Abstract
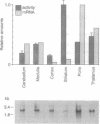
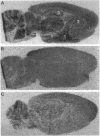
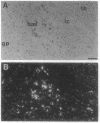
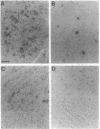
To investigate the molecular basis of regional variation in expression of brain acetylcholinesterase (AChE; EC 3.1.1.7), steady-state levels of AChE activity and mRNA were examined. Relative AChE activity in Triton extracts from six areas of the rat brain varied as follows: cortex < cerebellum < medulla < pons-midbrain < thalamus < striatum. In contralateral samples from the same brains, AChE mRNA was assessed by Northern blotting with random-primed 32P-labeled cDNA. The regional abundance of the major 2.4-kb AChE transcript differed from that of the enzyme activity: cortex < striatum < cerebellum < medulla < thalamus < pons-midbrain. In situ hybridization with a 33P-labeled antisense AChE oligonucleotide provided evidence for high levels of AChE message in cells of the nucleus basalis, nucleus accumbens, neostriatum, substantia nigra, motor nucleus of the facial nerve, and spinal nucleus of the trigeminal nerve. In the caudate-putamen, large, heavily labeled neurons were not numerous, but they were approximately as frequent as the cholinergic interneurons revealed by choline acetyltransferase immunocytochemistry. The relatively low number of these AChE-expressing cells probably explains the relative dearth of AChE mRNA-like material in the neostriatum.
Full text
PDF


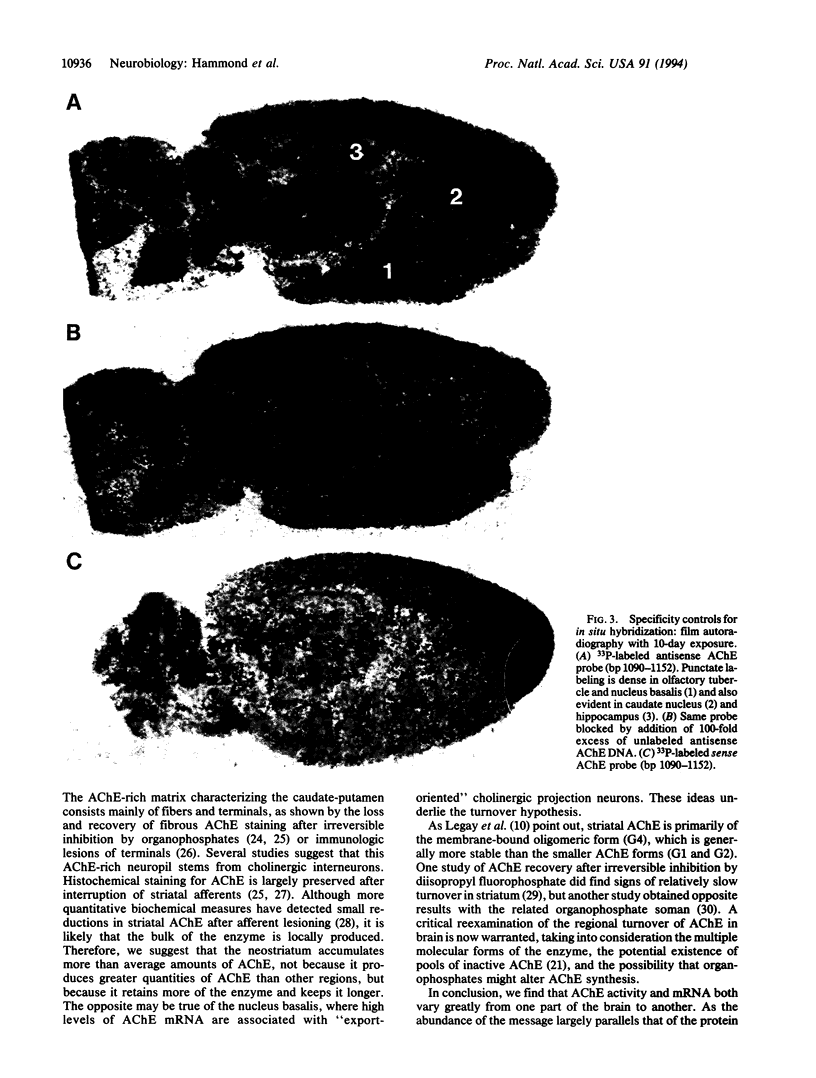
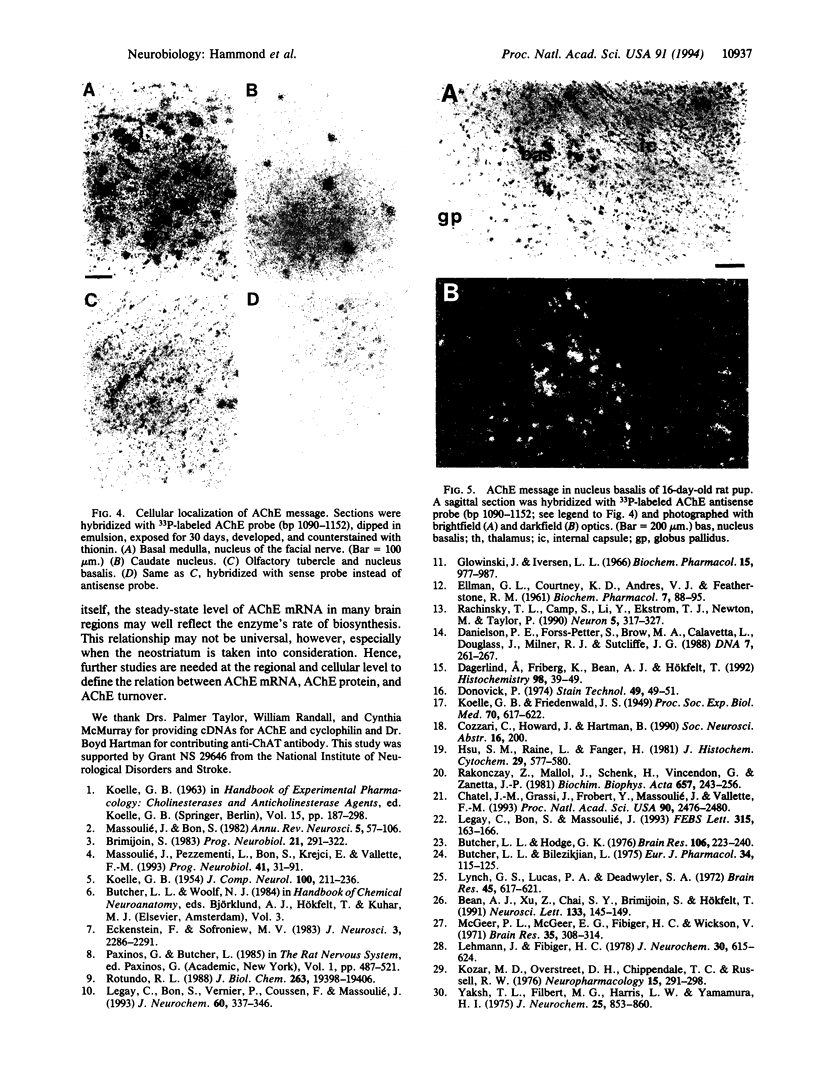
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean A. J., Xu Z., Chai S. Y., Brimijoin S., Hökfelt T. Effect of intracerebral injection of monoclonal acetylcholinesterase antibodies on cholinergic nerve terminals in the rat central nervous system. Neurosci Lett. 1991 Nov 25;133(1):145–149. doi: 10.1016/0304-3940(91)90078-8. [DOI] [PubMed] [Google Scholar]
- Brimijoin S. Molecular forms of acetylcholinesterase in brain, nerve and muscle: nature, localization and dynamics. Prog Neurobiol. 1983;21(4):291–322. doi: 10.1016/0301-0082(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Butcher L. L., Bilezikjian L. Acetylcholinesterase-containing neurons in the neostriatum and substantia nigra revealed after punctate intracerebral injection of di-isopropylfluorophosphate. Eur J Pharmacol. 1975 Nov;34(1):115–125. doi: 10.1016/0014-2999(75)90231-9. [DOI] [PubMed] [Google Scholar]
- Butcher L. L., Hodge G. K. Postnatal development of acetylcholinesterase in the caudate-putamen nucleus and substantia nigra of rats. Brain Res. 1976 Apr 23;106(2):223–240. doi: 10.1016/0006-8993(76)91022-2. [DOI] [PubMed] [Google Scholar]
- Chatel J. M., Grassi J., Frobert Y., Massoulié J., Vallette F. M. Existence of an inactive pool of acetylcholinesterase in chicken brain. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2476–2480. doi: 10.1073/pnas.90.6.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagerlind A., Friberg K., Bean A. J., Hökfelt T. Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry. 1992 Aug;98(1):39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Donovick P. J. A metachromatic stain for neural tissue. Stain Technol. 1974 Jan;49(1):49–51. doi: 10.3109/10520297409116937. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eckenstein F., Sofroniew M. V. Identification of central cholinergic neurons containing both choline acetyltransferase and acetylcholinesterase and of central neurons containing only acetylcholinesterase. J Neurosci. 1983 Nov;3(11):2286–2291. doi: 10.1523/JNEUROSCI.03-11-02286.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. Regional studies of catecholamines in the rat brain. 3. Subcellullar distribution of endogenous and exogenous catecholamines in various brain regions. Biochem Pharmacol. 1966 Jul;15(7):977–987. doi: 10.1016/0006-2952(66)90175-4. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical localization of cholinesterases in the central nervous system of the rat. J Comp Neurol. 1954 Feb;100(1):211–235. doi: 10.1002/cne.901000108. [DOI] [PubMed] [Google Scholar]
- Kozar M. D., Overstreet D. H., Chippendale T. C., Russell R. W. Changes of acetylcholinesterase activity in three major brain areas and related changes in behaviour following acute treatment with diisopropyl fluorophosphate. Neuropharmacology. 1976 May;15(5):291–298. doi: 10.1016/0028-3908(76)90131-3. [DOI] [PubMed] [Google Scholar]
- Legay C., Bon S., Massoulié J. Expression of a cDNA encoding the glycolipid-anchored form of rat acetylcholinesterase. FEBS Lett. 1993 Jan 4;315(2):163–166. doi: 10.1016/0014-5793(93)81155-s. [DOI] [PubMed] [Google Scholar]
- Legay C., Bon S., Vernier P., Coussen F., Massoulié J. Cloning and expression of a rat acetylcholinesterase subunit: generation of multiple molecular forms and complementarity with a Torpedo collagenic subunit. J Neurochem. 1993 Jan;60(1):337–346. doi: 10.1111/j.1471-4159.1993.tb05856.x. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Fibiger H. C. Acetylcholinesterase in the substantia nigra and caudate-putamen of the rat: properties and localization in dopaminergic neurons. J Neurochem. 1978 Mar;30(3):615–624. doi: 10.1111/j.1471-4159.1978.tb07816.x. [DOI] [PubMed] [Google Scholar]
- Lynch G. S., Lucas P. A., Deadwyler S. A. The demonstration of acetylcholinesterase containing neurones within the caudate nucleus of the rat. Brain Res. 1972 Oct 27;45(2):617–621. doi: 10.1016/0006-8993(72)90494-5. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Pezzementi L., Bon S., Krejci E., Vallette F. M. Molecular and cellular biology of cholinesterases. Prog Neurobiol. 1993 Jul;41(1):31–91. doi: 10.1016/0301-0082(93)90040-y. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G., Fibiger H. C., Wickson V. Neostriatal choline acetylase and cholinesterase following selective brain lesions. Brain Res. 1971 Dec 10;35(1):308–314. doi: 10.1016/0006-8993(71)90625-1. [DOI] [PubMed] [Google Scholar]
- Rachinsky T. L., Camp S., Li Y., Ekström T. J., Newton M., Taylor P. Molecular cloning of mouse acetylcholinesterase: tissue distribution of alternatively spliced mRNA species. Neuron. 1990 Sep;5(3):317–327. doi: 10.1016/0896-6273(90)90168-f. [DOI] [PubMed] [Google Scholar]
- Rakonczay Z., Mallol J., Schenk H., Vincendon G., Zanetta J. P. Purification and properties of the membrane-bound acetylcholinesterase from adult rat brain. Biochim Biophys Acta. 1981 Jan 15;657(1):243–256. doi: 10.1016/0005-2744(81)90148-0. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L. Biogenesis of acetylcholinesterase molecular forms in muscle. Evidence for a rapidly turning over, catalytically inactive precursor pool. J Biol Chem. 1988 Dec 25;263(36):19398–19406. [PubMed] [Google Scholar]
- Yaksh T. L., Filbert M. G., Harris L. W., Yamamura H. I. Acetylcholinesterase turnover in brain, cerebrospinal fluid and plasma. J Neurochem. 1975 Dec;25(6):853–860. doi: 10.1111/j.1471-4159.1975.tb04417.x. [DOI] [PubMed] [Google Scholar]