Abstract
Apoptotic lymphocytes are readily identified in murine lungs, both during the response to particulate antigen and in normal mice. Because apoptotic lymphocytes are seldom detected in other organs, we hypothesized that alveolar macrophages (AMø) clear apoptotic lymphocytes poorly. To test this hypothesis, we compared in vitro phagocytosis of apoptotic thymocytes by resident AMø and peritoneal Mø (PMø) from normal C57BL/6 mice. AMø were deficient relative to PMø both in percentage containing apoptotic thymocytes (19.1 ± 1.0% versus 96.0 ± 2.6% positive) and in phagocytic index (0.23 ± 0.02 versus 4.2 ± 0.67). This deficiency was not due to kinetic differences, was seen with six other inbred mouse strains, and was not observed using carboxylate-modified polystyrene microbeads. Annexin V blockade indicated that both Mø types cleared apoptotic T cells by a mechanism involving phosphatidylserine expression. By contrast, neither mAb blockade of a variety of receptors (CD11b, CD29, CD51, and CD61) known to be involved in clearance of apoptotic cells, nor the tetrapeptide RGDS, blocked ingestion by either type of Mø. To confirm these studies, apoptotic thymocytes were given intratracheally or intraperitoneally to normal mice and then AMø or PMø were recovered 30–240 min later. Ingestion of apoptotic thymocytes by AMø in vivo was significantly decreased at all times. Defective ingestion of apoptotic lymphocytes may preserve AMø capacity to produce proinflammatory cytokines in host defense, but could contribute to development of autoimmunity by failing to eliminate nucleosomes.
Keywords: Apoptosis, Phagocytosis, Lung, In Vivo Animal Models, Cell Surface Molecules
INTRODUCTION
Protective immune responses generate expanded clones of effector lymphocytes which are unneeded once the initiating threat has been controlled. Such obsolescent lymphocytes are eliminated by apoptosis in a process commonly called activation-induced cell death, although propriocidal regulation has been suggested as a better term (1). It is essential to clear these apoptotic lymphocytes before they advance to the necrotic state to prevent further inflammation, collateral tissue damage, and possibly development of autoimmunity (2). Macrophages (Mø) are believed to be the primary phagocytes responsible for ingestion of apoptotic cells in most organs (3). This process is generally highly efficient, so that even in tissues with known high rates of lymphocyte apoptosis such as the thymus, it has generally been difficult to demonstrate apoptotic lymphocytes in vivo (4). For this reason, it was somewhat surprising that we were previously able to detect large numbers of apoptotic lymphocytes in the lungs of mice, both during the immune response to particulate Ag and even in normal mice (5). As a potential explanation, we hypothesized that alveolar macrophages (AMø), the chief phagocyte of the gas exchanging regions of the lungs, might clear apoptotic T cells poorly relative to other types of Mø.
Although they all ultimately derived from common hematopoietic precursors, Mø within various tissues differ considerably in morphology, biochemistry, secretory products, surface phenotype, and function. In particular, alveolar Mø (AMø) are a distinctive cell type that reside in the unique environment of the pulmonary alveolus, where they are exposed to high ambient oxygen concentrations, to pulmonary surfactant which is rich in both lipids and unique opsonins, and to a large daily burden of inhaled particulates (6, 7). Because these particulates must be cleared without compromising gas exchange through excessive inflammation, it is likely that AMø are specialized phagocytes. Indeed, AMø differ from other Mø populations in expression of novel receptors (8), in antigen-presenting capacity (9) and in eicosanoid production (10, 11). The capacity of murine AMø to clear apoptotic lymphocytes has not been described previously, although resident AMø from normal rabbits have been shown to ingest apoptotic human neutrophils less avidly than inflammatory lung Mø recovered from lungs of rabbits undergoing immune complex injury (12).
Clearance of apoptotic cells by phagocytes is a complex and incompletely understood process that involves both recognition and ingestion steps (13). Multiple receptors appear to be involved in each of these steps, with the specific receptors used depending both on the apoptotic target and on the activation state of the phagocyte. Recognition of exposed phosphatidylserine (PS) on the surface of apoptotic cells in a stereo-specific fashion is an important mechanism for many apoptotic targets, especially lymphocytes (14–16). The receptors responsible for PS recognition have not yet been definitively identified (17), but candidates include CD14 (a glycosylphosphatidylinositol-linked receptor for lipopolysaccharide) (18), CD36 (a receptor for thrombospondin also known as the class B scavenger receptor), and CD68 (a receptor for oxidized LDL). Multiple adhesion receptors have also been implicated in phagocytosis of apoptotic cells in various systems. Phagocytosis of apoptotic neutrophils by human monocyte-derived Mø (HMDM) has been shown to involve the vitronectin receptor (VNR) (αvβ3 integrin, CD51/CD61), which together with CD36 on the Mø binds unidentified ligands on the neutrophil via thrombospondin (19). Thus, differential expression of a variety of surface receptors could underlie difference between Mø subtypes in clearance of apoptotic cells.
The goal of this study was to determine the ability of murine resident AMøs to ingest apoptotic lymphocytes. As a standard model of apoptotic T cells (20, 21), we used thymocytes induced to become apoptotic by exposure to dexamethasone. We found that in comparison to resident peritoneal Mø (PMø), resident murine AMø displayed a marked and specific deficiency in the ability to ingest apoptotic thymocytes both in vitro and in vivo.
MATERIALS AND METHODS
Antibodies
The following anti-murine mAbs were purchased from PharMingen (San Diego, CA): 2D7 (anti-CD11a); M1/70 (anti-CD11b); HL3 (anti-CD11c); rmC5–3 (anti-CD14); 2.4G2 (anti-CD16/CD32); C71/16 (anti-CD18); Ha2/5 (anti-CD29); R1–2 (anti-CD49d); 5H10–27 (anti-CD49e); H9.2B8 (anti-CD51); 2C9.G2 (anti-CD61); and R3–34 (control rat IgG1 kappa); R35–95 (control rat IgG2a kappa); A95-1 (control rat IgG2b kappa) G235–2356 (control hamster IgG1 lambda); A19–4 (control hamster IgG3 lambda); A19–3 (control hamster IgG1 kappa); G235-1(control hamster IgM). Paramagnetic microbeads coated with monoclonal anti-murine CD19 or anti-CD90 were purchased from Miltenyi Biotec (Auburn, CA).
Mice
Pathogen-free inbred female mice were used in all experiments. C57BL/6 (H-2 b) and C3H/HeNCrIRB (H-2 k) mice were purchased from Charles River Laboratory Inc. (Wilmington, MA); AKR/J (H-2 k), C57BL/10J (H-2 b), C3H/HeJ (H-2 k), and DBA/1J (H-2 q) mice were purchased from Jackson Laboratories (Bar Harbor, ME); BALB/c (H-2 d) mice were purchased from Taconic Laboratories (Germantown, NY). Mice were purchased at 7–8 weeks of age and used at 8–14 weeks of age. Mice were housed in the Animal Care Facility at the Ann Arbor VA Medical Center, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care, where they were fed standard animal chow (Rodent Lab chow 5001, Purina; St. Louis, MO) and chlorinated tap water ad libitum. This study complied with the NIH “Guide for the Care and Use of Laboratory Animals” {Department of Health, Education, & Welfare Publication No. (NIH) 80-23} and followed a protocol approved by the Animal Care Committee of the local Institutional Review Board.
Isolation and culture of Mø
Mice were euthanized by asphyxia in a high CO2 environment. AMø were collected by bronchoalveolar lavage (BAL) using a total of 10 ml Dulbecco’s phosphate-buffered saline (PBS) (GIBCO-BRL; Grand Island, NY) containing 0.5 mM EDTA. BAL was performed in 1-ml aliquots with gentle massage of the thorax as previously described (22). Greater than 95% of BAL cells were AMøs. PMø were collected by peritoneal lavage using the same type of PBS, which was administered in 2-ml aliquots to a total volume of 10 ml. PMø among the lavage cells were first enriched by negative selection using CD19- and CD90-conjugated paramagnetic beads according to the manufacturer’s instructions. Mø were plated at 2 ×105 cells/well in sterile 8-well Lab-Tek slides (Nalge Nunc International; Naperville, IL) and, after 1 hour incubation at 37° C, nonadherent cells were removed by gentle washing. Mø monolayers were cultured overnight in complete medium {RPMI 1640 containing 25 mM HEPES, 2 mM L-glutamine, 1 mM pyruvate, 100 units/ml penicillin/streptomycin (all obtained from GIBCO-BRL), 10% heat-inactivated FBS (triple filtered at 100 nm, ≤ 25 EU/ml endotoxin; ≤ 25 mg/dl hemoglobin) (Hyclone Laboratories, Logan, UT) and 55 µM 2-mercaptoethanol (Sigma; St. Louis, MO)} in a 5% CO2 environment at 37° C before use in the phagocytosis assay.
Isolation and apoptosis induction in thymocytes and cloned T cells
Thymuses were harvested from normal mice and minced to yield a single cell solution. To induce apoptosis, thymocytes were suspended with RPMI 1640 containing 10% heat-inactivated FBS at the concentration of 1 × 106/ml and incubated with a final concentration of 10−6 M dexamethasone (Sigma) overnight. Thymocytes were 50.9% early apoptotic and 42.1% late apoptotic as demonstrated by simultaneous annexin V and propidium iodide staining and flow cytometric analysis. CTLL-2 cells were induced to apoptosis by deprivation from IL-2 for 16 hours. The resulting preparation was 44.9% early apoptotic and 23.1% late apoptotic.
Isolation and apoptosis induction in neutrophils
Neutrophils were harvested from the peritoneum of mice that had been treated 16 hours and again 3 hours previously by IP injection of 1.0 ml of a 9% solution (w/v) of casein (Sigma) in PBS containing 0.9 mM CaCl2 and 0.5 mM MgCl2 (23). Neutrophils were purified from this peritoneal lavage using NIM™•2 (Cardinal Associates, Inc.; Santa Fe, NM) according to the manufacturer’s directions. Purity was 96.6% by differential cell count of a Giemsa-stained cytospin slide. Neutrophil apoptosis was induced by UV irradiation (254 nM) for 15 minutes followed by overnight incubation in complete medium at 37°C. The resulting preparation was 19.7% early apoptotic and 33.4% late apoptotic as judged by staining with annexin V-FITC plus propidium iodide and flow cytometric analysis.
Apoptosis assay
Leukocyte apoptosis was measured by flow cytometric analysis of surface expression of PS, a sensitive and specific measure of early apoptosis (15, 24). For this purpose, 100 µl aliquots were stained with annexin V-FITC (Apoptosis Detection Kit; R & D Systems; Minneapolis, MN) according to the manufacturer’s protocol. Cells were analyzed without fixation by flow cytometry within 1 hour of staining.
Phagocytosis assays
Phagocytosis of apoptotic thymocytes in vitro was assayed by adding 2 × 106 apoptotic thymocytes suspended in 400 µl of complete medium to each well of the Lab-Tek slides containing adherent Mø monolayers. Heat-inactivated serum (Hyclone) was included at a final concentration of 10% during the co-incubation, as phagocytosis of apoptotic thymocytes by resident PMø has been shown to be dependent on serum (21). The slides were incubated for 1.5 h at 37°C, washed with ice-cold PBS containing 0.5 mM EDTA, and stained using hematoxylin-eosin Y (H & E) (Richard-Allan; Kalamazoo, MI). Phagocytosis was evaluated by counting 200 to 300 macrophages per well at 1000 magnification under oil immersion. Results were expressed as percentage of Mø containing at least one ingested thymocyte (percent phagocytic), and as phagocytic index, which was generated by multiplying the percentage of phagocytosis by the mean number of phagocytosed cells per Mø.
As a control for the ability of Mø to ingest particles, 8 × 106 FITC-labeled carboxylate-modified polystyrene microbeads (1.7 µm mean diameter) (Catalogue # 17687; Polysciences, Inc.; Warrington, PA) were co-incubated with adherent AMø or PMø (2 × 105 cells in a final volume of 400 µl in complete medium) at 37° C in a 5% CO2 environment for 90 min. The slide was then washed three times with PBS containing 0.5 mM EDTA. Phagocytosis was determined immediately by fluorescence microscopy under oil immersion.
Assay of in vivo phagocytosis
To assay the ability of Mø to ingest apoptotic cells in vivo, 5 × 107 apoptotic thymocytes in 50 µl normal saline were injected either intratracheally or intraperitoneally using the methods previously described for Ag administration (25). BAL and peritoneal lavage were collected 2 h later. Slides of lavage cells were prepared by cytocentrifugation (Shandon; Pittsburgh, PA) and stained with H & E.
Inhibition of phagocytosis of apoptotic cells
For each blocking experiment, mAbs were used at final concentrations that were saturating as demonstrated by flow cytometry. Monolayer Møs were incubated with specified mAb for 30 min at 4°C. The cells were gently washed twice with PBS and then co-cultured with apoptotic thymocytes for 90 min at 37°C in complete medium. For annexin blocking experiments, apoptotic thymocytes were incubated with purified human annexin V (40 µg/105 cells) in binding buffer (10 mM HEPES/NaOH, pH 7.4, 0.14 M NaCl, 2.5 mM CaCl2) for 15 min at room temperature. The cells were added without washing to the Mø monolayers and were co-cultured for 90 min at 37°C.
Immunostaining and flow cytometry
Mø freshly isolated by BAL or peritoneal lavage were used to analyze expression of receptors potentially involved in clearance of apoptotic cells. Mø were washed twice in staining buffer (DIFCO Laboratories, Detroit, MI), resuspended in 100 µl staining buffer, and incubated for 30 min at 4°C in the dark with labeled antibodies diluted in 100 µl staining buffer. Final Ab concentrations were 1–2 µg/106 cells. FcR was blocked using mAb 2.4G2 (anti-CD16/32) for all primary mAbs except rmC5-3, as binding of this anti-CD14 mAb has been reported to be inhibited by 2.4G2 (26). After incubation, cells were washed twice in staining buffer, resuspended in 0.5 ml staining buffer, and analyzed immediately.
Flow cytometry was performed as previously described in detail (27) using a FACSCAN cytometer (Becton Dickinson; Mountain View, CA) running Cell Quest software on a PowerPC microcomputer (Apple; Cupertino, CA) for data collection and analysis. A minimum of 10,000 viable cells was analyzed to determine cell-surface receptor expression.
Purification of recombinant annexin V
To produce large quantities of annexin V for use in blocking experiments, recombinant human annexin was purified as described by Krahling (28). TG1 strain E. coli containing a plasmid encoding human placental annexin V (clone pRK6; American Type Culture Collection; Rockville, MD) was cultured overnight at 37°C in 200 ml LB medium containing 50 µg/ml ampicillin. After overnight incubation, this mixture was diluted fivefold into 1 L of fresh LB medium and was cultured for an additional 3 h. Next, isopropyl β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM. After 4 h of additional growth, bacteria were harvested by centrifugation (5,000 × g, 4°C, 15 min), and the pellet was resuspended in an equal volume of spheroplast buffer (0.5 mM EDTA, 750 mM sucrose, 200 mM Tris, pH 8.0). Lysozyme was added to a final concentration of 1 mg/ml immediately prior to the addition of 7-fold volume of spheroplast buffer diluted 1:1 with water and incubated for 30 min on ice. Spheroplasts were collected by centrifugation (14,000 × g, 4°C, 30 min) and the pellet was resuspended in 10 ml ultracentrifugation buffer (2 mM EDTA, 5 mM MgCl2, 100 mM NaCl, 0.1 mg/ml of RNAse, 0.1 mg/ml of DNase I, 2 mM phenylmethylsulfonylflouride, 0.5 µg/ml of pepstatin A, 0.1% (w/v) Triton X-100, 20 mM Tris, pH 8.0). The suspension was centrifuged overnight at 100,000 × g at 4°C, and then the supernatant was harvested.
Liposomes for use in purification of the annexin V were prepared by dissolving 2 mg PS and 1 mg phosphatidylcholine (Sigma) in chloroform and drying the mixture under nitrogen gas. The lipid mixture, resuspended in 5 ml of liposome buffer (100 mM NaCl, 3 mM MgCl2, 20 mM Tris, pH 8.0) by vortexing, was sonicated for 10 min using a probe sonicator to prepare liposomes. These liposomes were added to the bacterial culture supernatant and calcium content was adjusted to 5 mM by addition of CaCl2. The mixture was incubated on ice for 30 min, and then was centrifuged at 40,000 × g for 45 min at 4°C. The pellet was washed once in washing buffer (5 mM CaCl2, 100 mM NaCl, 3 mM MgCl2, 20 mM Tris, pH 8.0) and resuspended in extraction buffer (10 mM EDTA, 100 mM NaCl, 3 mM MgCl2, 20 mM Tris, pH 8.0). The liposomes were removed by centrifugation for 1 hour at 50,000 × g and 4°C. The supernatant was dialyzed in PBS pH 7.4 and concentrated using a Centricon filter (Millipore, Bedford, MA). The purity of the protein was tested by SDS-PAGE and Coomassie staining, which indicated the product to be >90% pure.
Statistical analysis
Data were expressed as mean ± SEM. Statistical calculations were performed using Statview and SuperANOVA programs (Abacus Concepts, Inc.; Berkeley, CA) on a Macintosh PowerPC G3 computer. Continuous ratio scale data were evaluated by unpaired Student t test (for two samples) or ANOVA (for multiple comparisons) with post hoc analysis by the Tukey-Kramer test or by the two-tailed Dunnett test, which compares treatment groups specifically to a control group (29). Use of these parametric statistics was deemed appropriate, as phagocytosis of apoptotic thymocytes by PMø has been shown to follow a Gaussian distribution (21). Percentage data were arcsine transformed before analysis to convert them from a binomial to a normal distribution using tables in Zar (29). Significant differences were defined as p<0.05.
RESULTS
AMø were deficient in phagocytosis of apoptotic thymocytes in vitro relative to PMø
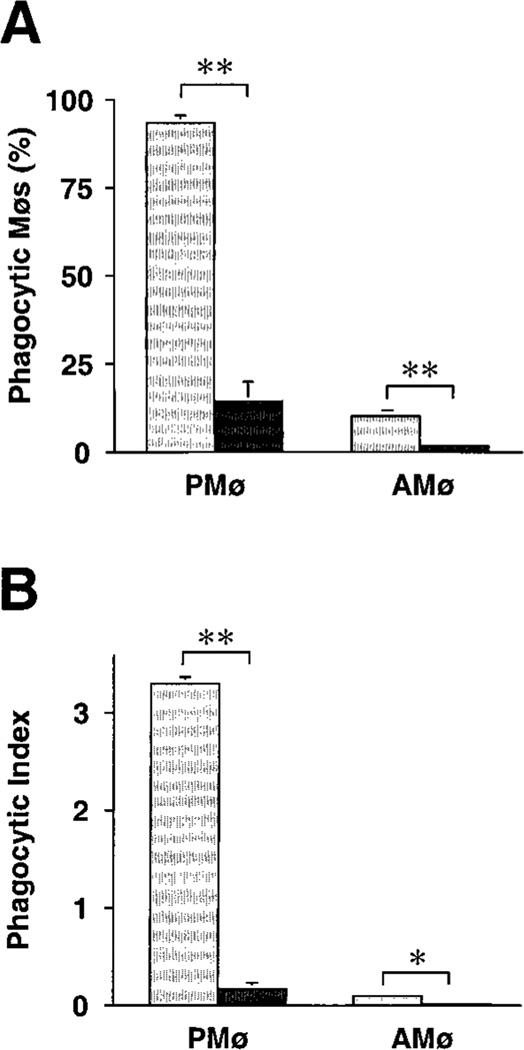
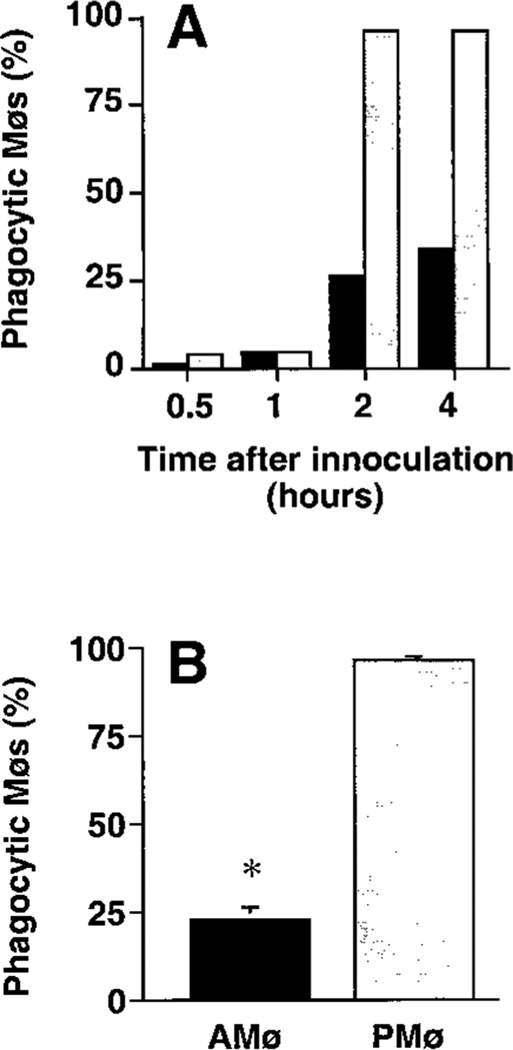
Co-culture of adherent AMø and PMø from normal C57BL/6 mice with a ten-fold greater number of apoptotic thymocytes for various times disclosed a marked deficiency in phagocytosis by AMø (Fig. 1). This deficiency was noted at all time-points, and was especially evident in the percentage of Mø that had ingested even a single thymocyte. Considering results of several experiments, 79–89% of PMø were positive for phagocytosis in 60 min versus only 3–12% of AMøs, and by 90–120 min a plateau in percentage of positive Mø had essentially been reached by both cell types, with over 90% of PMø, but only 6–28% of AMø, having ingested at least one apoptotic cell. Phagocytic index also showed a large difference between the two cell types, which continued to diverge through six h of assay. Most PMø ingested multiple apoptotic thymocytes, whereas virtually no AMø ingested more than a single thymocyte. Based on these results, we selected 90 minute for further analysis as a convenient but sufficiently long duration of assay to detect differences between the two Mø types.
FIGURE 1.
Phagocytosis of apoptotic thymocytes by mouse AMø and PMø in vitro. Adherent AMø and PMø obtained from normal C57BL/6 mice (2 × 105 in a final volume of 400 µl) were co-incubated at 37°C in 8-well chamber slides with apoptotic thymocytes (2 × 106 per well). After various times, slides were washed extensively with PBS containing 0.5 mM EDTA to remove non-ingested thymocytes and were stained with H & E. Phagocytosis was evaluated by counting 200ntrol hamster IgG3 lambda300 Mø per condition under oil immersion. (A) Percentage of phagocytic Mø; (B) phagocytic index. Open circles represent PMø and solid squares represent AMø. Data are from a single experiment which is representative of two experiments of identical design.
On microscopic analysis, ingested thymocytes had morphology typical of apoptosis, which is characterized by nuclear and cytoplasmic condensation (Fig. 2). Ingested cells appeared intact, suggesting that some surface charge promoted phagocytosis of apoptotic thymocytes before their lysis. The observed difference between the two Mø types could not be explained by differences in viability or apoptosis of the Mø themselves (96.3 ± 1.1% viable AMø by trypan blue exclusion versus 97.1 ± 0.5 % viable PMø, p = 0.51; 13.7 ± 1.9 % apoptotic AMø by annexin-FITC binding versus 13.0 ± 3.4 % apoptotic PMø; p = 0.87; mean ± SEM of four experiments). Nor was the deficiency due to inhalation of a high CO2 content during euthanasia, (which we used rather than the usual exsanguination while under deep anesthesia via IP pentobarbital to permit harvest of AMø and PMø from the same mice) (percentage of positive AMø after CO2 asphyxia 28.9 ± 3.2% versus 25.4 ± 1.7% positive after pentobarbital euthanasia, p = 0.37; phagocytic index after CO2 asphyxia 0.35 ± 0.04 versus 0.29 ± 0.025, p = 0.25; n = 5 mice in each group). Additional control experiments demonstrated that the relative deficiency of phagocytosis by AMø did not result from the overnight incubation step used (Table I). Further, varying the ratio of apoptotic thymocytes to macrophages to ratios as high 100:1 did not significantly increase phagocytosis by AMø (data not shown). Hence, artifacts of the in vitro assay did not appear to explain the differences in phagocytosis between the two types of Mø.
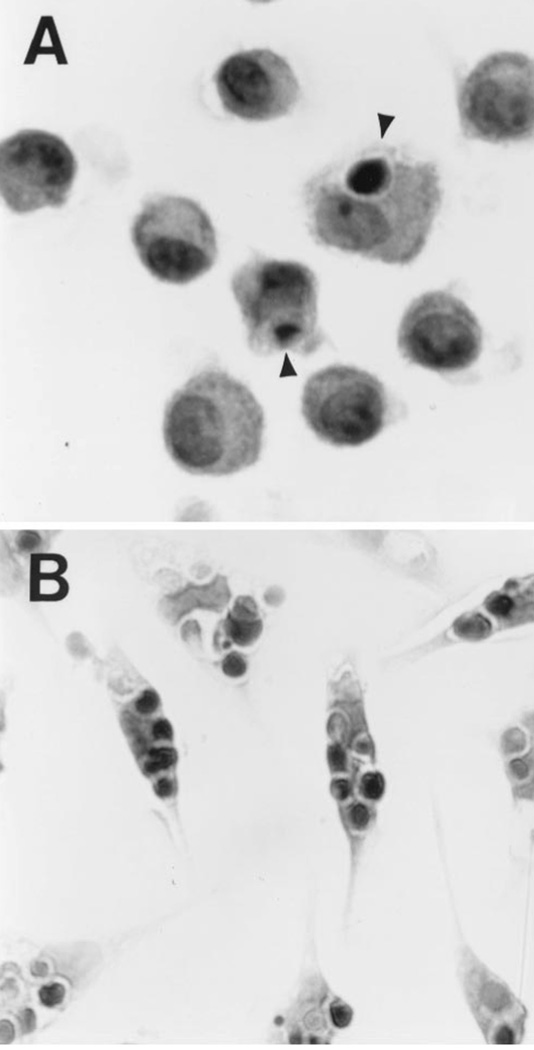
FIGURE 2.
Morphologic appearance of Mø containing ingested thymocytes. Adherent AMø and PMø were co-incubated with apoptotic thymocytes for 90 min as described in the legend to Figure 1, washed and stained with H & E. Representative fields of (A) AMø and (B) PMø are shown (1000 × magnification, oil immersion). Arrowheads indicate single ingested apoptotic thymocytes within AMø. By contrast, virtually all PMø contain several apoptotic thymocytes.
Table I.
Effect of duration of pre-incubation on Møphagocytosis of apoptotic thymocytes *
| Phagocytic Møs (%) |
Phagocytic Index |
|||
|---|---|---|---|---|
| 90 minutes |
overnight |
90 minutes |
overnight |
|
| AMø | 19.8 ± 1.5 | 23.1 ± 0.5 | 0.2 ± 0.0 | 0.3 ± 0.0 |
| PMø | 94.4 ± 1.3 | 88.1 ± 1.3 | 3.7 ± 0.1 | 3.1 ± 0.1 |
Resident AMø and PMø from normal C57BL/6 mice were cultured on Lab-Tek slides for 90 minutes or overnight, washed to remove non-adherent cells, and then incubated for an additional 90 minutes with apoptotic thymocytes. Phagocytosis was assayed on H&E stained slides. n = 3 mice per group assayed individually.
The observed defect in phagocytosis of apoptotic thymocytes was not limited to mice of the C57BL/6 strain, but was also noted with inbred mice of widely varying backgrounds and several H-2 haplotypes (Table II). In all cases, AMø showed highly significantly decreases in percentage of phagocytic Mø (p<0.001, all strains) and in phagocytic index (p<0.001, all strains) relative to PMø of the same strain. Interestingly, when results of AMø were compared between the mouse strains, both measures of phagocytosis by AMø of AKR and C57BL10 mice were slightly but significantly higher than those of AMø of all other strains except DBA/1 (ANOVA with Tukey-Kramer post hoc testing). No differences were seen between Mø of C3H/HeN mice and those of C3H/HeJ mice, which has a naturally occurring dominant-negative mutation in the innate immune receptor TLR4 (30). All remaining experiments were performed with Mø of the C57BL/6 stain, which gave an intermediate result.
Table II.
Phagocytosis of apoptotic thymocytes by macrophages of various mouse strains *
| Phagocytic Møs (%) |
Phagocytic Index |
|||
|---|---|---|---|---|
| Strain | AMø | PMø | AMø | PMø |
| AKR/J | 29.62 ± 2.74 | 97.17 ± 1.22 | 0.42 ± 0.06 | 4.85 ± 0.47 |
| BALB/c | 13.06 ± 1.21 | 95.86 ± 0.76 | 0.15 ± 0.01 | 5.07 ± 0.34 |
| C3H/HeJ | 20.91 ± 1.37 | 94.33 ± 1.11 | 0.27 ± 0.02 | 3.89 0.32 |
| C3H/HeN | 18.27 ± 2.10 | 96.84 ± 1.16 | 0.21 ± 0.03 | 3.94 ± 0.27 |
| C57Bl/6 | 19.12 ± 0.98 | 96.01 ± 2.57 | 0.23 ± 0.02 | 4.24 ± 0.67 |
| C57B10J | 29.92 2.21 | 93.26 0.66 | 0.42 ± 0.04 | 3.67 0.21 |
| DBA/1J | 22.44 ± 2.15 | 91.85 ± 5.01 | 0.29 ± 0.05 | 3.35 ± 0.79 |
Resident AMø and PMø from normal mice were cultured on Lab-Tek slides for 90 minutes or overnight, washed to remove non-adherent cells, and then were incubated for an additional 90 minutes with apoptotic thymocytes. Phagocytosis was assayed on H&E stained slides. n = 5 mice per group assayed individually. See text for statistical analysis.
To exclude the possibility that AMøs were globally deficient in phagocytic function, we examined the capacity of both types of Mø to ingest two types of particles: opsonized zymosan and carboxylate-modified polystyrene microbeads, a widely used model of opsonin-independent phagocytic activities in vitro (31). AMø and PMø had same ability to ingest opsonized zymosan (93.1 ± 2.4% phagocytic for AMø versus 98.6 ± 0.3% for PMø, p = 0.09, unpaired t test; phagocytic index 6.3 ± 0.5 for AMø versus 7.0 ± 0.3 for PMø, p = 0.3, unpaired t test). The two types of Mø also had identical phagocytosis of microbeads (62.4 ± 8.1% phagocytic for AMø versus 68.3 ± 4.6% for PMø, p = 0.56, unpaired t test; phagocytic index 3.1 ± 0.3 for AMø versus 3.2 ± 0.2 for PMø, p = 0.73, unpaired t test). Thus, it appeared that the phagocytic defect was specific for ingestion of apoptotic cells.
Because overnight incubation in dexamethasone allowed some of the thymocytes to progress to late apoptosis, potentially limiting the generalizability of our results, we performed a direct comparison with ingestion of early apoptotic thymocytes induced by 6 h dexamethasone incubation (42.0% annexin positive, 9.0% propidium iodide positive). Markedly deficient ingestion of apoptotic thymocytes was again seen using AMø but not PMø, with no difference between different durations of dexamethasone incubation (for AMø, 35.0 % positive Mø using 6 h treated thymocytes versus 33.8 ± 1.5% positive Mø using 18 h thymocytes, which were 57.1% propidium iodide positive, p = 0.55, unpaired t test; phagocytic index 0.50 ± 0.03 for 6 h thymocytes versus 0.53 ± 0.03 for 18 h thymocytes, p = 0.55, unpaired t test). In the remainder of the experiments in the current study we continued to use overnight dexamethasone incubation. Experiments using two other cell types as apoptotic targets indicated that the finding was not limited to thymocytes. Resident murine AMø also ingested apoptotic murine neutrophils poorly in comparison with resident murine PMø (8.4 ± 0.9% phagocytic for AMø versus 42.3 ± 11.8% for PMø, p < 0.02, unpaired t test; phagocytic index 0.09 ± 0.01 for AMø versus 0.52 ± 0.17 for PMø, p < 0.04, unpaired t test). The same was true for the cloned T cell line CTLL-2, induced to apoptosis by IL-2 deprivation (4.1 ± 1.7% phagocyte for AMø versus 41.3 ± 6.0% for PM, p< 0.001, unpaired t test; phagocytic index 0.04 ± 0.02 for AMø versus 0.49 ± 0.08 for PMø, p< 0.001, unpaired t test).
Phagocytosis of apoptotic thymocytes was blocked by binding annexin V to apoptotic cells
A variety of mononuclear phagocytes, including thioglycollate-elicited murine PMø and human Mø cell lines, have been shown to recognize apoptotic cells via expression of PS on the surface of the apoptotic cell shortly after commitment to cell death. Although the receptors responsible for this recognition event are currently unknown, it is possible to block the process using annexin V itself (28). Preliminary experiments showed that unconjugated annexin V at a 40 µg/105 thymocytes (final concentration 200 µg/ml) would totally inhibit subsequent binding of annexin-FITC, and that this concentration of annexin was not toxic to macrophages during incubation for up to 16–18 h (data not shown).
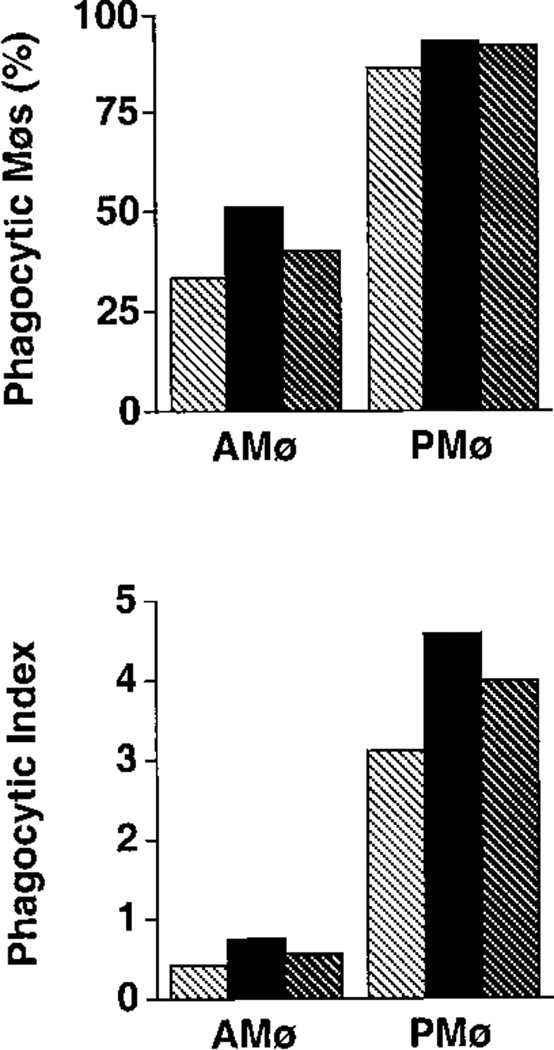
Pre-incubation of apoptotic thymocytes with annexin V substantially inhibited phagocytosis by adherent monolayers of both types of Møs (Fig. 3). Phagocytosis (expressed as percentage of positive Mø) was reduced by 84.7% in PMø and by 83.9% in AMø. The inhibitory effect of annexin was specific to recognition of apoptotic cells; pre-incubation of carboxylate microbeads with annexin V had no inhibitory effect on phagocytosis by either type of Mø (for AMø, 62.8% phagocytosis positive without annexin pre-incubation vs. 65.2%; phagocytic index 3.2 vs. 3.1; for PMø, 64.7% phagocytosis positive without annexin pre-incubation vs. 65.2; phagocytic index 2.9 vs. 3.0). Therefore, both types of murine Møs appeared to use recognition of PS expression on apoptotic T cells as a critical signal to initiate elimination.
FIGURE 3.
Mø phagocytosis of apoptotic thymocytes depends on recognition of PS. Apoptotic thymocytes were pre-incubated with recombinant human annexin V (40 µg/105 cells; final concentration 200 µg/ml)) for 15 min at room temperature. Next, without washing, these thymocytes were added to adherent Mø monolayers and co-incubated for 90 min at 37°C. Phagocytosis was determined on H & E stained slides. (A) percentage of phagocytosis; (B) phagocytic index. Light stippling represents control conditions and dark stippling represents thymocytes pretreated with annexin V. Values represent means ± SEM of four experiments. *, p <0.05, **, p <0.01, unpaired Student t test.
AMø and PMø differed in expression of surface receptors potentially involved in clearance of apoptotic cells
To attempt to define the reason for the marked difference between murine AMø and PMø in phagocytosis of apoptotic thymocytes, we next examined surface expression of VNR (CD51/CD61) and of several other integrins which might potentially be involved in clearance of apoptotic cells (Fig. 4). Expression of beta 1 and beta 2 integrins was examined in this context due to recent reports of their role in adherence to and phagocytosis of apoptotic leukocytes, respectively (32, 33). The current analysis demonstrated several differences in receptor expression between the two types of resident Mø (Table III). Both AMø and PM had detectable surface expression of CD51, although expression was more uniform and significantly greater on AMø. However, expression of CD61 was much lower on AMø than on PMø, suggesting that AMø must use an alternative integrin beta chain in conjunction with CD51. Both Mø expressed CD11a, although levels were again more uniform and significantly higher on AMø. AMø and PMø had nearly reciprocal expression of CD11b (which was high on PMø and nearly absent from AMø) and of CD11c (which had the converse expression). Total beta 2 integrin by the two Mø types was roughly equivalent, as judged by expression of the common beta chain CD18. Expression of CD29, the beta chain common to the beta 1 integrins, was equivalent on the two types of Mø. Relative to AMø, PM had higher expression of CD49d, the alpha chain of VLA-4, but not of CD49e, the alpha chain of VLA-6. Thus, differences in one or more of these receptors were potential explanations for the observed differences in phagocytosis of apoptotic thymocytes.
FIGURE 4.
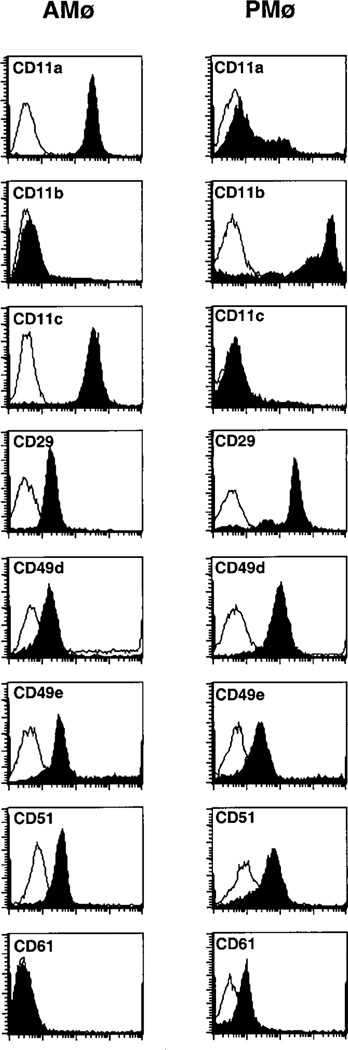
Mø expression of surface receptors potentially involved in clearance of apoptotic cells. Resident AMø and PMø from normal C57BL/6 mice were stained either with anti-mouse monoclonal antibodies against specific surface receptors (filled profile) or with isotype-matched control immunoglobulins (open profile). Representative histograms are shown.
Table III.
Mø expression of receptors potentially involved in phagocytosis of apoptotic cells *
| AMø |
PMø |
||||
|---|---|---|---|---|---|
| CD11a | 96.2 ± 1.5 † | 7‡ | 46.8 ± 6.3 | 10 | p< 0.001 § |
| CD11b | 6.6 ± 2.4 | 4 | 90.8 ± 0.9 | 8 | p< 0.001 |
| CD11c | 83.4 ± 4.8 | 4 | 6.4 ± 0.9 | 7 | p< 0.001 |
| CD14 | 7.7 ± 2.9 | 5 | 12.7 ± 2.4 | 8 | N.S. ¶ |
| CD18 | 90.8 ± 2.0 | 4 | 92.0 ± 2.5 | 7 | N.S. |
| CD29 | 94.2 ± 0.5 | 4 | 95.1 ± 0.9 | 8 | N.S. |
| CD49d | 66.4 ± 14.7 | 4 | 91.3 ± 3.1 | 11 | p = 0.02 |
| CD49e | 84.3 ± 2.8 | 6 | 78.1 ± 3.8 | 8 | N.S |
| CD51 | 90.9 ± 0.9 | 4 | 67.3 ± 3.8 | 8 | p = 0.0016 |
| CD61 | 2.7 ± 1.4 | 4 | 60.9 ± 2.7 | 8 | p< 0.001 |
Resident AMø and PMø from normal C57BL/6 mice were stained with a variety of biotinylated mAbs plus streptavidin-phycoerythrin. Expression was determined by flow cytometry
mean ± SEM % positive
n of experiments: for AMø, BAL from pairs of mice were pooled, for PMø, mice were assayed individually
unpaired Student t test comparing Mø types
not significant
To test the significance of these differences in integrin expression, we next performed blocking experiments using purified mAbs. For AMø, no single mAb significantly blocked either measurement of phagocytosis of apoptotic thymocytes compared to treatment with isotype control (Fig. 5A). Nor did several combinations of blocking mAbs (CD29 plus CD49e; CD29 plus CD51; CD51 plus CD61) have a significant effect (Fig. 5A). For PMø, small but statistically significant effects were seen in some experiments in percent of phagocytic Mø using anti-CD29 alone or the combination of anti-CD29 plus anti-CD49e and anti-CD29 plus anti-CD51 and in phagocytic index using mAb against CD29 or the combination of anti-CD29 plus anti-51 (Fig 5B). However, these changes were not seen in all experiments (data not shown). To further test the importance of integrins in the ingestion of apoptotic thymocytes by murine Mø, we preincubated adherent Mø with either of two tetrapeptides, Arginine-Glycine-Aspartic acid-Serine (RGDS) or Arginine-Gylcine-Glutamic acid-Serine (RGES). RGDS is well known to block a variety of integrins, whereas RGES serves as a non-blocking control peptide (34). No inhibition of phagocytosis was seen with either tetrapeptide (Fig. 6), providing further evidence against an obligate role for VNR (or other RGD-binding integrins) in this system.
FIGURE 5.
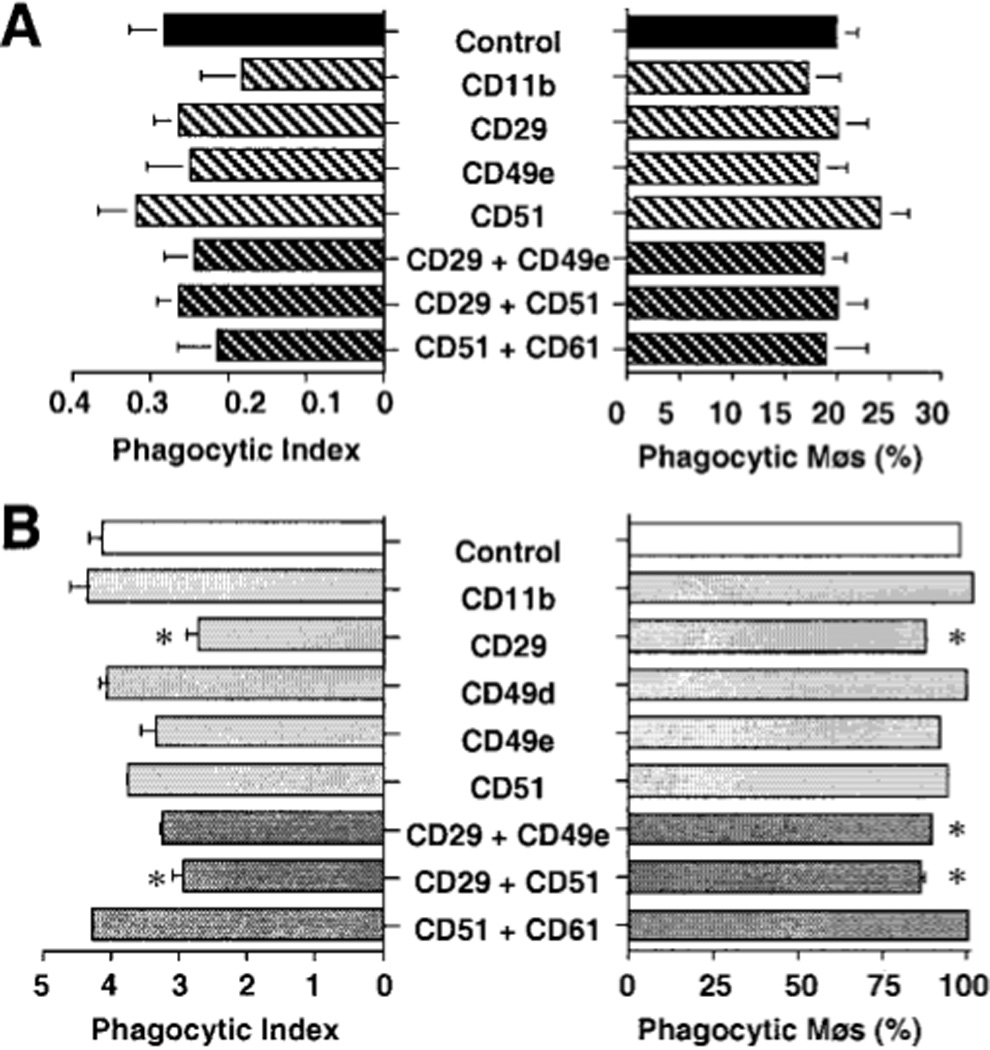
Effects of blocking antibodies on Mø phagocytosis of apoptotic thymocytes. AMø (A) and PMø (B) were incubated with saturating concentration of the designated mAbs for 30 min at 4°C. Next, treated Møs were co-incubated with apoptotic thymocytes for 90 min at 37°C. Phagocytosis was determined by inspection of H & E stained slides. Note differences in scales and in combinations of mAbs between the two type of Mø. Values represent means of three or more experiments. *, p < 0.05. ANOVA with two-tailed Dunnett post-hoc testing compared to control for that Mø type.
FIGURE 6.
Lack of inhibition of Mø phagocytosis of apoptotic thymocytes by RGDS tetrapeptide. AMø and PMø were incubated with medium (light cross-hatched), RGDS 1 mM (black bars), or RGES 1 mM (dark cross-hatched bars) for 30 min at 4°C. Next, without washing, apoptotic thymocytes were added to the treated Møs, co-incubated for 90 min at 37°C, and then phagocytosis was determined. A. Percentage of phagocytic Mø. B. Phagocytic index.
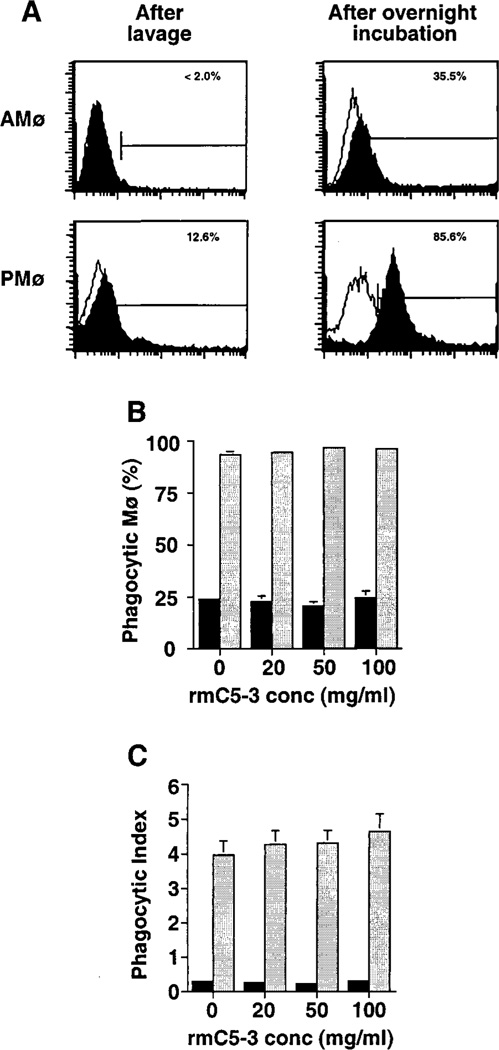
CD14 has been implicated in phagocytosis of apoptotic lymphoma cells by HMDM based on blocking experiments using the mAbs 61D3 and MEM18, both of which block the interaction of CD14 with LPS (18). Although expression of CD14 is a characteristic of human mononuclear phagocytes in a variety of tissues, detection of CD14 on murine phagocytes by mAb rmC5-3 is more variable. Both Mø types had low expression of CD14 immediately after isolation (Table III and Fig 7A). Because it was conceivable that this GPI-linked protein might have been released from the Mø surface during isolation, we analyzed surface expression after overnight incubation (i.e., at the time of assay for thymocyte ingestion) on Mø released from incubation by washing with ice-cold medium containing EDTA without enzyme treatment. This analysis showed that CD14 expression could be induced on virtually all PMø by overnight incubation, whereas only a minority of AMø expressed CD14 and that predominantly at levels only slightly above the staining with isotype control mAb (Fig 7A). No inhibitory effect on thymocyte ingestion was seen using mAb rmC5-3 at a variety of concentrations with either Mø type (Fig. 7B–C).
FIGURE 7.
mAb rmC5-3 does not inhibit murine Mø ingestion of apoptotic thymocytes. A. Flow cytometric analysis of CD14 expression immediately after recovery and after overnight incubation. Representative histograms are shown. (B-C) Effects of blocking mAb against CD14 on Mø phagocytosis of apoptotic thymocytes. AMø and PMø were incubated with various concentrations of mAb rmC5-3 against murine CD14 for 30 min at 4°C. Next, treated Møs were co-incubated with apoptotic thymocytes for 90 min at 37°C and then phagocytosis was evaluated by counting 200ntrol hamster IgG3 lambda300 Mø per condition under oil immersion. B. Percentage of phagocytic Mø. C. Phagocytic index. Data are mean ± SEM of Mø from three mice assayed individually.
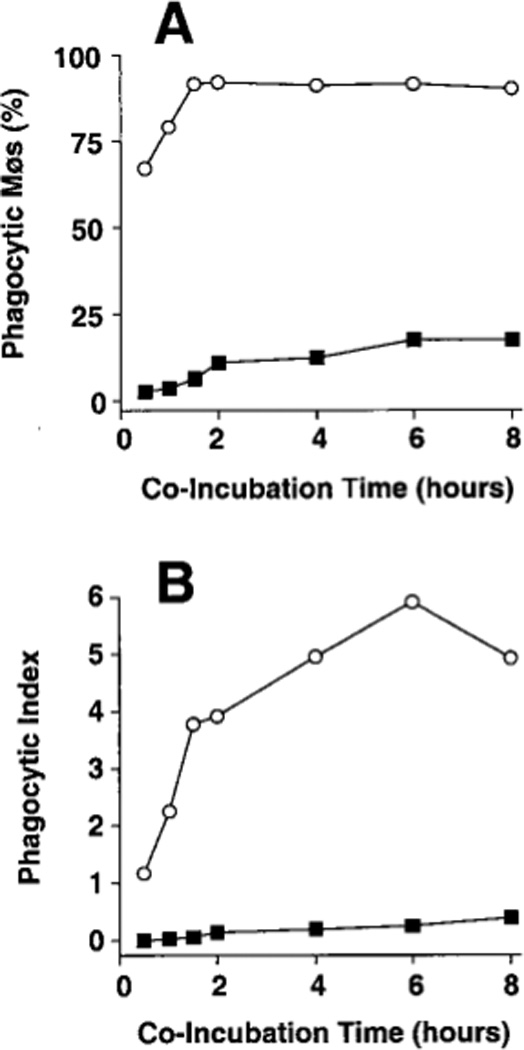
AMø and PMø had different ability to engulf apoptotic cells in vivo
Finally, to confirm the biologic significance of these in vitro results, we examined clearance of apoptotic thymocytes in vivo. Apoptotic thymocytes were administered intratracheally or intraperitoneally to mice, and then Mø were recovered by BAL or peritoneal lavage 30–240 min later, and phagocytosis was determined. Results showed that AMø ingested very few apoptotic thymocytes at any time point, whereas phagocytosis of apoptotic thymocytes by PMø was readily detected (Fig. 8). It was not feasible to calculate the phagocytic index in these experiments, as PMø appeared to degrade ingested cells more rapidly than was noted in vitro. Thus, AMø were also markedly deficient in ingestion of apoptotic cells relative to PMø in vivo.
FIGURE 8.
Phagocytosis of apoptotic thymocytes by Møs in vivo. Apoptotic thymocytes (5 × 107 in 50 µl normal saline) were injected either intratracheally or intraperitoneally into normal C57BL/6 mice. At various later times, cells were recovered by BAL or peritoneal lavage. Microscopic slides of cells recovered by lavages were prepared by cytocentrifugation, and stained with H & E, and then phagocytosis was determined. AMø, black bars, PMø lightly stippled bars. A. Time course. Values are from a single experiment; similar results were obtained in an additional experiment. B. Mean percentage of phagocytic Mø two h after in vivo inoculation. Values are mean ± SEM of three mice assayed individually for each cell type; *, p < 0.05, unpaired t test.
DISCUSSION
The major finding of this study is that compared to resident murine PMø, resident murine AMø are markedly deficient in ability to ingest apoptotic thymocytes cells both in vitro and in vivo. This deficiency appeared to be specific for apoptotic cells, as AMø ingested opsonized zymosan and carboxylate-modified polystyrene microbeads as well as did PMø. Annexin V blockade produced near total inhibition of ingestion by both Mø types, indicating that both Mø recognized the apoptotic thymocyte via its expression of PS. Although flow cytometry disclosed differences in several receptors known to be involved in clearance of apoptotic cells in other cell types, blockade of a variety of such receptors did not indicate an explanation for the deficiency of AMø in phagocytosis of apoptotic cells. In addition to explaining our previous observation that apoptotic lymphocytes are detected with surprising ease in the lungs, these results have important implications for the role of the lungs in development of autoimmunity.
The observation that murine AMø are markedly deficient in ability to ingest apoptotic thymocytes emphasizes the importance of examining the functional capacities of Mø in different organs. AMø patrol one of the body’s largest interfaces with the external environment, which they must keep free of inhaled particles and aspirated microbes without engendering excessive inflammation. The frequency of pneumonia, the most common lethal infection in hospitalized adults, on one hand, and the increasing frequency of asthma in industrialized nations on the other hand, illustrates the narrow balance needed to maintain this equilibrium. AMø are a highly differentiated type of Mø which have a predominantly suppressive role on the induction of immune responses (35, 36); however, AMø do permit local expression of T cell effector functions while inhibiting their proliferation (37). Although AMø ultimately derive from the bone marrow, there is considerable experimental evidence showing that steady state numbers of resident AMø derive largely from proliferation within the lung (38–40). Defective ingestion of apoptotic human neutrophils by resident rabbit AMø has been shown previously (12). Our results confirm and extend that finding to murine AMø and apoptotic lymphocytes (both thymocytes and a cloned T cell line). In addition, the results of our in vivo experiments demonstrate that this deficiency is not an artifact due to AMø removal from the unique environment of the lungs or of the in vitro assay.
These results are also significant because they extend to AMø recognition of apoptotic cells by their surface expression of PS. Because AMø are normally exposed to pulmonary surfactant, which is uniquely rich in PS, it was conceivable that they would eschew this recognition pathway. Instead, our annexin blocking experiments show a major contribution of PS recognition, as has previously been shown for a variety of other Mø types in humans and rodents (14–16). The negative results we obtained in blocking specific Mø integrins using both Mø types provide important data on resident tissue Mø which have previously received little attention for this function. Our results using resident murine AMø and PMø agree with those of Platt and associates who previously showed that antibodies against type 3 complement receptor (CD11b/CD18) or VNR (CD51/CD61) did not block ingestion of apoptotic thymocytes by thioglycollate-elicited murine PMø (41). These results are in contrast to the effect of blocking VNR by mAb or RGDS on ingestion of apoptotic human neutrophils by HMDM and by rat bone marrow-derived monocytes (BMDM), and of apoptotic murine thymocytes by the murine Mø cell line J774 (16, 19, 42). As discussed below, we believe this difference in results is chiefly due to the state of differentiation of the Mø studied. The mAbs against integrins and the RGDS tetrapeptide we used were in saturating concentrations and have been shown in other systems to block receptor function. Nevertheless, we cannot exclude the possibility that these antibodies did not block an epitope specific for recognition or phagocytosis of apoptotic cells. Moreover, the relatively late stage apoptotic thymocytes used may be an additional explanation for the lack of inhibition seen with anti-VNR mAbs. Our results using rmC5-3 should not be interpreted to exclude unequivocally a role for CD14 in ingestion of apoptotic lymphoid cells by resident murine AMø and PMø, as this mAb has been shown to enhance rather than block LPS-induced release of TNF-α by J774 cells (26). Indeed, only two anti-CD14 mAb among several tested have been found to inhibit uptake of apoptotic cells (18). Reagents to test additional receptors that have been implicated in clearance of apoptotic cells in other model systems (e.g., CD36, CD68) are not available in the mouse. Our results underscore the multiplicity of receptors used by Mø to ingest apoptotic cells and the likelihood that additional, uncharacterized receptors exist.
Several models have been presented to explain the interactions of this multiplicity of Mø receptors. Fadok, Savill and colleagues proposed that the receptors used for recognition of apoptotic targets depends primarily on the activation state of the Mø rather than on its species or site of origin, or the specific apoptotic target cell (14). This model is based on their observation that both HMDM and murine BMDM use VNR, whereas elicited inflammatory Mø are dependent on recognition of PS. An activated phenotype with use of PS recognition could be induced in murine BMDM by exposure to digestible particulates such as β-1,3-glucan via endogenous TGF-β elaboration (43). Interpreted in this regard, our results imply that even in the absence of inflammation, resident murine AMø and PMø also use this “inflammatory” pathway rather than the vitronectin pathway, either because of tissue differentiation or due to the burden of inhaled particles they routinely ingest. It could be argued that the initial adherence step used in our studies could have induced a switch from the vitronectin pathway to the use of PS recognition. Activation of some Mø functions by even brief adherence steps has been observed previously (44). This possibility is supported by our observation that surface expression of CD14 increased on PMø (but only slightly on AMø) after overnight incubation, which implies some degree of activation. However, the 12–16 hour period of Mø incubation we used was shorter than the 5–7 days in culture needed to mature HMDM or murine BMDM. Moreover, our in vitro results for resident PMø agree closely with those of Licht and associates who examined resident PMø of BALB/c mice and who completed their in vitro assay within 7 h of Mø harvest (21). Hence, we believe it more likely that resident AMø and PMø recognize apoptotic lymphocytes primarily via PS expression in vivo. Further experiments will be needed to test this possibility. More recently it has been observed that annexin V, unlike PS liposomes or analogues, blocks phagocytosis of apoptotic lymphocytes by both unactivated murine Mø (MBMDM and J774 cells) and elicited murine PMø (28). These findings, which agree with our results, have led Schlegel and associates to suggest that recognition of PS is a general feature in the recognition of apoptotic lymphocytes by murine Mø (28). The molecular nature of Mø receptors for PS remains undefined {reviewed in (17)}. None of these considerations detract from our major finding, that differences between resident murine Mø in maximal rate of phagocytosis of apoptotic lymphocytes depends on their organ of origin.
Impaired clearance of apoptotic lymphocytes from the lungs could contribute to development of autoimmunity in susceptible individuals by making available nucleosomes, which are highly immunogenic particles now recognized as a major auto-antigen in systemic lupus erythematosus (2). Nucleosomes are macromolecular complexes that form the basic units of chromatin. They are composed of eight core histones (four homodimers of H2A, H2B, H3, and H4), two superhelical turns of DNA, and a single encircling histone H1 molecule. Nucleosomes are polyclonal B cell activators in vitro (45, 46) and are recognized specifically by T cell clones (47). Nucleosome-specific CD4+ T cells are identifiable in the spleens of lupus-prone mice by 1 month of age, before other abnormalities develop (2). Because nucleosomes are formed in vivo exclusively by endonuclease digestion of chromatin during apoptosis, impaired clearance of apoptotic lymphocytes provides a potential mechanism for breaking peripheral self-tolerance. The extensive DNA fragmentation we have previously demonstrated in lung lymphocytes during a pulmonary immune response to a non-infectious agent (5) indicates that nucleosomes can be formed with ease in the lungs of mice. Even greater degrees of lymphocyte apoptosis, particularly of the greatly expanded CD8+ T cell effector populations, occur during the resolution of viral infections (48, 49). Several considerations suggest that the lungs are a key site of clearance of apoptotic lymphocytes. Normal lungs contain a large fraction of body’s total lymphocytes, primarily as single cells within alveolar capillaries and walls (50, 51). Lung lymphocytes are highly enriched for activated T cells (27, 52–54), many of which appear not to ever leave the lungs (55). Thus, the observed deficiency in phagocytosis of apoptotic lymphocytes by AMø even more surprising.
The reason for the characteristic of AMø is currently unknown. Given the extensive kinetic studies we performed, including relatively short time points both in vitro and in vivo, we have rejected the possibility that AMø actually ingested and digested apoptotic lymphocytes so rapidly that they could not be detected. We have considered three potential mechanisms. First, AMø ingestion of apoptotic cells may be down-regulated due to previous ingestion of digestible particles or even apoptotic cells themselves. The latter possibility was recently demonstrated in vitro using rat BMDM and apoptotic neutrophils (42). That study showed that an initial round of phagocytosis led subsequently (after 48 h) to decreased phagocytosis of apoptotic neutrophils, but not of opsonized erythrocytes, which would be recognized via Fc receptors. Such a specific defect in phagocytosis of apoptotic targets is just what we found. A second, teleological possibility is that a level of indifference to apoptotic cells is an evolutionary adaptation that preserves the capacity of AMø to produce pro-inflammatory cytokines for host defense of the lungs. Phagocytosis of apoptotic neutrophils has been shown to inhibit actively and specifically the production by HMDM of IL-1β, IL-8, IL-10, GM-CSF, and TNF-α, as well as leukotriene C4 and thromboxane B2 (56). This effect is mediated by autocrine/paracrine elaboration of TGF-β, PGE2, and platelet-activating factor (56). Uptake of apoptotic lymphocytes by murine peritoneal Mø has recently been shown to favor the growth of the protozoan pathogen Trypanosoma cruzi via mechanisms that depend on prostaglandins, TGF-β, and polyamines (57). A third possibility is that AMø are relatively deficient in currently unknown receptors for recognition or phagocytosis of apoptotic leukocytes. These possibilities are not mutually exclusive, and multiple mechanisms could underlie the phenotype we observed in murine AMø.
Two possible limitations of the current study should be considered. First, we used overnight (12–16 h) dexamethasone incubation for the majority of experiments because it resulted in very uniform thymocyte apoptosis, as evidenced by the annexin-V positivity of >95%. This treatment is longer than the 4–6 h treatment used in many other studies, and it resulted in a relatively late stage of apoptosis denoted by the 42% propidium iodide staining we found. Based on the experiments using 6 h dexamethasone treatment, CTLL-2 cells, and neutrophils, we do not believe that late apoptosis of the thymocytes alone explains the underlying deficiency by AMø. However, the duration of apoptosis should be considered in interpreting the inhibition experiments. Second, we examined only resident (i.e., non-elicited) AMø and PMø from normal mice. It is clear that the relative deficiency we have found in phagocytosis of apoptotic T cells by resident Mø from normal mice can be overcome at the level of the total lung mononuclear phagocyte population during lung infection or inflammation, when large numbers of dying T cells and other leukocytes must be cleared rapidly and specifically. Previous studies have, in fact, shown that mononuclear phagocytes recovered during resolving pneumonia have ingested apoptotic neutrophils (58–60). The capacity to recognize and ingest apoptotic cells is lacking in freshly isolated human blood monocytes (12, 19), but is induced rapidly (4 h) in a dose-dependent fashion by GM-CSF, TGF-β, IFN-γ, and IL-1-β (61). Physiological modification could occur via changes in resident AMø themselves, by altered differentiation of recruited blood monocytes in the inflammatory environment, or both. Thus, our findings are most relevant to the non-inflammed lungs.
In summary, we have demonstrated an unanticipated and pronounced deficiency in phagocytosis of apoptotic lymphocytes by resident murine AMø. Additional experiments are needed to define whether this defect exists in human AMø and to establish whether the defect can be overcome by AMø activation.
ACKNOWLEDGMENTS
We thank Drs. James M. Beck, Bethany Moore, Geneva Omann, Robert Paine III, Galen B. Toews, and Jo Rae Wright for helpful suggestions; Michael Hormuth and Carolyn White for assistance with the photomicrographs; Joyce O’Brien for secretarial support; and Dr. Paine for critiquing the manuscript.
Supported by RO1 HL56309 and RO-1 HL6157 from the USPHS; and by Merit Review funding and a Research Enhancement Award Program (REAP) grant from the Department of Veterans Affairs. Dr. Curtis is a Career Investigator of the American Lung Association of Michigan.
Abbreviations used
- AMø
alveolar macrophage
- BAL
bronchoalveolar lavage
- BMDM
bone marrow-derived macrophage
- IT
intratracheal
- HMDM
human monocyte-derived macrophage
- PBS
phosphate-buffered saline
- PMø
peritoneal macrophage
- PS
phosphatidylserine
- VNR
vitronectin receptor
Footnotes
Portions of these data were presented at the Midwest Autumn Immunology Meeting (Chicago, IL; November 21, 1999) and at the International Scientific Conference of the American Thoracic Society (Toronto, Ottawa; May 2000), and have been published in abstract form.
REFERENCES
- 1.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 2.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 5.Milik AM, Beuchner-Maxwell VA, Kim S, Sonstein J, Seitzman GD, Beals TF, Curtis JL. Lung lymphocyte elimination by apoptosis in the murine response to intratracheal particulate antigen. J Clin Invest. 1997;99:1082. doi: 10.1172/JCI119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipscomb MF, Russell SW. Lung macrophages and dendritic cells in health and disease. In: Lenfent C, editor. Lung biology in health and disease. Vol. 107. New York: Marcel Dekker; 1997. p. 855. [Google Scholar]
- 7.Brody AR. Whither goes the alveolar macrophage? Another small chapter is written on the localized response of this crucial cell. J Lab Clin Med. 1998;131:391. doi: 10.1016/s0022-2143(98)90138-x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa K, Suzuki Y, Kawai M, Fukada M, Yokochi T. Novel cell surface antigens expressed on mouse alveolar macrophages. Microbiol Immunol. 1991;35:803. doi: 10.1111/j.1348-0421.1991.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 9.Lipscomb MF, Lyons CR, Nunez G, Ball EJ, Stanstny P, Vial W, Lem V, Weissler JC, Miller LM, Toews GB. Human alveolar macrophages: HLA-DR-positive cells that are poor stimulators of a primary mixed leukocyte reaction. J Immunol. 1986;136:497. [PubMed] [Google Scholar]
- 10.Bigby TD, Holtzman MJ. Enhanced 5-lipoxygenase activity in lung macrophages compared to monocytes from normal subjects. J Immunol. 1987;138:1546. [PubMed] [Google Scholar]
- 11.Balter MS, Toews GB, Peters-Golden M. Different patterns of arachidonate metabolism in autologous human blood monocytes and alveolar macrophages. J Immunol. 1989;142:602. [PubMed] [Google Scholar]
- 12.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156:430. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlegel RA, Callahan M, Krahling S, Pradhan D, Williamson P. Mechanisms for recognition and phagocytosis of apoptotic lymphocytes by macrophages. Adv Exp Med Biol. 1996;406:21. doi: 10.1007/978-1-4899-0274-0_3. [DOI] [PubMed] [Google Scholar]
- 14.Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029. [PubMed] [Google Scholar]
- 15.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Braton DL, Henson PM. Exposure of phophatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207. [PubMed] [Google Scholar]
- 16.Pradhan D, Krahling S, Williamson P, Schlegel RA. Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol Biol Cell. 1997;8:767. doi: 10.1091/mbc.8.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 18.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 19.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 20.Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351. [PMC free article] [PubMed] [Google Scholar]
- 21.Licht R, Jacobs CW, Tax WJ, Berden JH. An assay for the quantitative measurement of in vitro phagocytosis of early apoptotic thymocytes by murine resident peritoneal macrophages. J Immunol Methods. 1999;223:237. doi: 10.1016/s0022-1759(98)00212-9. [DOI] [PubMed] [Google Scholar]
- 22.Kaltreider HB, Byrd PK, Curtis JL. Expression of Ia by murine alveolar macrophages is up-regulated during the evolution of a specific immune response in pulmonary parenchyma. Am Rev Respir Dis. 1988;137:1411. doi: 10.1164/ajrccm/137.6.1411. [DOI] [PubMed] [Google Scholar]
- 23.Lou Y, Dorf ME. Isolation of murine neutrophils. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current protocols in immunology. Vol. 1. Secaucus, NJ: John Wiley & Sons, Inc; 1997. p. 3.20.1. [Google Scholar]
- 24.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 25.Curtis JL, Kaltreider HB. Characterization of bronchoalveolar lymphocytes during a specific antibody-forming cell response in the lungs of mice. Am Rev Respir Dis. 1989;139:393. doi: 10.1164/ajrccm/139.2.393. [DOI] [PubMed] [Google Scholar]
- 26.Hisaka H, Kataoka M, Higuchi Y, Matsuura K, Yamamoto S. Close localization of mouse CD14 and CD32/16 in the cell surface of monocytic cell lines. Pathobiology. 1999;67:92. doi: 10.1159/000028056. [DOI] [PubMed] [Google Scholar]
- 27.Curtis JL, Kim S, Scott PJ, Buechner-Maxwell VA. Adhesion receptor phenotypes of murine lung CD4+ T cells during a pulmonary immune response to sheep erythrocytes. Am J Respir Cell Mol Biol. 1995;12:520. doi: 10.1165/ajrcmb.12.5.7537969. [DOI] [PubMed] [Google Scholar]
- 28.Krahling S, Callahan MK, Williamson P, Schlegel RA. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999;6:183. doi: 10.1038/sj.cdd.4400473. [DOI] [PubMed] [Google Scholar]
- 29.Zar JH. Biostatistical analysis. Prentice-Hall: Englewood Cliffs; 1974. [Google Scholar]
- 30.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 31.Perticarari S, Presani G, Mangiarotti MA, Banfi E. Simultaneous flow cytometric method to measure phagocytosis and oxidative products by neutrophils. Cytometry. 1991;12:687. doi: 10.1002/cyto.990120713. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz BR, Karsan A, Bombeli T, Harlan JM. A novel beta 1 integrin-dependent mechanism of leukocyte adherence to apoptotic cells. J Immunol. 1999;162:4842. [PubMed] [Google Scholar]
- 33.Moffatt OD, Devitt A, Bell ED, Simmons DL, Gregory CD. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J Immunol. 1999;162:6800. [PubMed] [Google Scholar]
- 34.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 35.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upham JW, Strickland DH, Bilyk N, Robinson BWS, Holt PG. Alveolar macrophages from humans and rodents selectively inhibit T-cell proliferation but permit T-cell activation and cytokine secretion. Immunology. 1995;84:142. [PMC free article] [PubMed] [Google Scholar]
- 38.van oud Alblas AB, van Furth R. Origin, kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979;149:1504. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shellito J, Esparza C, Armstrong C. Maintenance of the normal rat alveolar macrophage cell population: the roles of monocyte influx and alveolar macrophage proliferation in situ . Am Rev Respir Dis. 1987;135:78. doi: 10.1164/arrd.1987.135.1.78. [DOI] [PubMed] [Google Scholar]
- 40.Tarling JD, Lin H, Hsu S. Self-renewal of pulmonary alveolar macrophages: evidence from chimera studies. J Leukocyte Biol. 1987;42:443. [PubMed] [Google Scholar]
- 41.Platt N, da Silva RP, Gordon S. Class A scavenger receptors and the phagocytosis of apoptotic cells. Immunol Lett. 1999;65:15. doi: 10.1016/s0165-2478(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 42.Erwig LP, Gordon S, Walsh GM, Rees AJ. Previous uptake of apoptotic neutrophils or ligation of integrin receptors downmodulates the ability of macrophages to ingest apoptotic neutrophils. Blood. 1999;93:1406. [PubMed] [Google Scholar]
- 43.Fadok VA, Laszlo DJ, Noble PW, Weinstein L, Riches DW, Henson PM. Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells. J Immunol. 1993;151:4274. [PubMed] [Google Scholar]
- 44.Standiford TJ, Kunkel SL, Kasahara K, Milia MJ, Rolfe MW, Strieter RM. Interleukin-8 gene expression from human alveolar macrophages: the role of adherence. Am J Respir Cell Mol Biol. 1991;5:579. doi: 10.1165/ajrcmb/5.6.579. [DOI] [PubMed] [Google Scholar]
- 45.Bell DA, Morrison B, VandenBygaart P. Immunogenic DNA-related factors. Nucleosomes spontaneously released from normal murine lymphoid cells stimulate proliferation and immunoglobulin synthesis of normal mouse lymphocytes. J Clin Invest. 1990;85:1487. doi: 10.1172/JCI114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell DA, Morrison B. The spontaneous apoptotic cell death of normal human lymphocytes in vitro: the release of, and immunoproliferative response to, nucleosomes in vitro. Clin Immunol Immunopathol. 1991;60:13. doi: 10.1016/0090-1229(91)90108-m. [DOI] [PubMed] [Google Scholar]
- 47.Mysler E, Bini P, Drappa J, Ramos P, Friedman SM, Krammer PH, Elkon KB. The apoptosis-1/Fas protein in human systemic lupus erythematosus. J Clin Invest. 1994;93:1029. doi: 10.1172/JCI117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akbar AN, Savill J, Gombert W, Bofill M, Borthwick NJ, Whitelaw F, Grundy J, Janossy G, Salmon M. The specific recognition by macrophages of CD8+,CD45RO+ T cells undergoing apoptosis: A mechanism for T cell clearance during resolution of viral infections. J Exp Med. 1994;180:1943. doi: 10.1084/jem.180.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Razvi ES, Jiang Z, Woda BA, Welsh RM. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. Am J Pathol. 1995;147:79. [PMC free article] [PubMed] [Google Scholar]
- 50.Pabst R, Binns RM, Licence ST, Peter M. Evidence of a selective major vascular marginal pool of lymphocytes in the lung. Am Rev Respir Dis. 1987;136:1213. doi: 10.1164/ajrccm/136.5.1213. [DOI] [PubMed] [Google Scholar]
- 51.Nelson D, Strickland D, Holt PG. Selective attrition of non-recirculating T cells during normal passage through the lung vascular bed. Immunol. 1990;69:476. [PMC free article] [PubMed] [Google Scholar]
- 52.Saltini C, Kirby M, Trapnell BC, Tamura N, Crystal RG. Biased accumulation of T lymphocytes with “memory”-type CD45 leukocyte common antigen gene expression on the epithelial surface of the human lung. J Exp Med. 1990;171:1123. doi: 10.1084/jem.171.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coulson PS, Wilson RA. Pulmonary T helper lymphocytes are CD44hi, CD45RB- effector/memory cells in mice vaccinated with attenuated cercariae of Schistosoma mansoni . J Immunol. 1993;151:3663. [PubMed] [Google Scholar]
- 54.Seitzman GD, Sonstein J, Choy W, Kim S, Curtis JL. Lung lymphocytes proliferate minimally in the murine pulmonary immune response to intratracheal sheep erythrocytes. Am J Respir Cell Mol Biol. 1998;18:800. doi: 10.1165/ajrcmb.18.6.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Upham JW, McMenamin C, Schon-Hegrad MA, Robinson BW, Holt PG. Functional analysis of human bronchial mucosal T cells extracted with interleukin-2. Am J Respir Crit Care Med. 1994;149:1608. doi: 10.1164/ajrccm.149.6.7911708. [DOI] [PubMed] [Google Scholar]
- 56.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freire-de-Lima CG, Nacimento DO, Soares MBP, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 58.Shellito J, Sniezek M, Warnock M. Acquisition of peroxidase activity by rat alveolar macrophages during pulmonary inflammation. Am J Pathol. 1987;129:567. [PMC free article] [PubMed] [Google Scholar]
- 59.Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- 60.Ishii Y, Hashimoto K, Nomura A, Sakamoto T, Uchida Y, Ohtsuka M, Hasegawa S, Sagai M. Elimination of neutrophils by apoptosis during the resolution of acute pulmonary inflammation in rats. Lung. 1998;176:89. doi: 10.1007/pl00007597. [DOI] [PubMed] [Google Scholar]
- 61.Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol. 1995;154:2366. [PubMed] [Google Scholar]