Abstract
α-Tocopherol (vitamin E) has attracted considerable attention as a potential protective or palliative agent. In vitro, its free radical-scavenging antioxidant action has been widely demonstrated. In vivo, however, vitamin E treatment exhibits negligible benefits against oxidative stress. α-Tocopherol influences lipid ordering within biological membranes and its derivatives have been suggested to inhibit the multi-drug efflux pump, P-glycoprotein (P-gp). This study employs the fluorescent membrane probe, 1-(3-sulfonatopropyl)-4-[β[2-(di-n-octylamino)-6-naphthyl]vinyl] pyridinium betaine, to investigate whether these effects are connected via influences on the membrane dipole potential (MDP), an intrinsic property of biological membranes previously demonstrated to modulate P-gp activity. α-Tocopherol and its non-free radical-scavenging succinate analog induced similar decreases in the MDP of phosphatidylcholine vesicles. α-Tocopherol succinate also reduced the MDP of T-lymphocytes, subsequently decreasing the binding affinity of saquinavir for P-gp. Additionally, α-tocopherol succinate demonstrated a preference for cholesterol-treated (membrane microdomain enriched) cells over membrane cholesterol-depleted cells. Microdomain disruption via cholesterol depletion decreased saquinavir’s affinity for P-gp, potentially implicating these structures in the influence of α-tocopherol succinate on P-gp. This study provides evidence of a microdomain dipole potential-dependent mechanism by which α-tocopherol analogs influence P-gp activity. These findings have implications for the use of α-tocopherol derivatives for drug delivery across biological barriers.
Keywords: antioxidants, cholesterol, lipid rafts, saquinavir, vitamin E
The plasma membrane of mammalian cells has diverse functions, many of which are impaired by oxidative damage to its constituent lipids and proteins. The effects of such damage are seen in conditions ranging from natural ageing to type II diabetes, as well as specific diseases of the nervous and cardiovascular systems (1–4). It is not surprising, therefore, that antioxidants such as vitamin E have attracted considerable attention as potential protective or palliative agents. In vitro, vitamin E has been widely demonstrated as having a free radical-scavenging antioxidant action (5). However, its mechanism of action in vivo remains controversial (6, 7), as treatment with vitamin E has yielded negligible benefit against oxidative stress and in some instances has resulted in cytotoxic effects (8–11). In the present report, we demonstrate that vitamin E has the capability to modify the membrane dipole potential (MDP). We also show evidence that tocopherol-dependent changes of the MDP can lead to changes of the ligand binding capability of the multi-drug efflux pump, P-glycoprotein (P-gp). Such observations suggest, therefore, that it is worth considering that some of the reported effects of tocopherols may arise through such mechanisms [as outlined in (12)] rather than resulting solely from antioxidant properties.
α-Tocopherol is the form of vitamin E most preferentially absorbed by the human body (13), despite the β, γ, and δ isoforms and the tocotrienol counterparts, also exhibiting free radical-scavenging action in vitro (14). This observation, along with other evidence, supports the argument that α-tocopherol must have significant nonantioxidant roles not shared by other antioxidants (6), prompting investigations to identify the nonantioxidant physiological effects of α-tocopherol. α-Tocopherol succinate has received attention as an analog of α-tocopherol that demonstrates no in vitro free radical-scavenging action (15), but is reported to possess clinically relevant effects, such as improving the efficacy of chemotherapy when administered as a cotherapeutic agent (16, 17). This effect is likely to involve the multi-drug efflux pump, P-gp, but the underlying mechanism responsible is not well-understood.
Both α-tocopherol and its succinate ester analog, the structures of which are shown in Fig. 1, are reported to have a number of effects on model biological membranes. These include: imparting a negative curvature stress (18) and modifying the phase behavior of lipids; decreasing the enthalpy of the gel to liquid crystalline transition by influencing lipid organization (19, 20); and inducing phase separation as a result of preferential association with specific lipids (21, 22). The present study investigates the effect of α-tocopherol and α-tocopherol succinate on the MDP, which is both a sensitive indicator of changes in lipid organization and that of surrounding water molecules (12, 23–28), and directly influences the behavior of membrane proteins (25, 29, 30).
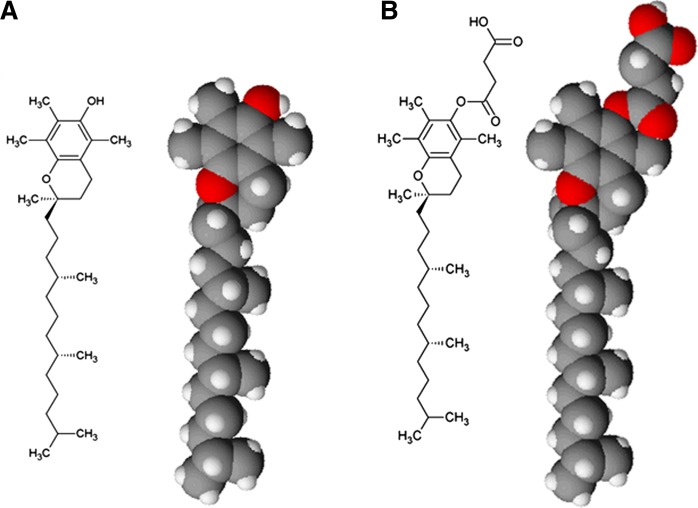
Fig. 1.
Planar schematic and space-filling model views of α-tocopherol (A) and α-tocopherol succinate (B). Images were prepared using ACD/ChemSketch (freeware), version C10E41 (2015) and ACD/3D Viewer (Freeware), version D10E41 (2014), Advanced Chemistry Development, Inc., Toronto, ON, Canada.
We utilize fluorescent probes developed in our laboratories as sensitive indicators of molecular interactions with membranes (30). The first, N-(fluorescein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycerol-3-phosphoethanolamine (FPE), is an indicator of the electrostatic surface potential, and so reports the addition or loss of electrical charge to the membrane surface. The second membrane-localized fluorescent probe, 1-(3-sulfonatopropyl)-4-[β[2-(di-n-octylamino)-6-naphthyl]vinyl] pyridinium betaine (Di-8-ANEPPS), reports the level of the MDP and its use as an indicator of molecular interactions is described in (12, 25, 29, 30). The virtue of both these indicators is that they can be used in exactly the same fashion in model membrane systems as in living cell membranes [e.g., (29)]. We use these techniques to show that in intact cells both α-tocopherol succinate and oxidized cholesterol, 7-ketocholesterol, decrease the dipole potential and influence the interaction of the saquinavir with P-gp.
MATERIALS AND METHODS
Reagents
D-α-tocopherol, D-α-tocopherol succinate, cholesterol, 7-ketocholesterol, and methyl-β-cyclodextrin were supplied by Sigma-Aldrich (UK). Egg phosphatidylcholine (PC) was supplied by Lipid Products (UK). Saquinavir was supplied by Roche (UK). Di-8-ANEPPS and FPE were obtained from Invitrogen (UK). Cell culture reagents, including RPMI 1640, Dulbecco’s PBS (DPBS), FBS, penicillin-streptomycin mixture, L-glutamine, and Trypan blue (0.4% solution) were obtained from Sigma-Aldrich. All other reagents were also supplied by Sigma-Aldrich at the highest purity available.
Model membrane vesicle preparation and labeling with Di-8-ANEPPS
Large unilamellar PC vesicles were prepared and labeled with Di-8-ANEPPS as described in (29). Briefly, egg PC, D-α-tocopherol, D-α-tocopherol succinate, cholesterol, and 7-ketocholesterol dissolved in 5:1 chloroform:methanol were combined at the desired proportions and the solvent evaporated by gentle rotation under a stream of oxygen-free nitrogen gas. The resulting lipid film was rehydrated with 280 mM sucrose and 10 mM Tris (pH 7.4), and this multilamellar solution was then subjected to five freeze-thaw cycles in liquid nitrogen to promote the formation of unilamellar vesicles. Finally, the vesicles were extruded 10 times through polycarbonate filters with 100 nm pores (Nucleopore Corp., Pleasanton, CA) at 45°C using an extruder (Lipex Biomembranes Inc., Vancouver, Canada) according to the extrusion procedure (31). This produced a monodisperse suspension of unilamellar vesicles. Vesicle compositions formed included: PC100%, PC95%X5%, PC90%X10%, PC85%X15%, PC80%X20%, PC75%X25%, and PC70%X30% (molar ratios; X: D-α-tocopherol, D-α-tocopherol succinate, cholesterol, or 7-ketocholesterol). Vesicles were labeled by incubation with 30 μM Di-8-ANEPPS dissolved in ethanol at 37°C, protected from light, for 2 h.
Labeling of Jurkat T-lymphocytes with Di-8-ANEPPS or FPE
Jurkat T-lymphocytes (E6-1 clone, ECACC) were cultured in RPMI 1640 supplemented with 10% v/v heat-inactivated FBS, 2 mM L-glutamine, 100 U ml−1 penicillin, and 100 U ml−1 streptomycin and maintained at 37°C with 5% CO2. The viable cell population was counted using a Trypan blue exclusion assay prior to harvesting by centrifugation (300 g, 5 min). Cells were labeled with Di-8-ANEPPS by incubating a suspension of 1 × 106 cells ml−1 with 0.5 μM Di-8-ANEPPS for 1.5 h at 37°C, as outlined by (29). Alternatively, cells were labeled with FPE according to the method of (32). Briefly, the solvent was evaporated from a volume of FPE (2 mM in 5:1 chloroform:methanol) by gentle rotation under a stream of oxygen-free nitrogen gas. The resulting FPE film was resolvated in a small volume of ethanol (not exceeding 0.5% of the total volume of labeling solution) and diluted in 280 mM sucrose and 10 mM Tris (pH 7.4) to give a 10 μM FPE labeling solution. Cells were resuspended in this labeling solution at 0.5 × 106 cells ml−1 and incubated at 37°C for 1 h, protected from light. After this time, cells were washed twice in DPBS by centrifugation (300 g, 5 min) to remove unbound FPE and resuspended at 0.5 × 106 cells ml−1 in 280 mM sucrose and 10 mM Tris (pH 7.4).
Depletion of cell membrane cholesterol
Methyl-β-cyclodextrin was used to remove cholesterol from the plasma membrane of cells using a method adapted from (33) reported to result in the removal of most of the cholesterol from membrane microdomains (rafts) with negligible effects on cell viability. Following labeling with Di-8-ANEPPS or FPE, cells were washed twice in DPBS by centrifugation (300 g, 5 min) before exposure to 10 mM methyl-β-cyclodextrin in 280 mM sucrose and 10 mM Tris (pH 7.4) for 1 min. Cells were then immediately centrifuged (500 g, 2 min) and resuspended in fresh 280 mM sucrose and 10 mM Tris (pH 7.4).
Cell viability
An alamarBlue resazurin reduction assay (Invitrogen, Carlsbad, CA) was used to assess cell viability and was performed according to the manufacturer’s instructions. After incubating cells with α-tocopherol succinate, cholesterol, or 7-ketocholesterol at the maximum concentration and for the maximum exposure time relevant to this study, 10% v/v alamarBlue was added to the cells in a 96-well plate. After a 4 h incubation at 37°C with 5% CO2, fluorescent measurements were made at 590 nm with 550 nm excitation using a LS-55 plate reader (Perkin Elmer). Baseline readings were taken from culture medium and alamarBlue reagent without cells.
Fluorescence spectroscopy measurements
The spectral sensitivity of membrane bound Di-8-ANEPPS to the dipole potential was used to determine relative differences in the dipole potential of phospholipid vesicles of varying composition and the relative changes in the dipole potential on the interaction of exogenously introduced α-tocopherol succinate and 7-ketocholesterol with vesicles and cell membranes. Di-8-ANEPPS excitation spectra of suspensions of vesicles (400 μM lipid) were taken between 400 and 550 nm and recorded at an emission wavelength of 590 nm using a Fluoromax4 spectrofluorometer (Horiba Jobin Yvon, Middlesex, UK). To visualize the extent of spectral shift, each excitation spectrum was normalized with respect to its integral prior to subtracting the normalized spectrum of the reference (pure egg PC vesicles) resulting in a difference spectrum. A measure of the relative dipole potential of vesicles of varying composition was achieved by exciting the vesicle suspension at 460 and 520 nm and taking the ratio of the emission intensities recorded at 580 nm. The relative difference in the dipole potential of vesicles resulting from increasing proportions of non-egg PC components was found by subtracting the ratio of the reference (pure egg PC vesicles) giving a change in the ratio.
Relative changes in the MDP on serial addition of α-tocopherol succinate, 7-ketocholesterol, or saquinavir to vesicle or cell suspensions (400 μM lipid or 40,000 cells ml−1) were observed in real time, using the above ratiometric technique. The time course of the ratio of Di-8-ANEPPS emission intensities on rapid alternate excitation of the sample at 460 and 520 nm was recorded using a Fluoromax4 spectrofluorometer (Horiba Jobin Yvon, Middlesex, UK) and the relative change in the dipole potential was found by calculating the change in the ratio above the baseline ratio recorded at the onset of the experiment. The effect of any photobleaching, of dilution, and of the solvent on the fluorescence signal was corrected by adding equivalent volumes of ethanol or dimethyl sulfoxide solvents (final concentration not exceeding 1% v/v) to equivalent vesicle or cell suspensions, over a comparable timeframe, and subtracting any resulting fluorescence ratio changes from those obtained on the addition of α-tocopherol succinate, 7-ketocholesterol, or saquinavir. These data were fitted to both equations 1 and 2, and the model best describing the data was determined using an extra sum-of-squares F-test.
| (Eq. 1) |
| (Eq. 2) |
Where y is the calculated change in the ratio; x is the cumulative concentration of α-tocopherol succinate, 7-ketocholesterol, or saquinavir added; Kd is the dissociation constant (concentration units) reflecting the affinity of the binding molecule for the membrane or receptor; Bmax is the 100% binding capacity of the membrane or receptor for the molecule (fluorescence units); and h represents the Hill coefficient, i.e., as an index of cooperativity.
The dissociation constants for the interaction of α-tocopherol succinate with Jurkat T-lymphocytes, with or without prior cholesterol treatment, were determined in a similar fashion by fitting equations 1 and 2 to the recorded change in FPE fluorescence (y) with increasing concentration of α-tocopherol succinate (x). The time course of the fluorescence of FPE at 520 nm with 488 nm excitation during serial addition of α-tocopherol succinate to a suspension of 40,000 cells ml−1 was recorded. The baseline fluorescence determined at the onset of the experiment was subtracted giving a cumulative change in fluorescence intensity per stepwise increase in α-tocopherol succinate concentration, representing the change in the surface potential. This data was likewise corrected for the effects of photobleaching, of dilution, and of the solvent by subtracting the change in fluorescence obtained on the addition of equivalent volumes of solvent over a comparable time frame. Dissociation constants (Kd) and cooperativity (Hill) coefficients were compared using unpaired two-tailed t-tests. Curve fitting and statistical analysis was performed using GraphPad Prism v5.04 (GraphPad Software).
RESULTS
Modulation of the MDP by α-tocopherol and α-tocopherol succinate
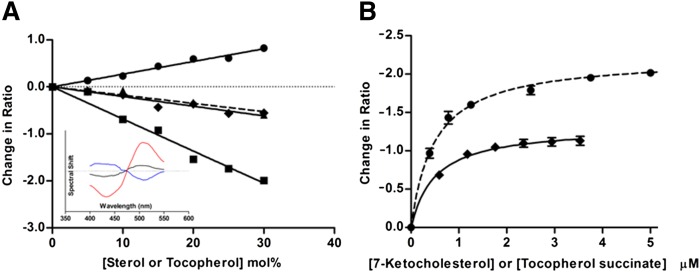
The influence of α-tocopherol and α-tocopherol succinate on the MDP was first explored in a lipid vesicle membrane system. Egg PC vesicles containing α-tocopherol or its analog α-tocopherol succinate were prepared using lipid film hydration followed by extrusion (31). The change in the MDP due to the incorporation of these forms of vitamin E was observed by the potential-dependant shift in the excitation spectrum of the electrochromic fluorescent probe, Di-8-ANEPPS (12). Figure 2A (inset) illustrates difference spectra for tocopherol-containing vesicles, obtained by subtracting the baseline excitation spectrum of Di-8-ANEPPS in PC vesicles from that in the α-tocopherol-containing vesicles. α-Tocopherol induced a spectral red-shift, indicative of a decrease in MDP. Also shown are the difference spectra for vesicles containing cholesterol and its oxidized derivative, 7-ketocholesterol, which have previously been demonstrated to increase and decrease the dipole potential, respectively (28, 29). The concentration dependence of the change in dipole potential induced by the sterols, α-tocopherol, and α-tocopherol succinate in PC vesicles is shown in Fig. 2A. α-Tocopherol produced a change in the dipole potential of PC vesicles similar in magnitude to that induced by cholesterol, but in the opposite direction, consistent with reports on mixed lipid membranes (22). α-Tocopherol succinate, the non-free radical-scavenging analog of α-tocopherol, was found to have a similar effect (P = 0.09, two-tailed t-test on slopes). The influence of 7-ketocholesterol and α-tocopherol succinate, the more soluble of the α-tocopherols, on the dipole potential of Jurkat T-lymphocyte cell membranes was then explored by exposing the intact cells to increasing concentrations of either substance (Fig. 2B). Both decreased the dipole potential with 7-ketocholesterol inducing the greater effect, as was seen in PC vesicles.
Fig. 2.
The relative dipole potential modulation of cell and vesicle membranes by α-tocopherols and sterols. A (inset): Difference spectra demonstrating the blue or red shift of the excitation spectrum of Di-8-ANEPPS in pure egg PC 100 nm vesicles in response to a relative increase or decrease of the dipole potential, respectively. Inclusion of 10 mol% cholesterol (blue line) in PC vesicles increased the dipole potential, whereas 10 mol% α-tocopherol (black line) or 7-ketocholesterol (red line) decreased the dipole potential. A: The change in ratio of Di-8-ANEPPS fluorescence intensity at 460 and 520 nm excitation (R = I460/I520) with increasing concentration of α-tocopherol (diamonds), α-tocopherol succinate (triangles), cholesterol (circles), or 7-ketocholesterol (squares). An increasing ratio reflects a blue-shifting of the excitation spectrum indicating an increasing dipole potential and vice versa. B: The decrease in MDP of T-lymphocyte cells on exposure to increasing concentrations of α-tocopherol succinate (solid line) or 7-ketocholesterol (dashed line). Values represented are mean ± SE for three replicate experiments.
The effect of α-tocopherol succinate on the interaction of saquinavir with the multi-drug efflux pump, P-gp
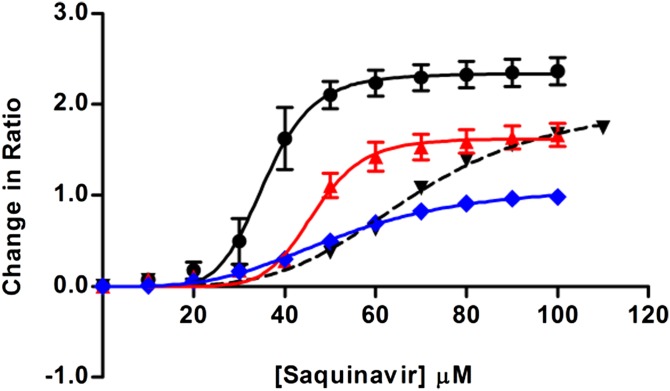
Saquinavir, an anti-retroviral agent used in HIV therapy, is known to act as a ligand of the multi-drug efflux pump, P-gp (34). These interactions can be observed in cell membranes, as they lead to small changes of the dipole potential (29). The binding of saquinavir to Jurkat T-lymphocytes shown in Fig. 3 follows a sigmoidal profile, indicating its interaction with a membrane-localized receptor in a manner very similar to that found in our earlier studies with Caco-2 cells (29). Pretreatment of T-lymphocytes with α-tocopherol succinate significantly reduced the affinity of saquinavir for P-gp, demonstrated by an increase in the dissociation constant (Kd, see Table 1) for the interaction (unpaired two-tailed t-test; P = 0.0012, R2 = 0.945). Similarly, 7-ketocholesterol, which likewise decreases the dipole potential, also decreased the affinity of saquinavir for P-gp (unpaired two-tailed t-test; P = 0.004, R2 = 0.90), consistent with reports on the influence of a change in the dipole potential on P-gp function (29). Interestingly, α-tocopherol succinate, which decreases the MDP to a lesser extent than 7-ketocholesterol, demonstrated the larger effect on the affinity of saquinavir for P-gp. The influence of 7-ketocholesterol or α-tocopherol succinate treatment on the shape of the binding profiles of saquinavir may provide an explanation for the apparent discrepancy between the magnitude of their influence on the dipole potential and P-gp activity. Following 7-ketocholesterol treatment, the Hill coefficient (see Table 1) of the sigmoidal binding profile, reflecting the extent of cooperativity in the interaction, is not significantly altered (unpaired two-tailed t-test; P = 0.42). Following treatment with α-tocopherol succinate, however, the Hill coefficient is significantly reduced (unpaired two tailed t-test; P = 0.02, R2 = 0.75). This observation suggests that α-tocopherol succinate is exerting a significant effect on the nature of the saquinavir-P-gp binding mechanism that is not induced by 7-ketocholesterol.
Fig. 3.
The interaction of saquinavir with T-lymphocytes following α-tocopherol succinate, 7-ketocholesterol, or methyl-β-cyclodextrin treatment. The binding of saquinavir to T-lymphocytes is observed through the change in ratio of Di-8-ANEPPS fluorescence intensity at 460 and 520 nm excitation (R = I460/I520) reflecting the change in the cell MDP induced by the interaction. The binding curves for saquinavir to untreated cells (black, solid line) and cells treated with methyl-β-cyclodextrin (black, dashed line) to disrupt cholesterol-rich membrane microdomains are shown in addition to the binding curves to cells pretreated with α-tocopherol succinate (blue) or 7-ketocholesterol (red). Values represented are mean ± SE for three replicate experiments.
TABLE 1.
The dissociation constants and Hill coefficients for the interaction of saquinavir with pretreated T-lymphocytes
| Treatment | Kd (μM) | Hill Coefficient |
| Untreated | 36 ± 1 | 7 ± 1 |
| 7-KC | 47 ± 1 | 9 ± 2 |
| α-TS | 52 ± 2 | 3.4 ± 0.2 |
| MβCD | 68 ± 2 | 4.4 ± 0.4 |
The dissociation constants (Kd) and Hill coefficients (±SEM) obtained from best fits of a sigmoidal binding model to the data presented in Fig. 3 for the interaction of saquinavir with T-lymphocytes, either untreated or pretreated with 7-ketocholesterol (7-KC), α-tocopherol succinate (α-TS), or methyl-β-cyclodextrin (MβCD).
The mechanism underlying the cooperative nature of the saquinavir-P-gp interaction is unknown, however, the activity of P-gp is thought to be membrane microdomain-associated (35, 36). The receptor is thought to show a preference for the elevated dipole potential associated with cholesterol-rich microdomains (29, 30) due to their higher lipid packing density and decreased hydration (28, 37). The treatment of T-lymphocytes with methyl-β-cyclodextrin to disrupt these microdomains via cholesterol depletion (33) significantly decreased the affinity of saquinavir for P-gp (unpaired two-tailed t-test; P = 0.0002, R2 = 0.98) (Fig. 3, Table 1), suggesting the activity of P-gp is membrane microdomain-dependant. The Hill coefficient for the interaction (see Table 1) was also much reduced suggesting that cholesterol-rich microdomains may have an important role in the underlying mechanism of the saquinavir-P-gp interaction. Interestingly, the effect of methyl-β-cyclodextrin and α-tocopherol succinate on the cooperativity of the saquinavir-P-gp interaction is similar (unpaired two tailed t-test on Hill coefficients; P = 0.09). We suggest that α-tocopherol succinate influences P-gp by decreasing the dipole potential of P-gp that is associated with membrane microdomains.
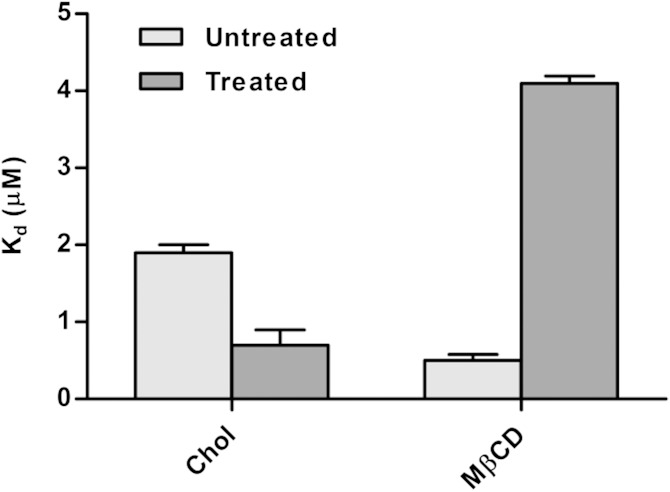
We frame this hypothesis as follows: α-Tocopherol succinate localizes in cholesterol-rich membrane microdomains where it acts to decrease the dipole potential. To explore this hypothesis, the interaction of α-tocopherol succinate with cells enriched with, or depleted of, cholesterol-rich microdomains was investigated. T-lymphocytes were treated with cholesterol to increase the extent of cholesterol-rich microdomains in the plasma membrane or with methyl-β-cyclodextrin to induce microdomain disruption. The dissociation constant for α-tocopherol succinate binding to these treated cells, determined from binding curves formed from measurements of the change in surface or dipole potential induced by the binding interaction (30), was compared with that for α-tocopherol succinate binding to untreated cells (Fig. 4). Cholesterol treatment significantly increased the affinity of α-tocopherol succinate for the membrane (unpaired two-tailed t-test on Kd values; P = 0.0058), whereas methyl-β-cyclodextrin treatment had the opposite effect (unpaired two-tailed t-test on Kd values; P < 0.0001). The increased affinity of α-tocopherol succinate for membranes rich in microdomains suggests it has a preference toward these structures and is likely to decrease the dipole potential with lateral heterogeneity across the membrane.
Fig. 4.
The influence of cholesterol-rich cell membrane microdomains on the binding affinity of α-tocopherol succinate. The dissociation constants for the interaction of α-tocopherol succinate with T-lymphocytes pretreated with cholesterol (increasing the extent of membrane microdomains) or methyl-β-cyclodextrin (reducing the extent of membrane microdomains) are compared with those for untreated cells. Treatment with cholesterol (Chol) significantly increased the affinity of α-tocopherol succinate for the membrane, whereas treatment with methyl-β-cyclodextrin (MβCD) had the opposite effect. The binding interaction was observed through the modulation of the membrane surface potential detected using the fluorescent probe, FPE, for the cholesterol treatment experiment and through the modulation of the MDP for the methyl-β-cyclodextrin treatment experiment. Values represented are mean ± SE for three replicate experiments.
DISCUSSION
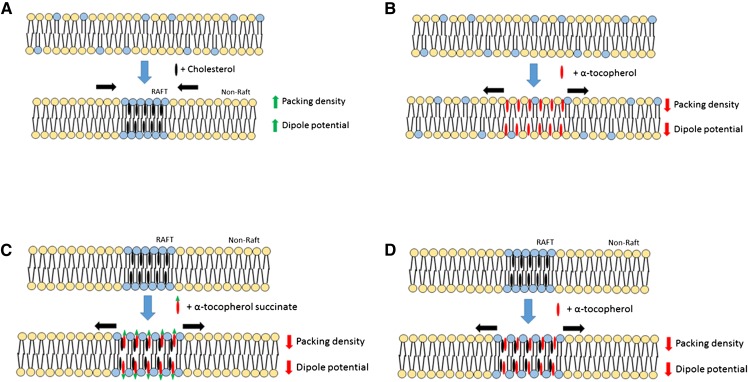
The present study demonstrates that the interaction of α-tocopherol and its water soluble derivative, α-tocopherol succinate, with biological membranes leads to changes of the MDP. A schematic representation of these interactions and their effects in relation to those of cholesterol is shown in Fig. 5. As α-tocopherol succinate is a non-free radical-scavenging analog of α-tocopherol, this effect is independent of, and may be additional to, any free radical-scavenging activity for α-tocopherol.
Fig. 5.
Schematic comparison of the interaction of cholesterol and α-tocopherol with biological membranes. A: Cholesterol is well-documented to form microdomains of increased packing density and elevated dipole potential in phospholipid membranes. B: In this study, we report that addition of α-tocopherol to egg PC membranes causes a reduction in the dipole potential. A possible explanation for this phenomenon is that α-tocopherol sits higher in the membrane, closer to the aqueous interface than cholesterol, thereby increasing phospholipid lipid headgroup spacing. C: In this model the insertion of α-tocopherol into cholesterol-rich microdomains reduces the lipid packing density and MDP. D: The observation that α-tocopherol succinate modulates the dipole potential to a similar extent as α-tocopherol suggests that both forms interact similarly with membranes and supports the hypothesis that they sit high in the membrane, such that the addition of the succinate group does not additionally perturb the dipole potential.
Supplementing membranes with α-tocopherol succinate appears to influence the interaction of the anti-retroviral agent, saquinavir, most likely through effects on the multi-drug efflux pump, P-gp. This membrane receptor is reported to be sensitive to change in the dipole potential (29) and its activity is linked here and elsewhere (29, 38, 39) with cholesterol-rich membrane microdomains. α-Tocopherol succinate is shown to have a preferential affinity for membranes rich in these microdomains, suggesting its possible localization within these structures. While direct evidence for the association of tocopherol succinate with cholesterol-enriched membrane microdomains remains elusive, this hypothesis is supported by previous observations that the membrane localization of tocopherol succinate influences the activity of microdomain-associated kinases, including protein kinase C (40, 41). In the present study, the membrane interactions of tocopherol succinate were also shown to influence the nature of the saquinavir-P-gp interaction with similar effect to microdomain disruption via membrane cholesterol depletion. We hypothesize that the mechanism by which α-tocopherol succinate influences P-gp is via its effect to decrease the dipole potential of membrane microdomains with which this receptor associates. In support of this notion, 7-ketocholesterol, an oxidized form of cholesterol known to localize in cholesterol-rich microdomains (42, 43) and demonstrated to decrease the dipole potential of the plasma membrane, was also shown to influence the interaction of saquinavir with P-gp. The apparent differences in the extent to which α-tocopherol succinate and 7-ketocholesterol influence both the dipole potential and P-gp are suggested to be due to differences in their heterogeneous lateral distribution within membranes and, in particular, their extent of colocalization with P-gp in microdomains. The specific influence of molecules on the microdomain profile of membranes and the resulting lateral heterogeneity in dipole potential modulation across the membrane are likely to be factors which correlate more directly with microdomain-associated receptor activity.
The implications of α-tocopherol succinate modulation of P-gp activity are significant. The cytotoxicity of many native and xenobiotic compounds could be increased due to the reduced ability of P-gp to remove them from the cell. In addition to the potential negative side effects this may cause, α-tocopherol succinate treatment may increase the efficacy of applied therapeutic agents by improved cellular retention. In addition, α-tocopherol succinate may also have a role in countering multi-drug resistance. This phenomenon is associated with P-gp which is able to remove a wide variety of chemically unrelated drugs from cells, including antibiotics, anti-cancer agents, and anti-HIV agents such as saquinavir (44–46). For example, increased expression of P-gp is observed in some cancerous tumor cells (47) and the consequent increase in drug resistance is associated with the failure of chemotherapy (45, 46). Interestingly, α-tocopherol succinate treatment has been shown to increase the efficacy of some chemotherapeutic agents (16, 17) and this effect could be explained, therefore, by a similar mechanism to that proposed in our present work.
Drug delivery across biological barriers is presently considered a major bottle-neck for the delivery of therapeutics to target tissues (48). Multidrug resistance transporters such as P-gp (MDR1) play an important role in maintaining epithelial and endothelial barrier function by actively transporting a wide variety of predominantly small hydrophobic xenobiotics and toxins out of cells (49). P-gp is expressed in multiple biological barriers, including the blood-brain (50), blood-retinal (51), corneal (52), and intestinal mucosal barriers (53). In addition, overexpression of this protein has also long been known to play a central role in the development of multi-drug resistant cancer (54). Inhibitors of P-gp are well-documented to improve drug delivery across biological barriers (55, 56), enhance bioavailability of orally administered therapeutics (57), and overcome multi-drug resistant cancers (58). For these reasons there has been considerable interest in the development of well-tolerated P-gp inhibitors (49). For example, α-tocopherol polyethylene glycol succinate is reported to enhance oral absorption of several therapeutic compounds, including cyclosporin, paclitaxel, doxorubicin, vinblastine, and colchicine, through P-gp inhibition (44, 59). This PEGylated α-tocopherol succinate is likely to influence the dipole potential in a similar manner to α-tocopherol and its nonPEGylated succinate ester and modulate P-gp activity via the same mechanism.
The dipole potential is a determinant of the functions of various membrane proteins in addition to the P-gp investigated in the present study, and many proteins have been identified as being present in membrane microdomains. Our observations may therefore apply to a variety of proteins and different cell types. α-Tocopherol, through its localization in membrane microdomains (43), may influence the function of microdomain-associated proteins via the same mechanism we propose for α-tocopherol succinate. Indeed the ubiquity of this mechanism may help to explain the diverse and sometimes apparently contradictory actions which have been ascribed to vitamin E.
In conclusion, the present study demonstrates that α-tocopherol and α-tocopherol succinate modulate the dipole potential of artificial and cell membranes. This effect may contribute toward an explanation of the diverse physiological actions of vitamin E. In addition, this effect forms the basis of a mechanism for the indirect inhibition of P-gp by α-tocopherol derivatives, highlighting the potential for the application of these compounds in countering multi-drug resistance and in facilitating drug delivery across biological barriers.
Acknowledgments
The authors are grateful to the anonymous referees, as some revisions to the manuscript following their comments have helped to clarify the message of the paper.
Footnotes
Abbreviations:
- Di-8-ANEPPS
- 1-(3-sulfonatopropyl)-4-[β[2-(di-n-octylamino)-6-naphthyl]vinyl] pyridinium betaine
- DPBS
- Dulbecco’s PBS
- FPE
- N-(fluorescein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycerol-3-phosphoethanolamine
- MDP
- membrane dipole potential
- PC
- phosphatidylcholine
- P-gp
- P-glycoprotein
This work was partially supported by the Engineering and Physical Sciences Research Council (EPSRC) (grant reference EP/J017566/1). The University of Exeter provided funds for consumables for some of this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Evans J. L., Maddux B. A., Goldfine I. D. 2005. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal. 7: 1040–1052. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T., Holbrook N. J. 2000. Oxidants, oxidative stress and the biology of ageing. Nature. 408: 239–247. [DOI] [PubMed] [Google Scholar]
- 3.Molavi B., Mehta J. L. 2004. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr. Opin. Cardiol. 19: 488–493. [DOI] [PubMed] [Google Scholar]
- 4.Montine T. J., Neely M. D., Quinn J. F., Beal M. F., Markesbery W. R., Roberts L. J., Morrow J. D. 2002. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic. Biol. Med. 33: 620–626. [DOI] [PubMed] [Google Scholar]
- 5.Kamal-Eldin A., Appelqvist L. A. 1996. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 31: 671–701. [DOI] [PubMed] [Google Scholar]
- 6.Azzi A. 2007. Molecular mechanism of alpha-tocopherol action. Free Radic. Biol. Med. 43: 16–21. [DOI] [PubMed] [Google Scholar]
- 7.Brigelius-Flohé R. 2009. Vitamin E: the shrew waiting to be tamed. Free Radic. Biol. Med. 46: 543–554. [DOI] [PubMed] [Google Scholar]
- 8.Brigelius-Flohé R., Kelly F. J., Salonen J. T., Neuzil J., Zingg J. M., Azzi A. 2002. The European perspective on vitamin E: current knowledge and future research. Am. J. Clin. Nutr. 76: 703–716. [DOI] [PubMed] [Google Scholar]
- 9.Golbidi S., Ebadi S. A., Laher I. 2011. Antioxidants in the treatment of diabetes. Curr. Diabetes Rev. 7: 106–125. [DOI] [PubMed] [Google Scholar]
- 10.Robinson I., de Serna D. G., Gutierrez A., Schade D. S. 2006. Vitamin E in humans: an explanation of clinical trial failure. Endocr. Pract. 12: 576–582. [DOI] [PubMed] [Google Scholar]
- 11.Saremi A., Arora R. 2010. Vitamin E and cardiovascular disease. Am. J. Ther. 17: e56–e65. [DOI] [PubMed] [Google Scholar]
- 12.O’Shea P. 2005. Physical landscapes in biological membranes: physico-chemical terrains for spatio-temporal control of biomolecular interactions and behaviour. Philos. Trans. A Math. Phys. Eng. Sci. 363: 575–588. [DOI] [PubMed] [Google Scholar]
- 13.Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O., Arai H., Inoue K. 1997. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 409: 105–108. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida Y., Niki E., Noguchi N. 2003. Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chem. Phys. Lipids. 123: 63–75. [DOI] [PubMed] [Google Scholar]
- 15.Neuzil J., Weber T., Gellert N., Weber C. 2001. Selective cancer cell killing by alpha-tocopheryl succinate. Br. J. Cancer. 84: 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad K. N., Kumar B., Yan X. D., Hanson A. J., Cole W. C. 2003. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. J. Am. Coll. Nutr. 22: 108–117. [DOI] [PubMed] [Google Scholar]
- 17.Ripoll E. A., Rama B. N., Webber M. M. 1986. Vitamin E enhances the chemotherapeutic effects of adriamycin on human prostatic carcinoma cells in vitro. J. Urol. 136: 529–531. [DOI] [PubMed] [Google Scholar]
- 18.Bradford A., Atkinson J., Fuller N., Rand R. P. 2003. The effect of vitamin E on the structure of membrane lipid assemblies. J. Lipid Res. 44: 1940–1945. [DOI] [PubMed] [Google Scholar]
- 19.Massey J. B. 2001. Interfacial properties of phosphatidylcholine bilayers containing vitamin E derivatives. Chem. Phys. Lipids. 109: 157–174. [DOI] [PubMed] [Google Scholar]
- 20.Quinn P. J. 2004. Is the distribution of alpha-tocopherol in membranes consistent with its putative functions? Biochemistry (Mosc). 69: 58–66. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz A., Aranda F. J., Gómez-Fernández J. C. 1987. A differential scanning calorimetry study of the interaction of alpha-tocopherol with mixtures of phospholipids. Biochim. Biophys. Acta. 898: 214–222. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Migallón M. P., Aranda F. J., Gómez-Fernández J. C. 1996. Interaction between alpha-tocopherol and heteroacid phosphatidylcholines with different amounts of unsaturation. Biochim. Biophys. Acta. 1279: 251–258. [DOI] [PubMed] [Google Scholar]
- 23.Bechinger B., Seelig J. 1991. Interaction of electric dipoles with phospholipid head groups. A 2H and 31P NMR study of phloretin and phloretin analogues in phosphatidylcholine membranes. Biochemistry. 30: 3923–3929. [DOI] [PubMed] [Google Scholar]
- 24.Brockman H. 1994. Dipole potential of lipid membranes. Chem. Phys. Lipids. 73: 57–79. [DOI] [PubMed] [Google Scholar]
- 25.Cladera J., O’Shea P. 1998. Intramembrane molecular dipoles affect the membrane insertion and folding of a model amphiphilic peptide. Biophys. J. 74: 2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke R. J. 1997. Effect of lipid structure on the dipole potential of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 1327: 269–278. [DOI] [PubMed] [Google Scholar]
- 27.Gawrisch K., Ruston D., Zimmerberg J., Parsegian V. A., Rand R. P., Fuller N. 1992. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys. J. 61: 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starke-Peterkovic T., Turner N., Vitha M. F., Waller M. P., Hibbs D. E., Clarke R. J. 2006. Cholesterol effect on the dipole potential of lipid membranes. Biophys. J. 90: 4060–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asawakarn T., Cladera J., O’Shea P. 2001. Effects of the membrane dipole potential on the interaction of saquinavir with phospholipid membranes and plasma membrane receptors of Caco-2 cells. J. Biol. Chem. 276: 38457–38463. [DOI] [PubMed] [Google Scholar]
- 30.O’Shea P. 2003. Intermolecular interactions with/within cell membranes and the trinity of membrane potentials: kinetics and imaging. Biochem. Soc. Trans. 31: 990–996. [DOI] [PubMed] [Google Scholar]
- 31.Mayer L. D., Hope M. J., Cullis P. R. 1986. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta. 858: 161–168. [DOI] [PubMed] [Google Scholar]
- 32.Cladera J., Martin I., O’Shea P. 2001. The fusion domain of HIV gp41 interacts specifically with heparan sulfate on the T-lymphocyte cell surface. EMBO J. 20: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouquette-Jazdanian A. K., Pelassy C., Breittmayer J. P., Aussel C. 2006. Revaluation of the role of cholesterol in stabilizing rafts implicated in T cell receptor signaling. Cell. Signal. 18: 105–122. [DOI] [PubMed] [Google Scholar]
- 34.Deeley R. G., Cole S. P. 2006. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 580: 1103–1111. [DOI] [PubMed] [Google Scholar]
- 35.Demeule M., Jodoin J., Gingras D., Beliveau R. 2000. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 466: 219–224. [DOI] [PubMed] [Google Scholar]
- 36.Hinrichs J. W., Klappe K., Hummel I., Kok J. W. 2004. ATP-binding cassette transporters are enriched in non-caveolar detergent-insoluble glycosphingolipid-enriched membrane domains (DIGs) in human multidrug-resistant cancer cells. J. Biol. Chem. 279: 5734–5738. [DOI] [PubMed] [Google Scholar]
- 37.Duggan J., Jamal G., Tilley M., Davis B., McKenzie G., Vere K., Somekh M. G., O’Shea P., Harris H. 2008. Functional imaging of microdomains in cell membranes. Eur. Biophys. J. 37: 1279–1289. [DOI] [PubMed] [Google Scholar]
- 38.Klappe K., Hummel I., Hoekstra D., Kok J. W. 2009. Lipid dependence of ABC transporter localization and function. Chem. Phys. Lipids. 161: 57–64. [DOI] [PubMed] [Google Scholar]
- 39.Orlowski S., Martin S., Escargueil A. 2006. P-glycoprotein and ‘lipid rafts’: some ambiguous mutual relationships (floating on them, building them or meeting them by chance?). Cell. Mol. Life Sci. 63: 1038–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuschieri J., Bulger E., Biligren J., Garcia I., Maier R. V. 2007. Vitamin E inhibits endotoxin-mediated transport of phosphatases to lipid rafts. Shock. 27: 19–24. [DOI] [PubMed] [Google Scholar]
- 41.Traber M. G., Atkinson J. 2007. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 43: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers S. J., Stanley K. K. 1999. Src family kinase activation in glycosphingolipid-rich membrane domains of endothelial cells treated with oxidised low density lipoprotein. Atherosclerosis. 143: 389–397. [DOI] [PubMed] [Google Scholar]
- 43.Royer M. C., Lemaire-Ewing S., Desrumaux C., Monier S., Pais de Barros J. P., Athias A., Neel D., Lagrost L. 2009. 7-Ketocholesterol incorporation into sphingolipid/cholesterol-enriched (lipid raft) domains is impaired by vitamin E: a specific role for alpha-tocopherol with consequences on cell death. J. Biol. Chem. 284: 15826–15834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dintaman J. M., Silverman J. A. 1999. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm. Res. 16: 1550–1556. [DOI] [PubMed] [Google Scholar]
- 45.Endicott J. A., Ling V. 1989. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem. 58: 137–171. [DOI] [PubMed] [Google Scholar]
- 46.Gottesman M. M., Pastan I. 1993. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62: 385–427. [DOI] [PubMed] [Google Scholar]
- 47.Gottesman M. M., Fojo T., Bates S. E. 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2: 48–58. [DOI] [PubMed] [Google Scholar]
- 48.Pardridge W. M. 2005. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amin M. L. 2013. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights. 7: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schinkel A. H. 1999. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 36: 179–194. [DOI] [PubMed] [Google Scholar]
- 51.Fujii S., Setoguchi C., Kawazu K., Hosoya K. 2014. Impact of P-glycoprotein on blood-retinal barrier permeability: comparison of blood-aqueous humor and blood-brain barrier using mdr1a knockout rats. Invest. Ophthalmol. Vis. Sci. 55: 4650–4658. [DOI] [PubMed] [Google Scholar]
- 52.Dey S., Patel J., Anand B. S., Jain-Vakkalagadda B., Kaliki P., Pal D., Ganapathy V., Mitra A. K. 2003. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 44: 2909–2918. [DOI] [PubMed] [Google Scholar]
- 53.Ho G. T., Moodie F. M., Satsangi J. 2003. Multidrug resistance 1 gene (P-glycoprotein 170): an important determinant in gastrointestinal disease? Gut. 52: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clarke R., Leonessa F., Trock B. 2005. Multidrug resistance/P-glycoprotein and breast cancer: review and meta-analysis. Semin. Oncol. 32(6, Suppl 7) S9–S15. [DOI] [PubMed] [Google Scholar]
- 55.Kemper E. M., van Zandbergen A. E., Cleypool C., Mos H. A., Boogerd W., Beijnen J. H., van Tellingen O. 2003. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin. Cancer Res. 9: 2849–2855. [PubMed] [Google Scholar]
- 56.Ostacolo C., Caruso C., Tronino D., Troisi S., Laneri S., Pacente L., Del Prete A., Sacchi A. 2013. Enhancement of corneal permeation of riboflavin-5′-phosphate through vitamin E TPGS: a promising approach in corneal trans-epithelial cross linking treatment. Int. J. Pharm. 440: 148–153. [DOI] [PubMed] [Google Scholar]
- 57.Woo J. S., Lee C. H., Shim C. K., Hwang S. J. 2003. Enhanced oral bioavailability of paclitaxel by coadministration of the P-glycoprotein inhibitor KR30031. Pharm. Res. 20: 24–30. [DOI] [PubMed] [Google Scholar]
- 58.Bansal T., Jaggi M., Khar R. K., Talegaonkar S. 2009. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharm. Sci. 12: 46–78. [DOI] [PubMed] [Google Scholar]
- 59.Constantinides P. P., Han J., Davis S. S. 2006. Advances in the use of tocols as drug delivery vehicles. Pharm. Res. 23: 243–255. [DOI] [PubMed] [Google Scholar]