Abstract
The purpose of this review is to summarize our current understanding of the physiological roles of apoA-IV in metabolism, and to underscore the potential for apoA-IV to be a focus for new therapies aimed at the treatment of diabetes and obesity-related disorders. ApoA-IV is primarily synthesized by the small intestine, attached to chylomicrons by enterocytes, and secreted into intestinal lymph during fat absorption. In circulation, apoA-IV is associated with HDL and chylomicron remnants, but a large portion is lipoprotein free. Due to its anti-oxidative and anti-inflammatory properties, and because it can mediate reverse-cholesterol transport, proposed functions of circulating apoA-IV have been related to protection from cardiovascular disease. This review, however, focuses primarily on several properties of apoA-IV that impact other metabolic functions related to food intake, obesity, and diabetes. In addition to participating in triglyceride absorption, apoA-IV can act as an acute satiation factor through both peripheral and central routes of action. It also modulates glucose homeostasis through incretin-like effects on insulin secretion, and by moderating hepatic glucose production. While apoA-IV receptors remain to be conclusively identified, the latter modes of action suggest that this protein holds therapeutic promise for treating metabolic disease.
Keywords: chylomicron, intestinal lipid transport, lymph fistula mouse model, glucose tolerance, incretins, diabetes, obesity
ApoA-IV was discovered 40 years ago as a major protein component of chylomicrons in postprandial lymph and plasma of both humans and rats (1–3). In plasma it is found on chylomicrons and on HDL, but 40–50% circulates lipid free (1–3). The presence of apoA-IV in the blood is uniquely linked to the absorption and secretion of dietary lipid. It is synthesized by the intestine and secreted into mesenteric lymph on chylomicrons. During hydrolysis of the chylomicron triglycerides in the circulation, most of the apoA-IV dissociates from the chylomicrons. Approximately 25% transfers to plasma HDL and the rest is found in the lipoprotein-free fraction of plasma (4, 5). The reason why apoA-IV detaches from chylomicrons during lipolysis is not fully known, but is likely due to the shrinking surface area of the particles plus competition for the remaining lipophilic surface with other exchangeable apolipoproteins such as apoE and the apoCs, as well as other changes in the environment of the chylomicron particle. apoA-IV is also found in bile and cerebrospinal fluid (6).
Over the years there has been an arduous search by several investigators to discover the physiological functions of this protein. Initial studies were focused on lipid absorption, lipoprotein metabolism, and cardiovascular diseases related to hyperlipidemia. Studies of genetically modified mice, as well as some clinical studies, suggested that apoA-IV protects against atherosclerosis (7–10). While a mechanism related to lipid absorption is possible, apoA-IV also promotes reverse cholesterol transport and has potent antioxidant and anti-inflammatory properties (10–16), which may be the relevant functions in those models (8–10). While these properties are potentially very important, they are not unique to apoA-IV and have been very well-characterized in the related apolipoproteins, apoA-I and apoE. The top half of Table 1 lists the proposed cardiovascular-related functions of apoA-IV with a brief summary of the supporting data. These functions will not be discussed in detail in this review but several references are included in Table 1 for the interested reader.
TABLE 1.
Summary of proposed physiological functions of apoA-IV
| Proposed Function | Nature of Evidence | References |
| Protects from atherosclerosis | Human genetic data correlated with large deletions in apoA1-apoA5 locus; reduced aortic lesions plus higher HDL in human or mouse apoA-IV transgenic mice; protection conferred even in -ull or LDL receptor-null animals | 7–10 |
| Anti-oxidant protein | Decreased copper-mediated LDL oxidation in vitro; decreased oxidation paramters in vivo in apoA-IV transgenic/apoE KO mice | 10, 15 |
| Anti-inflammatory protein | apoA-IV administration diminished severity of experimental colitis in WT and apoA-IV KO mice | 16 |
| Promotes reverse cholesterol transport | Enhanced cholesterol efflux from cultured macrophage and fibroblasts to medium with recombinant or purified apoA-IV, or lipid-free serum from apoA-IV transgenic mice | 11–14 |
| Affects intestinal lipid absorption | Changes in regional lipid absorption and gene expression in KO mice; minor changes in chylomicron size and composition; affects chylomicron hydrolysis and metabolism | 73, 74, 79–81a |
| Affects stomach emptying | Reduced rate of emptying and decreased acid secretion by icv administration of purified apoA-IV | 87–94a |
| Satiety factor | Reduced food intake with either peripheral or central apoA-IV administration | 55, 82–86a |
| Promotes insulin secretion | Decreased glucose tolerance and insulin secretion in KO mice; increased insulin secretion after ip apoA-IV administration | 17a |
| Modulates hepatic gluconeogenesis | apoA-IV decreases gluconeogenesis by primary hepatocytes; involvement of NR1D1 | 144a |
Discussed in this review.
We focus instead on properties more unique to apoA-IV among the apolipoproteins. These functions are listed in the bottom half of Table 1 and are discussed in detail later. apoA-IV has been shown to affect satiation and acute food consumption through interaction with other peripheral and central (nervous system) regulators. Recently our group has discovered that apoA-IV also contributes to glucose homeostasis through an incretin-like function (17), positioning this protein as a new and potentially very important therapeutic molecule for treatment of type II diabetes and metabolic disease. Which of these functions is (are) more important remains to be demonstrated, and will require continued investigation using animal models, especially genetically modified mice, as well as additional clinical studies designed to evaluate these newly discovered properties of apoA-IV.
In this review, we summarize several aspects of our current knowledge about apoA-IV genetics, regulation, protein structure, and function, and then individually discuss the various physiologic functions of apoA-IV by focusing on each of the pathways to which it may contribute, lipid absorption and metabolism, food intake and weight regulation, and glucose metabolism. Throughout, we discuss questions that remain unanswered in each of these areas and approaches that are needed for productive future investigations. Finally, we present a model for apoA-IV function that represents our current understanding, and close with some perspectives on future directions with the potential for translational application of apoA-IV to cardiometabolic diseases.
apoA-IV GENE, PROTEIN STRUCTURE, AND GENETIC VARIANTS
In humans, the APOA4 gene is located on chromosome 11q23 (chromosome 9 in mouse) as part of the gene cluster that also includes APOA1, APOC3, and APOA5 (18). It is located 6.6 kb downstream of APOC3 (which is oriented in the opposite direction) and 28 kb upstream of APOA5. It is comprised of three exons spread over 2.6 kb, which encode a 396 amino acid (376 after processing) 46 kDa protein. Similar to the related protein apoA-I, apoA-IV consists of 12 amphipathic helices that facilitate both lipid binding and aqueous interaction, resulting in a protein that binds to the surface of lipid particles but is readily exchangeable, consistent with its role in metabolism of both chylomicrons and HDL, as well as its abundance lipid-free in the circulation. Key features of the structure and function of these helices and other aspects of apoA-IV were determined by crystallography, cross-linking, and mutational analyses of lipid binding properties (19, 20). Analysis of point mutations and deletions revealed that the lipid binding properties are conferred primarily by the central helices. Regions near the N and C termini, on the other hand, raise the activation energy for lipid binding, apparently through direct interaction of the two ends that stabilizes the lipid-free form (20). Crystallography data revealed that lipid-free apoA-IV exists primarily as a homodimer with the two molecules aligned in anti-parallel fashion. The 12 helices are arranged in four helical bundles, a long central bundle of five helix segments flanked by a short helix on one end and three helix segments arranged into two bundles at the other end. The four bundles of each strand interact to shield the hydrophobic regions of the other strand. The current model is that lipid binds to this structure initially in a central hydrophobic pocket, and as more lipid is incorporated, the hydrophobic regions of the end and central bundles relax away from each other to accommodate, eventually forming a disc-like and ultimately spherical particle (19).
The APOA1/APOC3/APOA4/APOA5 gene cluster is important for lipoprotein metabolism and the maintenance of plasma lipid levels. The entire gene cluster has been a focus of clinical interest since a genetic study showed that deletion of a portion of this locus greatly increased the risk of cardiovascular events (7). A number of subsequent genetic studies have confirmed the importance of this locus for susceptibility to heart disease, especially dyslipidemias that involve high serum triglyceride and/or low HDL. A role for apoA-IV in protecting against heart disease was first implicated by transgenic mouse studies which showed that animals with elevated intestinal expression of the protein were protected from diet-induced atherosclerosis (8–10). Several isoforms of apoA-IV have been known since shortly after the identification of the protein in human plasma over 30 years ago (21). While at least five variants caused by point mutations or small insertions have been identified in various populations (21–23), there are three polymorphic sites in the APOA4 gene that are more common and have been of clinical interest: N147S, T347S, and Q360H. The first of these is located in the long central helix bundle B, while the other two are located in helix bundle D nearer the C terminus (19). Interestingly, the Q360H mutation introduces a charge near a region that is known to affect lipid binding.
While clinical correlates for APOA1, APOC3, and APOA5 mutations have been clearly documented, the clinical effects of these APOA4 mutations on cardiovascular risk have been more difficult to establish. This may result, in part, from linkage disequilibrium between APOA5 or APOC3 mutants and APOA4 SNPs of interest, such that apparent APOA4 effects are actually due to cosegregating risk alleles of one or more of the other genes in the cluster (24). However, the controversy could also result from sampling parameters used in the studies. Fasting serum is typically collected for lipid and lipoprotein measurements in clinical studies. Because apoA-IV function is related to chylomicron secretion and metabolism and response to dietary lipid load, which are not measured by fasting lipids, potential effects of mutant APOA4 alleles may have been missed in some studies. Gomez et al. (25) found that the T347S difference modified the effect of APOA1 mutations on LDL particle size in a manner that was diet dependent. An earlier study by Hockey et al. (26) suggested that the 360H allele, which was predicted to have greater lipid affinity, delays postprandial chylomicron clearance compared with 360Q, with some suggestion that the 347S allele may have the opposite effect. Other studies have also suggested linkage between the 347 and 360 polymorphisms and response to diet (27, 28). Interestingly, a report by Liu et al. (29) suggests that the N147S polymorphism may affect the response of individuals to fibrate treatment, further indicating the potential clinical importance of APOA4 genotype screening with respect to metabolic disease risk and for potential therapeutic indications. While these studies suggest a role for apoA-IV in lipid metabolic diseases, only a few clinical studies have investigated its linkage to diseases of glucose metabolism and energy utilization (30–32). Further studies of this type may now be warranted by the newer findings showing a role for apoA-IV in glucose metabolism, as will be discussed later.
GENE REGULATION
Each gene in the APOA1/APOC3/APOA4/APOA5 cluster displays distinct tissue-specific expression patterns. APOA1 is expressed in both liver and intestine; APOC3 is primarily expressed in liver and to a lesser degree in the intestine; and APOA5 appears to be exclusive to the liver (33). APOA4 is expressed primarily in the small intestine with minor amounts made in the liver (34, 35). As will be discussed later, the neurons in the hypothalamus and arcuate nucleus (ARC) are also capable of synthesizing apoA-IV and it has recently been reported in dendritic cells of the immune system, although its function in the latter remains to be investigated (36). The jejunum is the major site of apoA-IV synthesis, but it is also produced in the duodenum and ileum (37). apoA-IV expression can be regulated by a number of factors, including lipids, glucocorticoids, thyroid hormone, estrogen, insulin, and metabolic state (35, 38–42). This regulation is tissue specific and can also be species specific. For example, expression in the intestine is increased by estrogen in rats but decreased in mice (43). The effects of insulin and glucocorticoids on intestinal apoA-IV synthesis may be age dependent and may differ among species. Some reports indicate that apoA-IV does not respond to these effectors (35, 38–41), although jejunal explants from 2-day-old piglets were found to respond with increased apoA-IV production (44).
Hepatic apoA-IV expression is low and is strain dependent in mice (42). Expression is influenced by metabolic state as well as the various hormones mentioned above (35, 38, 39, 41). Whether or not this holds true for human liver expression is not known. Hepatic apoA-IV expression was thought to be rodent specific but its presence in human liver and several human hepatocyte cell lines has been documented (45, 46), and most DNA response elements are similar between the species. Some uncertainty arises because hepatocyte cell lines do not adequately reflect in vivo conditions. Conversely human liver samples from subjects in various metabolic states have not been tested, and current literature indicates that apoA-IV expression is very responsive to energy balance in the intestine, liver, and brain in rodents (42, 45, 46).
While extensive characterization of the physiological regulation of apoA-IV has been done in rats and genetically modified mice, DNA sequences and binding proteins involved in molecular regulation of APOA4 gene expression have been less thoroughly investigated. Nonetheless, at least two key regions have been identified, one being a hormone response element 300–700 bp upstream from the transcription start site that binds HNF-4α and confers villus-specific expression to APOA4 (47). Recently members of the cyclic AMP response element binding protein (CREB) family of transcription factors have been shown to increase APOA4 expression (36, 48) and specific binding sites for CREBH were identified proximal to both human and mouse APOA4 genes (48). Interestingly, one of the CREB members, LUMAN (CREB3), increased APOA4 expression ∼5-fold in dendritic cells without affecting other members of the gene cluster (36), but the physiological importance of apoA-IV protein in these cells remains to be investigated. Estrogen related receptor-α (ERR-α) has been shown to regulate APOA4, however this appears to act through the closely linked APOC3 promoter/enhancer region (49). A PPAR-α response element was also demonstrated ∼3 kb upstream of APOA4 and was shown to activate the gene in HepG2 cells (46). Whether it also functions in vivo is not known, and it is possible that it is primarily involved in APOC3 regulation.
PHYSIOLOGICAL REGULATION OF apoA-IV
Diet, fasting, and feeding
Of the major apolipoproteins secreted by the intestine, only apoA-IV is stimulated by lipid absorption; neither protein nor carbohydrate absorption affects apoA-IV secretion. Kalogeris, Fukagawa, and Tso (50) found that intestinal apoA-IV secretion is closely tied to the transport of long-chain fatty acids in chylomicrons, with higher doses of administered lipid eliciting a graded increase of apoA-IV secretion into lymph. Absorption of medium- or short-chain fatty acids does not stimulate apoA-IV synthesis or secretion (51). In addition to stimulating apoA-IV secretion (2, 52), lipid feeding also stimulates its synthesis several fold during active lipid absorption and chylomicron formation in the small intestine (53, 54). This stimulation of apoA-IV secretion occurs rapidly (within one-half hour following the onset of active lipid absorption) and is unique to apoA-IV among the apolipoproteins (55). Fasting markedly reduces circulating and central apoA-IV levels, with lowered apoA-IV gene expression in both the jejunum and the hypothalamus (56). Refeeding fasted rats with a low fat diet (LFD) (e.g., regular rodent chow) evokes a significant increase of apoA-IV mRNA in the jejunum but not in the hypothalamus, whereas refeeding them with a lipid-rich meal raises apoA-IV mRNA levels in both hypothalamus and jejunum (56). The mechanisms underlying this differential response remain to be investigated.
While acute consumption of a lipid meal upregulates apoA-IV synthesis and secretion, chronic consumption of a high fat diet (HFD) changes this response. Liu et al. (57) maintained rats on a HFD (20% by weight of fat, 19% butter fat, and 1% safflower oil to prevent essential fatty acid deficiency), LFD (4% fat, 3% by weight of butter fat, and 1% of safflower oil), or standard chow (CHOW) for 2, 4, 6, 8, or 10 weeks. Rats fed the HFD had significantly greater body weight (diet-induced obesity) than LFD or CHOW rats. Intestinal and plasma apoA-IV levels were comparable between different dietary groups and over time. LFD and CHOW rats had comparable hypothalamic apoA-IV mRNA across the course of the experiment. However, HFD rats had a slow progressive reduction in hypothalamic apoA-IV mRNA that became significantly lower than that of LFD or CHOW rats by 10 weeks. Intragastric infusion of a lipid emulsion to overnight-fasted animals significantly stimulated hypothalamic apoA-IV mRNA in LFD and CHOW rats, but had a minimal effect in HFD rats (57). These results imply that chronic consumption of a HFD and/or becoming obese significantly reduces apoA-IV mRNA levels and the responsiveness of apoA-IV gene expression to dietary lipids in the hypothalamus (57). In that study, we also tested to determine whether it was the obesity or the high fat content that was responsible for the decrease in hypothalamic apoA-IV. This was achieved by pair-feeding the HFD to match calories consumed by CHOW-fed animals. In that scenario, hypothalamic apoA-IV did not decrease, and so we concluded that the decrease in hypothalamic apoA-IV was caused by obesity and not the high fat content of the diet. Furthermore, we proposed that the dysregulation of hypothalamic apoA-IV could contribute to diet-induced obesity. Further supporting this concept is the observation that the attenuated responsiveness of apoA-IV to fasting and lipid feeding has also been observed in ob/ob mice (P. Tso et al., unpublished observations).
Interestingly, because of the effect of acute lipid feeding on apoA-IV synthesis and secretion (58), plasma apoA-IV is a good marker of triglycerides after fat ingestion. That is, plasma apoA-IV is positively correlated with plasma triglycerides during fasting and after a lipid-rich meal (59–61). It was once thought that apoA-IV would be a useful biomarker of fat intake in animals and in humans (60, 61). However, the muted response in obese subjects and HFD animals has greatly diminished the potential utility of apoA-IV as a marker of fat intake for this purpose (57, 62–66). Rather, this muted response may be a factor reducing the effects of apoA-IV in obese animals (and humans), including regulation of food intake and insulin secretion, as discussed later.
Circadian rhythm of apoA-IV synthesis and secretion by intestine and brain
Serum apoA-IV exhibits circadian rhythm (67) and it increases during the dark phase, corresponding to the most active feeding period of the rodents. The serum apoA-IV circadian rhythm was abolished by lymph diversion, suggesting that lymph apoA-IV flux into the blood stream was responsible for the serum apoA-IV circadian rhythm. These results were consistent with meal-stimulated intestinal apoA-IV synthesis and secretion being the major source of plasma apoA-IV. Bile diversion abolishes the circadian rhythm of lymphatic apoA-IV output, thus suggesting that bile is a major determinant of the circadian rhythm of lymph apoA-IV, probably due to its necessity for luminal lipid digestion and thus absorption and chylomicron secretion. It should also be noted that lymph apoA-IV output in the fasting state is maintained by bile flow, possibly as a supply of phospholipids as well as bile acids to support intestinal “VLDL” secretion during fasting.
The circadian rhythm of brain apoA-IV expression is quite different, however. Liu et al. (68) examined the diurnal pattern of hypothalamic apoA-IV gene and protein expression in ad libitum-fed as well as feed time-restricted (food provided 4 h daily during the light cycle) rats. In ad libitum-fed rats, hypothalamic apoA-IV mRNA and protein levels were highest during the light phase, peaking 3 h after lights on, and with a nadir 3 h after lights off. This pattern is opposite to the serum apoA-IV pattern. To determine whether the hypothalamic pattern of apoA-IV is related to food intake or to the diurnal cycle, we changed the feeding pattern of the rats by shifting feeding to a 4 h period during the lights-on cycle (diet restricted animals). With this new feeding regimen, the daily patterns of the fluctuation shifted with a marked decrease in hypothalamic apoA-IV mRNA and protein levels during the 4 h feeding period of the light phase. Although corticosterone (CORT) secretion temporally coincided with the decreasing level of apoA-IV in the hypothalamus, depletion of circulating CORT by adrenalectomy significantly decreased, rather than increased, hypothalamic apoA-IV mRNA and protein levels (68). This result indicated that the diurnal expression of hypothalamic apoA-IV is regulated by factors other than circulating CORT levels. The fact that hypothalamic apoA-IV level and food intake were inversely related during the normal diurnal cycle, as well as in the period of restricted feeding, demonstrates that hypothalamic apoA-IV is regulated by the timing of daily food intake and not by the light-dark cycle. Importantly, apoA-IV in the brain appears to decrease in anticipation of feeding. Collectively, the data suggest that hypothalamic apoA-IV is an important mediator of meal-related events, and its downregulation is involved with preparing certain neural circuits that influence peripheral metabolic organs to be ready for an impending meal.
ROLE OF apoA-IV IN INTESTINAL LIPID ABSORPTION AND SECRETION
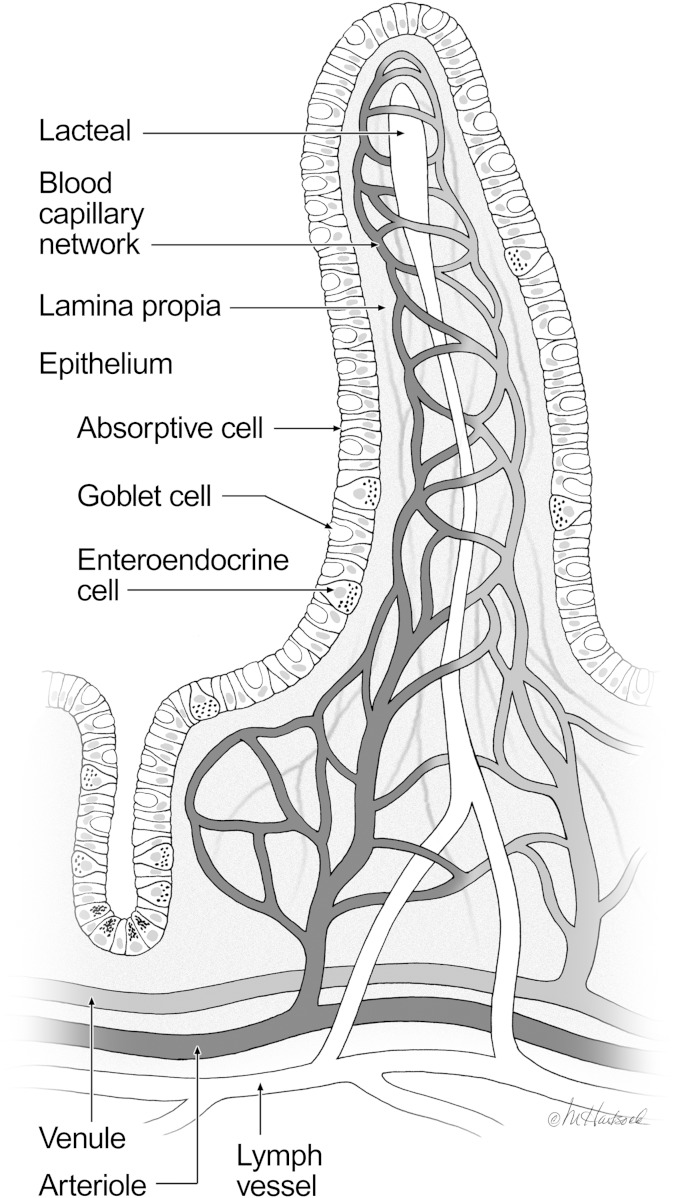
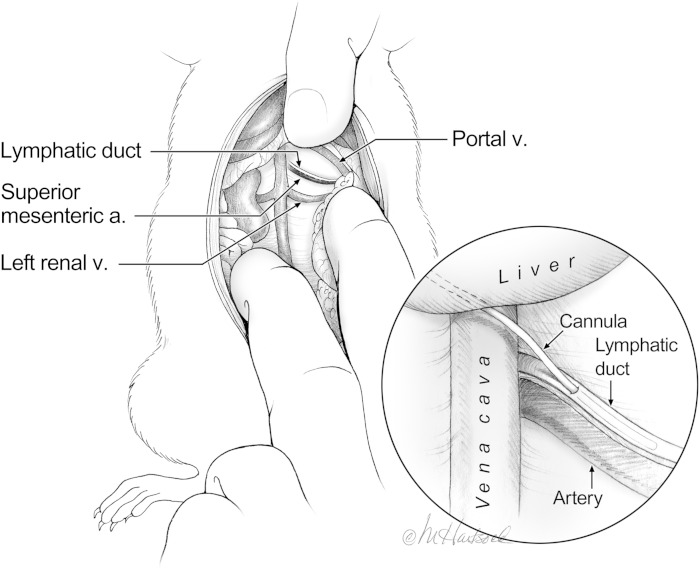
Although apoA-IV is most highly expressed in the intestine and is responsive to lipid absorption, whether or not it influences the transport of dietary fat across the intestine into the lymph has not been settled. Before describing the results of our studies and those of others, we give the following description of intestinal architecture and function to help the reader appreciate the utility of the conscious lymph fistula model. The villi and crypts forming the luminal surface of the small intestine are comprised of a continuous sheet of epithelial cells (enterocytes), together with a small number of scattered goblet and enteroendocrine cells. Beneath the enterocytes is the basement membrane, separating the enterocytes and the lamina propria. A lymphatic vessel (lacteal), which is surrounded by a blood capillary network, is located in the center of each intestinal villus. Dietary triglycerides are digested in the lumen, taken up by enterocytes as fatty acids and monoglycerides, resynthesized into triglycerides, and assembled into apoB-48-containing chylomicron particles. Because of the size of these particles, rather than entering the capillaries, dietary triglyceride and associated lipophilic compounds are transported through the absorptive enterocyte into the lacteals and eventually into the intestinal lymphatics (illustrated in Fig. 1). Thus, sampling intestinal lymph is the most direct way to assay the lipid absorptive process before the secreted lipids are diluted and metabolized in the plasma compartment. To this end, we have routinely and extensively used a lymph fistula procedure in rats and mice to measure absorption of triglyceride, cholesterol, and other lipids under a variety of experimental conditions. As illustrated in Fig. 2, mice are fitted with lymphatic cannulas for the collection of lymph secreted from the small intestine, and with a gastric or duodenal cannula for the delivery of lipid emulsion directly into the stomach or intestinal lumen. This conscious mouse model allows direct quantitation of the output of dietary (infused) lipids into the intestinal lymph, the amount of lipid remaining in the lumen (not yet taken up by enterocytes), and the amount taken up by enterocytes but not yet transported into lymph as chylomicrons (69, 70). The key advantages of this model are that: 1) the animal is conscious, enabling the efficient and rapid packaging and secretion of chylomicrons during active intestinal lipid infusion; 2) lymph can be continuously sampled over the course of many hours, both during and after lipid infusion; 3) animals can act as their own controls, thus reducing the number of animals needed for study; and 4) lymph is sampled prior to its entry into the blood, so there is no confounding effect of peripheral metabolism to obscure the results.
Fig. 1.
Structure of the intestinal villus. The surface of the small intestine is comprised of finger-like projections called villi and crypts. A continuous sheet of epithelial cells, which are the primary absorptive cells of the intestine, covers the villi and crypts. Below the absorptive epithelium is the intestinal lamina propria and a lymphatic capillary (lacteal), which is surrounded by a blood capillary network. Dietary triglyceride and lipophilic compounds are transported from the absorptive enterocyte into the lacteal and eventually into the mesenteric lymph duct (© 2010 Marcia Hartsock, MA, CMI) (69).
Fig. 2.
Lymph-fistula mouse model. After opening the abdominal cavity of the rat, the stomach, small intestine, and colon are retracted and gently pushed under the abdominal muscle walls. The surgeon’s fingers can be used to keep the liver out of the surgical plane. The inferior vena cava and left renal vein (v.) can now be exposed and the intestinal lymphatic duct can be visualized as a clear vessel running parallel to and lying directly above the superior mesenteric artery (a.). The inset shows the site of the cannula placement. The incision should be made proximal to the vena cava, allowing insertion of the cannula and advancement approximately 3–5 mm. Once secured by a drop of cyanoacrylate glue, the cannula is positioned beneath the liver and exteriorized through the right flank of the animal (© 2010 Marcia Hartsock, MA, CMI) (69, 80).
The concept that apoA-IV is important for chylomicron formation was first suggested by Hayashi et al. (54), who observed that when Pluronic L-81 [a potent inhibitor of intestinal chylomicron formation (71, 72)] was added to an infusion of lipid, apoA-IV secretion into lymph failed to increase, and that during the reversal of Pluronic L-81 inhibition, apoA-IV secretion increased rapidly along with chylomicron secretion. Lipid droplets isolated from intestinal epithelial cells during Pluronic L-81 treatment contain apoB and apoA-IV, indicating that apoA-IV associates with prechylomicrons during the early phases of their formation. This was further supported by Weinberg and colleagues, who suggested that apoA-IV may be important in stabilizing the surface of a forming prechylomicron particle inside the enterocyte (73, 74), enabling these prechylomicrons to grow larger with the incorporation of more triglyceride molecules. This concept was supported in studies of cultured pig intestinal epithelial cells where the production of large amounts of apoA-IV resulted in copious secretion of large chylomicron particles (73, 75–77). In the assembly of VLDL, Chauhan et al. (78) clearly demonstrated that the C-terminal α-helical domains adapt their surface conformation as the particle is expanded by the addition of triglyceride molecules. The authors speculated that because the gut produces only apoB-48, apoA-IV in enterocytes compensates for the lack of the C-terminal domains of apoB-48 in facilitating chylomicron assembly. Lu and colleagues proposed that in the newborn piglet, as well as in stably transfected IPEC-1 cells, elevated expression of apoA-IV greatly enhanced fat absorption by facilitating the formation of larger chylomicron particles (73, 75–77). More recently, Weinberg and Simon provided some evidence from KO mice that apoA-IV is important in regional intestinal lipid absorption and is associated with related changes in gene expression (79).
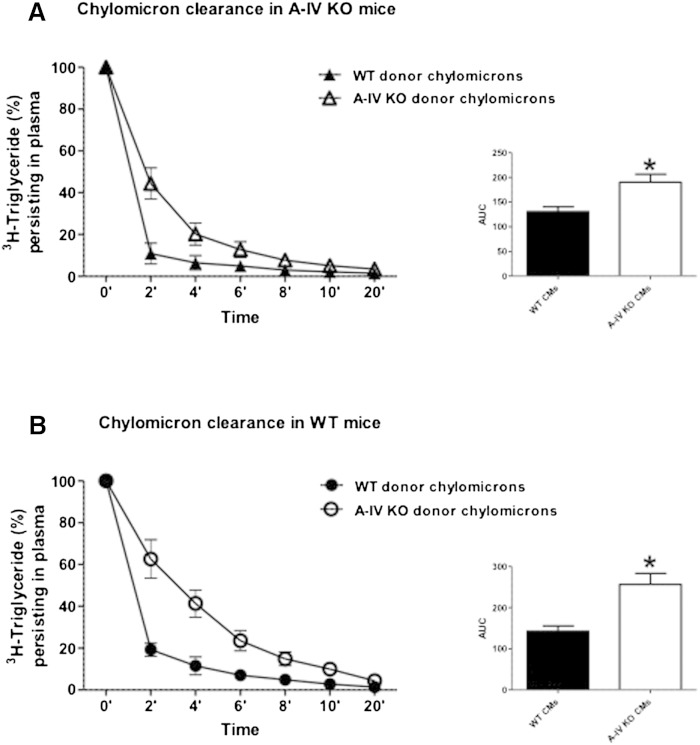
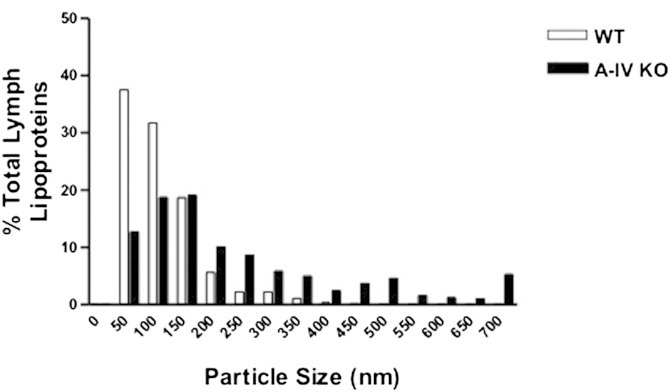
However, the importance of apoA-IV in intestinal lipid absorption is challenged by studies conducted by Kohan et al. (80, 81), which reported contradictory results. The in vivo role of intestinal apoA-IV in the formation and secretion of chylomicrons was tested directly in apoA-IV KO mice using the lymph-fistula model. Using this model, we found that apoA-IV KO mice have reduced plasma lipid levels that are not caused by a defect in lipid absorption, food intake, or adiposity. The apoA-IV KO mice also had normal hepatic lipid metabolism and VLDL secretion. Interestingly, the only major difference we observed between the WT and KO animals was that apoA-IV KO animals secreted larger chylomicrons from the intestine and they were metabolized more slowly than the WT chylomicrons (Figs. 3, 4) (80), documenting a previously unknown and unique property of chylomicrons lacking apoA-IV. This delay in chylomicron metabolism was not corrected by injecting KO-derived particles into WT animals, suggesting that it is the chylomicrons, and not the recipient animals, that are responsible for the slower metabolism of the CM particles lacking apoA-IV. Another potentially interesting conclusion from this study is that circulating apoA-IV in lipid-free form or on HDL does not readily associate with chylomicrons. Despite an abundance of apoA-IV in the circulation of the WT animals, it may be difficult to add it to the apoA-IV free chylomicrons. This would be consistent with the “closed” structure of lipid-free apoA-IV described earlier. It is also possible that chylomicrons from apoA-IV KO mice differ from WT chylomicrons in ways other than the absence of apoA-IV, and this is responsible for the slower chylomicron metabolic rate. apoA-IV KO mice do not have a gross defect in dietary lipid absorption, and the cumulative secretion of triglyceride into lymph in apoA-IV KO mice is not different from that of their WT counterparts under a variety of lipid infusion regimens (81). Collectively, these data indicate that apoA-IV is not required for normal overall dietary lipid absorption. However, the conflicting data described above do suggest a subtle but potentially important effect on chylomicron assembly and transport that warrants further investigation for clarification.
Fig. 3.
Chylomicrons from apoA-IV mice exhibit delayed clearance rates from plasma. Radiolabeled chylomicrons (containing 3H-triglyceride) were freshly isolated from either A-IV KO or WT donor mice and then given iv to either A-IV KO (A) or WT (B) mice (n = 6). Blood was collected 0 to 20 min postinjection. Values are means ± SE. Area under the curve (AUC) analysis was performed. *P < 0.05 versus mice receiving WT donor chylomicrons (CMs) (80).
Fig. 4.
apoA-IV KO mice secrete larger chylomicrons. Size distribution of lymph lipoproteins in A-IV KO and WT mice (n = 6). Lymph samples were stained with 2% phosphotungstic acid prior to transmission electron microscopy, and 800 particles were counted from each group (80).
apoA-IV AS A SATIETY FACTOR
Exogenous apoA-IV reduces food intake. Fujimoto et al. (55, 82) first provided evidence that apoA-IV is a satiety signal whose production by the small intestine is markedly increased as a result of active lipid absorption. This was inferred when rodents receiving chylous lymph intravenously ate less than those receiving chylous lymph without apoA-IV (55). It was subsequently found that iv administration of a physiological dose of native apoA-IV inhibits food intake (55, 82). Fujimoto et al. (82) also provided evidence that the potential site of action of apoA-IV is in the central nervous system. Consistent with this, central or peripheral administration of recombinant apoA-IV reduces food intake without causing malaise (55, 83–85), while administration of apoA-I at comparable doses has no effect on satiation (55). The latter is an important control because apoA-1 and apoA-IV have related structures and comparable actions on some aspects of lipoprotein metabolism. Coupled with our observation that both the lymphatic and serum apoA-IV concentrations are elevated during the dark phase, this evidence lends support to the concept that apoA-IV’s physiological role may be as a fat-induced satiety signal to regulate food intake.
To assess the possibility that endogenous apoA-IV has a satiating action, Fujimoto et al. (82) injected apoA-IV antibodies into the third cerebral ventricle of rats and observed significantly increased food intake during the light cycle when animals normally do not eat much. Later work by Liu et al. (84) suggested that the apoA-IV antibody likely neutralized endogenous apoA-IV produced by neurons in the ARC of the hypothalamus. Both peripherally and centrally administered apoA-IV cause satiety, the question is, “how does it operate?” We proposed that peripheral apoA-IV secreted in response to the feeding of fat stimulates vagal afferent fibers sending signals to the ARC. In turn, vagal efferents send signals from the hypothalamus to cause satiety by slowing both gastric and intestinal motility. Indeed, total vagotomy abolishes the satiety effect of peripherally administered apoA-IV (86).
apoA-IV REGULATION OF GASTRIC EMPTYING AND SECRETION
Lipids, and particularly long-chain triglycerides, inhibit gastric motor and secretory function (87), and free fatty acids of chain length 12 or greater are more effective at inhibiting gastric motility than C10 fatty acids (87). Intestinally absorbed long-chain fatty acids are resynthesized into triglyceride in the enterocytes and are subsequently secreted in chylomicrons containing apolipoproteins, including apoA-IV (88). Mesenteric lymph, following lipid absorption, reduces gastric motor function in recipient animals (89), and this is attenuated when the chylomicrons are removed from the lymph. In subsequent studies, purified recombinant apoA-IV was found to significantly inhibit gastric motility (89, 90). apoA-IV KO mice have significantly faster gastric emptying and greater secretion of gastric acid after an ingested meal (91). The action of apoA-IV to reduce gastric emptying is due to its being released from chylomicrons and initiating a negative feedback on gastric motility via the cholecystokinin-1 receptor (CCK-1R) on capsaicin-sensitive vagal afferent nerve terminals (91). In addition to gastric emptying, centrally administered apoA-IV inhibits gastric acid secretion. Okamura and colleagues published a series of interesting articles on this subject from 1994 to 1996 (92–95). Okamura also showed that apoA-IV administered centrally has an anti-ulcer action (94).
INTERACTION OF apoA-IV WITH LEPTIN AND CHOLECYSTOKININ
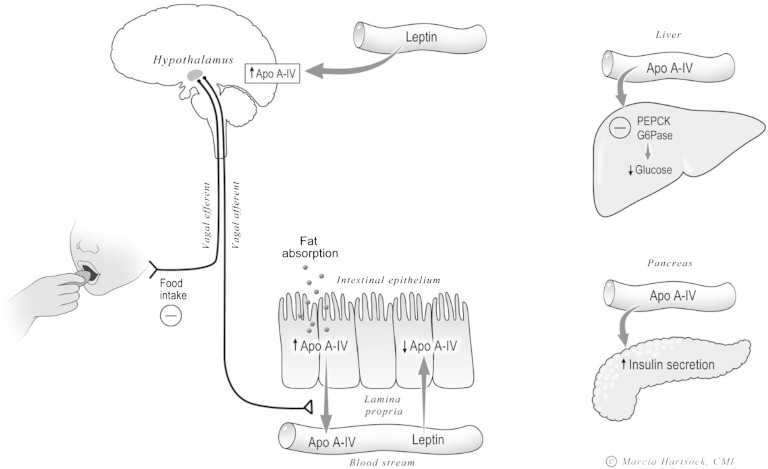
Leptin is secreted from adipocytes in direct proportion to stored fat (96–98). Leptin is involved with many aspects of energy metabolism and neuroendocrine function (99) and, like apoA-IV, exogenous leptin reduces food intake and increases energy expenditure (100, 101), with the two hormones interacting synergistically in this regard (102, 103). Consumption of a HFD increases plasma leptin levels in humans and rodents (97, 102, 103). Leptin acts on its receptor in enterocytes (104–110) to attenuate lipid-induced apoA-IV synthesis and secretion (103). Consumption of a HFD causes an initial increase of plasma apoA-IV levels in rodents, but over time the increase is attenuated (57, 63, 66). It is not known whether this damping of the apoA-IV response in HFD-fed animals is due to the elevation of circulating leptin, although animal studies support this notion. Administration of leptin ip to rats reduces the lipid-stimulated expression of apoA-IV (103). Circulating leptin also affects intestinal apoA-IV synthesis and secretion in mice, as evidenced by the result that in leptin-deficient obese (ob/ob) mice, the expression of intestinal apoA-IV is markedly elevated during fasting relative to WT animals with intact circulating leptin. Similarly, the responsiveness of gut apoA-IV to fasting and lipid feeding is also lost in ob/ob mice (102).
Central leptin has a very different effect on hypothalamic apoA-IV gene expression. Unlike what occurs in the small intestine, leptin upregulates apoA-IV gene and protein expression in the hypothalamus (73), and ob/ob mice have significantly reduced hypothalamic apoA-IV mRNA compared with WT (102). Intragastric infusion of a lipid emulsion significantly stimulated hypothalamic apoA-IV gene expression in lean controls but not in ob/ob mice, and daily ip administration of leptin for 5 days significantly increased hypothalamic apoA-IV mRNA levels of ob/ob mice relative to pair-fed controls. Centrally administered leptin increased the apoA-IV gene expression in ob/ob mice, even in the fasting state. Thus, leptin acts in the brain to enhance apoA-IV synthesis. The molecular mechanisms underlying leptin’s downregulation of apoA-IV in the gut and upregulation in the hypothalamus is potentially important and warrants further investigation.
Using immunohistochemistry, Shen et al. (102) further demonstrated that apoA-IV is expressed in leptin-sensitive phosphorylated signal transducer and activator of transcription-3 (pSTAT3)-positive cells of the hypothalamic ARC. Knockdown of STAT3 expression by siRNA significantly attenuated the stimulatory effect of leptin on apoA-IV protein expression in cultured primary hypothalamic neurons, implying that apoA-IV is regulated by leptin in the hypothalamus, probably through the STAT3 signaling pathway. Leptin and apoA-IV potentiate each other in the reduction of food intake. Thus sub-effective doses of leptin and apoA-IV, when administered together, significantly reduced food intake (102).
Leptin is also produced by gastric mucosal chief cells (cells that also secrete HCl) and other endocrine cells in rodents and humans (111–114). Gastric leptin can be secreted into the blood stream as well as into the gastric lumen, where it is resistant to the proteolysis in the stomach (115). The secretion of gastric leptin is stimulated by nutrients, vagal stimulation, and several hormones, including cholecystokinin (CCK) and insulin (111, 116, 117). Whether luminal gastric leptin has any effect on intestinal apoA-IV expression, and the relative importance of the luminal (gastric) versus circulating leptin (mostly derived from the adipose tissue) on intestinal apoA-IV expression, is unknown and further investigation is needed to understand the relationship between apoA-IV and leptin and their role in regulating food intake and energy metabolism.
CCK is secreted by intestinal I cells after lipid and protein consumption. Fat-induced stimulation is dependent on the formation and secretion of chylomicrons (118–120). CCK is involved in the initiation of gallbladder contraction and bile flow, stimulation of pancreatic enzyme secretion, modulating intestinal motility, and mediating satiation (121–123). Experimental rats administered exogenous CCK have reduced meal size (122, 124), an effect that is attenuated by either vagotomy, deactivation of vagal afferents with capsaicin, or administration of CCK-1R antagonists, thus leading to the conclusion that the satiation signals are relayed to the brain via CCK-1R on vagal afferent nerves (119, 125–127). There are several similarities between CCK and apoA-IV. Secretion of each is induced by fat absorption, and this lipid-induced stimulation is dependent on chylomicron formation (54, 120, 128). In addition, both act peripherally (after either ip or iv administration) as well as centrally to reduce food intake (82, 129, 130).
Combinations of sub-threshold doses of CCK and apoA-IV produce a short-term satiation, and this satiation effect is attenuated by the CCK-1R antagonist, lorglumide (85). Combinations of higher doses of CCK and apoA-IV cause a prolonged satiating effect (up to 4 h) that is not seen by either alone. apoA-IV-elicited satiation is greatly attenuated in CCK-KO mice (86), and in contrast, CCK-induced satiation is greater in apoA-IV KO mice than WT animals due to increased CCK-1R expression in the nodose ganglia and nucleus of the solitary tract (131). These findings suggest that endogenous apoA-IV and CCK normally interact with each other to reduce food intake via CCK-1R, and that systemic apoA-IV requires an intact CCK system in order for it to work physiologically. This working model was first proposed by Raybould and colleagues who proposed that the stimulation of apoA-IV release induced by lipid absorption results in the stimulation of CCK secretion followed by activation of the vagal nerves via a CCK-1R-dependent pathway (91). Consistent with this, we have found that apoA-IV administered ip inhibits food intake in WT but not CCK-KO mice (86).
Although circulating apoA-IV does not cross the blood-brain barrier (132), it is able to increase CCK-elicited activity in vagal afferent fibers, which discharge via a CCK-1R-dependent pathway in vitro (90). Blockade of CCK-1R using lorglumide significantly reduces apoA-IV-induced satiation, suggesting that CCK-1R on vagal afferent nerves is important for relaying apoA-IV-induced anorectic signals to the brain (86). Subdiaphragmatic vagal deafferentation is a surgical procedure which eliminates all neuronal signals mediated via vagal afferent fibers from the abdominal viscera, including the intestines and liver, while leaving many vagal efferent fibers intact (133, 134). Long-Evan rats with subdiaphragmatic vagal deafferentation have attenuated apoA-IV-elicited satiation effect (86). Our current model is that systemic apoA-IV and CCK work codependently to suppress food intake, and that peripheral apoA-IV requires an intact CCK system, including CCK-1R on vagal afferent nerves, to exert its satiation effects.
INTERACTION OF BRAIN apoA-IV WITH OTHER NEUROPEPTIDES IN REGULATING FOOD INTAKE AND ENERGY METABOLISM
Administration of apoA-IV into the third cerebral ventricle significantly reduces food intake in a dose-dependent manner without eliciting signs of toxicity (82), and blocking the action of endogenous apoA-IV locally within the brain with its antibody increases meal size, implying that endogenous apoA-IV within the brain exerts an inhibitory tone on feeding (82). In 2001, Liu et al. (56) provided the first direct evidence that apoA-IV mRNA and protein are present in rat hypothalamus. Using immunohistochemical techniques, Shen et al. (132) further characterized the distribution of apoA-IV in different brain areas involved in energy homeostasis. Intense apoA-IV staining was detected in the ARC and ventromedial hypothalamic nuclei with less staining in cells in the paraventricular and dorsomedial nuclei. In the brainstem, apoA-IV staining was found in the nucleus of the solitary tract. Double-staining immunohistochemistry in the ARC revealed coexistence of apoA-IV with neuronal nuclei (a neuronal marker), but less with glial fibrillary acidic protein (GFAP, a glial marker), suggesting that apoA-IV is largely present in neurons. Carefully controlled studies with radiolabeled apoA-IV demonstrated that circulating apoA-IV does not cross the blood-brain barrier, confirming that brain apoA-IV is neuronally derived (132).
Food intake and body adiposity are controlled by multiple neuropeptides within the central nervous system, and these can be conceptually divided into anabolic and catabolic categories. When activated, anabolic systems, e.g., neuropeptide Y (NPY), elicit an increase in food intake, a decrease in energy expenditure, and, consequently, an increase in stored energy in the form of adipose tissue. Catabolic effector systems, e.g., the melanocortin system, decrease food intake, increase energy expenditure, and, therefore, decrease adipose tissue mass. The balance between the anabolic and catabolic circuits ultimately determines the level of adiposity in the body.
NPY is a hypothalamic neuropeptide with broad regulatory actions in the central nervous system. In 2003, Liu et al. (135) demonstrated that icv NPY dose-dependently increases apoA-IV mRNA in the hypothalamus. Intraduodenal infusion of lipid also stimulated the gene expression of hypothalamic apoA-IV, but no further significant increment occurred when icv injection of NPY was combined with lipid infusion. Thus, both NPY and lipid regulate hypothalamic apoA-IV gene expression. One possible explanation of this observation is that the administration of NPY, which prepares the body to ingest food (136), stimulates central apoA-IV, which also helps in the anticipation of food, and which ultimately suppresses food intake to prevent overeating. Like NPY, the hypothalamic melanocortin system is important in the central regulation of body weight, but with an opposite action. α-Melanocyte-stimulating hormone (α-MSH), which is derived from proopiomelanocortin (POMC) neurons in the ARC, is the primary agonist for melanocortin type 3 and 4 receptors (MC3/4-Rs) in the brain. α-MSH exerts a tonic inhibitory influence over feeding. Agouti-related protein (AgRP) is a peptide produced in nonPOMC cells in the ARC, many of which also coexpress NPY, and antagonizes MC3/4-R. Consistent with this, AgRP elicits hyperphagia when it is centrally administered (137). The administration of metallothionein-II (MT-II), a synthetic MC3/4-R agonist, into the third ventricle potently reduces feeding (139), whereas the administration of SHU9119, a synthetic MC3/4-R antagonist, blocks the anorectic effect of MT-II and elicits feeding when administered alone at higher doses (139).
Shen et al. found that apoA-IV colocalizes with POMC in the ARC, and that apoA-IV administration stimulates ARC POMC gene expression, suggesting that one way by which the brain apoA-IV system suppresses food intake is by stimulating the ARC POMC system (132). Gotoh et al. (139) further demonstrated that third-ventricular administration of a low dose of apoA-IV potentiated the MT-II-elicited suppression of feeding in rats, and a low dose of SHU9119 (a melanocortin receptor antagonist) completely attenuated the anorectic effect of icv apoA-IV (139). These data support a synergistic interaction between apoA-IV and the melanocortins that reduces food intake by acting downstream of the ARC.
ROLE OF apoA-IV IN GLUCOSE HOMEOSTASIS AND INSULIN SECRETION
A novel role for apoA-IV in glucose metabolism and pancreatic β-cell insulin secretion has recently been identified (17). Exogenous apoA-IV stimulates insulin secretion in a dose-dependent manner (17). apoA-IV KO mice fed a chow diet are glucose intolerant, resulting from an attenuated insulin secretion in response to an oral or ip glucose challenge, suggesting that apoA-IV is essential for physiological blood glucose control. The male apoA-IV KO mice are more glucose intolerant than the female mice, and both sexes became more glucose intolerant as they aged. The glucose intolerance of the KO mice persisted on a HFD. It should be noted that in the C57BL/6J background, the apoA-IV KO mice respond to HFD similarly to the WT animals. Administration of exogenous apoA-IV to apoA-IV KO mice dramatically improved glucose tolerance and restored insulin secretion of animals on a chow diet as well as those on a HFD. apoA-IV enhanced insulin secretion and glucose tolerance in WT animals. ApoA-IV secretion increases rapidly within 15 min in response to intragastric lipid and remains significantly elevated for at least 30 min (140). The magnitude of the increase comprises about 10–15% of total plasma apoA-IV protein, and is comparable to the dose of exogenous apoA-IV we used in apoA-IV KO mice (17). Therefore, it is likely that the rapid secretion of apoA-IV after consumption of a fat-rich meal is sufficient to stimulate insulin secretion. We believe that it is not the absolute level of circulating apoA-IV to which the body responds, but rather the change in concentration triggers the physiological action.
These data identify apoA-IV as an important component of the enteroinsular axis and the relationship between insulin secretion and the ingestion of glucose. apoA-IV has a half-life of about 8 h, which is much longer than that of better-known incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) (141) secreted by the gut enteroendocrine cells. As a result of its long half-life, it has been reported that a single injection of apoA-IV normalized blood sugar for about 12 h in KKAy diabetic mice (17).
apoA-IV induced a dose-dependent augmentation of insulin release when isolated islets were exposed to 20 mM glucose, but not with 3 mM glucose (17). Membrane depolarization by closure of the KATP channels activates voltage-gated Ca2+ channels and leads to Ca2+ influx, which triggers the exocytosis of insulin. Although the functional KATP and calcium channels are required for the action of apoA-IV on insulin secretion, apoA-IV did not act directly to stimulate calcium influx. Instead, the action of apoA-IV is downstream of Ca2+ influx and amplifies insulin exocytosis (20). While these data suggest that apoA-IV acts on the later stages of insulin secretion, the precise molecular mechanism remains to be elucidated. However, we do know that the pancreas is not capable of synthesizing apoA-IV and so the pancreatic β-cells rely on circulating apoA-IV to enhance their insulin secretion in response to elevated blood glucose.
ApoA-IV also regulates gluconeogenesis. apoA-IV suppresses the gene expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphate (Glc-6-Pase) in hepatocytes, and as a result decreases hepatic glucose production (142). Importantly, the transcriptional suppression of PEPCK and Glc-6-Pase by apoA-IV is mediated by nuclear receptor subfamily 1 group D member 1 (NR1D1), because when NR1D1 expression is suppressed in hepatocytes by siRNA, apoA-IV loses its effect on PEPCK and Glc-6-Pase gene expression (142). As one would expect, there is higher hepatic glucose production and higher gluconeogenic gene expression in apoA-IV KO mice. We conclude that apoA-IV inhibits hepatic gluconeogenesis by decreasing Glc-6-Pase and PEPCK gene expression through NR1D1, and that this novel regulatory pathway links an influx of energy as fat from the gut (and subsequent apoA-IV secretion) with inhibition of hepatic glucose production to normalize blood sugar. As with the pancreas, the mechanism by which circulating apoA-IV affects hepatic NR1D1 remains to be elucidated. We do know that apoA-IV exhibits high-affinity binding to isolated human hepatocellular plasma membranes; the binding is saturable, reversible, and specific, suggesting that a membrane protein is involved in binding (143). Although a specific receptor for apoA-IV has not been identified as yet, apoA-IV apparently is able to enter the hepatocyte and interact with transcription factor NR1D1 (142).
POTENTIAL CLINICAL IMPLICATIONS: BARIATRIC SURGERY
Roux-en-Y gastric bypass (RYGB) is an effective treatment for the dramatic and sustainable weight loss in morbidly obese subjects. Moreover, type II diabetes is resolved in over 80% of obese patients receiving RYGB (144). While the mechanisms behind the amelioration of metabolic abnormalities are largely unknown, growing evidence suggests that the rapid bypassing of the foregut by ingested nutrients alters the normal hormonal output of the intestine, including many factors which regulate food intake, glucose homeostasis, and energy metabolism (145). A recent report has suggested a potential role for apoA-IV in ameliorating diabetes after gastric bypass. Initially, plasma apoA-IV decreases markedly a short time after bypass surgery or in patients undergoing weight loss, but the level rises after several months (146). In agreement with other studies, body weight, obesity-related comorbidities, and medication needs decreased in these obese patients who had elevated apoA-IV following RYGB, and their mortality was decreased by 40% (147). Another study investigated the effect of RYGB on body weight and cardiovascular risk factors one year after surgery (148). HDL-cholesterol and apoA-IV protein were significantly increased after RYGB, and there was a strict linear relationship between weight loss and plasma apoA-IV. apoA-IV could now be added to the list of gastrointestinal hormones whose plasma concentrations rise after RYGB, along with peptide tyrosine-tyrosine and GLP-1 (149).
The significant association between weight change and apoA-IV level in these studies is consistent with an effect of apoA-IV on food intake reduction and weight loss. However, additional work is needed to assess whether higher apoA-IV has a causative role or is a consequence of weight loss. Notably, changes in gastrointestinal hormones following RYGB typically occur within days to weeks, and reduced food intake and improved insulin secretion also occur soon after surgery (149). Because apoA-IV decreases immediately after RYGB surgery and then rises again much later, it is probably unlikely that apoA-IV is involved in the relatively quick improvement in glucose homeostasis in diabetic patients. However, as pointed out earlier, the absolute level of circulating apoA-IV may not be as important as the changes in the response of apoA-IV to fasting and feeding. As regards cardiovascular risk, elevation of apoA-IV may be protective due to its contribution to reverse cholesterol transport and/or its anti-oxidative and anti-inflammatory properties (10–16). Thus, it is reasonable to speculate that increased circulating apoA-IV not only contributes to weight loss but also to the improvements in inflammation and cardiovascular disease after RYGB. Future clinical studies of metabolic changes after RYGB surgery need to be designed that assay for and assess whether apoA-IV changes are involved with improved weight loss and glucose homeostasis. apoA-IV KO mice could provide a powerful tool to test for the importance of apoA-IV in mediating the effect of bariatric surgery by determining whether the improvement of obesity and diabetes is blunted by the absence of apoA-IV.
SUMMARY AND PROSPECTUS
Increasing evidence indicates that apoA-IV is very important in the integrated control of food intake, metabolism, and glucose homeostasis. Intestinal apoA-IV responds uniquely to the ingestion of lipid, a high-energy food substrate (Fig. 5). It does not respond to the ingestion of the other macronutrients. The rapid rise of circulating apoA-IV after fat ingestion is consistent with its involvement in the short-term regulation of satiation and glucose homeostasis by stimulation of insulin secretion by the pancreas (as shown in Fig. 5). The satiety effect of apoA-IV released by the gut is mediated via vagal afferent nervous signals to the brain which are blocked by the CCK-1R antagonist. In addition, satiety caused by apoA-IV can be mediated centrally by apoA-IV-containing neurons in the hypothalamus (Fig. 5). Thus overall, the satiety effect of apoA-IV is probably regulated centrally through the hypothalamic apoA-IV-containing neurons. In addition to active fat absorption, apoA-IV synthesis and secretion by the gut is also stimulated by peptide tyrosine-tyrosine, an ileal brake hormone. Both rodent and human studies suggest that while intestinal apoA-IV synthesis and secretion are initially increased during consumption of a HFD, the system becomes less responsive to fat feeding after chronic consumption of a HFD (63, 66). This attenuation of apoA-IV secretion in response to chronic lipid feeding is probably mediated through increased circulating leptin, which may blunt its regulation of food intake and body weight. A clinically relevant question that remains to be tested is whether diet modification, exercise, or bariatric surgery can restore the normal apoA-IV response to lipid feeding.
Fig. 5.
A summary of proposed functions for apoA-IV. apoA-IV synthesis and secretion by the enterocytes of the small intestine is markedly stimulated by fat absorption, but not by the absorption of other macronutrients. Circulating leptin downregulates intestinal apoA-IV synthesis but upregulates hypothalamic apoA-IV synthesis. Intestinal apoA-IV induces satiety by activating the vagal afferent nerve to the central nervous system and the vagal efferent from the brain induces satiety. Circulating apoA-IV plays an important role on glucose homeostasis by stimulating insulin secretion by the pancreatic islets and the downregulation of glucose secretion by the liver. This is mediated through the inhibition of hepatic PEPCK and G6Pase (© 2010 Marcia Hartsock, MA, CMI).
In terms of glucose homeostasis, we have clearly established the incretin properties of apoA-IV in promoting insulin secretion with elevated glucose. With apoA-IV having a much longer half-life than GLP-1 and GIP (∼8 h vs. a few minutes), it will be important to determine the relative importance of these three incretins and the pathways involved in maintaining glucose homeostasis. We do have preliminary data strongly suggesting that apoA-IV’s action on pancreatic islets is not mediated through the GLP-1 receptor, though determining apoA-IV’s mechanisms of action on islets and on gluconeogenesis remain high priorities given the current prevalence of type II diabetes.
Although we currently have no information on apoA-IV’s receptor(s), it is tempting to speculate that it has multiple receptors, perhaps depending on the particular function with which apoA-IV is involved or the tissue being affected. The situation may be analogous to the histamine receptors, where one ligand interacts with four different types of receptors. Initial structure-function studies have indicated that this may indeed be the case. For example, a region near the N terminus is required for apoA-IV’s effect on satiation and food intake (17), but this region is not involved with islet interactions (P. Tso et al., unpublished observations). It is clear that identifying the apoA-IV receptor(s) and the cellular and molecular pathways involved represents the next frontier for understanding the functions and translational applications of apoA-IV. We predict that apoA-IV has great potential to serve as a therapeutic agent for the treatment of glucose intolerance, diabetes, and obesity, as well as having potential for treating lipid disorders and atherosclerosis.
Acknowledgments
The authors are extremely grateful to the National Institutes of Health for the support of this study.
Footnotes
Abbreviations:
- ARC
- arcuate nucleus
- CCK
- cholecystokinin
- CCK-1R
- cholecystokinin-1 receptor
- CHOW
- standard chow
- CORT
- corticosterone
- CREB
- cyclic AMP response element binding protein
- GIP
- glucose-dependent insulinotropic peptide
- Glc-6-Pase
- glucose 6-phosphatase
- GLP-1
- glucagon-like peptide-1
- HFD
- high-fat diet
- LFD
- low-fat diet
- MC3/4-R
- melanocortin type 3 and 4 receptor
- MT-II
- metallothionein-II
- NPY
- neuropeptide Y
- NR1D1
- nuclear receptor subfamily 1 group D member 1
- PEPCK
- phosphoenolpyruvate carboxykinase
- POMC
- proopiomelanocortin
- RYGB
- Roux-en-Y gastric bypass
This work was supported by National Institutes of Health Grants DK076928 and DK059630 (University of Cincinnati MMPC) to P.T., F32-091173-01 to A.B.K., DK92779 to M.L., and DK83550 and DK97436 to C-M.L.
REFERENCES
- 1.Utermann G., Beisiegel U. 1979. Apolipoprotein A-IV: a protein occurring in human mesenteric lymph chylomicrons and free in plasma. Isolation and quantification. Eur. J. Biochem. 99: 333–343. [DOI] [PubMed] [Google Scholar]
- 2.Green P. H., Glickman R. M., Riley J. W., Quinet E. 1980. Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J. Clin. Invest. 65: 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu A. L., Windmueller H. G. 1978. Identification of circulating apolipoproteins synthesized by rat small intestine in vivo. J. Biol. Chem. 253: 2525–2528. [PubMed] [Google Scholar]
- 4.Ghiselli G., Krishnan S., Beigel Y., Gotto A. M., Jr 1986. Plasma metabolism of apolipoprotein A-IV in humans. J. Lipid Res. 27: 813–827. [PubMed] [Google Scholar]
- 5.Ohta T., Fidge N. H., Nestel P. J. 1985. Studies on the in vivo and in vitro distribution of apolipoprotein A-IV in human plasma and lymph. J. Clin. Invest. 76: 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghini I., Barja F., Pometta D., James R. W. 1995. Characterization of subpopulations of lipoprotein particles isolated from human cerebrospinal fluid. Biochim. Biophys. Acta. 1255: 192–200. [DOI] [PubMed] [Google Scholar]
- 7.Ordovas J. M., Cassidy D. K., Civeira F., Bisgaier C. L., Schaefer E. J. 1989. Familial apolipoprotein A-I, C–III, and A-IV deficiency and premature atherosclerosis due to deletion of a gene complex on chromosome 11. J. Biol. Chem. 264: 16339–16342. [PubMed] [Google Scholar]
- 8.Cohen R. D., Castellani L. W., Qiao J. H., Van Lenten B. J., Lusis A. J., Reue K. 1997. Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J. Clin. Invest. 99: 1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duverger N., Tremp G., Caillaud J. M., Emmanuel F., Castro G., Fruchart J. C., Steinmetz A., Denèfle P. 1996. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 273: 966–968. [DOI] [PubMed] [Google Scholar]
- 10.Ostos M. A., Conconi M., Vergnes L., Baroukh N., Ribalta J., Girona J., Cailloud J. M., Ochoa A., Zakin M. M. 2001. Antioxidative and antiatherosclerotic effects of human apolipoprotein A-IV in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 11.Stein O., Stein Y., Lefevre M., Roheim P. S. 1986. The role of apolipoprotein A-IV in reverse cholesterol transport studied with cultured cells and liposomes derived from an ether analog of phosphatidylcholine. Biochim. Biophys. Acta. 878: 7–13. [DOI] [PubMed] [Google Scholar]
- 12.von Eckardstein A., Huang Y., Wu S., Sarmadi A. S., Schwarz S., Steinmetz A., Assman G. 1995. Lipoproteins containing apolipoprotein a-IV but not apolipoprotein A-I take up and esterify cell-derived cholesterol in plasma. Arterioscler. Thromb. Vasc. Biol. 15: 1755–1763. [DOI] [PubMed] [Google Scholar]
- 13.Fournier N., Atger V., Paul J. L., Sturm M., Duverger N., Rothblatt G. H., Moatti N. 2000. Human apoA-IV overexpression in transgenic mice induces cholesterol efflux from J774 macrophages to whole serum. Arterioscler. Thromb. Vasc. Biol. 20: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 14.Gomaraschi M., Putt W. E., Pozzi S., Iametti S., Barbiroli A., Bonomi F., Favari E., Bernini F., Franceschini G., Talmud P. J., et al. 2010. Structure and function of the apoA-IV T347S and Q360H common variants. Biochem. Biophys. Res. Commun. 393: 126–130. [DOI] [PubMed] [Google Scholar]
- 15.Qin X., Swertfeger D. K., Zheng S., Hui D. Y., Tso P. 1998. Apolipoprotein AIV: a potent endogenous inhibitor of lipid oxidation. Am. J. Physiol. 274: H1836–H1840. [DOI] [PubMed] [Google Scholar]
- 16.Vowinkel T., Mori M., Krieglstein C. F., Russell J., Saijo F., Bharwani S., Turnage R. H., Davidson W. S., Tso P., Granger D. N., et al. 2004. Apolipoprotein A-IV inhibits experimental colitis. J. Clin. Invest. 114: 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F., Kohan A. B., Kindel T. L., Corbin K. L., Nunemaker C. S., Obici S., Woods S. C., Davidson W. S., Tso P. 2012. Apolipoprotein A-IV improves glucose homeostasis by enhancing insulin secretion. Proc. Natl. Acad. Sci. USA. 109: 9641–9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karathanasis S. K. 1985. Apolipoprotein multigene family: Tandem organization of human apolipoprotein Ai, CIII, and AIV genes. Proc. Natl. Acad. Sci. USA. 82: 6374–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng X., Morris J., Dressmen J., Tubb M. R., Tso P., Jerome W. G., Davidson W. S., Thompson T. B. 2012. The structure of dimeric apolipoprotein A-IV and its mechanism of self-association. Structure. 20: 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng X., Morris J., Chaton C., Schröder G. F., Davidson W. S., Thompson T. B. 2013. Small-angle X-ray scattering of apolipoprotein A-IV reveals the importance of its termini for structural stability. J. Biol. Chem. 288: 4854–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohse P., Kindt M. R., Rader D. J., Brewer H. B., Jr 1991. Three genetic variants of human plasma apolipoprotein A-IV. apoA-IV-1(Thr347—Ser), apoA-IV-0(Lys167—Glu,Gln360—His), and apoA-IV-3(Glu165—Lys). J. Biol. Chem. 266: 13513–13518. [PubMed] [Google Scholar]
- 22.Menzel H. J., Dieplinger H., Sandholzer C., Karádi I., Utermann G., Császár A. 1995. Apolipoprotein A-IV polymorphism in the Hungarian population: gene frequencies, effect on lipid levels, and sequence of two new variants. Hum. Mutat. 5: 58–65. [DOI] [PubMed] [Google Scholar]
- 23.Bai H., Saku K., Liu R., Oribe Y., Yamamoto K., Arakawa K. 1996. Polymorphism of the apolipoprotein A-IV gene and its significance in lipid metabolism and coronary heart disease in a Japanese population. Eur. J. Clin. Invest. 26: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 24.Talmud P. J., Hawe E., Martin S., Olivier M., Miller G. J., Rubin E. M., Pennacchio L. A., Humphries S. E. 2002. Relative contribution of variation within the APOC3/A4/A5 gene cluster in determining plasma triglycerides. Hum. Mol. Genet. 11: 3039–3046. [DOI] [PubMed] [Google Scholar]
- 25.Gomez P., Perez-Martinez P., Marin C., Camargo A., Yubero-Serrano E. M., Garcia-Rios A., Rodriguez F., Delgado-Lista J., Perez-Jimenez F., Lopez-Miranda J. 2010. APOA1 and APOA4 gene polymorphisms influence the effects of dietary fat on LDL particle size and oxidation in healthy young adults. J. Nutr. 140: 773–778. [DOI] [PubMed] [Google Scholar]
- 26.Hockey K. J., Anderson R. A., Cook V. R., Hantgan R. R., Weinberg R. B. 2001. Effect of the apolipoprotein A-IV Q360H polymorphism on postprandial plasma triglyceride clearance. J. Lipid Res. 42: 211–217. [PubMed] [Google Scholar]
- 27.Hubacek J. A., Bohuslavova R., Skodova Z., Pitha J., Bobkova D., Poledne R. 2007. Polymorphisms in the APOA1/C3/A4/A5 gene cluster and cholesterol responsiveness to dietary change. Clin. Chem. Lab. Med. 45: 316–320. [DOI] [PubMed] [Google Scholar]
- 28.Delgado-Lista J., Perez-Jimenez F., Ruano J., Perez-Martinez P., Fuentes F., Criado-Garcia J., Parnell L. D., Garcia-Rios A., Ordovas J. M., Lopez-Miranda J. 2010. Effects of variations in the APOA1/C3/A4/A5 gene cluster on different parameters of postprandial lipid metabolism in healthy young men. J. Lipid Res. 51: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Ordovas J. M., Gao G., Province M., Straka R. J., Tsai M. Y., Lai C. Q., Zhang K., Borecki I., Hixson J. E., et al. 2009. Pharmacogenetic association of the APOA1/C3/A4/A5 gene cluster and lipid responses to fenofibrate: the genetics of lipid-lowering drugs and diet network study. Pharmacogenet. Genomics. 19: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi L., Liu S., Rifai N., Hunter D., Hu F. B. 2007. Associations of the apolipoprotein A1/C3/A4/A5 gene cluster with triglyceride and HDL cholesterol levels in women with type 2 diabetes. Atherosclerosis. 192: 204–210. [DOI] [PubMed] [Google Scholar]
- 31.Guclu-Geyik F., Onat A., Coban N., Komurcu-Bayrak E., Sansoy V., Can G., Erginel-Unaltuna N. 2012. Minor allele of the APOA4 gene T347S polymorphism predisposes to obesity in postmenopausal Turkish women. Mol. Biol. Rep. 39: 10907–10914. [DOI] [PubMed] [Google Scholar]
- 32.Kretowski A., Hokanson J. E., McFann K., Kinney G. L., Snell-Bergeon J. K., Maahs D. M., Wadwa R. P., Eckel R. H., Ogden L. G., Garg S. K., et al. 2006. The apolipoprotein A-IV Gln360His polymorphism predicts progression of coronary artery calcification in patients with type 1 diabetes. Diabetologia. 49: 1946–1954. [DOI] [PubMed] [Google Scholar]
- 33.Pennacchio L. A., Olivier M., Hubacek J. A., Cohen J. C., Cox D. R., Fruchart J. C., Krauss R. M., Rubin E. M. 2001. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 294: 169–173. [DOI] [PubMed] [Google Scholar]
- 34.Karathanasis S. K., Yunis I., Zannis V. I. 1986. Structure, evolution and tissue-specific synthesis of human apolipoprotein A-IV. Biochemistry. 25: 3962–3970. [DOI] [PubMed] [Google Scholar]
- 35.Staels B., van Tol A., Verhoeven G., Auwerx J. 1990. Apolipoprotein A-IV messenger ribonucleic acid abundance is regulated in a tissue-specific manner. Endocrinology. 126: 2153–2163. [DOI] [PubMed] [Google Scholar]
- 36.Sanecka A., Ansems M., van Hout-Kuijer M. A., Looman M. W., Prosser A. C., Welten S., Gilissen C., Sama I. E., Huynen M. A., Veltman J. A., et al. 2012. Analysis of genes regulated by the transcription factor LUMAN identifies ApoA4 as a target gene in dendritic cells. Mol. Immunol. 50: 66–73. [DOI] [PubMed] [Google Scholar]
- 37.Kalogeris T. J., Tsuchiya T., Fukagawa K., Wolf R., Tso P. 1996. Apolipoprotein A-IV synthesis in proximal jejunum is stimulated by ileal lipid infusion. Am. J. Physiol. 270: G277–G286. [DOI] [PubMed] [Google Scholar]
- 38.Hanniman E. A., Lambert G., Inoue Y., Gonzalez F. J., Sinal C. J. 2006. Apolipoprotein A-IV is regulated by nutritional and metabolic stress: involvement of glucocorticoids, HNF-4 alpha, and PGC-1 alpha. J. Lipid Res. 47: 2503–2514. [DOI] [PubMed] [Google Scholar]
- 39.Davidson N. O., Carlos R. C., Drewek M. J., Parmer T. G. 1988. Apolipoprotein gene expression in the rat is regulated in a tissue-specific manner by thyroid hormone. J. Lipid Res. 29: 1511–1522. [PubMed] [Google Scholar]
- 40.Elshourbagy N. A., Boguski M. S., Liao W. S., Jefferson L. W., Gordon J. I., Taylor J. M. 1985. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc. Natl. Acad. Sci. USA. 82: 8242–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inui Y., Hausman A. M., Nanthakumar N., Henning S. J., Davidson N. O. 1992. Apolipoprotein B messenger RNA editing in rat liver: developmental and hormonal modulation is divergent from apolipoprotein A-IV gene expression despite increased hepatic lipogenesis. J. Lipid Res. 33: 1843–1856. [PubMed] [Google Scholar]
- 42.Williams S. C., Bruckheimer S. M., Lusis A. J., LeBoeuf R. C., Kinniburgh A. J. 1986. Mouse apolipoprotein A-IV gene: nucleotide sequence and induction by a high-lipid diet. Mol. Cell. Biol. 6: 3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srivastava R. A., Kitchens R. T., Schonfeld G. 1994. Regulation of the apolipoprotein AIV gene expression by estrogen differs in rat and mouse. Eur. J. Biochem. 222: 507–514. [DOI] [PubMed] [Google Scholar]
- 44.Black D. D., Ellinas H. 1992. Apolipoprotein synthesis in newborn piglet intestinal explants. Pediatr. Res. 32: 553–558. [DOI] [PubMed] [Google Scholar]
- 45.Barbosa S., Fasanella G., Carriera S., Llarena M., Fox R., Barreca C., Andrew D., O’Hare P. 2013. An orchestrated programme regulating secretory pathway genes and cargos by the transmembrane transcription factor CREB-H. Traffic. 14: 382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagasawa M., Hara T., Kashino A., Akasaka Y., Ide T., Murakami K. 2009. Identification of a functional peroxisome proliferator-activated receptor (PPAR) response element (PPRE) in the human apolipoprotein A-IV gene. Biochem. Pharmacol. 78: 523–530. [DOI] [PubMed] [Google Scholar]
- 47.Sauvaget D., Chauffeton V., Citadelle D., Chatelet F. P., Cywiner-Golenzer C., Chambaz J., Pinçon-Raymond M., Cardot P., Le Beyec J., Ribeiro A. 2002. Restriction of apolipoprotein A-IV gene expression to the intestine villus depends on a hormone-responsive element and parallels differential expression of the hepatic nuclear factor 4alpha and gamma isoforms. J. Biol. Chem. 277: 34540–34548. [DOI] [PubMed] [Google Scholar]
- 48.Xu X., Park J., So J., Hur K. Y., Lee A. 2014. Transcriptional regulation of apolipoprotein A-IV by the transcription factor CREBH. J. Lipid Res. 55: 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrier J. C., Deblois G., Champigny C., Levy E., Giguère V. 2004. Estrogen-related receptor alpha (ERRalpha) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. J. Biol. Chem. 279: 52052–52058. [DOI] [PubMed] [Google Scholar]
- 50.Kalogeris T. J., Fukagawa K., Tso P. 1994. Synthesis and lymphatic transport of intestinal apolipoprotein A-IV in response to graded doses of triglyceride. J. Lipid Res. 35: 1141–1151. [PubMed] [Google Scholar]
- 51.Kalogeris T. J., Monroe F., Demichele S. J., Tso P. 1996. Intestinal synthesis and lymphatic secretion of apolipoprotein A-IV vary with chain length of intestinally infused fatty acids in rats. J. Nutr. 126: 2720–2729. [DOI] [PubMed] [Google Scholar]
- 52.Imaizumi K., Fainaru M., Havel R. J. 1978. Composition of proteins of mesenteric lymph chylomicrons in the rat and alterations produced upon exposure of chylomicrons to blood serum and serum proteins. J. Lipid Res. 19: 712–722. [PubMed] [Google Scholar]
- 53.Hayashi H., Fujimoto K., Cardelli J. A., Nutting D. F., Bergstedt S., Tso P. 1990. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am. J. Physiol. 259: G709–G719. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi H., Nutting D. F., Fujimoto K., Cardelli J. A., Black D., Tso P. 1990. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J. Lipid Res. 31: 1613–1625. [PubMed] [Google Scholar]
- 55.Fujimoto K., Cardelli J. A., Tso P. 1992. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am. J. Physiol. 262: G1002–G1006. [DOI] [PubMed] [Google Scholar]
- 56.Liu M., Doi T., Shen L., Woods S. C., Seeley R. J., Zheng S., Jackman A., Tso P. 2001. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280: R1382–R1387. [DOI] [PubMed] [Google Scholar]
- 57.Liu M., Doi T., Shen L., Woods S. C., Seeley R. J., Zheng S., Jackman A., Tso P. 2004. Obesity induced by a high-fat diet downregulates apolipoprotein A-IV gene expression in rat hypothalamus. Am. J. Physiol. Endocrinol. Metab. 287: E366–E370. [DOI] [PubMed] [Google Scholar]
- 58.Yoshioka M., Bolduc C., Raymond V., St-Amand J. 2008. High-fat meal-induced changes in the duodenum mucosa transcriptome. Obesity (Silver Spring). 16: 2302–2307. [DOI] [PubMed] [Google Scholar]
- 59.Ferrer F., Bigot-Corbel E., N’Guyen P., Krempf M., Bard J. M. 2002. Quantitative measurement of lipoprotein particles containing both apolipoprotein AIV and apolipoprotein B in human plasma by a noncompetitive ELISA. Clin. Chem. 48: 884–890. [PubMed] [Google Scholar]
- 60.Ferrer F., Nazih H., Zaïr Y., Krempf M., Bard J. M. 2003. Postprandial changes in the distribution of apolipoprotein AIV between apolipoprotein B- and non apolipoprotein B-containing lipoproteins in obese women. Metabolism. 52: 1537–1541. [DOI] [PubMed] [Google Scholar]
- 61.Vergès B., Guerci B., Durlach V., Galland-Jos C., Paul J. L., Lagrost L., Gambert P. 2001. Increased plasma apoA-IV level is a marker of abnormal postprandial lipemia: a study in normoponderal and obese subjects. J. Lipid Res. 42: 2021–2029. [PubMed] [Google Scholar]
- 62.Apfelbaum T. F., Davidson N. O., Glickman R. M. 1987. Apolipoprotein A-IV synthesis in rat intestine regulation by dietary triglyceride. Am. J. Physiol. 252: G662–G666. [DOI] [PubMed] [Google Scholar]
- 63.Kalogeris T. J., Painter R. G. 2001. Adaptation of intestinal production of apolipoprotein A-IV during chronic feeding of lipid. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280: R1155–R1161. [DOI] [PubMed] [Google Scholar]
- 64.Kuller L. H. 1997. Dietary fat and chronic diseases: epidemiologic overview. J. Am. Diet. Assoc. 97: S9–S15. [DOI] [PubMed] [Google Scholar]
- 65.Liu M., Doi T., Tso P. 2003. Regulation of intestinal and hypothalamic apolipoprotein A-IV. Exp. Biol. Med. (Maywood). 228: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 66.Weinberg R. B., Dantzker C., Patton C. S. 1990. Sensitivity of serum apolipoprotein A-IV levels to changes in dietary fat content. Gastroenterology. 98: 17–24. [DOI] [PubMed] [Google Scholar]
- 67.Fukagawa K., Gou H. M., Wolf R., Tso P. 1994. Circadian rhythm of serum and lymph apolipoprotein AIV in ad libitum-fed and fasted rats. Am. J. Physiol. 267: R1385–R1390. [DOI] [PubMed] [Google Scholar]
- 68.Liu M., Shen L., Liu Y., Tajima D., Sakai R., Woods S. C., Tso P. 2004. Diurnal rhythm of apolipoprotein A-IV in rat hypothalamus and its relation to food intake and corticosterone. Endocrinology. 145: 3232–3238. [DOI] [PubMed] [Google Scholar]
- 69.Kohan A. B., Yoder S. M., Tso P. 2011. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiol. Behav. 105: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nutting D. F., Kumar N. S., Siddiqi S. A., Mansbach C. B., 2nd 2002. Nutrient absorption. Curr. Opin. Gastroenterol. 18: 168–175. [DOI] [PubMed] [Google Scholar]
- 71.Tso P., Balint J. A., Bishop M. B., Rodgers J. B. 1981. Acute inhibition of intestinal lipid transport by Pluronic L-81 in the rat. Am. J. Physiol. 241: G487–G497. [DOI] [PubMed] [Google Scholar]
- 72.Tso P., Balint J. A., Rodgers J. B. 1980. Effect of hydrophobic surfactant (Pluronic L-81) on lymphatic lipid transport in the rat. Am. J. Physiol. 239: G348–G353. [DOI] [PubMed] [Google Scholar]
- 73.Lu S., Yao Y., Cheng X., Mitchell S., Leng S., Meng S., Gallagher J. W., Shelness G. S., Morris G. S., Mahan J., et al. 2006. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J. Biol. Chem. 281: 3473–3483. [DOI] [PubMed] [Google Scholar]
- 74.Weinberg R. B., Anderson R. A., Cook V. R., Emmanuel F., Denefle P., Hermann M., Steinmetz A. 2000. Structure and interfacial properties of chicken apolipoprotein A-IV. J. Lipid Res. 41: 1410–1418. [PubMed] [Google Scholar]
- 75.Gonzalez-Vallina R., Wang H., Zhan R., Berschneider H. M., Lee R. M., Davidson N. O., Black D. D. 1996. Lipoprotein and apolipoprotein secretion by a newborn piglet intestinal cell line (IPEC-1). Am. J. Physiol. 271: G249–G259. [DOI] [PubMed] [Google Scholar]
- 76.Weinberg R. B., Gallagher J. W., Fabritius M. A., Shelness G. S. 2012. ApoA-IV modulates the secretory trafficking of apoB and the size of triglyceride-rich lipoproteins. J. Lipid Res. 53: 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao Y., Lu S., Huang Y., Beeman-Black C. C., Lu R., Pan X., Hussain M. M., Black D. D. 2011. Regulation of microsomal triglyceride transfer protein by apolipoprotein A-IV in newborn swine intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 300: G357–G363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chauhan V., Wang X., Ramsamy T., Milne R. W., Sparks D. L. 1998. Evidence for lipid-dependent structural changes in specific domains of apolipoprotein B100. Biochemistry. 37: 3735–3742. [DOI] [PubMed] [Google Scholar]
- 79.Simon T., Cook V. R., Rao A., Weinberg R. B. 2011. Impact of murine intestinal apolipoprotein A-IV expression on regional lipid absorption, gene expression, and growth. J. Lipid Res. 52: 1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kohan A. B., Wang F., Li X., Bradshaw S., Yang Q., Caldwell J. L., Bullock T. M., Tso P. 2012. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am. J. Physiol. Gastrointest. Liver Physiol. 302: G628–G636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kohan A. B., Wang F., Li X., Vandersall A. E., Huesman S., Xu M., Yang Q., Lou D., Tso P. 2013. Is apolipoprotein A-IV rate limiting in the intestinal transport and absorption of triglyceride? Am. J. Physiol. Gastrointest. Liver Physiol. 304: G1128–G1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujimoto K., Fukagawa K., Sakata T., Tso P. 1993. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J. Clin. Invest. 91: 1830–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujimoto K., Machidori H., Iwakiri R., Yamamoto K., Fujisaki J., Sakata T., Tso P. 1993. Effect of intravenous administration of apolipoprotein A-IV on patterns of feeding, drinking and ambulatory activity of rats. Brain Res. 608: 233–237. [DOI] [PubMed] [Google Scholar]
- 84.Liu M., Maiorano N., Shen L., Pearson K., Tajima D., Zhang D. M., Woods S. C., Seeley R. J., Davidson W. S., Tso P. 2003. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol. Behav. 78: 149–155. [DOI] [PubMed] [Google Scholar]
- 85.Lo C. M., Zhang D. M., Pearson K., Ma L., Sun W., Sakai R. R., Davidson W. S., Liu M., Raybould H. E., Woods S. C., et al. 2007. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293: R1490–R1494. [DOI] [PubMed] [Google Scholar]
- 86.Lo C. C., Langhans W., Georgievsky M., Arnold M., Caldwell J. L., Cheng S., Liu M., Woods S. C., Tso P. 2012. Apolipoprotein AIV requires cholecystokinin and vagal nerves to suppress food intake. Endocrinology. 153: 5857–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunt J. N., Knox M. T. 1968. A relation between the chain length of fatty acids and the slowing of gastric emptying. J. Physiol. 194: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tso P., Balint J. A. 1986. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am. J. Physiol. 250: G715–G726. [DOI] [PubMed] [Google Scholar]
- 89.Glatzle J., Kalogeris T. J., Zittel T. T., Guerrini S., Tso P., Raybould H. E. 2002. Chylomicron components mediate intestinal lipid-induced inhibition of gastric motor function. Am. J. Physiol. Gastrointest. Liver Physiol. 282: G86–G91. [DOI] [PubMed] [Google Scholar]
- 90.Glatzle J., Darcel N., Rechs A. J., Kalogeris T. J., Tso P., Raybould H. E. 2004. Apolipoprotein A-IV stimulates duodenal vagal afferent activity to inhibit gastric motility via a CCK1 pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287: R354–R359. [DOI] [PubMed] [Google Scholar]
- 91.Whited K. L., Lu D., Tso P., Kent Lloyd K. C., Raybould H. E. 2005. Apolipoprotein A-IV is involved in detection of lipid in the rat intestine. J. Physiol. 569: 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okumura T., Fukagawa K., Tso P., Taylor I. L., Pappas T. N. 1994. Intracisternal injection of apolipoprotein A-IV inhibits gastric secretion in pylorus-ligated conscious rats. Gastroenterology. 107: 1861–1864. [DOI] [PubMed] [Google Scholar]
- 93.Okumura T., Fukagawa K., Tso P., Taylor I. L., Pappas T. N. 1995. Mechanism of action of intracisternal apolipoprotein A-IV in inhibiting gastric acid secretion in rats. Gastroenterology. 109: 1583–1588. [DOI] [PubMed] [Google Scholar]
- 94.Okumura T., Taylor I. L., Fukagawa K., Tso P., Pappas T. N. 1995. Apolipoprotein A-IV acts centrally in the brain to reduce the severity of gastric ulceration in the rat. Brain Res. 673: 153–156. [DOI] [PubMed] [Google Scholar]
- 95.Okumura T., Fukagawa K., Tso P., Taylor I. L., Pappas T. N. 1996. Apolipoprotein A-IV acts in the brain to inhibit gastric emptying in the rat. Am. J. Physiol. 270: G49–G53. [DOI] [PubMed] [Google Scholar]
- 96.Collins S., Kuhn C. M., Petro A. E., Swick A. G., Chrunyk B. A., Surwit R. S. 1996. Role of leptin in fat regulation. Nature. 380: 677. [DOI] [PubMed] [Google Scholar]
- 97.Frederich R. C., Hamann A., Anderson S., Löllmann B., Lowell B. B., Flier J. S. 1995. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1: 1311–1314. [DOI] [PubMed] [Google Scholar]
- 98.Considine R. V., Sinha M. K., Heiman M. L., Kriauciunas A., Stephens T. W., Nyce M. R., Ohannesian J. P., Marco C. C., McKee L. J., Bauer T. L. 1996. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334: 292–295. [DOI] [PubMed] [Google Scholar]
- 99.Kelesidis T., Kelesidis I., Chou S., Mantzoros C. S. 2010. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann. Intern. Med. 152: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Havel P. J. 2000. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 59: 359–371. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz M. W., Woods S. C., Porte D., Seeley R. J., Baskin D. G. 2000. Central nervous system control of food intake. Nature. 404: 661–671. [DOI] [PubMed] [Google Scholar]
- 102.Shen L., Tso P., Woods S. C., Sakai R. R., Davidson W. S., Liu M. 2007. Hypothalamic apolipoprotein A-IV is regulated by leptin. Endocrinology. 148: 2681–2689. [DOI] [PubMed] [Google Scholar]
- 103.Doi T., Liu M., Seeley R. J., Woods S. C., Tso P. 2001. Effect of leptin on intestinal apolipoprotein AIV in response to lipid feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281: R753–R759. [DOI] [PubMed] [Google Scholar]
- 104.Banks W. A., Coon A. B., Robinson S. M., Moinuddin A., Shultz J. M., Nakaoke R., Morley J. E. 2004. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 53: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 105.Collins S., Surwit R. S. 1996. Pharmacologic manipulation of ob expression in a dietary model of obesity. J. Biol. Chem. 271: 9437–9440. [DOI] [PubMed] [Google Scholar]
- 106.Barrenetxe J., Villaro A. C., Guembe L., Pascual I., Muñoz-Navas M., Barber A., Lostao M. P. 2002. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut. 50: 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guilmeau S., Buyse M., Tsocas A., Laigneau J. P., Bado A. 2003. Duodenal leptin stimulates cholecystokinin secretion: evidence of a positive leptin-cholecystokinin feedback loop. Diabetes. 52: 1664–1672. [DOI] [PubMed] [Google Scholar]
- 108.Hansen G. H., Niels-Christiansen L-L., Danielsen E. M. 2008. Leptin and the obesity receptor (OB-R) in the small intestine and colon: a colocalization study. J. Histochem. Cytochem. 56: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Plaisancie P., Ducroc R., El Homsi M., Tsocas A., Guilmeau S., Zoghbi S., Thibaudeau O., Bado A. 2006. Luminal leptin activates mucin-secreting goblet cells in the large bowel. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G805–G812. [DOI] [PubMed] [Google Scholar]
- 110.Morton N. M., Emilsson V., Liu Y. L., Cawthorne M. A. 1998. Leptin action in intestinal cells. J. Biol. Chem. 273: 26194–26201. [DOI] [PubMed] [Google Scholar]
- 111.Bado A., Levasseur S., Attoub S., Kermorgant S., Laigneau J. P., Bortoluzzi M. N., Moizo L., Lehy T., Guerre-Millo M., Le Marchand-Brustel Y., et al. 1998. The stomach is a source of leptin. Nature. 394: 790–793. [DOI] [PubMed] [Google Scholar]
- 112.Sobhani I., Bado A., Vissuzaine C., Buyse M., Kermorgant S., Laigneau J. P., Attoub S., Lehy T., Henin D., Mignon M., et al. 2000. Leptin secretion and leptin receptor in the human stomach. Gut. 47: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cinti S., Matteis R. D., Picó C., Ceresi E., Obrador A., Maffeis C., Oliver J., Palou A. 2000. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int. J. Obes. Relat. Metab. Disord. 24: 789–793. [DOI] [PubMed] [Google Scholar]
- 114.Oliver P., Picó C., De Matteis R., Cinti S., Palou A. 2002. Perinatal expression of leptin in rat stomach. Dev. Dyn. 223: 148–154. [DOI] [PubMed] [Google Scholar]
- 115.Buyse M., Berlioz F., Guilmeau S., Tsocas A., Voisin T., Peranzi G., Laburthe M. C., Lewin M. J. M., Roze C., Bado A. 2000. Intestinal epithelial cells express leptin receptors that modulate the oligopeptide transporter pept-1. Gastroenterology. 118: A604. [Google Scholar]
- 116.Cammisotto P. G., Bendayan M. 2007. Leptin secretion by while adipose tissue and gastric mucosa. Histol. Histopathol. 22: 199–210. [DOI] [PubMed] [Google Scholar]
- 117.Sobhani I., Buyse M., Goïot H., Weber N., Laigneau J. P., Henin D., Soul J. C., Bado A. 2002. Vagal stimulation rapidly increases leptin secretion in human stomach. Gastroenterology. 122: 259–263. [DOI] [PubMed] [Google Scholar]
- 118.Liddle R. A., Goldfine I. D., Rosen M. S., Taplitz R. A., Williams J. A. 1985. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Invest. 75: 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raybould H. E. 1991. Capsaicin-sensitive vagal afferents and CCK in inhibition of gastric motor function induced by intestinal nutrients. Peptides. 12: 1279–1283. [DOI] [PubMed] [Google Scholar]
- 120.Raybould H. E., Meyer J. H., Tabrizi Y., Liddle R. A., Tso P. 1998. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am. J. Physiol. 274: R1834–R1838. [DOI] [PubMed] [Google Scholar]
- 121.Debas H. T., Farooq O., Grossman M. I. 1975. Inhibition of gastric emptying is a physiological action of cholecystokinin. Gastroenterology. 68: 1211–1217. [PubMed] [Google Scholar]
- 122.Gibbs J., Young R. C., Smith G. P. 1973. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. 84: 488–495. [DOI] [PubMed] [Google Scholar]
- 123.Zieve L., Mulford B., McHale A. 1966. Secretion of pancreatic enzymes. II. Comparative response following test meal or injection of secretin and pancreozymin. Am. J. Dig. Dis. 11: 685–694. [DOI] [PubMed] [Google Scholar]
- 124.Mueller K., Hsiao S. 1977. Specificity of cholecystokinin satiety effect: reduction of food but not water intake. Pharmacol. Biochem. Behav. 6: 643–646. [DOI] [PubMed] [Google Scholar]
- 125.Moran T. H., Baldessarini A. R., Salorio C. F., Lowery T., Schwartz G. J. 1997. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 272: R1245–R1251. [DOI] [PubMed] [Google Scholar]
- 126.Deleted in proof. [Google Scholar]
- 127.Reidelberger R. D., Hernandez J., Fritzsch B., Hulce M. 2004. Abdominal vagal mediation of the satiety effects of CCK in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286: R1005–R1012. [DOI] [PubMed] [Google Scholar]
- 128.Sayegh A. I., Ritter R. C. 2000. Vagus nerve participates in CCK-induced Fos expression in hindbrain but not myenteric plexus. Brain Res. 878: 155–162. [DOI] [PubMed] [Google Scholar]
- 129.Green G. M., Taguchi S., Friestman J., Chey W. Y., Liddle R. A. 1989. Plasma secretin, CCK, and pancreatic secretion in response to dietary fat in the rat. Am. J. Physiol. 256: G1016–G1021. [DOI] [PubMed] [Google Scholar]
- 130.Deleted in proof. [Google Scholar]
- 131.Melville L. D., Smith G. P., Gibbs J. 1993. Comparison of the satiating potencies of cholecystokinin-33 and cholecystokinin-8. Pharmacol. Biochem. Behav. 45: 85–87. [DOI] [PubMed] [Google Scholar]
- 132.Wang L., Martínez V., Barrachina M. D., Taché Y. 1998. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res. 791: 157–166. [DOI] [PubMed] [Google Scholar]
- 133.Yoshimichi G., Lo C. C., Tamashiro K. L., Ma L., Lee D. M., Begg D. P., Liu M., Sakai R. R., Woods S. C., Yoshimatsu H., et al. 2012. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302: G1336–G1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen L., Pearson K. J., Xiong Y., Lo C. M., Tso P., Woods S. C., Davidson W. S., Liu M. 2008. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol. Behav. 95: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arnold M., Mura A., Langhans W., Geary N. 2006. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J. Neurosci. 26: 11052–11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Norgren R., Smith G. P. 1994. A method for selective section of vagal afferent or efferent axons in the rat. Am. J. Physiol. 267: R1136–R1141. [DOI] [PubMed] [Google Scholar]
- 137.Liu M., Shen L., Doi T., Woods S. C., Seeley R. J., Tso P. 2003. Neuropeptide Y and lipid increase apolipoprotein AIV gene expression in rat hypothalamus. Brain Res. 971: 232–238. [DOI] [PubMed] [Google Scholar]
- 138.Chambers A. P., Woods S. C. 2012. The role of neuropeptide Y in energy homeostasis. Handb. Exp. Pharmacol. 209: 23–45. [DOI] [PubMed] [Google Scholar]
- 139.Ebihara K., Ogawa Y., Katsuura G., Numata Y., Masuzaki H., Satoh N., Tamaki M., Yoshioka T., Hayase M., Matsuoka N., et al. 1999. Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes. 48: 2028–2033. [DOI] [PubMed] [Google Scholar]
- 140.Hagan M. M., Benoit S. C., Rushing P. A., Pritchard L. M., Woods S. C., Seeley R. J. 2001. Immediate and prolonged patterns of Agouti-related peptide-(83–132)-induced c-Fos activation in hypothalamic and extrahypothalamic sites. Endocrinology. 142: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 141.Gotoh K., Liu M., Benoit S. C., Clegg D. J., Davidson W. S., D’Alessio D., Seeley R. J., Tso P., Woods S. C. 2006. Apolipoprotein A-IV interacts synergistically with melanocortins to reduce food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290: R202–R207. [DOI] [PubMed] [Google Scholar]
- 142.Rodriguez M. D., Kalogeris T. J., Wang X. L., Wolf R., Tso P. 1997. Rapid synthesis and secretion of intestinal apolipoprotein A-IV after gastric fat loading in rats. Am. J. Physiol. 272: R1170–R1177. [DOI] [PubMed] [Google Scholar]
- 143.Kim W., Egan J. M. 2008. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 60: 470–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li X., Xu M., Wang F., Kohan A. B., Haas M. K., Yang Q., Lou D., Obici S., Davidson W. S., Tso P. 2014. Apolipoprotein A-IV reduces hepatic gluconeogenesis through the nuclear receptor NR1D1. J. Biol. Chem. 289: 2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weinberg R. B., Patton C. S. 1990. Binding of human apolipoprotein A-IV to human hepatocellular plasma membranes. Biochim. Biophys. Acta. 1044: 255–261. [DOI] [PubMed] [Google Scholar]
- 146.Tam C. S., Berthoud H. R., Bueter M., Chakravarthy M. V., Geliebter A., Hajnal A., Holst J., Kaplan L., Pories W., Raybould H., et al. 2011. Could the mechanisms of bariatric surgery hold the key for novel therapies? Report from a Pennington Scientific Symposium. Obes. Rev. 12: 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beckman L. M., Beckman T. R., Sibley S. D., Thomas W., Ikramuddin S., Kellogg T. A., Ghatei M. A., Bloom S. R., le Roux C. W., Earthman C. P. 2011. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN J. Parenter. Enteral Nutr. 35: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Culnan D. M., Cooney R. N., Stanley B., Lynch C. J. 2009. Apolipoprotein A-IV, a putative satiety/antiatherogenic factor, rises after gastric bypass. Obesity (Silver Spring). 17: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adams T. D., Gress R. E., Smith S. C., Halverson R. C., Simper S. C., Rosamond W. D., Lamonte M. J., Stroup A. M., Hunt S. C. 2007. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 357: 753–761. [DOI] [PubMed] [Google Scholar]
- 150.Raffaelli M., Guidone C., Callari C., Iaconelli A., Bellantone R., Mingrone G. 2014. Effect of gastric bypass versus diet on cardiovascular risk factors. Ann. Surg. 259: 694–699. [DOI] [PubMed] [Google Scholar]
- 151.Morínigo R., Moizé V., Musri M., Lacy A. M., Navarro S., Marín J. L., Delgado S., Casamitjana R., Vidal J. 2006. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab. 91: 1735–1740. [DOI] [PubMed] [Google Scholar]