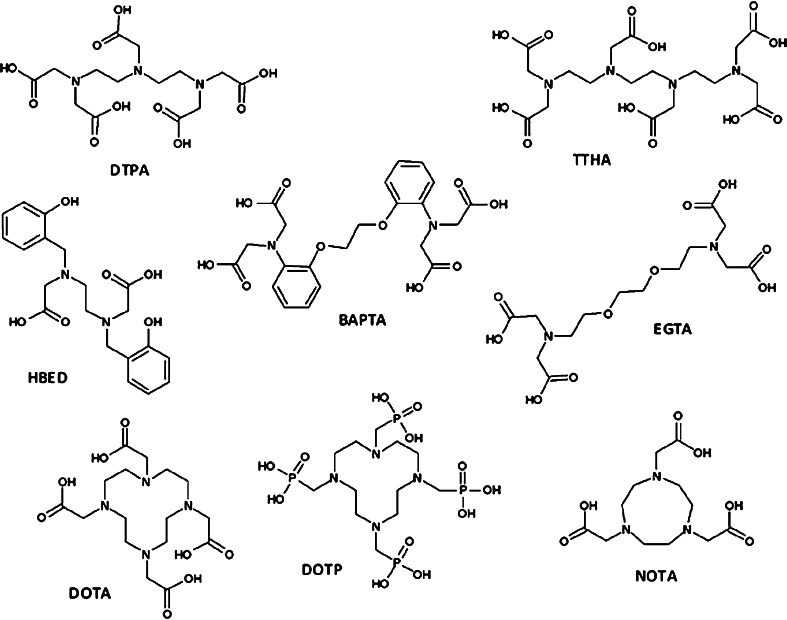
We have chosen the following acyclic ligands for the studies: 8-dentate diethylenetriaminepentaacetic acid (DTPA), 6-dentate N,N-bis(2-hydroxybenzyl)ethylenediamine-N,N-diacetic acid (HBED), 6-dentate 1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (BAPTA), 8-dentate ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) and 10-dentate triethylenetetraamine-N,N′,N″,N″′-hexaacetic acid (TTHA). From the cyclic ligands we have chosen 8-dentate 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), 8-dentate 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrayl-tetrakis(methylphosphonic acid) (DOTP) and 6-dentate 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA).
Synthesis of radiolabeled complexes
The experimental conditions for labeling, such as the metal-to-ligand molar ratio, pH, time of reaction and temperature, were optimized to achieve a high complexation efficiency. The 212Pb complexes with the studied ligands were synthesized by mixing 50 μl of non-carrier-added 212Pb in 0.01 M HNO3 with 5 μl of either 10−1 or 10−2 M solution of the respective ligand. The volume of solution was adjusted to 500 μl by adding 0.01 M CH3COONH4 solution, and pH were settled at pH 6 or 7 using 2 M NaOH. Complexes with acyclic ligands were prepared at room temperature in 2 h.
Determination of labeling efficiency and assay
The determination of labeling efficiency was achieved in accordance with the modified procedure proposed by Mirzadeh et al. [9] by isolation of uncomplexed cations by the use of chelating Chelex 100 resin in a small column (d = 3 mm, h = 10 mm). In preliminary experiments we found that when solution containing nca 212Pb and 212Bi was loaded on the column all activity remained on the column, even after elution with 0.1 M NH4NO3. In next step the 212Pb and 212Bi radionuclides were quantitatively eluted with 2 ml of 5 M HCl. We assumed that under the same conditions the negatively charged complexes of Pb2+ and Bi3+ would be eluted from the column by 0.1 M NH4NO3. This separation procedure was tested on Pb and Bi complexes formed by 0.01 M DOTA and DTPA ligands, and we found that these complexes were completely eluted by 2 ml of 0.1 M NH4NO3.
Assay of 212Bi after decay of 212Pb–L complexes
The complexes were prepared as described above. Concentration of the synthesized complexes was decreased using isotonic solution of sodium chloride (0.9 % NaCl solution), in order to obtain 0.5 ml samples. Solutions were incubated for 4 h to attain 212Pb–212Bi radioactive equilibrium and then in order to separate complexes from the uncomplexed cations the solution was loaded on the column filled with Chelex 100 resin (3 × 10 mm). To achieve the separation the column was washed with 2 ml of 0.1 M NH4NO3 solution which eluted the complexes. The retained uncomplexed 212Pb and 212Bi cations were next eluted with 2 ml of 5 M HCl. The activities of the eluted fractions were measured over 5 h time period.
Results and discussion
The labeling of biomolecules with 212Pb instead of 212Bi or 213Bi has the advantage of obtaining a conjugate with a half-life of 10 h, instead of 60 min for 212Bi or 46 min for 213Bi.
Therefore, when 212Pb labeled conjugate is used, the delivered dose is much greater per unit of administered activity than in the case of 212/213Bi conjugates [7]. As noted in [12] a dose of 10 mCi of 212Pb was equally effective as a 500 mCi injected dose of 213Bi. However, as reported by Mirzadeh et al. [9] and Miao et al. [13] approximately one-third of the radioactivity escaped from the DOTA chelator due to ionization associated with the decay of 212Pb to 212Bi. In the case of radiobioconjugate Fu-Min Su et al. [14] found that 212Pb–DOTA-biotin was initially stable, but 30 % of 212Bi activity was released from the DOTA-biotin in 4 h. This result is in agreement with that reported by Mirzadeh et al. [9] who found that 36 % of 212Bi activity was released from 212Pb–DOTA in the decay.
Redistribution was not a concern for 212Pb internalized in tumor cells, since diffusion of metal ions across the cell membrane would be very slow. However, loss of 212Bi from circulating 212Pb-bioconjugate could allow 212Bi to redistribute and irradiate normal organs.
In the previous studies, DOTA and its N,N,N,N-tetraamide analog were used for binding 212Pb to biomolecules [11]. In our opinion, because formation of kinetically inert Bi3+–DOTA complex is very slow, the released 212Bi from the 212Pb–DOTA complex very poorly reassociates with DOTA. In our studies, we examined selected acyclic and cyclic polyaminopolicarboxylate ligands, which form complexes with bismuth cations more rapidly than does DOTA. The ligands demonstrating high affinity for 3+ metal cations like Fe3+ and lanthanides were selected for our studies. The structure of the ligands is presented in the Fig. 1.
Fig. 1.
Structure of the ligand studied
From the studied ligands DOTP and BAPTA are the only two, which can be taken into consideration for designing new applicable radioconjugates, because they demonstrate sufficient labeling yields Table 1. The high yield of labeling can be achieved only in the case, when the ligand concentration exceeds 10−4 M. The remaining ligands form complexes with 212Pb with too low efficiency. Therefore, only the 212Pb–DOTP and 212Pb–BAPTA complexes were selected for studying stability in isotonic solution of sodium chloride (0.9 % NaCl).
Table 1.
Labeling yield of the ligands by 212Pb. Solution—in 0.01 M CH3COONH4, pH = 6
| Ligand (concentration M) | Labeling yield (%) |
|---|---|
| DOTP (10−4 M) | 89.5 |
| DOTP (10−5 M) | 62.7 |
| DTPA (10−4 M) | 47.2 |
| TTHA (10−4 M) | 68.3 |
| EGTA (10−4 M) | 73.1 |
| BAPTA (10−4 M) | 85.8 |
| HBED (10−4 M) | 27.2 |
| NOTA (10−4 M) | <10 |
As shown in Table 2 the 212Pb–DOTP complex is stable in isotonic solution of sodium chloride, because at DOTP concentration of 10−4 M only very small amount of 212Pb escapes into solution. The radioactivity level of released 212Bi is under the limit of detection. Comparison of our results with those on 212Pb–DOTA, described by Mirzadeh et al. [9], shows that DOTA forms with 212Pb kinetically inert complexes. Unfortunately, 212Bi the decay product of 212Pb, released to solution very poorly reassociates with DOTA. On the contrary, DOTP forms with 212Pb more labile complexes, for which the escaped 212Bi easily reassociates with the ligand. It should be emphasized that 212Pb–DOTP is stable only in the case when concentration of the free ligand exceeds 10−4 M.
Table 2.
Stability of 212Pb-DOTP and 212Pb-BAPTA complexes in isotonic solution of sodium chloride
| Ligand | Ligand concentration (M) | Free 212Pb activity (%) | Free 212Bi activity (%) |
|---|---|---|---|
| DOTP | 10−4 | 2 | 0 |
| 10−5 | 20 | 0 | |
| BAPTA | 10−4 | 80 | 70 |
The activity of the 212Pb-DOTP solution was 2.6 × 104 cpm and that of 212Pb-BAPTA 2.5 × 104 cpm
The results obtained show that DOTP could be used as a ligand in designing 212Pb/212Bi in vivo generators, but only in the case when high specific activity of the radiopharmaceutical is not required, as it happens in palliation therapy of bone metastasis.
Acknowledgments
This study was supported by grant No. N 204 143 32/3547 from Ministry of Science and Higher Education, Poland and by European Cooperation in Science and Technology Action BM 0607, Contract No. 53/040/2009, Dec. 368/N-COST/2008/0.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Hall EJ. Radiobiology for the radiologist. Philadelphia: Lippincott; 1988. pp. 161–177. [Google Scholar]
- 2.Humm JL, Cobb LM. Nonuniformity of tumor dose in radioimmunotherapy. J Nucl Med. 1990;31:75–83. [PubMed] [Google Scholar]
- 3.Zalutsky MR, Pozzi OR. Radioimmunotherapy with α-particle emitting radionuclides. Q J Nucl Med. 2004;48:1–7. [PubMed] [Google Scholar]
- 4.Cordier D, Forrer F, Bruchertseifer F, Morgenstern A, Apostolidis C, Good S, Müller-Brand J, Mäcke H, Reubi JC, Merlo A. Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8, Met(O2)11]-substance P: a pilot trial. Eur J Nucl Med Mol Imaging. 2010;37:1335–1344. doi: 10.1007/s00259-010-1385-5. [DOI] [PubMed] [Google Scholar]
- 5.Zalutsky MR, Reardon DA, Pozzi OR, Vaidyanathan G, Bigner DD (2007) Targeted α-particle radiotherapy with 211At-labeled monoclonal antibodies. Nucl Med Biol 34:779–785 [DOI] [PMC free article] [PubMed]
- 6.Yong K, Brechbiel MW. Towards translation of 212Pb as a clinical therapeutic; getting the lead in! Dalton Trans. 2011;40:6068. doi: 10.1039/c0dt01387k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leichner PK. Radiation dosimetry of monoclonal antibodies: practical considerations. In: Henkin RE, Boles MA, Dillehay GL, Halama JR, Karesh SM, Wagner RH, Zimmer AM, editors. Nuclear Medicine. Mosby: St Louis; 1996. pp. 558–562. [Google Scholar]
- 8.Henriksen G, Schoultz BW, Hoff P, Larsen RH. Potential in vivo generator for alpha-particle therapy with Bi-212: presentation of a system to minimize escape of daughter nuclide after decay of Pb-212 to Bi-212. Radiochim Acta. 2003;91:109–113. doi: 10.1524/ract.91.2.109.19988. [DOI] [Google Scholar]
- 9.Mirzadeh S, Kumar K, Gansow OA (1993) The chemical fate 212Bi-DOTA formed by β-decay of 212Pb(DOTA)2− complex. Radiochim Acta 60:1–10
- 10.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma DS, Abdulla A, Brechbiel MW. Alpha-particle radioimmunotherapy of disseminated peritoneal disease using a Pb-212-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm. 2005;20:557–568. doi: 10.1089/cbr.2005.20.557. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Gallazzi F, Quinn T, Deutschera S. Pb-212-labeled α-MSH analogue modified with a nuclear localization sequence for melanoma targeting. Nucl Med Biol. 2010;37:693. doi: 10.1016/j.nucmedbio.2010.04.064. [DOI] [Google Scholar]
- 12.Milenic DE, Garmestani K, Brady ED, Baidoo KE, Albert PS, Wong K, Flynn J, Brechbiel MW. Multimodality therapy: potentiation of high-LET radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin Cancer Res. 2008;14:5108–5115. doi: 10.1158/1078-0432.CCR-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman T, Quinn TP. Melanoma therapy via peptide targeted alpha-radiation. Clin Cancer Res. 2005;11:5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 14.Fu-Min Su, Beaumier P, Axworthy D, Atcher R, Fritzberg A. Pretargeted radioimmunotherapy in tumored mice using an in vivo 212Pb/212Bi generator. Nucl Med Biol. 2005;32:741–747. doi: 10.1016/j.nucmedbio.2005.06.009. [DOI] [PubMed] [Google Scholar]