Highlight
Increases in seed yield must occur without impacting seed or plant quality. Specific CKX gene family members were identified as targets for breeders along with transporter and metabolic genes as quality markers.
Key words: Cell division, cytokinin, cytokinin oxidase/dehydrogenase, food security, forage, homoeologues, isopentenyl transferase, seed, sink, source, transcriptome, yield.
Abstract
Forage brassica (Brassica napus cv. Greenland) is bred for vegetative growth and biomass production, while its seed yield remains to be improved for seed producers without affecting forage yield and quality. Cytokinins affect seed yield by influencing flower, silique and seed number, and seed size. To identify specific cytokinin gene family members as targets for breeding, as well as genes associated with yield and/or quality, a B. napus transcriptome was obtained from a mixed sample including leaves, flower buds and siliques of various stages. Gene families for cytokinin biosynthesis (BnIPT1, 2, 3, 5, 7, 8 and 9), cytokinin degradation (BnCKX1 to BnCKX7), cell wall invertase (BnCWINV1 to BnCWINV6), sugar transporter (BnSUT1 to BnSUT6) and amino acid permease (BnAAP1 to BnAAP8) were identified. As B. napus is tetraploid, homoeologues of each gene family member were sought. Using multiple alignments and phylogenetic analysis, the parental genomes of the two B. napus homoeologues could be differentiated. RT-qPCR was then used to determine the expression of gene family members and their homoeologues in leaves, flowers, siliques and seeds of different developmental stages. The expression analysis showed both temporal and organ-specific expression profiles among members of these multi-gene families. Several pairs of homoeologues showed differential expression, both in terms of level of expression and differences in temporal or organ-specificity. BnCKX2 and 4 were identified as targets for TILLING, EcoTILLING and MAS.
Introduction
Food security is reliant on security of seed production. Seed of many different species will need to be produced in increasing amounts to meet the demands for direct human and animal consumption, for the production of forage for animals, and also for the production of vegetables. A step change is needed in plant breeding to meet the demand for seed under a changing environment of reduced inputs (water, fertilizer, herbicides and pesticides), and a changing global climate.
The Brassicaceae represents the most diverse family used as a vegetable for human consumption and as forage for animal grazing, and consistency of yield and quality of seed are traits not yet optimized by the Brassica seed industry in New Zealand (Hampton et al., 2012). In this research, we have focused on Brassica napus cv. Greenland, a forage brassica used for winter grazing of cattle in New Zealand. Forage brassica is purposely bred for optimized vegetative growth and biomass production, while its seed yield remains to be improved for seed producers without affecting forage yield and quality.
The cytokinins have been implicated as a limiting factor in the establishment of sink number and sink size in legumes, cereals and Arabidopsis. Ectopic expression of an IPT gene has been shown to increase seed yield in a variety of plants (see review by Guo and Gan, 2014). Notwithstanding the implication that cytokinin is limiting yield, high levels of endogenous cytokinins are routinely detected in developing fruits and seeds. However, the peak of cytokinin is often transitory. For instance, the amounts of endogenous cytokinin in developing cereal grains have been shown to change rapidly (Morris et al., 1993). In wheat, the endogenous changes are compressed into just a few days after anthesis (Jameson et al., 1982), at the time when sink size is established during the phase of free nuclear divisions and cellularization of the endosperm (Bennett et al., 1973). The rapid changes in endogenous levels of cytokinins in wheat have been linked to the expression of specific members of the cytokinin biosynthesis (IPT), degradation (CKX), O-glucosylation and ß-glucosidase gene families (Song et al., 2012).
Similarly in maize, Brugière et al. (2008) suggested that the coincidence of the expression of ZmIPT2, activity of its enzyme, peak endogenous cytokinin levels and the phase of cell division is indicative of cytokinin as a necessary component of endosperm cell division. However, they also showed, using immunolocalization, ZmIPT protein not only in the endosperm/embryo during the cell division phase, but also located in the endosperm transfer cell layer both during the phase of cell division and later in seed development.
In legumes, cytokinin is limiting even to pod set. In lupin, where the majority of flowers will abort, application of cytokinin prevented this abortion (Aitkins and Pigeaire, 1993). Emery et al. (2000) correlated cytokinin form with flower/ovary abortion, and increased pod set in plants by ectopically expressing an IPT gene (Atkins et al., 2011). Moreover, while xylem-supplied cytokinin does reach the pod wall of lupins (Jameson et al., 1987), little of the translocated cytokinin crossed the apoplastic space between the seed coat and embryo (Singh et al., 1988; Letham, 1994), whereas adenosine did (Noodén and Letham, 1984). Detailed GC-MS data on developing white lupin showed a high, transient peak of cytokinin in the liquid endosperm of developing seeds (Emery et al., 2000). The conclusions from work on legumes have been that maternally supplied cytokinin via xylem and/or phloem is limiting to pod and seed set (Emery et al., 2000), but that the developing embryo is dependent on cytokinin biosynthesis in the filial tissues (Singh et al., 1988; Emery et al., 2000). In Arabidopsis, Day et al. (2008) showed elevated levels of core cell cycle genes and genes involved in cytokinin biosynthesis (IPT8) and signalling in the syncytial endosperm, and suggested that the chalazal endosperm was directing syncytial endosperm development via cytokinin signalling (Day et al., 2008).
The seminal work of Ashikari et al. (2005) on yield of rice has shown the possibility that the cytokinins could be a direct target for plant breeders. They showed that a QTL for increased grain number was associated with the gene for cytokinin oxidase/dehydrogenase (CKX), which codes for the enzyme that inactivates cytokinin. Indeed, Ashikari et al. (2005) showed that the rice cultivars with increased seed number had mutated forms of a specific OsCKX gene family member—OsCKX2. Seed number was increased in lines in which a mutation for reduced activity or a null mutation in OsCKX2 had occurred (Ashikari et al., 2005). Additionally, down-regulation of HvCKX1 or HvCKX9 by RNA interference in barley led to both increased seed number and seed size (Zalewski et al., 2010, 2012, 2014). Moreover, the significant yield increase caused by the down-regulation of HvCKX1, which was shown to be the more highly expressed gene family member during kernel development, was inherited across four generations (Zalewski et al., 2014). Recently, Zhang et al. (2012) suggested that variants of TaCKX6-D1, a wheat orthologue of rice OsCKX2, were significantly associated with grain weight but not grain number. In Eudicots, a double ckx mutant of Arabidopsis showed a 55% increase in yield (Bartrina et al., 2011) leading to the suggestion that the role of CKX genes in determining seed yield has been evolutionarily conserved and is of functional significance for all or most flowering plants (Bartrina et al., 2011).
The original work of Mothes and Engelbrecht (1963), in which cytokinin applied to a leaf was shown to attract nutrients to the site of application, established a role for cytokinin in enhancing sink activity independent of a role in cell division. Subsequently, cross-talk between cytokinin and genes involved in sink activity was shown by Ehneß and Roitsch (1997) using cultured cells in vitro, where cytokinin enhanced the simultaneous expression of a cell wall invertase (CWINV) (which irreversibly catalyses the breakdown of sucrose to fructose and glucose) and a hexose transporter gene in Chenopodium rubrum (Amaranthaceae). Subsequently, the induction of CWINV as an essential component of cytokinin-induced delay of senescence was also shown (Balibrea et al., 2004) with Hwang et al. (2012) suggesting that cytokinin-mediated senescence delay is caused by an increased sink activity via the direct activation of CWINV activity.
Significantly, a gene coding for a CWINV has been shown to be necessary for normal seed development. Research has shown that the maize cell wall invertase-deficient mutant, miniature1 (mn1), has impaired development of the endosperm and pedicel cells (Cheng et al., 1996; Rijavic et al., 2009) and that seeds of the mutant have altered hormone levels (LeClere et al., 2010; Rijavic et al., 2009). Rijavic et al. (2009) suggested that the effect of cytokinin on seed size is both direct, through an effect on cell cycle-related genes, and indirect, through the action of sugar signalling. They also suggested that cross-talk between cytokinin and CWINV might enhance phloem unloading and sugar import into the maize endosperm (Rijavic et al., 2009).
Wang and Ruan (2012) showed that CWINV in both cotton seeds and Arabidopsis seeds was more abundant in the chalazal endosperm undergoing endoreduplication but much weaker in cellularizing endosperm. They suggest that CWINV may be promoting nuclear division probably through sugar signalling and that, by cross-talk between hormones, invertase-mediated sugar signalling may regulate the expression of cell cycle control genes (Wang and Ruan, 2012). Both sugars and cytokinin have been directly implicated in control of the plant cell cycle. Sugar availability has been shown to play a major role during the G1 phase of the cell cycle by controlling the expression of D-type cyclins. Both AtCYCD2 and D3 responded to sucrose availability (Riou-Khamlichi et al., 1999, 2000). While CYCD2 expression was independent of hormones, a continuing response of CYCD3 required both sucrose and cytokinin. Both the sucrose and the cytokinin responses were direct and did not require protein synthesis (Riou-Khamlichi et al., 2000).
If cytokinin levels are increased in seeds of forage brassica by selecting for reduced activity of CKX, a concomitant increase in CWINV activity might be anticipated. However, if seed yield is to be increased and quality maintained in both seed and forage, then a co-ordinated increase in activity of transporters is also required to supply the carbon and nitrogen backbones for growth and metabolism, and eventually storage. Assimilate translocation from source to sink tissues depends on transporter genes including sucrose transporters (SUTs). Indeed, Li et al. (2011) suggested that assimilate translocation is the most critical limiting factor for seed yield in Brassica, and showed that BnSUT1 was associated with a QTL for yield in rapeseed (Li et al., 2007).
In addition to a supply of carbon, developing pods and seeds have competitive requirements for nitrogen, initially for metabolic enzymes in the expanding pod and developing seeds and subsequently for the formation of seed storage proteins. Tegeder (2014) suggests that sink development and sink nitrogen levels depend on the amounts of nitrogen (and carbon) that are transported in the phloem. Transport of nitrogen from the leaves to sink tissues is in the form of amino acids in non-leguminous plants and involves the activity of amino acid permeases (AAPs) (Tegeder, 2014).
In this work we had two key aims. The first was to identify CKX gene family members expressing specifically in developing siliques and seeds, as a preliminary to identifying mutant CKX forms for breeding. From a breeding perspective utilizing TILLING and EcoTILLING strategies, detection of loss of function mutants of CKX should be easier than detection of mutants with increased expression of IPT. The second aim was to obtain an overall picture of the expression of IPT, CKX, SUT, INV and AAP gene family members during development of forage brassica plants. A B. napus transcriptome was obtained from a mixed sample of plant material including leaves, flower buds and siliques of various stages. This was mined for the gene families of interest. Subsequently, we determined, using RT-qPCR, the expression of the BnIPT and BnCKX gene family members as well as of genes likely to be involved in source-sink relationships during leaf, flower and silique development in forage brassica. As B. napus is tetraploid, homoeologues of each gene family member were sought.
Materials and methods
Plant material
Forage brassica, Brassica napus cv. Greenland, was sourced from a commercial seed field in Tinwald, South Canterbury, New Zealand (December 2010). Tissues at three stages of leaf and flower and seven stages of silique development were collected from six plants at similar developmental and physiological status, and were immediately placed into liquid nitrogen and then stored at −80°C.
Leaf 1 were identified as being small, very young leaves near the top of the extending plant. Leaf 2 were larger, fully expanded functional leaves nearer to the middle of the plant. Leaf 3 were senescing leaves nearest the base of the plant. Flower 1 were young, closed flower buds of 3–4mm in length, with no yellow petals visible. Flower 2 were still just closed flower buds of 6–8mm in length, with the yellow petals tips visible. Flower 3 were newly opened flowers ready to be pollinated. The seven stages of silique were taken from two days after pollination (DAP) through to fully expanded siliques, with lengths of P1=15–20mm, P2=25–30mm, P3=35–45mm, P4=50–55mm, P5=60–65mm, P6=70–75mm, and P7=70–80mm.
RNA isolation and cDNA synthesis
Total RNA was extracted from up to 100mg of frozen samples using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions and immediately stored at 20°C. The integrity and quality of isolated RNA was assessed by running 1 μl samples on a 1% (w/v) agarose gel. The concentration and purity of the total RNA was assessed using a Nanodrop™ spectrophotometer.
Extracted RNA was converted to cDNA through reverse transcription. Approximately 1 µg of total RNA, 50U Expand Reverse Transcriptase (Roche, Mannheim, Germany), 50 pmol oligo (dT) primers and 100 pmol random hexamer (pdN6) primers were used in a 20 μl reaction. The final reaction mix was incubated at 42°C for 2h, and then at 70°C for 15min to deactivate the enzyme. The cDNA was diluted 10-fold with nanopure water and stored at −20°C.
Gene isolation and sequence analysis
Sequences of family members from candidate genes of interest and their homoeologues were isolated from RNA-Seq transcriptome data. An RNA pool of combined RNA samples extracted separately from multiple developmental stages of leaves, flowers and siliques was used to construct the cDNA library, which was then sequenced using an Illumina HiSeq2000 genome analyzer at the Beijing Genomic Institute (BGI) customer service.
Orthologue sequences of IPT, CKX, SUT, CWINV and AAP from Arabidopsis and Brassica species available in the GenBank database were used as query sequences to BLAST search our B. napus transcriptome data using prfectBLAST 2.0. The putative sequences were verified via BLAST searching the GenBank database and multiple sequence alignment with representative orthologue sequences in closely related species.
The newly identified sequences and their orthologues in Arabidopsis and other Brassica species were used to construct Neighbor-Joining (NJ) phylogenetic trees using ClustalX software with 1000 bootstrap replicates. Each tree was rooted with an out group orthologue sequence. The GenBank accession numbers for the nucleotide sequences are listed in Supplementary Table S1.
Quantitative reverse transcription polymerase chain reaction
Quantitative RT-PCR was used to measure relative gene expression of the individual family members across the various plant tissues as they developed. Specific PCR primers were designed for each family member of the five genes of interest and their homoeologues within the subgenomes A and C using Primer Premier 6.0. In most cases, four primer pairs were designed and the best one was chosen for gene expression analysis after PCR testing (Supplementary Table S2). A volume of 20 µl was used for all qPCR reactions in a Rotor-Gene Q (Qiagen) real-time PCR instrument, using home-made SYBR Green master mix or a KAPA SYBR® FAST qPCR Kit (Kapa Biosystems, Boston, USA). PCR products for each target sequence were Sanger sequenced to confirm homology to genes already identified in various gene databases (e.g. NCBI). PCR systems were then optimized and the amplification efficiency determined. Two reference genes, elongation factor gene EF and GAPDH, were used as internal controls. For each cDNA sample, the Ct values of each target gene were corrected using a correction factor (CF) calculated as described previously (Song et al., 2012). Three technical replicates for each of two biological replicates were carried out for each sample set. The expression values relative to EF and GAPDH were calculated based on the methods of Pfaffl (2001) and modified as described in Song et al. (2012). Data for the second biological replicate are shown in Supplementary Table S3.
Results
Silique and seed development
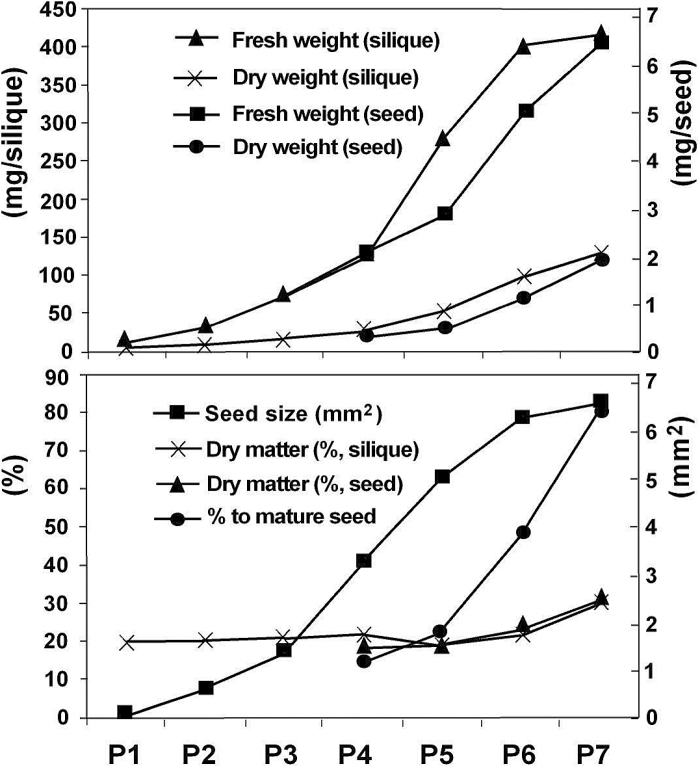
Siliques were collected at different stages of maturity (based on length), from secondary or tertiary branches with the most mature siliques collected from the base and the least developed from the upper branches of indeterminate forage brassica plants. Siliques were separated into size classes with the largest included in P6 and P7 samples. Simultaneous with silique elongation, seed size was also increasing to a maximum in P6 and P7 siliques (Fig. 1). Immature seeds observed in P1–P3 siliques were mostly seed coat and liquid endosperm. Seeds from P1 and P2 siliques were transparent, seeds from P3 siliques were yellowish, those from P4 slightly green, and those from P5–P7 were bright green. Embryo dissection showed that the embryos were at heart to torpedo stages in P4 siliques. Early linear cotyledon stage to curled cotyledon stage embryos were found in P5 siliques, and well formed green cotyledons occupied the whole of the seed from P6 siliques. Seeds from P4–P7 were able to be dissected from the siliques for gene expression analysis.
Fig. 1.
Description of developmental stages of B. napus silique and seed samples used for gene expression analysis. Silique length (mm): P1, 15–20; P2, 25–30; P3, 35–45; P4, 50–55; P5, 60–65; P6, 70–75; P7, 70–80. Embryo developmental stages: P4, heart to torpedo; P5, linear to curled cotyledons; P6, green cotyledons.
Fresh weight and dry weight of siliques increased noticeably from P4. Dry weight of the seeds was assessed against a commercial seed lot: seeds removed from P6 siliques had accumulated approximately 50% of final dry matter, and in P7 siliques up to 80%. While the rate of fresh weight increase of siliques decreased between P6 and P7, the fresh weight of seeds in P7 siliques was still increasing, indicating that the seeds had not reached the stage of desiccation (Fig. 1).
Transcriptome analysis
The RNA-Seq from a cDNA library constructed using pooled RNA samples from leaves, flowers and siliques generated a transcriptome of 4.46 G clean data and 31 552 000 of 50-bp pair-end reads, which were assembled into 466 800 contigs. In total, 43.350 unigene sequences were obtained, with a median length of 598bp. This suggests that the transcriptome data represented adequate sequencing depth and genome coverage to meet the requirement of identifying candidate gene sequences expressed in the tissue samples in the current study.
Sequence and phylogeny analysis
By using all of the annotated family members of IPT, CKX, SUT, CWINV and AAP in Arabidopsis and Brassica species available in the GenBank database including a large number of those uploaded after September 2014 (Brassica rapa Annotation Release 100) as query sequences to BLAST-search our B. napus transcriptome data, we identified most of the putative IPT, CKX, SUT, CWINV and AAP orthologous sequences in forage brassica. Results of sequence verification via BLAST-searching the GenBank database showed that most of the identified sequences were confirmed to be the target gene sequences. Multiple sequence alignments and phylogenetic analyses showed that two or three sequences with very high similarity were identified for the majority of the members of the multigene families. Up to four sequences were identified for some family members (Supplementary Table S4). In the case where three or four sequences were identified in the B. napus genome, one of the sequences shared high similarity with the orthologue in the C genome of B. oleraceae, while each of the others was more similar to an orthologue in the A genome of B. rapa.
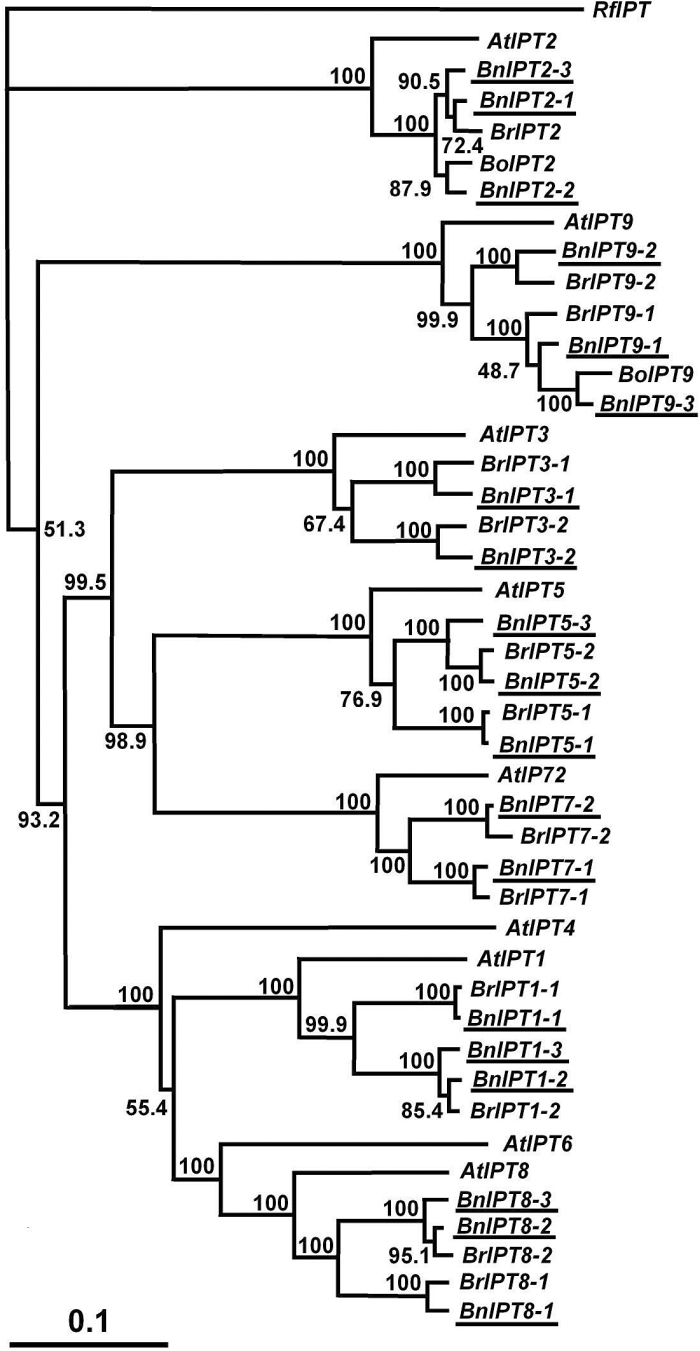
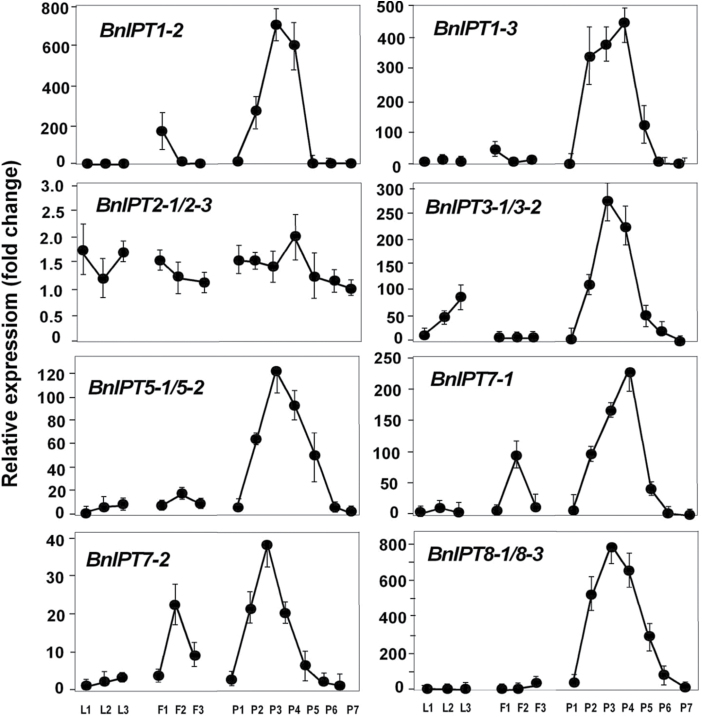
Nineteen sequences for seven of the nine IPT gene family members in B. napus were identified, with two distinct sequences for BnIPT5 and BnIPT7, and three each for IPT1, 2, 3, 8 and 9. Twelve of the sequences were highly similar to their counterparts in the A genome, two to the C genome, and the remaining five may align with the C genome but these sequences are not yet available in GenBank. However, sequences for BnIPT4 and BnIPT6 were not found. In addition to the two tRNA-associated IPT clades, orthologues of IPT3, 5 and 7 formed a distinct clade while those of IPT1 and 8 formed another clade (Fig. 2).
Fig. 2.
Neighbor-Joining phylogenetic tree for IPT gene sequences in Brassica napus and closely related species. At, Arabidopsis thaliana; Bn, B. napus; Bo, B. oleraceae; Br, B. rapa. The tree was rooted using Rhodococcus fascians IPT gene sequence. Node values are percentages of bootstraps generated with 1000 bootstrap replicates. Sequences identified in this study are underlined.
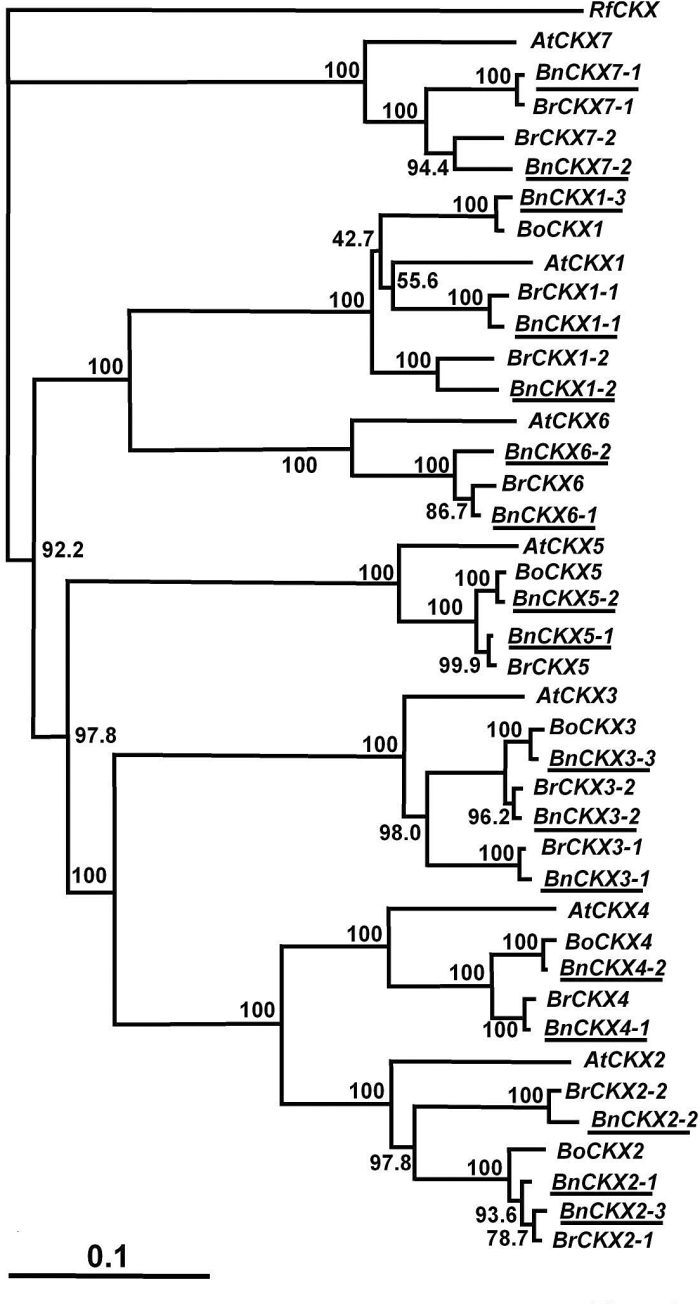
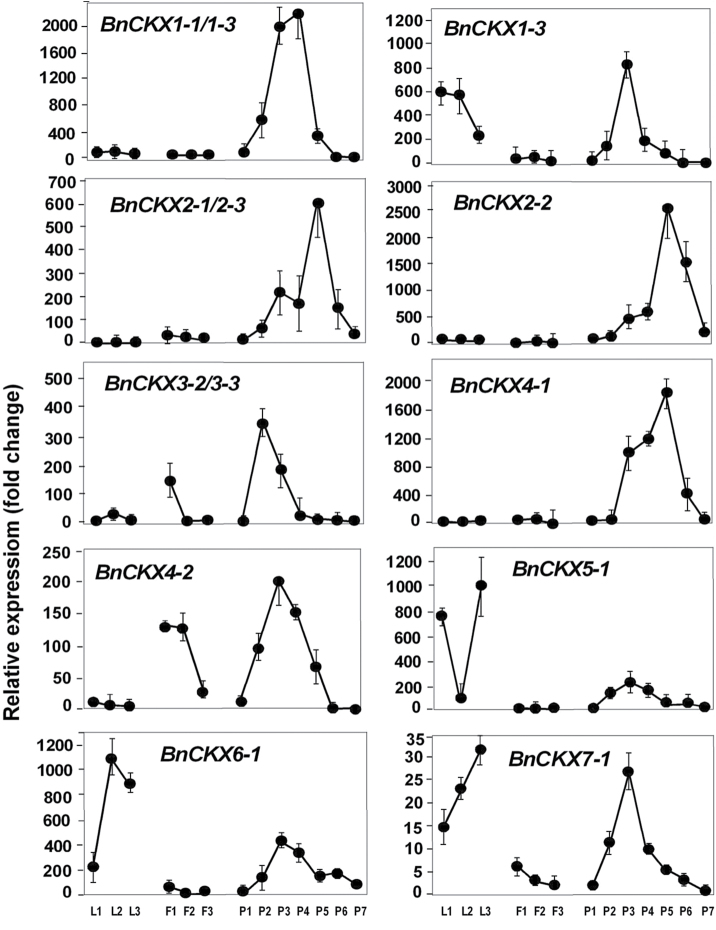
Sequences for all the seven CKX gene family members in B. napus were identified. Two distinct sequences for BnCKX4, 5, 6 and 7, and three for BnCKX1, 2 and 3 were identified (Supplementary Table S4) and allocated to distinct sub-clades with their orthologues from Arabidopsis, B. rapa and B. oleraceae (Fig. 3).
Fig. 3.
Neighbor-Joining phylogenetic tree for CKX family members in B. napus and closely related species. The tree was rooted using Rhodococcus fascians CKX gene sequence. See Fig. 2 for other information.
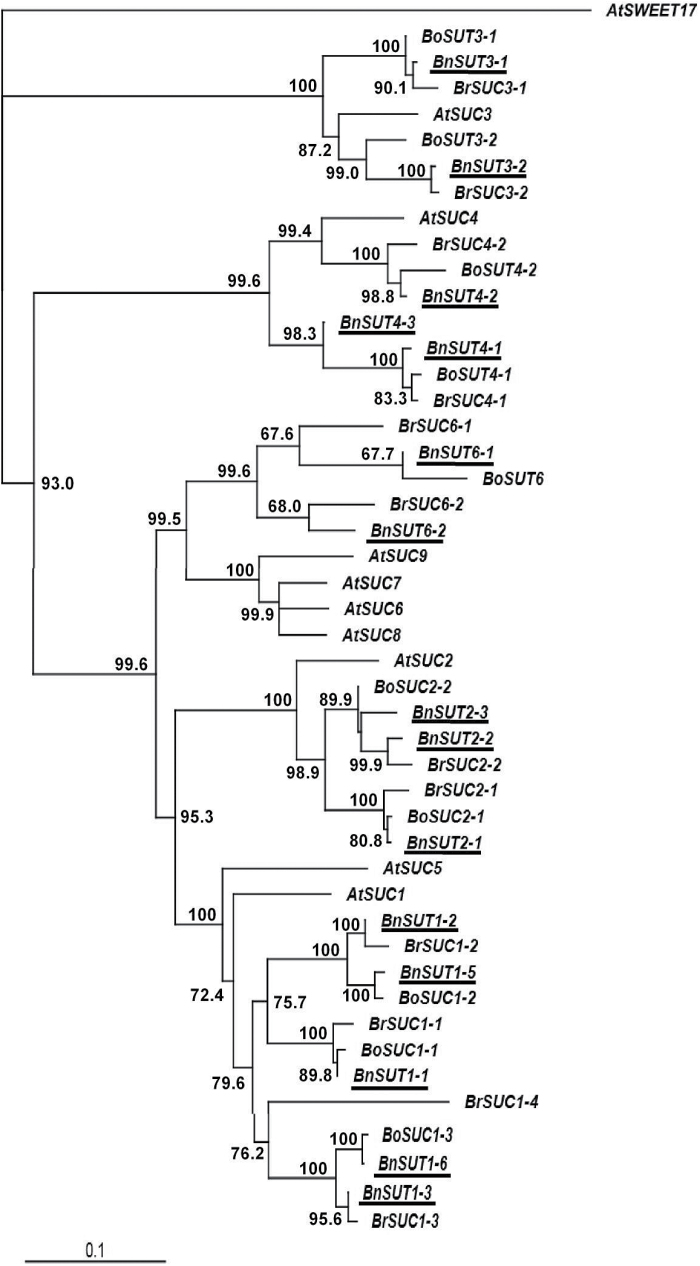
Sequences of SUT gene family members were identified from each of the three clades to which Arabidopsis and B. rapa SUTs are allocated. Duplicate and triplicate sequences were identified (Supplementary Table S4), which grouped together with their orthologues from B. rapa or B. oleraceae (Fig. 4). No sequences of the Arabidopsis SUC7, 8 and 9 orthologues were identified in our transcriptome data, or for B. rapa in the current publically available databases.
Fig. 4.
Neighbor-Joining phylogenetic tree for sucrose transporter (SUT) gene sequences in Brassica napus and closely related species. The tree was rooted using maize SUT1 gene sequence. See Fig. 2 for other information.
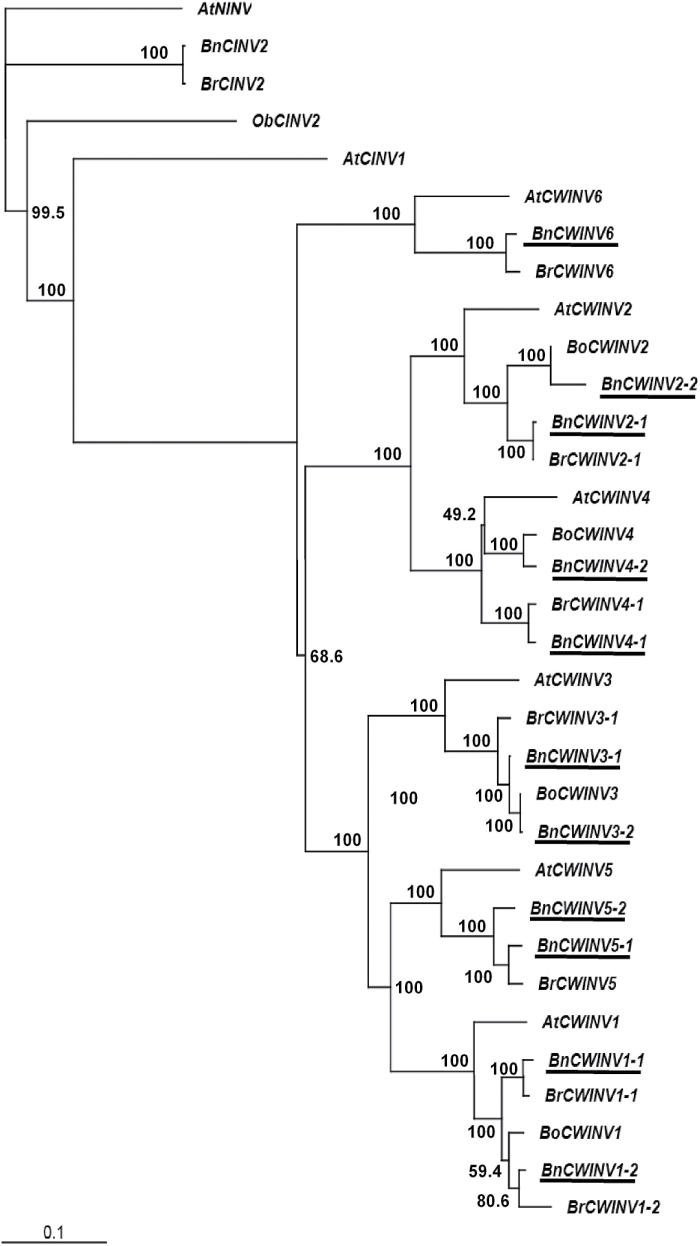
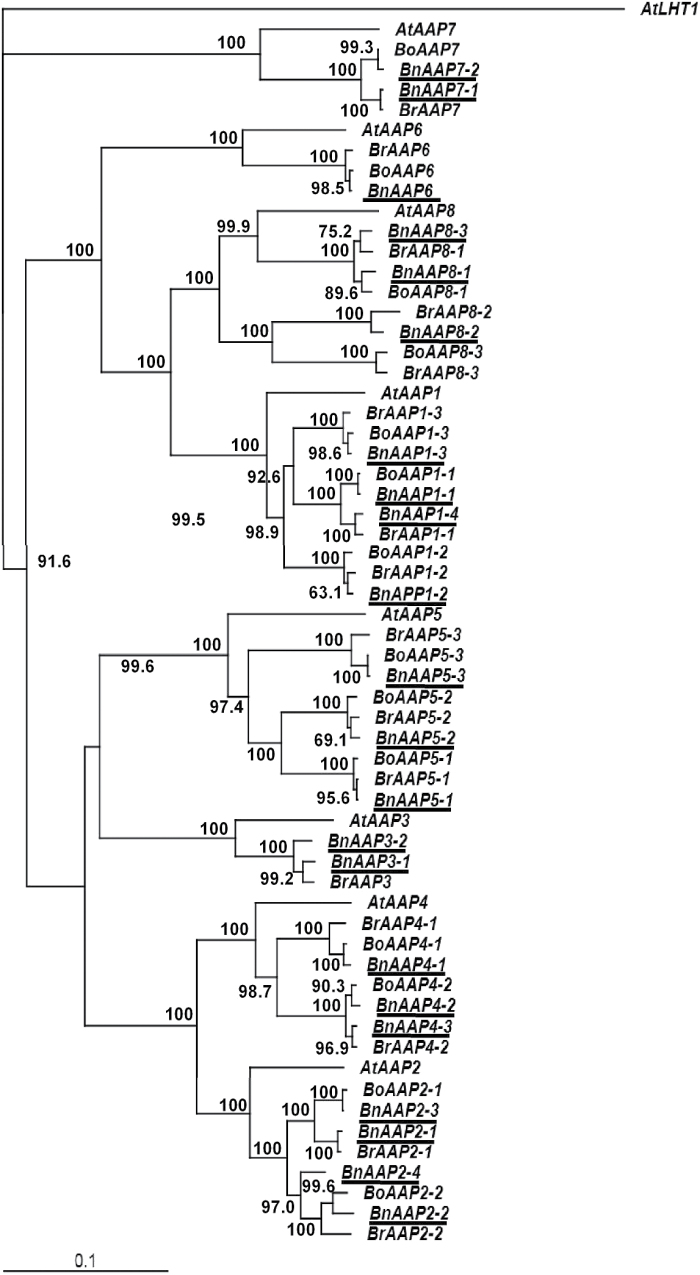
Six BnCWINV members were identified (Fig 5, Supplementary Table S4), with one of the paired sequences sharing high similarity with its orthologue in B. rapa and the other with B. oleraceae in the case of CWINV2, 3, and 4 (Fig. 5). Multiple sequences were identified for most of the AAP family members: four for BnAAP1 and 2, three for BnAAP4, 5 and 8 but only one for BnAAP6 (Supplementary Table S4). Each sequence allocated well to sequences from either B. rapa or B. oleraceae (Fig. 6).
Fig. 5.
Neighbor-Joining phylogenetic tree for cell wall invertase (CWINV) gene family members in B. napus and closely related species. The tree was rooted using an Arabidopsis alkaline/neutral invertase AtNINV gene sequence. See Fig. 2 for other information.
Fig. 6.
Neighbor-Joining phylogenetic tree for amino acid permease (AAP) gene family members in B. napus and closely related species. The tree was rooted using a closely related lysine histidine transporter 1(AtLHT1) gene sequence. See Fig. 2 for other information.
Expression in leaves
Analysis focused on leaves as expanding sink, mature source and senescing leaves. BnIPT expression in leaves was low, particularly relative to developing siliques. Interestingly, BnIPT3 showed expression in senescing leaves (Fig. 7). Strong differences were seen in expression between BnCKX gene family members in the leaves (Fig. 8). Whereas BnCKX1-3 showed a decreasing expression as leaves aged, BnCKX5-1 expression was high in sink leaves, reduced in source leaves, and high again in senescing leaves. In contrast, BnCKX6-1 showed strong expression in both mature and senescing leaves. BnCKX2-1, 2-2, and 4-1 showed no expression in leaves at any of the stages examined, and expression of 4-2 and 7-1 was minimal (Fig. 8).
Fig. 7.
Expression profiles of selected isopentenyl transferase (IPT) gene family members in Brassica napus. Y-axis values are relative expression (fold-changes) averaged from 3–4 technical replicates calculated relative to the lowest expressed sample of the same sample set, and were corrected using the geometric means of reference genes α-EF1 and GAPDH. Error bars represent standard deviation. L1, young expanding leaves; L2, expanded leaves; L3, senescing leaves; F1, flower buds 3–4mm; F2, flower buds 6–8mm; F3, open flowers; P1–P7, siliques at different developmental stages, from 15–20mm to full size of 70–80mm.
Fig. 8.
Expression profiles of selected cytokinin oxidase/dehydrogenase (CKX) gene family members in Brassica napus. See Fig. 7 for legend.
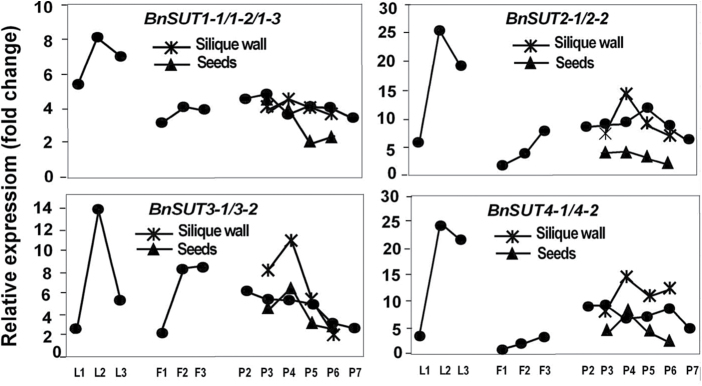
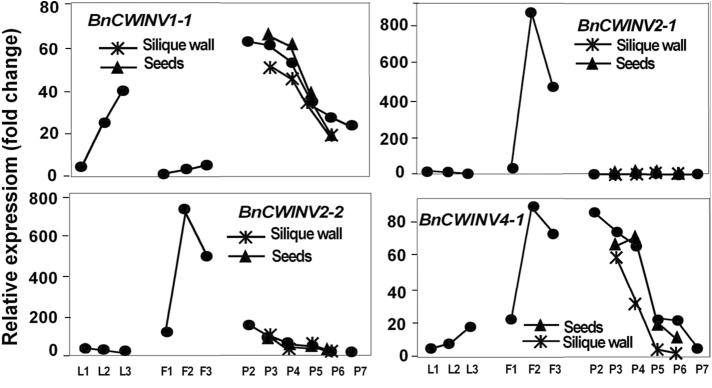
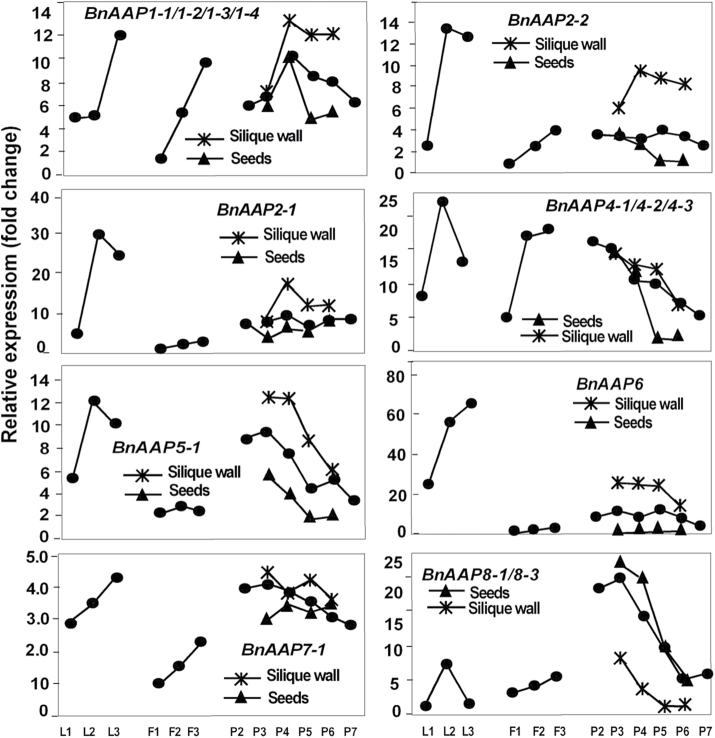
All four BnSUT gene family members showed the same pattern of expression in leaves: relatively low in the young expanding leaves, at a peak in mature leaves, and decreased expression in the senescing leaves (Fig. 9). Differential expression among the cell wall invertase gene family members was evident with increasing expression during leaf development being shown by BnCWINV1-1, and to a lesser extent by 4-1. BnCWINV2-1 and 2-2 showed little expression in leaves (Fig. 10). Amino acid permease expression was relatively low in expanding leaves but was greater in most cases in the mature source leaves (Fig. 11). The gene family members showed variable expression in senescing leaves with the expression of BnAAP1, 6 and 7-1 continuing to increase and that of 2-1, 4, 5-1, and 8 decreasing. Greatest expression overall was shown by BnAAP6.
Fig. 9.
Expression profiles of selected sucrose transporter (SUT) gene family members in Brassica napus. See Fig. 7 for legend.
Fig. 10.
Expression profiles of selected cell wall invertase (CWINV) gene family members in Brassica napus. See Fig. 7 for legend.
Fig. 11.
Expression profiles of selected amino acid permease (AAP) gene family members in Brassica napus. See Fig. 7 for legend.
Expression in flowers
Variation between the BnIPT gene family members was evident with little or no expression in flower buds or flowers detected for BnIPT3, 5 or 8. BnIPT1-2 and 1–3 showed expression in the smallest flower buds whereas BnIPT7-1 and 7-2 showed peaks of expression in the medium-sized flower buds (Fig. 7). Expression of BnCKX gene family members varied, with little or no expression detectable for CKX1-1, 1–3, 2-1/ 2–3, 2-2, 5-1, 6-1 or 7-1. Both 3-2/3-3 and 4-2 showed elevated expression in small buds, and this was maintained by BnCKX4-2 as buds expanded. Overall there was minimal expression of BnCKX in open flowers (Fig. 8).
As flowers developed there was increasing expression of the BnSUT gene family members (Fig. 9). Similarly, the expression of most BnAAP gene family members also increased (Fig. 11). Relative to their expression in other plant parts, both BnINV2-1 and 2-2 were very strongly expressed as flowers developed with peak expression in the larger buds. BnINV4-1 showed a similar pattern, whereas 1-1 was only weakly expressed (Fig. 10).
Expression in siliques, silique walls and seeds
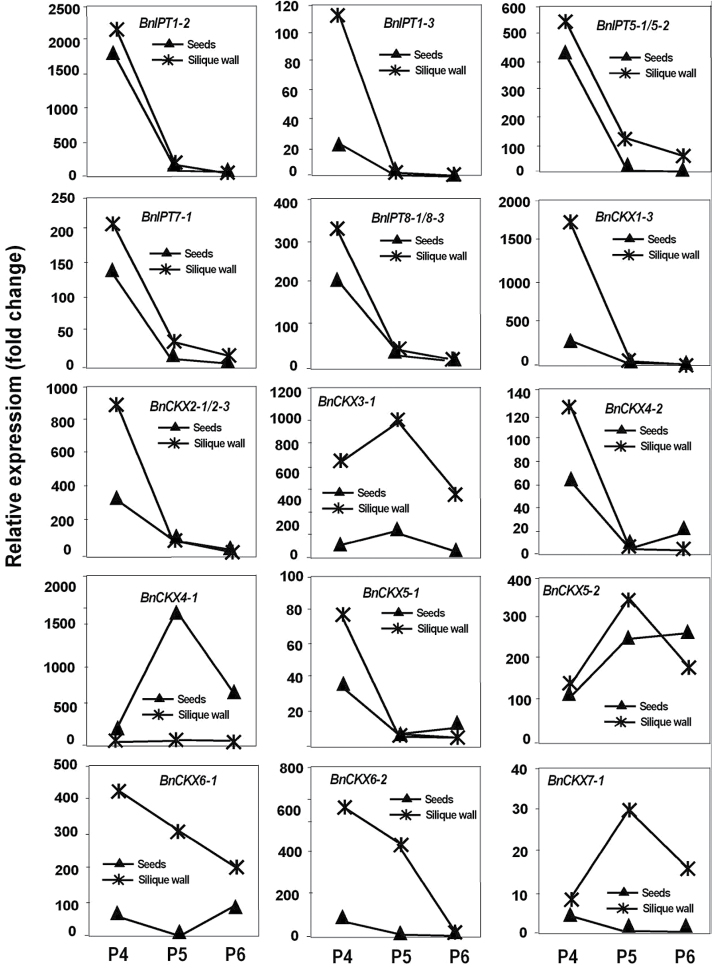
BnIPT expression rose sharply in P2 siliques, reaching a peak either in P3 or P4 siliques (Fig. 7). BnIPT1-2 and 1–3, and BnIPT8 were the most abundantly expressed gene family members. BnIPT2 also showed a small change in expression. Most BnIPT gene expression had decreased substantially in P5 siliques. Expression of most BnCKX family members showed significant changes during silique development: CKX3-2/3-3 peaked at P2; CKX1-3, 4-2, 5-1, 6-1 and 7-1 peaked at P3; BnCKX1-1/1–3 peaked around P3/P4. BnCKX2-2, CKX2-1/2–3, and CKX4-1 peaked at P5. BnCKX2-2 showed the strongest expression and BnCKX7-1 showed the weakest (Fig. 8).
It was difficult to cleanly separate seed at the early stages of silique development but, even with some degree of cross contamination allowed for, it is clear that BnIPT expression was decreasing rapidly in both siliques and seeds between P4 and P5 (Fig. 12). Much of the BnCKX expression was located in the silique walls with the exception of BnCKX4-1 where peak expression occurred at P5 and was located in the seed.
Fig. 12.
Expression profiles of selected IPT and CKX gene family members in seeds and silique walls of Brassica napus. Y-axis values are fold-changes averaged from 3–4 technical replicates. P4, silique length 50–55mm; P5, silique length 60–65mm; P6, silique length 70–75mm.
Transcript expression patterns of the four BnSUT family members were not strongly differentiated in the siliques and seeds; expression generally decreased after P4 (Fig. 9). Compared to flowers, the transcripts of BnCWINV were fewer, with both BnCWINV1-1 and 4-1 reducing sharply during development (Fig. 10). Strong differential expression patterns were shown by the BnAAP family members. BnAAP4 and BnAAP8 were the most strongly expressed in the developing seeds and transcript levels decreased sharply as siliques expanded and seeds developed (Fig. 11).
Genomic contribution
The contribution of the B. napus genes derived from the A genome of B. rapa and the C genome of B. oleraceae differed quantitatively and in some cases were also differentially expressed on an organ-specific basis. However, no fixed pattern of this genome-specific expression was observed. For example, there was a marked difference in tissue-specific expression of BnCKX4-1 and 4-2, with 4-1 (A genome) very strongly expressed in developing seeds, and 4-2 (C genome) strongly expressed in flower buds. BnCKX5-2 (C genome) was more strongly expressed in silique walls and seeds compared to 5-1 (A genome), as well as developmentally later. Both the BnCWINV gene family members expressing in siliques and seeds (BnCWINV1-1 and BnCWINV4-1) derived from the A genome. In contrast, the two homoeologues of BnCWINV2, which were highly expressed in developing flowers but which had low expression in leaves, siliques and seeds, derived from different genomes: BnCWINV2-1 derived from the A genome whereas the BnCWINV-2 derived from the C genome.
Discussion
Phylogenetic and genomic analyses reveal both amplification and loss of gene family members
Whole-genome duplication followed by functional divergence in the event of polyploidization is thought to be a prominent evolutionary force in eukaryotes and a major contributor to evolutionary novelties (Dun et al., 2014). The Brassica genomes have undergone a whole genome triplication after speciation from Arabidopsis (Lysak et al., 2005), leading to significant expansion of gene numbers. In general, for each ancestral gene in Arabidopsis, three syntenic copies could be identified in diploid Brassica species such as B. rapa (Wang et al., 2011; Cheng et al., 2013; Dun et al., 2014). However, due to genome shrinkage and loss of function of some genes, the triplicated B. rapa genome contains only 1.5–2 times the number of genes as in Arabidopsis (Mun et al., 2009; Wang et al., 2011). For instance, Liu et al. (2013) were able to identify only 13 IPTs and 12 CKXs from the Chinese cabbage B. rapa genome draft, instead of 3 × nine IPTs and 3 × seven CKXs, respectively. This is also the case for the gene families investigated in the current study.
Multiple sequence comparison and phylogenetic analysis of IPT, CKX, SUT, CWINV and AAP gene families in forage brassica and its genome contributors revealed that the amplification and/or decrease of gene sequences was family member-specific. In the majority of cases, two sequence variants in B. rapa could be identified from public databases for each of the Arabidopsis gene family members. This is the case for IPT1-3, 5, 7-9, CKX1-3 and 7, SUT2-4, 6, CWINV1, and AAP2 and 4. However, four sequence variants of SUT1 and three sequence variants of AAP1, 5 and 8 were found in B. rapa. These stronger sequence amplifications/retentions may be due to the functional importance of these family members. SUT1 is a dicot specific Type I sucrose transporter which is suggested to be associated with evolution of vascular cambium and phloem transport (Peng et al., 2014). In contrast, sequence variants in some family members may have decreased or even been completely lost during evolution due to loss of function. For instance, no sequences of IPT4 and 6, or of SUT5, 7, 8 and 9 were identified from our transcriptome data, or from the B. rapa genome draft by Li et al. (2013) and Peng et al. (2014).
B. napus is a tetraploid containing A and C genomes derived from B. rapa and B. oleraceae, respectively. While there is limited sequence data available for B. oleraceae, we expected to identify two homoeologues for each sequence variant in B. rapa. However, this was true only for CKX4-6, CWINV2-5, and AAP2, 3 and 7, for which we were able to identify two sequence variants in B. napus for each B. rapa sequence variant. In most cases, fewer than twice the sequence variants were identified. In some extreme scenarios, only one B. napus sequence was identified for each B. rapa sequence. This was the case for IPT3, 7, CKX7, SUT3, 6, CWINV1, 6, and AAP5, 6 and 8. As there is no whole genome sequence available for forage brassica we were not able to determine the actual reason for this sequence decrease. We suggest that this may be due to several factors including the fact that these variants may not have been expressed in our RNA-seq sample, or were not detectable due to low sequencing depth, or, more likely, due to loss of function or complete loss of gene sequences, particularly from B. oleraceae.
Silique and seed development occurred concurrently
Using terminology as defined by Locascio et al. (2014), seeds from P1–P4 siliques were at the first phase of seed development—morphogenesis—with the seed undergoing endosperm development, cell divisions, and embryo and cotyledon differentiation. Seeds from P5–P7 siliques were at the second phase of development—maturation—including embryo growth by cell expansion, absorption of the endosperm by the embryo and dry matter accumulation.
However, the seeds were not fully mature as they had not begun to desiccate (Locascio et al., 2014). As microscopic dissection of seeds from P1, 2 and 3 siliques failed to detect a developing embryo, most metabolic activity in these seeds would be associated with the syncytial cell divisions occurring in the endosperm. As heart and torpedo stage embryos were present in seeds from P4 siliques, it is likely, as in Arabidopsis and oilseed rape, that cellularization of the endosperm was essentially completed in P4 siliques, and that cell proliferation in seeds from P5 siliques was complete as the cotyledons were expanding and the endosperm was being absorbed (Morley-Smith et al., 2008; Dante et al., 2014).
However, competition for resources between the elongating silique and the seeds inside was likely to be occurring as the siliques were steadily elongating during both phases of seed development. As the siliques were green they potentially contributed to carbohydrate metabolism, as shown for B. napus siliques (King et al., 1997), but competition for nitrogen would have been occurring.
Silique walls and seeds are active sites for cytokinin biosynthesis
While it was established early on that filial tissues of the developing seed of legumes were reliant on in situ cytokinin biosynthesis (e.g. Jameson et al., 1987; Singh et al., 1988; Letham 1994), this was less clear for pod walls and seed coats as both were shown to have significant amounts and diversity of cytokinin forms (Davey and van Staden, 1977, 1978, 1979; Summons et al., 1981; Zhang and Letham 1990), and were in receipt of xylem- and/or phloem-supplied cytokinin (Jameson et al., 1987; Singh et al., 1988; Taylor et al., 1990; Zhang and Letham, 1990).
Differential spatio-temporal expression patterns of IPT gene family members have been shown in Arabidopsis (Miyawaki et al., 2004; Belmonte et al., 2013) and other Brassicaceae: in Rapid Cycling B. rapa (RCBr) (O’Keefe et al., 2011) and in Chinese cabbage (B. rapa ssp. perkinensis) (Liu et al., 2013). Using RT-PCR, Miyawaki et al. (2004) showed, between all seven AtIPT gene family members, that cytokinin biosynthesis could occur in most organs of the plant. However, using the GUS reporter gene construct, quite distinct tissue specificity was shown although each family member expressed at more than one location. Liu et al. (2013) showed that while BrIPT1, 3, 5 and 7 were most strongly expressed in the roots, BrIPT8-1 was expressed in immature siliques, and BrIPT8-2 in stamens. This aligns well with Arabidopsis, where AtIPT8 (but also AtIPT4) was shown to be localized to the chalazal region in developing Arabidopsis seeds (Miyawaki et al., 2004; Day et al., 2008; Belmonte et al., 2013), but with significantly decreased expression in seeds at the linear and mature green cotyledonary stage (Belmonte et al. 2013).
In forage brassica BnIPT8 was strongly expressed in the developing siliques, and transcript levels were reduced to low levels during seed maturation. However, unlike Arabidopsis (Miyawaki et al., 2004; Belmonte et al., 2013), neither homoeologue of BnIPT4 was shown to express in any of the tissues analysed, whereas BnIPT1-2/1-3 was as strongly expressed as BnIPT8-1/8-3 in siliques, and more so in developing seeds. AtIPT1 was detected in siliques and in the integument and seed coat of immature Arabidopsis seeds (Miyawaki et al., 2004), but BnIPT1 from Chinese cabbage was not detected in immature siliques (Liu et al., 2013). It is noteworthy that neither IPT4 nor 6 were detected by us, Ando et al. (2005) or Liu et al. (2013) in Chinese cabbage (B. rapa). While AtIPT6 and OsIPT6 are regarded as pseudogenes in some accessions of Arabidopsis and cultivars of rice respectively (Kakimoto et al., 2001), clearly the loss of IPT4 from Brassica sp. indicates that Arabidopsis is not an infallible model for gene expression in the Brassicaceae.
What is clear from forage brassica is that both silique wall and seed are capable of cytokinin biosynthesis, but that this capability has declined in both organs by the end of phase one. In forage brassica, cytokinin biosynthesis is, therefore, associated with the morphogenesis phase of free nuclear divisions, cell division and differentiation. Notable also is that BnIPT expression was not detected in the smallest of the siliques sampled, leaving those dependent on a maternal source of cytokinin, as suggested for white lupin where xylem/phloem accounted for most of the cytokinin supply during early pod set (Emery et al., 2000).
While a degree of tissue specificity was shown for the expressed BnIPT family members, tRNA-associated BnIPT2 was expressed at a low level in the leaves, flower buds, flowers and siliques, but with slightly more transcript measured during the stages of rapid silique and seed development. Based on the fact that the tRNA-associated IPTs are constitutively expressed and the functional similarity between IPT9 and IPT2, BnIPT9 expression in forage brassica was not investigated in this work although two homoeologue gene sequences were identified from our transcriptome data. This is in agreement with Miyawaki et al. (2004), who showed that both AtIPT2 and 9 were expressed constitutively, but more strongly in proliferating tissues (Miyawaki et al., 2004). Liu et al. (2013) also showed low, constitutive expression of both BrIPT2 and 9 in Chinese cabbage.
CKX limits endogenous cytokinin levels during development
Cytokinin oxidases/dehydrogenases (CKX) catalyse the irreversible degradation of the active cytokinins, isopentenyladenine and zeatin, and their ribosides (Werner et al., 2006). Tissue-specific expression has been reported for both Arabidopsis (Werner et al., 2006) and B. rapa (Liu et al., 2013), the latter reporting differential expression of BrCKX gene family members across a variety of tissues, although the peaks of BnCKX activity that were detected in developing siliques and seeds of forage brassica would not have been observed by Liu et al. (2013). While BrCKX2-2 was the most highly expressed in Chinese cabbage but limited predominantly to reproductive tissues, BnCKX2-1 and 2-2 expression in forage brassica was restricted to siliques and seeds.
Hirose et al. (2008) diagrammatically summarized the expression of AtIPT gene family members (from Miyawaki et al., 2004) and AtCKX gene family members (from Werner et al., 2003) and showed that the expression patterns of AtIPT and AtCKX gene family members often overlapped. There are now numerous reports suggesting that whenever the expression of the IPT genes occurs, or the endogenous cytokinin levels are elevated, expression of CKX genes and/or increased CKX activity occurs (O’Keefe et al., 2011 and references therein; Liu et al., 2013). Indeed, there are suggestions that CKX activity acts to limit seed development (Brugière et al., 2003; Li et al., 2013) or has a controlling influence on seed yield (Bartrina et al., 2011). This is a situation reflected in forage brassica, where the expression of many of the BnIPT and BnCKX gene family members appears to be co-ordinately controlled during silique and seed development. The silique- and seed-specific expression of BnCKX2, and that also of BnCKX4, make these gene family members our likely targets for mutant selection and functional MAS, along with the fact that AtCKX2 was identified as an integrator of genetic and epigenetic regulation of endosperm growth (Li et al., 2013).
However, in leaves, biosynthesis of cytokinin appears relatively limited. We show, with the exception of BnIPT3 in mature and senescent leaves, that there is limited BnIPT expression in leaves, as also shown for maize by Vyroubalová et al. (2009) and in Chinese cabbage by Liu et al. (2013). Consequently, a supply of cytokinin from the roots to leaves is implicated (Letham 1994; Hirose et al., 2008) and the strong BnCKX expression detected in sink (BnCKX1-3 and 4-1), source (BnCKX1-1 and 6-1) and senescing forage brassica leaves (BnCKX5-1 and 6-1) can be interpreted as a response to the presence of endogenous cytokinin in developing and mature leaves, and the destruction of cytokinin to enable the final remobilization of resources from senescing leaves to the sinks (Werner et al., 2006).
In forage brassica flowers, as in leaves, there was limited expression of BnIPT, and what there was in the flower buds was matched by BnCKX4-2 expression. Similarly, in Chinese cabbage, while there was limited BnIPT expression in flowers there was detectable BnCKX expression (Liu et al., 2013). This supports the contention that the developing flower and ovule is also dependent on maternally supplied cytokinin, in contrast to the expanding silique and developing seeds, but with CKX providing a moderating influence in all tissues.
Sucrose transporters are active in multiple tissues
SUTs belong to a small gene family and are essential for the export and efficient movement of sucrose from source leaves to sink organs (Peng et al., 2014). They function to load sucrose into the phloem and to unload sucrose into sink tissues such as seeds and flowers (Reinders et al., 2012). Braun et al. (2014) suggested that the function of the plasma membrane-located SUTs is critical in apoplastic loading species. Based on our multiple sequence alignment and phylogenetic analysis, forage brassica BnSUT1, 2 and 3 are most likely plasma membrane-located, and BnSUT4 tonoplast-located (Fig. 4). Li et al. (2011) reported that rapeseed BnSUT1 is associated with a QTL for yield. Spatial and developmental profiling of the rapeseed BnSUT1 showed abundance of transcript in source leaves and stems, and lower levels in reproductive organs. However, in these latter organs, BnSUT1 was more strongly expressed in the pistil and when the siliques were rapidly elongating, reducing to lower levels when the dry weight of the siliques reached a maximum (Li et al., 2013). This pattern is reflected not only by forage brassica BnSUT expression in the developing siliques and seeds but also in the sharp increase in expression as leaves developed from being sinks to being source leaves, and subsequently senescing. The requirement of developing flowers for sucrose is reflected also by an increase in BnSUT expression.
Interestingly, the heat map compiled by Peng et al. (2014) shows high activity for only three of the seven Type I AtSUTs, with AtSUC2 being expressed in both vegetative and reproductive tissues, AtSUC1 similarly but only in tissues in which AtSUC2 was not expressed, and AtSUC9 expression restricted to pollen tubes (in which neither AtSUC2 nor AtSUC1 were expressed). The single Type II and III AtSUTs showed substantially lesser expression. Similarly, in forage brassica the number of expressed Type I SUTs is also limited as only BnSUT1, 2 and 6 sequences were identified from the transcriptome data. However, the Type I, Type II and III BnSUTs showed relatively similar expression patterns to each other, both in terms of organ expression and developmentally.
Cell wall invertase gene family members show strong tissue specificity
Ruan et al. (2010) suggested that invertase has a wide range of regulatory functions in plant growth and development in addition to its major role in primary carbon metabolism, and that CWINV is essential for flower, seed and fruit production and may even be rate-limiting to seed development through its effects on cell division in filial tissue. Wang and Ruan (2012) showed AtCWIN4 to express more strongly than AtCWIN2, and to express both in the syncytial endosperm near the chalazal region as well as in the embryo from globular to torpedo stage. The transition from cell division and expansion to storage activities in seeds is usually associated with a decrease in invertase expression and activity (Wang and Ruan, 2013). The expression profile of forage brassica BnCWINV1 and 4 in seeds is in accord with this. However, the strongest expression of forage brassica BnCWINV was of both homoeologues of BnCWINV2 in developing and open flowers, suggesting that BnCWINV is supplying hexoses, possibly to the developing anthers and ovaries (Ruan et al., 2010) and expanding petals (Bihmidine et al., 2013).
Amino acid permease gene family members are spatio-temporally differentiated
Activity of AAP gene family members has been shown variously in roots, xylem parenchyma, transport phloem and sink tissues including cotyledon and endosperm (Tegeder, 2014). Tegeder (2012) assigned the eight Arabidopsis gene family members to three clusters. AtAAP2, 3, 4 and 5 were all assigned to Cluster 3A, and all are considered to be transporters generally associated with loading into the phloem: AtAAP3 function was restricted to the roots; AtAAP5 was associated with loading into the companion cells of both roots and leaves; AtAAP4 with leaf phloem loading and AtAAP2 with phloem loading along the transport pathway (reviewed by Tegeder, 2012). Expression of BnAAP3 was not detected in forage brassica with the implication that it may also be confined to root expression in this species. However, transcripts for BnAAP2-1, 2-2, 4-1/4-2/4-3 and 5-1 were detected not only in leaves, but also in developing flower buds, open flowers, siliques and developing seeds, implicating these transporters not just in phloem loading but also in importation into sink tissues.
Gene family members AtAAP1, 6 and 8 are in cluster 4B (Tegeder, 2012). While AtAAP6 has been associated with exchange of nitrogen between xylem and phloem, AtAAP1 and 8 have been associated with seed loading (reviewed by Tegeder, 2012). In forage brassica, transcript levels of BnAAP8 were greatest at the earliest stages analysed and reduced sharply as seeds developed implying a similar role in early seed development to AtAAP8.
Tilsner et al. (2005) isolated and characterized three amino acid permeases, BnAAP1, BnAAP2, and BnAAP6, in rape seed. With work focused on the mobilization of nitrogen reserves from senescing leaves, Tilsner et al. (2005) showed that all three transporters were expressed in leaves and the expression was still detectable during leaf senescence, with BnAAP1 and BnAAP2 mRNA levels increasing from mature to old leaves. In leaves of forage brassica, most of which are senescent at the later stages of silique maturity, expression of BnAAP1, 2 and 6 increased or stabilized between mature and senescing leaves. Expression of AAPs in mature and senescing leaves is not unexpected as these are the main source of nitrogen for the developing siliques and seeds. However, the decrease in expression of the BnAAPs in seeds before the main phase of storage protein accumulation indicates transporters other than AAPs must be active.
Tegeder (2012) does note that the expression of AtAAPs generally is not that phloem- or seed-specific, and the data for forage brassica are in agreement with this, indicating a high degree of functional redundancy throughout much of the plant.
Conclusions
We have shown how a single transcriptome of mixed tissues can provide the information to detect expressed genes for subsequent profiling work. While the amplification of genes within clades provides a phylogenetic challenge, careful primer design allows for the detection of expression of individual gene family members and their homoeologues.
The co-ordinated import of sucrose and amino acids is required for successful silique and seed development and requires the integrated expression of different genes (Zhang et al., 2010). However, Morandini (2013) suggests that: ‘if one desires to increase [metabolic] flux, it seems more sensible to try to increase sink strength rather than tampering with signals or signal transduction events’. To increase sink strength requires an increase in seed number and/or seed size. The CKX gene family are an appropriate target as potentially BnCKX restricts BnIPT activity during and to the first phase of seed development.
A direct consequence of decreased CKX activity in the silique and seed is anticipated to be increased cytokinin levels. An indirect consequence should be increased CWINV activity, providing the sugar signals for enhanced cell cycle activity. The strong positive correlation between expression of BnIPT and two BnINV gene family members identifies these as good markers for such a response. Clear differential gene expression within the BnAAP gene family members as well as identification of the four expressed BnSUC family members allows for these to be used as markers for quality changes in the plant following the selection and breeding of plants with mutant CKX2 and/or 4 genes, identified from TILLING and EcoTILLING populations.
Supplementary Data
Supplementary data are available at JXB online.
Supplementary Table S1. GenBank accession numbers of the gene sequences isolated in this study.
Supplementary Table S2. Sequences of qPCR primers used in this work (F, forward; R, reverse).
Supplementary Table S3. Gene expression profiles during development (Biological replicate 2).
Supplementary Table S4. Gene family member(s) which could be amplified by the primer pairs used in this work.
Acknowledgements
The Advanced Seed Production Systems programme was funded by FRST/MSI/Ministry for Business, Innovation and Employment (LINX0803). We thank J Trethewey for collection of B. napus samples.
Glossary
Abbreviations:
- MAS
marker assisted selection
- QTL
quantitative trait loci
- TILLING
targeting induced local lesions in genomes
- RT-qPCR
quantitative reverse transcription polymerase chain reaction.
References
- Aikins CA, Pigeaire A. 1993. Application of cytokinins to flowers to increase pod set in Lupinus angustifolius . Australian Journal of Agricultural Research 44, 1799–1819. [Google Scholar]
- Ando S, Asano T, Tsushima S, Kamachi S, Hagio T, Tabei Y. 2005. Changes in gene expression of putative isopentenyltransferase during clubroot development in Chinese cabbage (Brassica rapa L.). Physiological and Molecular Plant Pathology 67, 59–67. [Google Scholar]
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M. 2005. Cytokinin oxidase regulates rice grain production. Science 309, 741–745. [DOI] [PubMed] [Google Scholar]
- Atkins CA, Emery RJN, Smith PMC. 2011. Consequences of transforming narrow leafed lupin (Lupinus angustifolius [L.]) with an ipt gene under control of a flower-specific promoter. Transgenic Research 20, 1321–1332. [DOI] [PubMed] [Google Scholar]
- Balibrea ME, Gonzalez Garcia MC, Fatima T, Ehness R, Lee T. 2004. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16, 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. 2011. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation and thus seed yield in Arabidopsis thaliana . Plant Cell 23, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MF, Kirkbride RC, Stone SL, et al. 2013. Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proceedings of the National Academy of Sciences 110, E435–E444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MD, Rao MK, Smith JB, Bayliss MW. 1973. Cell development in the anther, the ovule and the young seed of Triticum aestivum L. var. Chinese Spring. Philosophical Transactions Royal Society London Series B 266, 39–81. [Google Scholar]
- Bihmidine S, Hunter III CT, Johns CE, Koch KE, Braun DM. 2013. Regulation of assimilate import into sink organs: update on molecular drivers of sink strength. Frontiers in Plant Science 4, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DM, Wang L, Ruan Y-L. 2014. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. Journal of Experimental Botany 65, 1713–1735. [DOI] [PubMed] [Google Scholar]
- Brugière N, Humbert S, Rizzo N, Bohn J, Habben JE. 2008. A member of the maize isopentenyl transferase gene family, Zea mays isopentenyl transferase 2 (ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development. Plant Molecular Biology 67, 215–229. [DOI] [PubMed] [Google Scholar]
- Brugière N, Jiao S, Hanke S, Zinselmeier C, Roessler JA, Niu X, Jones RJ, Habben JE. 2003. Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress. Plant Physiology 132, 1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Mandáková T, Wu J, Xie Q, Lysak MA, Wang X. 2013. Deciphering the diploid ancestral genome of the mesohexaploid Brassica rapa . Plant Cell 25, 1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Taliercio EW, Chourey PS. 1996. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8, 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dante RA, Larkins BA, Sabelli PA. 2014. Cell cycle control and seed development. Frontiers in Plant Science 5, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey JE, van Staden J. 1977. A cytokinin complex in the developing fruits of Lupinus albus . Physiologia Plantarum 39, 221–224. [Google Scholar]
- Davey JE, van Staden J. 1978. Cytokinin activity in Lupinus albus III. Distribution in fruits. Physiologia Plantarum 43, 87–93. [Google Scholar]
- Davey JE, van Staden J. 1979. Cytokinin activity in Lupinus albus L. IV Distribution in seeds. Plant Physiology 63, 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RC, Herridge RP, Ambrose BA, Macknight RC. 2008. Transcriptome analysis of proliferating Arabidopsis endosperm reveals biological implications for the control of syncytial division, cytokinin signaling, and gene expression regulation. Plant Physiology 148, 1964–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun X, Shen W, Hu K, et al. 2014. Neofunctionalization of duplicated Tic40 genes caused a gain-of-function variation related to male fertility in Brassica oleracea lineages. Plant Physiology 166, 1403–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehneß R, Roitsch T. 1997. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. The Plant Journal 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Emery RJN, Ma Q, Atkins CA. 2000. The forms and sources of cytokinins in developing white lupine seeds and fruits. Plant Physiology 123, 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gan S. 2014. Translational researches of leaf senescence for enhancing plant productivity and quality. Journal of Experimental Botany 65, 3901–3913. [DOI] [PubMed] [Google Scholar]
- Hampton JG, Rolston MP, Pyke NB, Green W. 2012. Ensuring the long term viability of the New Zealand seed industry. Agronomy New Zealand 42, 129–140. [Google Scholar]
- Hirose N, Takei K, Huroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. 2008. Regulation of cytokinin biosynthesis, compartmentalization and translocation. Journal of Experimental Botany 59, 75–83. [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J, Müller B. 2012. Cytokinin signaling networks. Annual Review of Plant Biology 63, 353–380. [DOI] [PubMed] [Google Scholar]
- Jameson PE, Letham DS, Zhang R, Parker CW, Badenoch-Jones J. 1987. Cytokinin translocation and metabolism in lupin species. I. Zeatin riboside introduced into the xylem at the base of Lupinus angustifolius stems. Australian Journal of Plant Physiology 14, 695–718. [Google Scholar]
- Jameson PE, McWha JA, Wright GJ. 1982. Cytokinins and changes in their activity during the development of grains of wheat (Triticum aestivum L.). Zeitschrift für Pflanzenphysiologie 106, 27–36. [Google Scholar]
- Kakimoto T. 2001. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant Cell Physiology 42, 677–685. [DOI] [PubMed] [Google Scholar]
- King SP, Lunn JE, Furbank RT. 1997. Carbohydrate content and enzyme metabolism in developing canola siliques. Plant Physiology 114, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clere S, Schmelz EA, Chourey PS. 2010. Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiology 153, 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letham DS. 1994. Cytokinins as Phytohormones – sites of biosynthesis, translocation, and function of translocated cytokinins. In: Mok DWS, Mok MC, eds. Cytokinins: Chemistry, Activity and Function, Vol. 1 Boca Raton: CRC Press, 57–80. [Google Scholar]
- Li F, Ma C, Wang X, Gao C, Zhang J, Wang Y, Cong N, Li X, Wen J, Yi B, Shen J, Tu J, Fu T. 2011. Characterization of sucrose transporter alleles and their association with seed yield-related traits in Brassica napus L. BMC Plant Biology 11, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nie X, Tan JLH, Berger F. 2013. Integration of epigenetic and genetic controls of seed size by cytokinin in Arabidopsis . Proceedings of the National Academy of Sciences 110, 15479–15484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Shen JX, Wang TH, Chen QF, Zhang XG, Fu T, Meng JL, Tu JX. 2007. QTL analysis of yield-related traits and their association with functional markers in Brassica napus L. Australian Journal of Agricultural Reseach 58, 759–766. [Google Scholar]
- Liu Z, Lv Y, Zhang M, Liu Y, Kong L, Zou M, Lu G, Cao J, Yu X. 2013. Identification, expression, and comparative genomic analysis of the IPT and CKX gene families in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genomics 14, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio A, Roig-Villanova I, Bernardi J, Varotto S. 2014. Current perspectives on the hormonal control of seed development in Arabidopsis and maize: a focus on auxin. Frontiers in Plant Science 5, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysak MA, Koch MA, Pecinka A, Schubert I. 2005. Chromosome triplication found across the tribe Brassicaceae. Genome Research 15, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Kakimoto T, Matsumoto-Kitano M. 2004. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin and nitrate. Plant Journal 37, 128–138. [DOI] [PubMed] [Google Scholar]
- Morandini P. 2013. Control limits for accumulation of plant metabolites: brute force is no substitute for understanding. Plant Biotechnology Journal 11, 253–267. [DOI] [PubMed] [Google Scholar]
- Morley-Smith ER, Pike MJ, Findlay K, Köckenberger W, Hill LM, Smith AM, Rawsthorne S. 2008. The transport of sugars to developing embryos is not via the bulk endosperm in oilseed rape seeds. Plant Physiology 147, 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RD, Blevins DG, Dietrich JT, et al. 1993. Cytokinins in plant pathogenic bacteria and developing cereal grains. Australian Journal Plant Physiology 20, 621–637. [Google Scholar]
- Mothes K, Engelbrecht L. 1963. On the activity of a kinetin-like root factor. Life Science 11, 852–857. [Google Scholar]
- Mun JH, Kwon SJ, Yang TJ, et al. 2009. Genome-wide comparative analysis of the Brassica rapa gene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biology 10, R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén LD, Letham DS. 1984. Translocation of zeatin riboside and zeatin in soybean explants. Journal of Plant Growth Regulation 2, 265–279. [Google Scholar]
- O’Keefe D, Song J, Jameson PE. 2011. Isopentenyl transferase and cytokinin oxidase/dehydrogenase gene family members are differentially expressed during pod and seed development in Rapid-cycling Brassica . Journal of Plant Growth Regulation 30, 92–99. [Google Scholar]
- Peng D, Gu X, Xue L-J, Leebens-Mack JH, Tsai C-J. 2014. Bayesian phylogeny of sucrose transporters: ancient origins, differential expansion and convergent evolution in monocots and dicots. Frontiers in Plant Science 5, 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A, Sivitz AB, Ward JM. 2012. Evolution of plant sucrose uptake transporters. Frontiers in Plant Science 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijavec T, Kovač M, Kladnik A, Chourey PS, Dermastia M. 2009. A comparative study on the role of cytokinins in caryopsis development in the maize miniature1 seed mutant and its wild type. Journal of Integrative Plant Biology 51, 840–849. [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH. 1999. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Menges M, Healy J.M.S, Murray JAH. 2000. Sugar control of the plant cell cycle: Differential regulation of Arabidopsis D-type cyclin gene expression. Molecular Cellular Biology 20, 4513–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y-L, Jin Y, Yang Y-J, Li G-J, Boyer JS. 2010. Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Molecular Plant 3, 942–955. [DOI] [PubMed] [Google Scholar]
- Singh S, Letham DS, Jameson PE, Zhang R, Parker CW, Badenoch-Jones J, Noodén LD. 1988. Cytokinin biochemistry in relation to leaf senescence. IV. Cytokinin metabolism in soybean explants. Plant Physiology 88, 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Jiang L, Jameson PE. 2012. Co-ordinate regulation of cytokinin gene family members during flag leaf and reproductive development in wheat. BMC Plant Biology 12, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summons RE, Letham DS, Gollnow BI, Parker CW, Entsch B, Johnson LP, MacLeod JK, Rolfe BG. 1981. Cytokinin translocation and metabolism in species of the Leguminoseae: studies in relation to shoot and nodule development. In: Guern J, Peaud-Lenoel P, eds. Metabolism and Molecular Activities of Cytokinins . Berlin, New York: Springer-Verlag, 69–79. [Google Scholar]
- Taylor JS, Thompson B, Pate JS, Atkins CA, Pharis RP. 1990. Cytokinins in the phloem sap of white lupin (Lupinus albus L.). Plant Physiology 94, 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder M. 2012. Transporters for amino acids in plant cells: some functions and many unknowns. Current Opinion in Plant Biology 15, 315–321. [DOI] [PubMed] [Google Scholar]
- Tegeder M. 2014. Transporters involved in source to sink partitioning of amino acids and ureides: opportunities for crop improvement. Journal of Experimental Botany 65, 1865–1878. [DOI] [PubMed] [Google Scholar]
- Tilsner J, Kassner N, Struck C, Lohaus G. 2005. Amino acid contents and transport in oilseed rape (Brassica napus L.) under different nitrogen conditions. Planta 221, 328–338. [DOI] [PubMed] [Google Scholar]
- Vyroubalová S, Václavíková K, Turečková V, Novák O, Šmehilová M, Hluska T, Ohnoutková L, Frébort I, Galuszka P. 2009. Characterization of new maize genes putatively involved in cytokinin metabolism and their expression during osmotic stress in relation to cytokinin levels. Plant Physiology 151, 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ruan Y-L. 2012. New insights into roles of cell wall invertase in early seed development revealed by comprehensive spatial and temporal expression patterns of GhCWIN1 in cotton. Plant Physiology 160, 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ruan Y-L. 2013. Regulation of cell division and expansion by sugar and auxin signaling. Frontiers in Plant Science 4, 163, doi:10.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Wang J, et al. 2011. The genome of the mesopolyploid crop species Brassica rapa . Nature Genetics 43, 1035–1039. [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15, 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Köllmer I, Bartrina I, Holst K, Schmülling T. 2006. New insights into the biology of cytokinin degradation. Plant Biology doi: 10.1055. [DOI] [PubMed] [Google Scholar]
- Zalewski W, Galuszka P, Gasparis S, Orczyk W, Nadolska-Orczyk A. 2010. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity. Journal of Experimental Botany 61, 1839–1851. [DOI] [PubMed] [Google Scholar]
- Zalewski W, Gasparis S, Boczkowska M, Rajchel I, Orczyk W, Nadolska-Orczyk A. 2014. Expression patterns of HvCKX genes indicate their role in growth and reproductive development of barley. PLOS ONE doi: 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski W, Orczyk W, Gasparis S, Nadolska-Orczyk A. 2012. HvCKX2 gene silencing by biolistic or Agrobacterium-mediated transformation in barley leads to different phenotypes. BMC Plant Biology 12, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhao Y-L, Gao L-F, Zhao G-Y, Zhou R-H, Zhang B-S, Jia J-Z. 2012. TaCKX6-D1, the ortholog of rice OsCKX2, is associated with grain weight in hexaploid wheat. New Phytologist 195, 574–584. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tan Q, Lee R, Trethewy A, Lee Y-H, Tegeder M. 2010. Altered xylem-phloem transfer of amino acids affects metabolism and leads to increased seed yield and oil content in Arabidopsis . The Plant Cell 22, 3603–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Letham DS. 1990. Cytokinin translocation and metabolism in lupin species. III. Translocation of xylem cytokinin into the seeds of lateral shoots of Lupinus angustifolius . Plant Science 70, 65–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.