Abstract
Background
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is a treatment commonly applied to peritoneal surface disease from low-grade mucinous tumors of the appendix. Some centers have extended this therapy to carcinomatosis from more aggressive malignancies. Therefore, we reviewed our experience with CRS/HIPEC for patients with goblet cell carcinomatosis.
Methods
Patients with carcinomatosis from appendiceal primaries with goblet cell features were identified in a prospectively maintained database of 1198 CRS/HIPEC procedures performed between 1991 and 2014. Patient demographics, disease characteristics, morbidity, mortality, and survival were reviewed.
Results
A total of 31 patients with carcinomatosis originating from appendiceal goblet cell tumors underwent CRS/HIPEC during the study period. Patients were generally young (mean age, 53 y) and otherwise healthy (84% without comorbidities) with good performance status (94% Eastern Cooperative Oncology Group 0 or 1). The mean number of visceral resections was 3.5, and complete cytoreduction of macroscopic disease was accomplished in 36%. Major 90-d morbidity and mortality rates were 38.7% and 9.7%, respectively. Median overall survival (OS) for all patients was 18.4 mo. Patients with negative nodes had better survival than those with positive nodes (median OS, 29.2 versus 10.2 mo), respectively (P = 0.002). Although complete cytoreduction was associated with longer median OS after CRS/HIPEC (R0/R1 28.6 versus R2 17.2 mo, P = 0.47), the observed difference did not reach statistical significance.
Conclusions
CRS/HIPEC may improve survival in patients with node negative goblet cell carcinomatosis when a complete cytoreduction is achieved. Patients with disease not amenable to complete cytoreduction should not be offered CRS/HIPEC.
Keywords: Goblet cell, Appendiceal cancer, Carcinomatosis, HIPEC, Cytoreductive surgery, Appendiceal tumor, Neuroendocrine tumors
1. Introduction
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is a viable oncologic treatment option for well-selected patients suffering from carcinomatosis. Although high volume centers have extended this therapy to a variety of primary sites including the ovaries, stomach, colon, and rectum as well as to peritoneal mesothelioma, it is most commonly applied to the peritoneal dissemination of appendiceal tumors [1–5]. Within the subset of appendiceal tumors, however, a spectrum of histologies exist.
Goblet cell carcinomas are rare malignancies that may arise at any location along the gastrointestinal tract, although frequently occur at the appendix. Neuroendocrine tumors of the gastrointestinal tract have been associated with a variety of biologic behaviors, and goblet cell appendiceal carcinomas typically share features of both adenocarcinoma and carcinoids. Because appendiceal goblet cell carcinomas possess a relatively aggressive nature and are capable of early peritoneal seeding, a close examination of the peritoneum has been suggested when these tumors are encountered [6]. Unfortunately, the peritoneal dissemination of appendiceal goblet cell carcinomas is usually a rapidly fatal process with very few long-term survivors listed in the literature.
To better define the impact of CRS/HIPEC as a treatment strategy for appendiceal goblet cell carcinomatosis, we decided to review our experience with patients suffering from this disease. Specifically, we aimed to identify the effects of tumor biology as manifested by nodal involvement and the impact of completion of CRS in patients with goblet cell carcinomatosis undergoing CRS/HIPEC.
2. Methods
2.1. Patients
Using a prospectively maintained database of 1198 CRS/HIPEC procedures performed between 1991 and 2014, we identified patients with carcinomatosis and a final pathologic diagnosis of appendiceal cancer with goblet cell features. We limited our search to tumors originating from the appendix. Neuroendocrine tumors without goblet cell features were not included. In general, patients were highly selected based on their ability to tolerate an aggressive surgical procedure and on the feasibility of obtaining a complete cytoreduction. More specifically, retroperitoneal disease, extraperitoneal disease, unresectable primary, or volume or distribution of disease not amenable to cytoreduction functioned as exclusion criteria. Patients with low volume disease presumably amenable to a complete cytoreduction were taken to surgery upfront if they were referred before receiving systemic chemotherapy. Patients with a larger volume of disease or aggressive features on pathology (i.e., signet ring cells) were referred for chemotherapy first and taken to CRS/HIPEC if their disease seemed resectable at completion. There were no cut offs based on peritoneal carcinomatosis index (PCI).
2.2. Procedures
CRS/HIPEC was performed as has been previously described by our institution [7]. Patients deemed appropriate for the procedure were explored through a generous midline incision. When complete cytoreduction was considered feasible, all involved visceral organs and peritoneal surfaces were resected. The omentum was routinely removed when present. This was followed with HIPEC using the closed-abdominal technique [8]. HIPEC agents included mitomycin C or oxaliplatin. Resections were considered complete, R0/R1, if all gross disease were removed before HIPEC. Incomplete resections (R2) were subdivided based on the diameter of the largest lesions remaining (R2a ≤ mm, R2b > 5 mm and ≤2 cm, and R2c > 2 cm). R2a resections were not included in the complete cytoreduction group. Morbidity and mortality were graded according to the Clavien-Dindo classification system [9].
2.3. Statistical analyses
Descriptive statistics included frequency and percent for categorical variables and mean and range for continuous variables. Recurrence was only evaluated in patients after an R0 or R1 resection. Survival was estimated with the Kaplan-Meier method and compared using the log-rank test. Overall survival (OS) was measured from the date of CRS/HIPEC (not the date of diagnosis) to the date of death or of last recorded follow-up. Univariate and multivariate analyses were performed using Cox proportional hazard models. Multivariate analysis included all variables from univariate analysis with P values <0.1. Analyses were performed with SAS 9.3 (SAS, Cary, NC) and statistical significance was defined as a P value <0.05.
3. Results
3.1. Patient characteristics
A total of 31 patients with appendiceal goblet cell carcinomatosis underwent CRS/HIPEC during the study period. No patient had more than one CRS/HIPEC procedure. The number of procedures performed increased in each quartile of the study period with 61.3% occurring in the last quartile and 96.8% occurring in the latter half. Patients were relatively young (mean age, 53 y) and generally healthy (84% without comorbidities). Eastern Cooperative Oncology Group performance status was also good with 94% of patients scored 0–1 (Table 1). Lymph node data were available for 28 patients and 60.7% of these had lymph node metastases. Most patients received chemotherapy before HIPEC (21 of 31 or 67.7%), and the most common regimen was folinic acid, 5-fluorouracil, and Oxaliplatin (FOLFOX) with or without Avastin (18 of 21 or 85.7%). The mean length of preoperative chemotherapy was 4.6 mo (range, 1.5–9.5 mo). The agent used at HIPEC was mitomycin C in 26 (83.9%) and oxaliplatin in five patients (16.1%). Only eight patients (25.8%) received systemic chemotherapy after CRS/HIPEC. No patients were lost to follow-up and median follow-up time was 9.9 mo.
Table 1.
Patient and procedure characteristics.
| Characteristic | All Patients (n = 31) |
|---|---|
| Age, mean (range) (y) | 52.6 (36–72) |
| Female gender, n (%) | 12 (38.7%) |
| Race, n (%) | |
| White | 28 (90.3) |
| Black | 3 (9.7) |
| Heart disease, n (%) | 1 (3.2) |
| Lung disease, n (%) | 2 (6.5) |
| History of smoking, n (%) | 12 (38.7) |
| Diabetes, n (%) | 2 (6.5) |
| Body mass index, mean (range) | 26.6 (18.5–39.5) |
| ECOG performance status, n (%) | |
| 0 | 15 (48.4) |
| 1 | 14 (45.2) |
| 2 | 2 (6.5) |
| Preoperative ascites, n (%) | 5 (16) |
| Preoperative albumin, mean (range) (g/dL) | 3.9 (2.4–4.4) |
| Time period performed, n (%) | |
| 1991–1997 | 0 (0) |
| 1998–2002 | 1 (3.2) |
| 2003–2008 | 11 (35.5) |
| 2009–2014 | 19 (61.3) |
| Preoperative chemotherapy, n (%) | 21 (67.7) |
| Postoperative chemotherapy, n (%) | 8 (25.8) |
| Peritoneal carcinomatosis index, mean (range) (n = 20) | 15.3 (0–32) |
| No. of organs resected, mean (range) | 3.5 (1–6) |
| Length of operation, mean (range) (h) (n = 28) | 8.1 (4.1–12.0) |
| Positive lymph nodes, n (%) (n = 28) | 17 (60.7) |
| Tumor grade, n (%) (n = 18) | |
| Low | 2 (11.1) |
| High | 16 (88.9) |
| Resection status | |
| R0/R1 | 11 (35.5) |
| R2a | 16 (51.6) |
| R2b | 3 (9.7) |
| R2c | 1 (3.2) |
ECOG = Eastern Cooperative Oncology Group.
3.2. Perioperative outcomes
The median number of visceral resections per CRS was 3 (range, 1–6), and complete cytoreduction of all macroscopic disease was accomplished in 36% before HIPEC. The mean PCI in all patients was 15.3 (range, 0–32). The mean PCI in patients receiving a complete cytoreduction was 4.0 compared with 18.1 in patients ultimately receiving an incomplete cytoreduction (P < 0.001). The most common organs resected were the colon (24 of 31 or 77.4%), gallbladder (15 of 31 or 48.3%), small bowel (13 of 31 or 41.9%), and spleen (10 of 31 or 32.3%). Organs less commonly resected as part of the cytoreduction included the uterus, ovaries, rectum, stomach, and pancreas. Postoperatively patients spent an average of 4 d in the intensive care unit (range, 0–26) and 21 d in the hospital (range, 4–76). Major (Clavien-Dindo III and IV) morbidity was 38.7%. Specific complications are listed in Table 2. Thirty- and 90-d mortality rates were 6.5% and 9.7%, respectively. Cause of death was intraabdominal sepsis from enteric leaks in two patients and respiratory failure in a third patient. Nine patients (29%) were readmitted within 30 d of discharge.
Table 2.
Complications in patients with appendiceal goblet cell carcinomatosis following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy.
| Complications in all patients (n = 31) | n (%) |
|---|---|
| Reexploration | 8 (25.8) |
| Infectious complications | |
| Surgical site infection | 3 (9.7) |
| Abdominal abscess | 9 (29.0) |
| Urinary tract infection | 1 (3.2) |
| Clostridium difficile colitis | 1 (3.2) |
| Bacteremia | 3 (9.7) |
| Pneumonia | 6 (19.4) |
| Enteric leak | 5 (16.1) |
| Other leak* | 3 (9.7) |
| Enterocutaneous fistula | 2 (6.5) |
| Fascial dehiscence | 2 (6.5) |
| Gastroparesis | 1 (3.2) |
| Severe noninfectious diarrhea | 4 (12.9) |
| Pleural effusion requiring drainage | 3 (9.7) |
| Pneumothorax | 2 (6.5) |
| Anemia requiring transfusion | 10 (32.3) |
| Neutropenia requiring filgrastim | 6 (19.4) |
| Deep vein thrombosis | 1 (3.2) |
| Pulmonary embolus | 0 (0) |
| Acute renal failure | 1 (3.2) |
| Acute respiratory failure | 5 (16.1) |
Biliary leak, ureteral leak, and pancreatic leak (n = 1 each).
3.3. Recurrence
Of the 11 patients who received a complete macroscopic cytoreduction, five (45.5%) were without evidence of disease at the conclusion of the study, five (45.5%) had died, and one (9.1%) was lost to follow-up. Four of the deceased patients died of disease, and at least 50% of these had recurrence in the peritoneum. The remainder of the data regarding cause of death and distribution of recurrence was unavailable.
3.4. Survival
Median OS for the entire cohort regardless of R status of cytoreduction was 18.4 mo after CRS/HIPEC (Fig. 1). Significantly increased survival was observed in patients with negative nodes compared with patients with positive nodes (29.2 versus 10.2 mo), respectively (P = 0.002) (Fig. 2). Although complete cytoreduction was associated with longer survival after CRS/HIPEC in all patients (R0/R1 median OS 28.6 mo versus R2 median OS 17.2 mo, P = 0.47) and in those with nodal metastases in addition to peritoneal disease (R0/R1 median OS 13.4 mo versus R2 median OS 9.5 mo, P = 0.25), we were underpowered to detect statistical significance. At the conclusion of the study, no patient with negative nodes and a complete cytoreduction had died. On univariate analysis, positive lymph node status conferred significantly worse survival. After adjusting for variables with P values <0.1 on univariate analysis, lymph node status remained a significant predictor of survival in patients with appendiceal goblet cell carcinomatosis (Table 3).
Fig. 1.
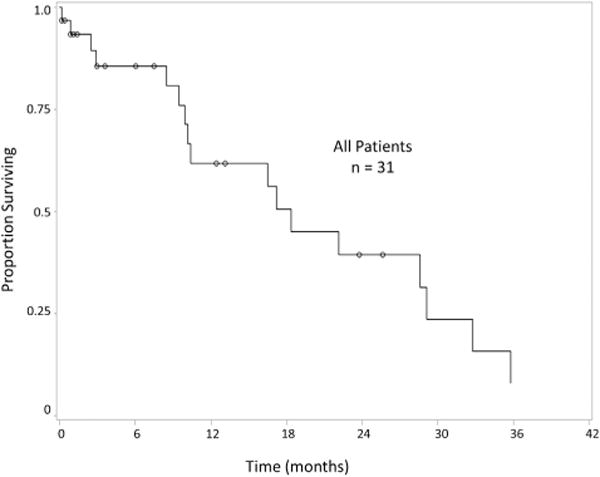
Overall survival of patients with appendiceal goblet cell carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
Fig. 2.
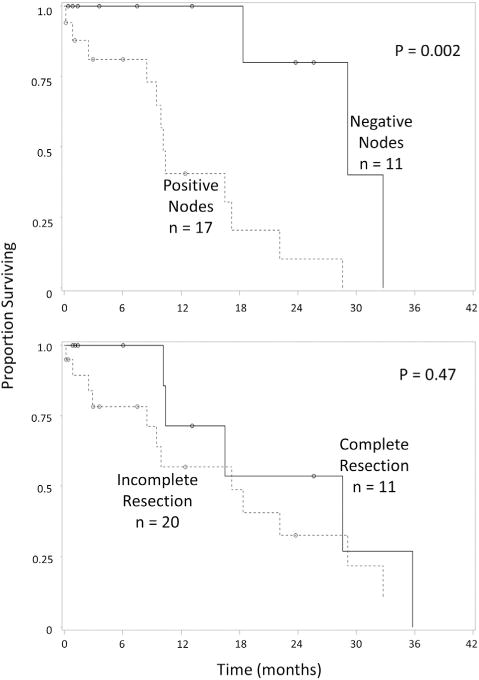
Overall survival of patients with appendiceal goblet cell carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy based on (A) lymph node status and (B) completeness of cytoreduction.
Table 3.
Univariate and multivariate survival analyses of patients with appendiceal goblet cell carcinomatosis after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy.
| Characteristic | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age, 5-y intervals | 1.0 (0.8–1.3) | 0.88 | ||
| Sex | 0.5 (0.2–1.4) | 0.17 | ||
| Race | 0.3 (0.0–2.6) | 0.26 | ||
| Body mass index | 0.6 (0.4–1.1) | 0.12 | ||
| ECOG performance status | 34.6 (0.9–1410.6) | 0.06 | 1.7 (0.7–3.9) | 0.21 |
| Preoperative albumin | 0.1 (0.0–5.0) | 0.28 | ||
| Preoperative chemotherapy | 2.5 (0.7–9.0) | 0.15 | ||
| Postoperative chemotherapy | 1.9 (0.2–17.6) | 0.56 | ||
| HIPEC agent (oxaliplatin versus mitomycin C) | 1.4 (0.5–4.5) | 0.54 | ||
| No. of organs resected | 3.5 (0.5–23.5) | 0.20 | ||
| Peritoneal carcinomatosis index | 0.1 (0.0–6.4) | 0.32 | ||
| Positive lymph nodes | 13.7 (1.8–107.9) | 0.01 | 10.8 (1.3–88.8) | 0.02 |
| Incomplete resection (versus complete resection) | 1.5 (0.5–4.2) | 0.48 | ||
| Length of operation | 0.8 (0.3–2.3) | 0.66 | ||
CI = confidence interval; HIPEC = hyperthermic intraperitoneal chemotherapy; OR = odds ratio.
4. Discussion
Tumor biology and operative intervention are strong determinants of survival in the treatment of patients with carcinomatosis. This is reflected by the fact that the type of primary and the completeness of cytoreduction are among the most important prognostic factors identified in patients undergoing CRS/HIPEC [7]. As such, the success of CRS/HIPEC is largely tied to proper patient selection. Herein, we sought to determine the outcomes of patients with appendiceal goblet cell carcinomatosis undergoing CRS/HIPEC and identify the impact of tumor biology, as defined by the presence of nodal metastasis, and the extent of cytoreduction on survival.
Certain considerations must be made when interpreting the survival reported in the present study. Meaningful survival conclusions can be derived only by the group of patients who achieved a complete cytoreduction and not by including patients with variable levels of residual macroscopic disease. In addition, survival in the current article is measured from the date of CRS/HIPEC, not the date of diagnosis, and 67% of our patients presented for consideration of CRS/HIPEC only after multiple courses of systemic chemotherapy. The demographics of our study population closely mirrored the US population of patients with appendiceal goblet cell tumors [10], yet patients proceeding to CRS/HIPEC are highly selected based on their Eastern Cooperative Oncology Group performance status and on the feasibility of providing a complete cytoreduction. Our cohort was relatively young, generally without comorbidities, and the OS experienced by our study cohort is similar to the 18.5 mo OS previously reported by Mahteme and Sugarbaker [11] in 13 patients with appendiceal adenocarcinoid receiving HIPEC. Subgroup analysis based on completeness of cytoreduction also yielded median OS that closely matched theirs [11,12]. McConnell et al. [13], who achieved a higher rate of complete cytoreduction, reported a 63.4% 3-y OS. A direct comparison between the results of McConnell et al. and those in the present study may not be valid, considering that their selection criteria for CRS/HIPEC was not disclosed. Given the retrospective nature of both studies, patient selection is of key importance.
Morbidity, mortality, intensive care unit stay, and hospital stay were disproportionally higher than what we have reported for our first 1000 CRS/HIPEC procedures [7]. In addition, we did observe a lower rate of complete cytoreduction (35.5% versus 44.4%) than we have published in patients with peritoneal carcinomatosis from appendiceal primaries other than goblet cell [14]. We attribute these deviations from our previously reported outcomes to the notoriously desmoplastic nature specific to goblet cell tumors, making surgical resection challenging. Additionally, these patients are usually treated with several cycles of systemic chemotherapy and referred to surgical evaluation only on progression of disease volume. The observation that so few patients received systemic chemotherapy postoperatively is likely multifactorial but may be partially attributed to the aforementioned prolonged recovery time and relatively high complication rates noted in this cohort.
Although all cases of carcinomatosis are considered stage IV disease, the behavior of different primary tumors varies greatly [7]. Furthermore, the biology of carcinomatosis originating from the same primary malignancy can vary as well [11]. It stands to reason that when lymph node status does not affect the stage of patients with carcinomatosis, lymph node metastases may serve as a marker for more aggressive tumor biology. These tumors display the potential to spread both directly throughout the peritoneum and systemically through the lymphatics. We were able to identify significantly worse survival in patients when lymph node metastases were present. This observation remained even after adjusting for other important prognosticators. McConnell et al. identified that patients with appendiceal goblet cell carcinomatosis had a prognosis that was intermediate between similar patients with carcinomatosis from low- and high-grade appendiceal adenocarcinomas. They also found that the rate of lymph node metastases in patients with appendiceal goblet cell carcinomatosis was similar to patients with high-grade appendiceal carcinoma and a worse prognosis but significantly higher than those with low-grade appendiceal cancer and a better prognosis [13].
Complete cytoreduction of all gross disease has been shown to correlate with better outcomes in patients receiving CRS/HIPEC for a variety of different primary tumors [7]. We were unable to show significantly increased survival in patients with goblet cell carcinomatosis after a complete cytoreduction. A power analysis revealed that we were considerably underpowered to detect a survival difference based on completeness of cytoreduction. To identify a difference in survival with 80% power at 3 y we would have needed 75 patients in each group. We did, however, identify a trend toward increased median OS in patients receiving a complete cytoreduction. Mahteme and Sugarbaker, on the other hand, did identify a significant difference in survival based on the completeness of cytoreduction score in patients receiving CRS and intraperitoneal chemotherapy (median OS 28.5 mo for CC0-1, 18.1 mo for CC2, and 5.9 for CC3, P = 0.007) [11,12]. In a larger cohort of patients with appendiceal carcinomatosis, our group identified completeness of cytoreduction as an independent prognosticator, and we believe that given increased power in the present study, we would have been able to identify a significant difference in survival based on completeness of cytoreduction [14].
We currently approach goblet cell carcinomatosis in the same way that we approach carcinomatosis from high-grade appendiceal primary tumors. Patients with very low volume disease amenable to complete cytoreduction (preferably a PCI < 10) and those with perforated appendiceal tumors are treated initially with CRS/HIPEC inclusive of a right hemicolectomy followed by systemic chemotherapy. All others, including those with nodal disease, will be referred for systemic chemotherapy and then restaged with imaging. CRS/HIPEC will then be offered if their functional status remains good and their disease appears resectable [14].
Although 31 patients represent a relatively large cohort of patients with a rare disease treated at a single institution with uniform selection criteria and treatment protocols, this study is unavoidably limited by a small size. It is possible that we were unable to identify important prognostic factors for patients with appendiceal goblet cell carcinomatosis treated with CRS/HIPEC. Secondly, our cohort does reflect a cautiously selected patient population and cannot be considered a reflection of all patients presenting with goblet cell appendiceal carcinomatosis. We do not know the number of patients that presented with this disease process and were not considered good candidates for HIPEC nor do we know the number that were explored for potential HIPEC and were not perfused with intraperitoneal chemotherapy. The most common reason for the latter is disease not amenable to a meaningful cytoreduction as determined at laparotomy. Lastly, this study is not designed to determine any benefit or risk HIPEC adds to meticulous cytoreduction.
In spite of the limitations, these data add a significant value to the existing literature regarding CRS/HIPEC in patients with goblet cell carcinomatosis. Firstly, the current article presents results in a very highly selected cohort of patients, and we have suggested possible reasons to explain the deviations of our outcomes from previously published reports in similar but not equivalent cohorts. Additionally, based on the reported outcomes and on the relatively poor survival obtained when a complete cytoreduction is not obtained, we suggest refining the selection process even more. PCI can be used to identify patients whose disease is not amenable to a complete cytoreduction, and lymph node status can be used as a marker of more aggressive disease and allow clinicians to accurately identify candidates who stand to benefit the most from CRS/HIPEC. Lastly, because we have disclosed our selection process and outcomes both as a whole and in certain subgroups, our results should be fairly well generalizable to similar patients with goblet cell carcinomatosis being considered for CRS/ HIPEC.
5. Conclusions
CRS/HIPEC is a reasonable treatment option for carefully selected patients with goblet cell carcinomatosis. This procedure may improve survival in patients with node negative appendiceal goblet cell carcinomatosis when a complete cytoreduction is achieved. Patients with nodal disease identified on preoperative imaging should be referred first for systemic chemotherapy and then proceed to CRS/HIPEC only if their disease has a good response and appears resectable assuming their functional status also remains acceptable. For patients with disease not amenable to a complete cytoreduction, CRS/HIPEC should not be offered.
Acknowledgments
This work was supported by Wake Forest University Biostatistics shared resource NCI CCSG P30CA012197.
Footnotes
Presented at the 10th Annual Academic Surgical Congress in Las Vegas, Nevada, in February 2015.
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Yan TD, Welch L, Black D, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol. 2007;18:827. doi: 10.1093/annonc/mdl428. [DOI] [PubMed] [Google Scholar]
- 2.Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14:484. doi: 10.1245/s10434-006-9182-x. [DOI] [PubMed] [Google Scholar]
- 3.Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24:4011. doi: 10.1200/JCO.2006.07.1142. [DOI] [PubMed] [Google Scholar]
- 4.Yonemura Y, Bando E, Kawamura T, et al. Cytoreduction and intraperitoneal chemotherapy for carcinomatosis from gastric cancer. Cancer Treat Res. 2007;134:357. doi: 10.1007/978-0-387-48993-3_23. [DOI] [PubMed] [Google Scholar]
- 5.Blackham A, Levine E. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma. Eur J Clin Med Oncol. 2012;4:25. [PMC free article] [PubMed] [Google Scholar]
- 6.Pahlavan PS, Kanthan R. Goblet cell carcinoid of the appendix. World J Surg Oncol. 2005;3:36. doi: 10.1186/1477-7819-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine EA, Stewart JH, Shen P, Russell GB, Loggie BL, Votanopoulos KI. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnaik AA, Sussman JJ, Ahmad SA, McIntyre BC, Lowy AM. Technology for the delivery of hyperthermic intraoperative intraperitoneal chemotherapy: a survey of techniques. Recent Results Cancer Res. 2007;169:75. doi: 10.1007/978-3-540-30760-0_6. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer. 2002;94:3307. doi: 10.1002/cncr.10589. [DOI] [PubMed] [Google Scholar]
- 11.Mahteme H, Sugarbaker PH. Treatment of peritoneal carcinomatosis from adenocarcinoid of appendiceal origin. Br J Surg. 2004;91:1168. doi: 10.1002/bjs.4609. [DOI] [PubMed] [Google Scholar]
- 12.Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol. 1998;14:254. doi: 10.1002/(sici)1098-2388(199804/05)14:3<254::aid-ssu10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.McConnell YJ, Mack LA, Gui X, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: an emerging treatment option for advanced goblet cell tumors of the appendix. Ann Surg Oncol. 2014;21:1975. doi: 10.1245/s10434-013-3469-5. [DOI] [PubMed] [Google Scholar]
- 14.Votanopoulos KI, Russell G, Randle RW, Shen P, Stewart JH, Levine EA. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol. 2015;22:1274. doi: 10.1245/s10434-014-4147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


