Abstract
Vaccines targeting pathogens are generally effective and protective because based on foreign non-self antigens which are extremely potent in eliciting an immune response. On the contrary, efficacy of therapeutic cancer vaccines is still disappointing. One of the major reasons for such poor outcome, among others, is the difficulty of identifying tumor-specific target antigens which should be unique to the tumors or, at least, overexpressed on the tumors as compared to normal cells. Indeed, this is the only option to overcome the peripheral immune tolerance and elicit a non toxic immune response. New and more potent strategies are now available to identify specific tumor-associated antigens for development of cancer vaccine approaches aiming at eliciting targeted anti-tumor cellular responses. In the last years this aspect has been addressed and many therapeutic vaccination strategies based on either whole tumor cells or specific antigens have been and are being currently evaluated in clinical trials. This review summarizes the current state of cancer vaccines, mainly focusing on antigen-specific approaches.
Keywords: cancer vaccine, clinical trials, epitopes, immunotherapeutics, tumor-associated antigens
Abbreviations
- MHC
major histocompatibility complex
- BCG
Bacille Calmette-Guerin
- GM-CSF
granulocyte macrophage-colony stimulating factor
- DCs
dendritic cells
- APCs
antigen-presenting cell
- NSCLC
non-small-cell lung carcinoma
- TAAs
tumor-associated antigens
- MAGE-A1
Melanoma-associated antigen 1
- CT
Cancer-testis
- SSX-2
Synovial sarcoma X breakpoint 2
- PSA
Prostate-specific antigen
- hTERT
human Telomerase reverse transcriptase
- TACAs
Tumor-associated carbohydrate antigens
- WGS
whole genome sequencing
- WES
whole exome sequencing
- HLA
human leukocyte antigen
- Ig Id
immunoglobulin idiotype
- BCR
B-cell receptor
- TPA
transporter associated with antigen processing
- MS
mass spectrometry
- GB
glioblastoma
- RCR
renal cell cancer
- CRC
colorectal cancer
- FDA
Food & drug administration
- TLRs
Toll-Like Receptors
- HER2
human epidermal growth factor receptor 2
- PRRs
Pattern Recognition Receptors
- HSPs
stress/heat shock proteins
- TARP
T-cell receptor gamma alternate reading frame protein
- LPs
long peptides
- CTL
cytotoxic T-lympocites
- IFNg
interferon gamma
- HPV
human papillomavirus
- CDCA1
cell division cycle associated 1
- PAP
prostatic acid phosphatase
- mCRPC
metastatic castrate-resistant prostate cancer
- EGT
electro-gene-transfer
- MVA
modified vaccinia strain Ankara
Tumor Cell Vaccines
Tumors accumulate several genetic modifications in somatic cells1,2 which provide selective growth advantage to cancer cells in order to initiate clonal expansion.3
Considering the high number of potential tumor antigens for each individual cancer, vaccination with whole tumor cells has been considered the optimal strategy to include all potentially relevant antigens. Moreover, such vaccine approach circumvents the major histocompatibility complex (MHC)- restriction and the need for specific patient-tailored epitope identification.
Autologous tumor vaccines prepared using patient-derived tumor cells represent one of the first types of cancer vaccines that have been tested.4 The efficacy of such approach has been evaluated during the years in several clinical trials targeting different tumor types, including lung cancer,5,6 colorectal cancer,7-9 melanoma,10-12 renal cell cancer13,14 and prostate cancer.15 However, sufficient amount of tumor specimen is needed for preparation of such autologous tumor cell vaccines, restraining its application to a limited number of tumor types or stages.
To overcome the limitations of patient-tailored vaccines, allogeneic whole tumor cell vaccines have been developed based on 2 or 3 established human tumor cell lines. In particular, they allow standardization of large-scale production, quality and composition of the vaccines as well as comparative analysis of clinical outcome. Moreover, they can be easily manipulated for expression of immunostimulatory molecules.
The first allogeneic whole-cell vaccine was the Canvaxin™, consisting of 3 melanoma lines combined with BCG as an adjuvant16 which, after promising results in phase II clinical trials,17,18 failed in 2 multi-institutional randomized phase III trials.19
However, the effectiveness of such vaccine strategy is dramatically hampered by the immune system's inherent tolerance to several antigens expressed in the whole tumor cell preparation, as they may be expressed by normal tissues or presented to T cells in a non-stimulatory context. In order to break tolerance and contain immune suppression, antigens should be combined to strong immunological adjuvants (reviewed in20,21). To this aim, whole tumor cell vaccines (autologous or allogeneic) can be genetically modified to express co-stimulatory molecules and/or cytokines, such as granulocyte macrophage-colony stimulating factor - GM-CSF (GVAX). GVAX has proven to be more effective than others in inducing recruitment, maturation, and function of dendritic cells (DCs), the most potent antigen-presenting cell (APC).22–24
The clinical activity of GVAX based on allogeneic whole-cell vaccine has been evaluated for treatment of recurrent prostate cancer,25,26 breast cancer27 and pancreatic cancer.28 However, the use of allogeneic cells as a vaccine can generate strong anti-MHC immune reactions that can interfere with the anti-tumor response and recent observations suggest a potential detrimental effect of GM-CSF due to induction of immune suppression in cancer patients (reviewed in29,30).
An alternative strategy to improve immunogenicity of allogeneic tumor cell vaccines is to engineer cell lines to secret antisense oligonucleotide for inhibiting expression of the immunosuppressive cytokine TGF-β2. This strategy is the principle of LucanixTM, targeting non-small-cell lung carcinoma – NSCLC, which in 2 independent phase 2 trials has induced significant improvement in overall survival in advanced disease.31,32 The phase 3 STOP (Survival, Tumor-free, Overall, and Progression-free) trial is in progress enrolling patients with locally advanced or advanced NSCLC without progression after first-line chemotherapy or chemoradiation (NCT00676507).
Tumor-Associated Antigens - TAAs
Shared TAAs
Cancer vaccines based on defined specific tumor antigens should elicit a very specific effector and memory cell response. However, such approach may result in selection and expansion of tumor variants which lack the target tumor antigen and are resistant to the vaccine-induced immune response. Nevertheless, the newly expressed antigens on tumor variants may elicit a broader anti-tumor immune response, in a process defined “epitope spreading."33,34
MAGE-1 was the first gene reported to encode a human tumor antigen recognized by T cells.35 Since then, a large number of tumor-associated antigens (TAAs) have been described and are divided into shared and unique TAAs.36 A complete and update list of shared TAAs is available at http://www.cancerimmunity.org/peptide/.
Shared TAAs can be classified in 3 main groups: 1) cancer-testis; 2) tissue differentiation; and 3) widely occurring over-expressed antigens. Cancer-testis (CT) antigens result from re-activation of genes which are normally silent in adult tissues,37 but are transcriptionally activated in different tumor histotypes.38 Many CT antigens have been identified and tested in clinical trials, although little is known about their specific functions, especially with regards to malignant transformation. Such group of TAAs includes the MAGE-A1,39,40 NY-ESO-141 and SSX-2.42 Tissue differentiation antigens are shared between tumors and the normal tissue of origin; they are mostly found in melanomas and normal melanocytes (Gp100, Melan-A/Mart-1, Tyrosinase).43-48 as well as in epithelial tissues and tumors such as prostate (PSA)49,50 and breast carcinomas (Mammaglobin-A).51 Widely occurring overexpressed TAAs are over-expressed in tumor cells compared to normal tissues, reaching the threshold for T cell recognition to break the immunological tolerance and trigger an anticancer response. The antiapoptotic proteins livin and survivin,52,53 hTERT,54-56 and tumor suppressor proteins (e.g., p53)57,58 belong to such group. Mucin 1 (MUC1) belongs to the “overexpressed TAA” category, although it is the combination of overexpression and modification of glycosylation status in tumor cells to make MUC1 highly immunogenic and, thus, an interesting target in cancer immunotherapy.59
Tumor-associated carbohydrate antigens (TACAs) represent an additional class of shared tumor antigens. They are glycans uniquely or overexpressed by tumors60 correlating also with various stages of cancer development.61,62
Unique personalized TAAs
Unique TAAs result from random somatic point mutations induced by physical or chemical carcinogens, and therefore represent neo-antigens uniquely expressed by individual tumors (reviewed in63,64). Cancer genome instability and subsequent selective pressure lead to accumulation of mutations which may give rise to non-synonymous mutations. Interestingly, the number of such non-synonymous mutations shows a significant variability between different tumor types (10 to 400).2,65 Given that neo-antigens are tumor – specific, their immunogenicity is not hampered by central T-cell tolerance and the elicited T-cell responses are not expected to result in autoimmune toxicity. Indeed, mutated epitopes identified in a murine melanoma cells have been shown to elicit a stronger T-cell response in vivo in a side-by-side comparison with corresponding wild type epitopes.66 Moreover, neo-antigens should be more resistant to immune-selection being crucial to the oncogenic process and thus indispensable for maintaining the neoplastic state. Most of the studies focused on cancer mutation discovery have been performed using broad assays like whole genome (WGS) and whole exome sequencing (WES) on each individual tumor,67,68 in order to identify mutated genes and select peptides whose motifs are predicted to be presented by the patient's HLA alleles. However, only a small fraction of such mutated peptides are indeed presented by MHC or recognized by T cells, and this seems to directly correlate with the tumor-specific mutation load.66,69-72 Therefore, prediction of MHC presentation calculated by software algorithms needs to be confirmed by experimental procedures. Moreover, each tumor bears highly heterogeneous sets of defects in dozens of different genes73-77 which need to be further verified for their substantial contribution to the tumor development and progression and, consequently, for their relevance as vaccine target.78
On the contrary, identification of unique TAAs for hematological tumors as B cell lymphomas requires sequencing analysis focused only on immunoglobulin idiotype (Ig Id) included in the B-cell receptor (BCR), which represents the target antigen.79,80
Selection of antigens for cancer vaccine development
TAAs may be used as vaccine administering the full-length protein, which contains all potential MHC class I and MHC class II epitopes capable of stimulating CD8+ and CD4+ T cells, respectively. Therefore, the full-length protein can be considered as an “off-the-shelf” vaccine ready-to-use for any eligible cancer patient regardless his/her HLA allele background. On the contrary, vaccines based on epitopes derived from TAAs require the identification and selection of specific epitopes that interact with specific MHC complexes in order to stimulate a T-cell-associated immune response. Such epitopes represent an “off-the-shelf” vaccine ready-to-use for any eligible cancer patient characterized by that specific HLA allele background. In the last years, this has been performed by predictive immune-informatics algorithms.81-85 Prediction algorithms have been constantly updated in order to take into considerations all the biological variables related to the complexity of the intra-cellular process governing the peptide fragmentation by the proteasome and the transportation to HLA class I molecules in the endoplasmic reticulum, via the transporter associated with antigen processing (TAP) (http://www.cbs.dtu.dk/services/). Nevertheless, immunological experimental validation of predicted epitopes is required to ultimately confirm the selection of epitopes.
Recently, strategies based on high resolution mass spectrometry (MS) have been developed for directly sequencing peptides presented by HLA molecules (HLA ligandome) on tumor cells, to identify naturally processed class I and class II tumor-associated peptides.86 This strategy, indeed, allows the identification of T cell epitopes in fact presented by the tumor cells, thus representing a valid target of the T cells, and it has been employed to identify the HLA ligandome for glioblastoma (GB),87 renal cell cancer (RCC) and colorectal cancer (CRC) (reviewed in88).
In the quest of the most specific tumor-associated antigens, a personalized approach is currently feasible based on the individual features of tumors. Next-generation sequencing and computation prediction allow the identification of genetic alterations in cancer cells of each cancer patient (the mutanome) encoding unique mutated peptides (m-peptides) that can be used as vaccine to elicit specific anti-tumor T cells.66,89 The latter approaches represent the very last frontier of the immunotherapy and their translation into clinical application is currently used in 2 projects funded by the European Union, within the Framework Program 7, focused on glioma (www.gapvac.eu) and on hepatocellular carcinoma (www.hepavac.eu).
Peptide-protein based cancer vaccines
Peptide-protein based vaccines are cost effective, compared to other vaccine approaches including multiple antigens. For such reason, most of cancer vaccine clinical trials have been performed with peptide-protein based vaccines including cancer-testis or differentiation TAAs but, despite the induction of strong T-cell immunity, clinical outcomes have been disappointingly limited90-95 (Table 1 and 2).
Table 1.
Cancer vaccines in Phase 1/2 clinical trials based on peptide/protein strategies.
| CANCER | Antigen | STRATEGY | NCT NUMBER | PHASE |
|---|---|---|---|---|
| Bile duct | URLC10 | Peptide | NCT00624182 | Phase 1 |
| Bladder | NY-ESO-1 | Peptide | NCT00070070 | Phase 1 |
| Brain | GAA | DC | NCT00612001 | Phase 1 |
| Breast | OFA | DC | NCT00715832 | Phase 1 |
| cyclin B1/WT-1/CEF | DC | NCT02018458 | Phase 1/2 | |
| VEGFR1 and VEGFR2 | Peptide | NCT00677326 | Phase 1/2 | |
| TTK | Peptide | NCT00678509 | Phase 1/2 | |
| Multiple | Peptide | NCT00674791 | Phase 1 | |
| MUC1-KLH | Protein | NCT00004156 | Phase 1 | |
| OFA | DC | NCT00879489 | Phase 1/2 | |
| HER2 | Protein | NCT00952692 | Phase 1/2 | |
| Cervical | HPV16 E7 | DC | NCT00155766 | Phase 1 |
| Colorectal | CEA | DC | NCT00228189 | Phase 1/2 |
| VEGFR1 and VEGFR2 | Peptide | NCT00677612 | Phase 1/2 | |
| Multiple | Peptide | NCT00677287 | Phase 1/2 | |
| Multiple IMA910 | Peptide | NCT00785122 | Phase 1/2 | |
| Esophageal | URLC10 | Peptide | NCT00753844 | Phase 1 |
| URLC10, VEGFR1 and VEGFR2 | Peptide | NCT00681421 | Phase 1/2 | |
| URC10, TTK, KOC1 | Peptide | NCT00681330 | Phase 1/2 | |
| Multiple | Peptide | NCT00669292 | Phase 1/2 | |
| Gastric | URLC10 | Peptide | NCT00845611 | Phase 1/2 |
| URLC10, VEGFR1 and VEGFR2 | Peptide | NCT00681252 | Phase 1/2 | |
| URLC10, KOC1, VEGFR1 and VEGFR2 | Peptide | NCT00681577 | Phase 1/2 | |
| Gliobalstoma | not specified | DC | NCT00576641 | Phase 1 |
| not specified | Peptide | NCT01854099 | Phase 1 | |
| SL-701 | Peptide | NCT02078648 | Phase 1/2 | |
| Multiple IMA950 | Peptide | NCT01403285 | Phase 1 | |
| Multiple IMA950 | Peptide | NCT01920191 | Phase 1/2 | |
| Multiple | Peptide | NCT02149225 | Phase 1 | |
| Hematological | WT1 | Peptide | NCT00672152 | Phase 1 |
| Leukemia | WT1 | DC | NCT00923910 | Phase 1/2 |
| Melanoma | p53; survivin; telomerase | DC | NCT00197912 | Phase 1/2 |
| MART-1; gp100; tyrosinase | Peptide | NCT00005841 | Phase 1 | |
| MART-1; gp100; Tyrosinase; NY-ESO-1 | DC | NCT00313508 | Phase 1 | |
| NY-ESO-1 | Protein | NCT01079741 | Phase 1/2 | |
| GSK2302025A | Protein | NCT01149343 | Phase 1 | |
| MART-1, gp100; tyrosinase | Peptide | NCT00028431 | Phase 1 | |
| gp100; tyrosinase | DC | NCT01530698 | Phase 1/2 | |
| tyrosinase | Protein | NCT01331915 | Phase 1/2 | |
| Multiple | DC | NCT00124124 | Phase 1 | |
| gp100 | Peptide | NCT00003229 | Phase 1/2 | |
| MART-1; MAGE-3.1; survivin | DC | NCT00074230 | Phase 1/2 | |
| MART-1, gp100 | Peptide | NCT00470015 | Phase 1 | |
| MART-1; MAGE-3.1 | Peptide | NCT00002952 | Phase 1/2 | |
| MART-1, gp100; tyrosinase | DC | NCT00003665 | Phase 1 | |
| MAGE-10.A2; MART-1; NY-ESO-1; tyrosinase | Peptide | NCT00037037 | Phase 1 | |
| MART-1; gp100 | Peptide | NCT00091338 | Phase 1 | |
| gp100 | Peptide | NCT00091143 | Phase 1 | |
| MART-1; gp100 | Peptide | NCT00019214 | Phase 1/2 | |
| MART-1; gp100 | Peptide | NCT00010309 | Phase 1/2 | |
| OVA BiP; gp209–2M; tyrosinase peptide | Peptide | NCT00005633 | Phase 1 | |
| MART-1; gp100; MAGE-3.1; tyrosinase | Peptide | NCT00003792 | Phase 1 | |
| gp100; MART-1 | Peptide | NCT00004025 | Phase 1/2 | |
| gp100 | Peptide | NCT00003897 | Phase 1 | |
| MAGE-1/MAGE-3; tyrosinase; MART-1; gp100 | DC | NCT01082198 | Phase 1/2 | |
| Melan-A | Peptide | NCT00324623 | Phase 1 | |
| MART-1; NY-ESO-1; gp100 | Peptide | NCT01176461 | Phase 1 | |
| gp100(g209–2M) | Peptide | NCT00960752 | Phase 2 | |
| gp100; tyrosinase | DC | NCT00243529 | Phase 1/2 | |
| MAGE-3.A1; NA17.A2 | Peptide | NCT01191034 | Phase 1/2 | |
| Multiple | KOC1, TTK, CO16, DEPDC1, MPHOSPH1 | Peptide | NCT00676949 | Phase 1 |
| HER2, NY-ESO-1 | Peptide | NCT00291473 | Phase 1 | |
| MAGE-12 | Peptide | NCT00020267 | Phase 1 | |
| NY-ESO-1 | Peptide | NCT01584115 | Phase 1/2 | |
| ONT-10 | glycolipopeptide | NCT01556789 | Phase 1 | |
| ONT-10 | glycolipopeptide | NCT01978964 | Phase 1 | |
| CEA | Peptide | NCT00057915 | Phase 1 | |
| Neuroblastoma | GD2L and GD3L | Protein | NCT00911560 | Phase 1/2 |
| Non Small Cell Lung | GSK2302032A | Protein | NCT01159964 | Phase 1 |
| URLC10; CDCA1; VEGFR1; VEGFR2 | Peptide | NCT00874588 | Phase 1 | |
| URLC10; TTK; KOC1 | Peptide | NCT00674258 | Phase 1/2 | |
| URLC10; VEGFR1; VEGFR2 | Peptide | NCT00673777 | Phase 1/2 | |
| Ovarian | Survivin | Peptide | NCT01416038 | Phase 1/2 |
| Multiple | Peptide | NCT01095848 | Phase 1 | |
| Pancreatic | MUC1 | Peptide | NCT00008099 | Phase 1 |
| Prostate | TF | Protein | NCT00003819 | Phase 1 |
| rsPSMA | Protein | NCT00705835 | Phase 1 | |
| PSA | DC | NCT00005992 | Phase 1 | |
| MUC-2 | Protein | NCT00004929 | Phase 1 | |
| MUC-2 | Protein | NCT00036933 | Phase 1 | |
| PSA; PAP; KLH | DC | NCT01171729 | Phase 1/2 | |
| Renal cell | Survivin; TERT | DC | NCT00197860 | Phase 1/2 |
| Sarcoma | NY-ESO-1; MAGE-A1; MAGE-A3 | DC | NCT01241162 | Phase 1 |
| NY-ESO-1; MAGE-A1; MAGE-A3 | DC | NCT00944580 | Phase 1 | |
| NY-ESO-1 | Peptide | NCT00027911 | Phase 1 |
Table 2.
Cancer vaccines in Phase 2 or 3 clinical trials based on peptide/protein strategies.
| CANCER | Antigen | STRATEGY | NCT NUMBER | PHASE |
|---|---|---|---|---|
| Bladder | MPHOSPH1 and DEPDC1 | Peptide | NCT00633204 | Phase 2 |
| Breast | MUC1 | Peptide | NCT00925548 | Phase 3 |
| Cervical | HPV16/18 | Protein | NCT01356823 | Phase 2 |
| HPV16/18 | Protein | NCT01735006 | Phase 3 | |
| Colorectal | not specified | DC | NCT01348256 | Phase 2 |
| not specified | DC | NCT01413295 | Phase 2 | |
| Esophageal | STF-II | Peptide | NCT01267578 | Phase 2 |
| G17DT | Peptide | NCT00020787 | Phase 3 | |
| Glioblastoma | ICT-107 | DC | NCT01280552 | Phase 2 |
| Hodgkin/Non-Hodgkin | LMP2A | DC | NCT02115126 | Phase 2 |
| Melanoma | tyrosinase | Peptide | NCT01989572 | Phase 3 |
| gp100; tyrosinase; MAGE-3.1 | Peptide | NCT00085189 | Phase 2 | |
| gp100; tyrosinase; MART-1 | Peptide | NCT00089063 | Phase 2 | |
| MART-1; NA17-A; gp100; tyrosinase | Peptide | NCT00036816 | Phase 3 | |
| MART-1; gp100; tyrosinase | Peptide | NCT00031733 | Phase 2 | |
| gp100; tyrosinase | Peptide | NCT00003339 | Phase 2 | |
| MART-1; gp100; tyrosinase | DC | NCT00334776 | Phase 2 | |
| gp100 | Peptide | NCT00032045 | Phase 2 | |
| MART-1; gp100; tyrosinase | Peptide | NCT00019396 | Phase 2 | |
| MART-1; gp100 | Peptide | NCT00295958 | Phase 2 | |
| MART-1, gp100 and tyrosinase | Peptide | NCT00001685 | Phase 2 | |
| MART-1; gp100 | Peptide | NCT00020475 | Phase 2 | |
| MART-1, gp100; tyrosinase | Peptide | NCT00059475 | Phase 2 | |
| gp100 antigen | Peptide | NCT00080353 | Phase 2 | |
| MART-1, gp100; tyrosinase | Peptide | NCT00006113 | Phase 2 | |
| MART-1, gp100; tyrosinase | Peptide | NCT00006385 | Phase 2 | |
| MART-1; gp100 | Peptide | NCT00019721 | Phase 2 | |
| MART-1; gp100 | Peptide | NCT00019994 | Phase 2 | |
| gp100 | Peptide | NCT00072085 | Phase 2 | |
| gp100; tyrosinase | Peptide | NCT00003222 | Phase 2 | |
| gp100; tyrosinase | Peptide | NCT00003362 | Phase 2 | |
| gp100; tyrosinase | Peptide | NCT00003274 | Phase 2 | |
| gp100 | Peptide | NCT00003568 | Phase 2 | |
| multi-epitope | Peptide | NCT00071981 | Phase 2 | |
| gp100; tyrosinase | Peptide | NCT00020358 | Phase 2 | |
| gp209–2M | Peptide | NCT00019487 | Phase 2 | |
| NY-ESO-1 | Peptide | NCT00079144 | Phase 2 | |
| multi-epitope | Peptide | NCT00004104 | Phase 2 | |
| gp100 | Peptide | NCT00077532 | Phase 2 | |
| NY-ESO-1 | Peptide | NCT00020397 | Phase 2 | |
| gp100 | Peptide | NCT00019682 | Phase 3 | |
| gp100; MART-1 | Peptide | NCT00303836 | Phase 2 | |
| NA17.A2; MAGE-3.1; MART-1 | Peptide | NCT01307618 | Phase 2 | |
| gp100; MART-1 | DC | NCT00019890 | Phase 2 | |
| Multiple | CEA | Peptide | NCT00012246 | Phase 2 |
| Non Small Cell Lung | Dex2 | Peptide | NCT01159288 | Phase 2 |
| Pancreatic | hTERT | Peptide | NCT00358566 | Phase 3 |
| Prostate | PSA | Peptide | NCT00109811 | Phase 2 |
| PAP | Sipuleucel-T | NCT01477749 | Phase 2 | |
| PAP | Sipuleucel-T | NCT00005947 | Phase 3 | |
| PAP | Sipuleucel-T | NCT00715078 | Phase 2 | |
| PAP | Sipuleucel-T | NCT01338012 | Phase 2 | |
| PAP | Sipuleucel-T | NCT00065442 | Phase 3 | |
| PAP | Sipuleucel-T | NCT00901342 | Phase 2 | |
| PSA | Peptide | NCT00030602 | Phase 2 | |
| PAP | Sipuleucel-T | NCT01431391 | Phase 2 | |
| Renal Cell | gp100; MART-1; tyrosinase | Peptide | NCT00019396 | Phase 2 |
| Multiple IMA901 | Peptide | NCT00523159 | Phase 2 | |
| Multiple IMA901 | Peptide | NCT01265901 | Phase 3 |
Indeed, with exception of the 2 cancer vaccine clinical trials based on Sipuleucel-T which have allowed the licensing by FDA for the treatment of asymptomatic metastatic castrate-resistant prostate cancer (see below),96,97 the other 8 Phase 3 clinical trials completed or terminated have not provided satisfactory results and no further implementation for licensing has been pursued (Table 3).
Table 3.
Cancer vaccines in Phase 3, completed or terminated, based on peptide/protein strategies.
| CANCER | Antigen | STRATEGY | NCT NUMBER | STATUS | Outcome |
|---|---|---|---|---|---|
| Breast | MUC1 | Peptide | NCT00925548 | Terminated | Following the clinical hold, EMD Serono has decided to permanently terminate the trial EMR 200038–010 (STRIDE) in the indication of breast cancer |
| Esophageal/Gastric | G17DT | Peptide | NCT00020787 | Completed | Data not available |
| Melanoma | tyrosinase | Peptide | NCT01989572 | Completed | Data not yet available |
| MART-1; NA17-A; gp100; tyrosinase | Peptide | NCT00036816 | Terminated | Low accrual | |
| gp100 | Peptide | NCT00019682 | Completed | In patients with advanced melanoma, the response rate was higher and progression-free survival longer with vaccine and interleukin-2 than with interleukin-2 alone. | |
| Pancreatic | hTERT | Peptide | NCT00358566 | Completed | Preliminary data showed no survival benefit in the GV1001 group compared to the gemcitabine group |
| Prostate | PAP | Sipuleucel-T | NCT00005947 | Completed | Data for FDA registration |
| PAP | Sipuleucel-T | NCT00065442 | Completed | Data for FDA registration |
Among many possible reasons for such unsatisfactory results, one could be the induction of a restricted T cell immune response that may not be sufficient and ultimately cause a selection of tumor cells lacking or down-regulating the targeted antigen. The use of multiple peptides derived from different TAAs could overcome such a drawback, eliciting a T cell response against multiple targets which may counteract tumor heterogeneity and enhance the probability of tumor eradication. The feasibility of such multi-epitope approach has been confirmed by in vivo and in vitro studies showing that multiple peptides do not mutually compete for MHC presentation and are able to induce a multi-specific T-cell response.98-100 Furthermore, studies have also clearly demonstrated that a potent and sustained CD8+ T-cell response can be induced only combining HLA class I and II-restricted peptides, due to the helper function provided by CD4+ T helper (TH) cells.101-103 Vaccines based on a multi-peptide cocktail have been developed and evaluated in phase I/II clinical trials for glioblastoma (IMA950, NCT01920191), renal cell carcinoma (IMA901, NCT00523159) and colo-rectal cancer (IMA910, NCT00785122) showing feasibility, safety and immunogenicity. IMA901 is currently in a world-wide phase 3 trial in patients receiving Sunitinib for advanced/metastatic RCC (NCT01265901).
Strategies to improve immunogenicity of peptide-based vaccines
Several strategies have been adopted to improve clinical outcome of peptide-based vaccines, mainly aiming at potentiating the innate immune response. Toll-Like Receptors (TLRs) agonists are being tested in clinical trials evaluating peptide/protein-based cancer vaccines. TLR3 agonists currently evaluated in human clinical trials are the poly(I) poly(C12U) (Ampligen®), in a phase I-II study of HER2 vaccination in breast cancer patients (NCT01355393) and the Poly-ICLC (Hiltonol®) in a multipeptide vaccine in melanoma patients (NCT01585350), in a MAGE-A3 ASCI peptide vaccine in melanoma patients (NCT01437605) as well as in a MUC1 peptide vaccine in patients with advanced colorectal adenoma (NCT00773097). The TLR7/8 agonist Resiquimod is currently evaluated in a gp100(g209–2M) and MAGE-3 peptide vaccine in patients with melanoma (NCT00960752). Additional agonists for Pattern Recognition Receptors (PRRs) are evaluated for their adjuvant activity in therapeutic cancer vaccines. In particular, stress/heat shock proteins (HSPs) can be utilized as immunostimulatory agents for cancer immunotherapy.104–106 Chaperoning technology has been generated to formulate recombinant HSP vaccines including clinically relevant tumor antigens (e.g.,, gp100, HER-2/Neu) (reviewed in107). Such strategy may be used to develop many different antigen targets108 and 2 phase I clinical trials of recombinant chaperone vaccine targeting melanoma have been designed, one completed (NCT00005633) and one currently recruiting patients (NCT01744171).
A phase III clinical trial has shown that melanoma patients in the M1a and M1b substages, receiving a larger number of immunizations with vitespen (autologous, tumor- derived heat shock protein gp96 peptide complexes), have a longer survival than those receiving fewer such treatments.109
Additional strategies to improve immunogenicity of peptides aims to generate peptide variants of TAAs, including mimotopes, heteroclitic peptides, altered-peptide ligands (reviewed in110) as well as introducing amino acid substitutions in the peptide-MHC-binding surface.111-113 A clinical trial based on a novel prostate and breast cancer antigen TARP, designed as an “epitope-enhanced” or “anchor-modified” peptide,114 is currently conducted in stage D0 prostate cancer patients (NCT00972309) with promising early clinical results.115 In addition, the above mentioned gp100(g209–2M) peptide evaluated in a clinical trial in melanoma patients (NCT00960752) is, indeed, an “epitope-enhanced” or “anchor-modified” peptide.116
Furthermore, long peptides (LPs) have been shown to be more immunogenic than individual MHC class I-restricted short peptide.117 Indeed, LPs do not bind directly to MHC class I but only through processing by DCs,118-120 resulting in a significant reduction of transient CTL response or tolerance.121,122 Moreover, LPs may persist longer in inflamed lymph nodes sustaining the clonal expansion of IFNg-producing effector T cells with improved anti-tumor CTL response.118 LPs have been generated linking CTL and Th epitopes, as shown for several TAAs including human papillomavirus (HPV) E6-E7 antigens,123,124 the CT antigen NY-ESO-1125 and HER-2/neu126,127 and, very recently, the novel cancer-testis antigen, cell division cycle associated 1 (CDCA1).128 Five clinical trials have been designed using LPs, 2 targeting melanoma based on NY-ESO-1 (NCT00112242) and on multiple TAAs (NCT02126579), 2 targeting ovarian cancer based on p53 TAA (NCT00844506 and NCT01639885) and one targeting cervical cancer based on HPV E6/E7 proteins (NCT02128126).
In the last years, it has been shown that the blockade of immune checkpoints by antibodies or modulated by recombinant forms of ligands or receptors (such as MAbs to PD-1, PDL-1, CTLA4) represents one of the most promising approaches to improve therapeutic antitumour immunity, amplifying antigen-specific T-cell responses.129 Therefore, the combination of a vaccine and blockade of immune checkpoints could result in elicitation of a stronger immune response with a more potent control of tumor growth. A clinical trial of patients with advanced melanoma evaluated the effect of a peptide vaccine of melanoma-specific gp100 combined with humanized CTLA4 antibody ipilimumab, showing a 3.5 month survival benefit compared with the group receiving the gp100 peptide vaccine alone.130 Few clinical trials have been or are currently conducted to investigate the combinatorial effect of TAA-based cancer vaccine and ipilimumab in patients with melanoma (MART-1 - NCT00090896; gp100 - NCT00094653; Tyrosinase/gp100/MART-1 - NCT00025181) or pancreatic cancer (PSA - NCT00113984). Similarly, few clinical trials are currently conducted to investigate the combinatorial effect of TAA-based cancer vaccine and anti-PD-1 antibody BMS-936558 in patients with melanoma (multiple epitopes - NCT01176461 and NCT01176474).
Dendritic Cells as Antigen Delivery System
Increased immunogenicity of peptides for cancer vaccine can be achieved by loading autologous dendritic cells (DCs) either ex vivo or in vivo with the peptide.131-133 Indeed, DCs are the professional antigen-presenting cells (APCs) bridging innate and adaptive immunity.134 Their role in the periphery is to uptake pathogen- or host-derived antigenic proteins, which are processed and presented to naïve T lymphocytes at the lymphoid organs in the context of major histocompatibility (MHC) molecules.135
Several cancer immunotherapeutic strategies have been developed based on DCs (reviewed in132) stemming from the original works on generation of ex vivo DCs from mice, starting from bone marrow precursors,136 and later on from humans, starting from CD34+ haematopoietic progenitors or from peripheral blood–derived monocytes.137 Ex vivo generated DCs have been loaded with different sources of antigens mostly targeting melanoma, including whole tumor cells138-141 and tumor-derived proteins or peptides.142-144 Several clinical trials have been conducted along the years with DCs loaded with tumor-derived specific targeting melanoma,145-147 renal cell carcinoma148 and glioma,149,150 resulting in contrasting clinical outcomes.
The Sipuleucel-T (Provenge™) is an “immune cell”-based cancer vaccine targeting prostate cancer consisting of autologous whole immune cell population incubated with PA2024 that contains prostatic acid phosphatase (PAP, a prostate antigen) fused to GMCSF.97,96 In 2010 it was the first therapeutic cancer vaccine ever approved by the US FDA and its application is for the treatment of asymptomatic metastatic castrate-resistant prostate cancer (mCRPC).151 However, no difference in time to progression is observed and a modest 4.1-month improvement in median survival in the active arm with respect to the placebo arm was observed (25.8 vs. Twenty-one.7 months).
Although the registration of Sipuleucel-T as therapeutic cancer vaccine represents a great advancement in the cancer immunotherapy field, the modest efficacy urges improvements and optimizations of the DC-based strategy. Increasing expression of activating molecules or, on the other side, reducing expression of inhibitory molecules would result in improved capacity of DCs in stimulating T cell activation and, ultimately, in anti-tumor efficacy. Over-expression of CD40L in human DCs results in increased elicitation of T cell response to tumor antigens, such as glycoprotein 100 (gp100) and Melan A.152,153 Similarly, enhanced DC functions in stimulating antigen-specific Th1 and CTL responses can be achieved by modulation of other costimulatory molecules or proinflammatory factors.154-159 Conversely, silencing of the ubiquitin-editing enzyme A20 or the scavenger receptor SRA/CD204 in human DCs facilitates the development of IFN-γ producing Th1 cells and antigen specific CD8+ T cells.160-163 These findings suggest that the potency of current DC vaccines can be efficiently optimized resulting in improved clinical outcomes.
Additional strategies for antigen-specific vaccines
Alternative strategies to deliver antigen or antigen fragments in vivo is to utilize genetic vaccines or viral vectors (Table 4 and 5). These strategies, indeed, allow the delivery of multiple antigens with the activation of various arms of immunity (reviewed in164,165).
Table 4.
Cancer vaccines in clinical trials based on nucleic acids strategies.
| CANCER | Antigen | STRATEGY | NCT NUMBER | PHASE |
|---|---|---|---|---|
| Acute Myeloid Leukemia | WT-1 | RNA-pulsed DC | NCT01686334 | Phase 2 |
| WT-1 | RNA-pulsed DC | NCT00834002 | Phase 1 | |
| Breast | Multiple antigens | DNA vaccine | NCT02157051 | Phase 1 |
| CEA | RNA-pulsed DC | NCT00003432 | Phase 1/2 | |
| Colorectal | CEA | RNA-pulsed DC | NCT00003433 | Phase 1/2 |
| Kidney | hPSMA | DNA | NCT00096629 | Phase 1 |
| Lymphoma | Idiotype | DNA | NCT01209871 | Phase 1 |
| Melanoma | Multiple | RNA | NCT00204516 | Phase 1/2 |
| tyrosinase-related peptide 2 (TRP2) | RNA-pulsed DC | NCT01456104 | Phase 1 | |
| Neo-antigens | RNA | NCT01684241 | Phase 1 | |
| Neo-antigens | RNA | NCT02035956 | Phase 1 | |
| gp100 and tyrosinase | RNA-pulsed DC | NCT00940004 | Phase 1/2 | |
| gp100 and tyrosinase | RNA-pulsed DC | NCT00243529 | Phase 1/2 | |
| Multiple | RNA-pulsed DC | NCT01216436 | Phase 1 | |
| gp100 and tyrosinase | RNA-pulsed DC | NCT01530698 | Phase 1/2 | |
| Multiple | RNA-pulsed DC | NCT00672542 | Phase 1 | |
| Multiple | CEA | RNA-pulsed DC | NCT00004604 | Phase 1 |
| NY-ESO-1 | DNA | NCT00199849 | Phase 1 | |
| Non Small Cell Lung | Multiple | RNA | NCT00923312 | Phase 1/2 |
| Multiple | RNA | NCT01915524 | Phase 1 | |
| Prostate | Multiple | RNA | NCT00906243 | Phase 1/2 |
| PSA | DNA | NCT00859729 | Phase 1/2 | |
| PSA | RNA-pulsed DC | NCT00004211 | Phase 1/2 |
Table 5.
Cancer vaccines in clinical trials based on viral vector strategies.
| CANCER | ANTIGEN | STRATEGY | NCT NUMBER | PHASE |
|---|---|---|---|---|
| Bladder | PANVAC | Vaccinia/Fowlpox | NCT02015104 | Phase 2 |
| Brain/CNS | CEA | measles virus | NCT00390299 | Phase 1 |
| Breast | CEA & MUC-1 | Vaccinia/Fowlpox | NCT00179309 | Phase 2 |
| HER-2/Neu | Adenovirus | NCT00197522 | Phase 1 | |
| Melanoma | gp100 antigen | Fowlpox | NCT00019175 | Phase 1 |
| gp100 antigen | Fowlpox | NCT00019669 | Phase 2 | |
| tyrosinase | Fowlpox | NCT00019734 | Phase 2 | |
| tyrosinase | Fowlpox | NCT00054535 | Phase 2 | |
| multiple | ALVAC | NCT00613509 | Phase 2 | |
| Multiple | MUC-1 | MVA | NCT00004881 | Phase 1 |
| EBNA1/LMP2 | MVA | NCT01147991 | Phase 1 | |
| HER-2/Neu | Adenovirus | NCT01730118 | Phase 1 | |
| CEA | Fowlpox | NCT00217373 | Phase 1 | |
| Nasopharyngeal | EBNA1/LMP2 | MVA | NCT01256853 | Phase 1 |
| Non Small Cell Lung | MUC-1 | MVA | NCT01383148 | Phase 2b/3 |
| Ovarian | NY-ESO-1 | Fowlpox | NCT00112957 | Phase 2 |
| NY-ESO-1 | ALVAC | NCT00803569 | Phase 1 | |
| CEA | Measles virus | NCT00408590 | Phase 1 | |
| NY-ESO-1 | ALVAC | NCT01982487 | Phase 1/2 | |
| Pancreatic | CEA, MUC1, and TRICOM | Vaccinia/Fowlpox | NCT00088660 | Phase 3 |
| Prostate | 5T4 | Poxvirus | NCT01194960 | Phase 2 |
| PSA | Fowlpox | NCT00005039 | Phase 2 | |
| PSA | Fowlpox | NCT00450463 | Phase 2 | |
| PSA | Fowlpox | NCT00045227 | Phase 2 | |
| PSA | Adenovirus | NCT00583024 | Phase 2 | |
| PSA | Fowlpox | NCT00020254 | Phase 2 | |
| PSA | Fowlpox | NCT00003871 | Phase 2 | |
| PSA | Vaccinia | NCT00001382 | Phase 1 | |
| PSA, TRICOM | Vaccinia/Fowlpox | NCT01322490 | Phase 3 |
DNA vaccine platforms have shown promise in preclinical studies166 which, however, do not hold when translated to non-human primates and humans167,168 due to lack of efficacy. New constructs and methods of administration may enhance their efficacy. Indeed, Phase I/II trials for melanoma and other cancers are currently testing the efficacy of DNA vaccines injected directly into the lymph nodes, aiming at increasing antigen uptake by APCs and promote local inflammatory signals.169,170 However, the in vivo nucleic acid electro-gene-transfer (EGT) appears to be the most promising strategy to enhance immunogenicity of nucleic acid immunizations for cancer vaccine protocols171 and a list of the ongoing cancer vaccine clinical trials with use of electro-gene-transfer is reviewed in.172
Similar to DNA vaccines and viral vectors, RNA vaccines may induce both CD4+ and CD8+ T cell responses and candidates targeting cancer antigens have been evaluated.173–175
mRNA vaccine candidates have been tested in human clinical trials using either whole tumor cell transcriptome176 to target metastatic melanoma, or specific TAAs to target metastatic melanoma177 and renal cell carcinoma,178 eliciting tumor antigen-specific antibody and T cell responses. More recently, trials targeting prostate and non-small cell lung cancer have shown mRNA vaccines to be safe, well tolerated and immunogenic.179
The first and most extensively evaluated viral-based vectors in cancer vaccine trials are from the poxviridae family, such as vaccinia, modified vaccinia strain Ankara (MVA), and the avipoxviruses (fowlpox and canarypox; ALVAC).180,181 PROSTVAC is a cancer vaccine to prostate cancer based on a replication-competent vaccinia prime and a replication-incompetent fowlpox boost. Each vector contains transgenes for PSA and 3 costimulatory molecules (CD80, CD54 and CD58), designated TRICOM.182 In 2 independent phase II trials, PROSTVAC improved median overall survival relative to the control vector183,184 and a phase III trial is currently ongoing (NCT01322490).
The MVA vector-based cancer vaccine TG4010 targeting the MUC1 antigen has been tested in a phase II trial for renal cell carcinoma combined with interferon-α2a and IL-2, resulting in improved overall survival.185 A separate phase II trial of TG4010 combined with first-line chemotherapy (cisplatin plus gemcitabine) in advanced NSCLC demonstrated a significant 6 months increase in median survival.186 A confirmatory phase IIb/III trial of TG4010 for treatment of advanced stage (IV) NSCLC is ongoing (NCT01383148).
A phase III clinical trial has been conducted and terminated to evaluate the efficacy of PANVAC-VF, a vaccine composed of recombinant vaccinia virus and fowlpox virus expressing CEA, MUC1, and TRICOM, in patients with advanced pancreatic cancer (NCT00088660). Vaccinated patients failed to show an advantage in overall survival over standard palliative chemotherapy.187
Adenovirus vectors expressing various TAAs (PSA, HER-2/Neu) are currently being tested for their immunological and clinical efficacy (NCT00583024, NCT00197522). Moreover, an adenovirus expressing the extracellular and transmembrane domains of HER2 is currently evaluated in patients with any HER2-expressing tumor, aiming at inducing neutralizing antibodies against HER2, not T cells (NCT01730118).
Conclusions and Future Directions
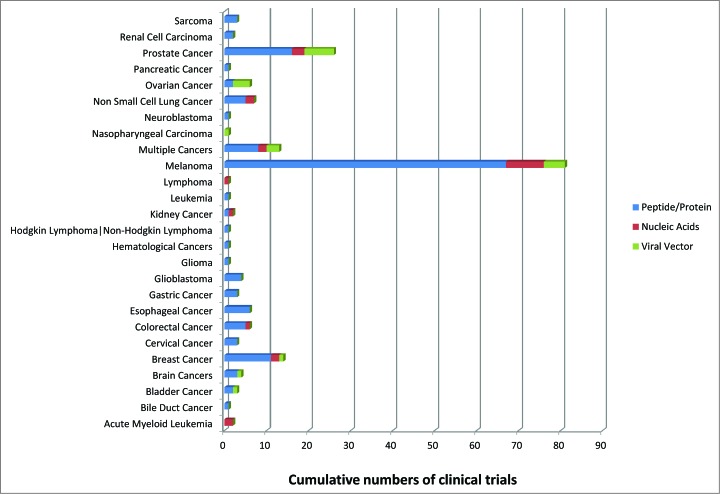
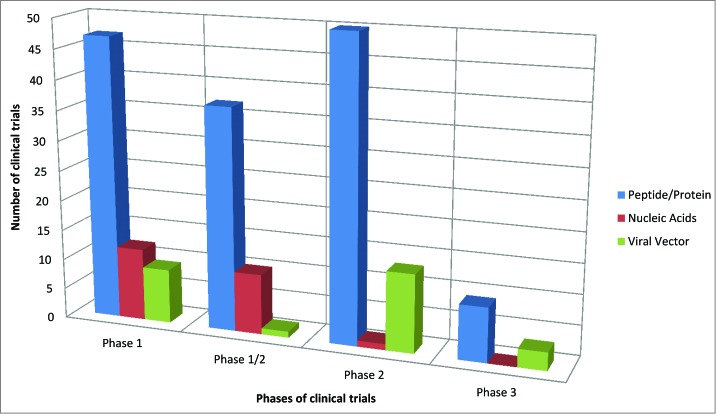
Several cancer vaccines clinical trials have been conducted in the last years based on the different type of antigens described in the present review (peptide vs. genetis vs. viral vectors). The vast majority of such clinical trials have been based on peptides mostly targeting melanoma (Fig. 1). The prevalence of peptide-based clinical trials is observed also in the different phases of clinical trials (Fig. 2). To date, only few clinical trials have reached the efficacy Phase III evaluation, based only on peptides and viral vectors. Evaluation of cancer vaccines on an increased number of target cancers using diverse vaccine strategies would definitely be highly beneficial to improve the knowledge in the field and, ultimately, clinical outcome in cancer patients.
Figure 1.
Cumulative numbers of cancer vaccine clinical trials for each cancer and each vaccination strategy.
Figure 2.
Number of cancer vaccine clinical trials in each experimental phase for each vaccination strategy.
Indeed, the first therapeutic cancer vaccine approved by FDA for the treatment of asymptomatic metastatic castrate-resistant prostate cancer (Sipuleucel-T (Provenge™), represents a landmark. However, Sipuleucel-T shows a modest increase in overall survival and other large scale clinical trials do not prove yet to be as efficacious as needed for complete tumor regression.
Several reasons account for these disappointing results. Identification of the appropriate target antigens, represents one the most relevant aspects and currently available high – throughput strategies make this goal accomplishable.
Along this path, identification of peptides naturally processed and presented by HLA molecules (HLA ligandome) on tumor cells as well as the personalized immunotherapy, to identify target tumor-associated antigens specific for each individual cancer patient, is further raising the bar in the quest of eliciting tumor specific immunity.
Efficacy in clinical application of cancer vaccine approaches based on cocktails of specific epitopes identified with high – throughput technologies is very promising and is currently being further evaluated in a broader range of tumors.
In general, besides target antigen identification, chances of success may increase only if a multi-faceted strategy is undertaken, including 1) addressing the tolerogenic environment and tumor suppressive mechanisms by combinatorial immunotherapy; 2) selecting optimal antigen presentation and delivery system; 3) adding a potent immune modulator able to increase the immunogenicity of the vaccine and to specifically elicit the more appropriate arm of the immune response (i.e. Th1 vs. Th2); and 4) employing multiparametric analyses to identify prediction markers of immunogenicity for selection of best responding vaccinees.
The combination of all such approaches will represent a great advancement in cancer vaccinology, enabling the development of vaccines with enhanced therapeutic efficacy to hopefully improve the quality of life of cancer patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The study was funded by EU FP7 Project Cancer Vaccine development for Hepatocellular Carcinoma – HEPAVAC (Grant Nr. 602893) and Italian Ministry of Health through Institutional “Ricerca Corrente”. M.T. and A.P. are HEPAVAC fellows.
References
- 1.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004; 10:789-799; PMID:15286780; http://dx.doi.org/ 10.1038/nm1087/ [DOI] [PubMed] [Google Scholar]
- 2.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, et al. Patterns of somatic mutation in human cancer genomes. Nature 2007; 446:153-158; PMID:17344846; http://dx.doi.org/ 10.1038/nature05610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57-70; PMID:10647931; http://dx.doi.org/ 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 4.Hanna MG, Jr. Peters LC. Specific immunotherapy of established visceral micrometastases by BCG-tumor cell vaccine alone or as an adjunct to surgery. Cancer 1978; 42:2613-2625; PMID:728864; http://dx.doi.org/ 10.1002/1097-0142(197812)42:6%3c2613::AID-CNCR2820420617%3e3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 5.Ruttinger D, van den Engel NK, Winter H, Schlemmer M, Pohla H, Grutzner S, Wagner B, Schendel DJ, Fox BA, Jauch KW, et al. Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical experience and evidence of an immune response. J Transl Med 2007; 5:43-; PMID:17868452; http://dx.doi.org/ 10.1186/1479-5876-5-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemunaitis J, Nemunaitis J. Granulocyte-macrophage colony-stimulating factor gene-transfected autologous tumor cell vaccine: focus[correction to fcous] on non-small-cell lung cancer. Clin Lung Cancer 2003; 5:148-157 [DOI] [PubMed] [Google Scholar]
- 7.Harris JE, Ryan L, Hoover HC, Jr., Stuart RK, Oken MM, Benson AB, III, Mansour E, Haller DG, Manola J, Hanna MG, Jr. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol 2000; 18:148-157; PMID:10623705; . [DOI] [PubMed] [Google Scholar]
- 8.de WV, Turksma AW, Voorham QJ, Euler Z, Bril H, van den Eertwegh AJ, Bloemena E, Pinedo HM, Vermorken JB, van TH, et al. Clinical effects of adjuvant active specific immunotherapy differ between patients with microsatellite-stable and microsatellite-instable colon cancer. Clin Cancer Res 2012; 18:882-889; PMID:22156611; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1716 [DOI] [PubMed] [Google Scholar]
- 9.Hanna MG, Jr., Hoover HC, Jr., Vermorken JB, Harris JE, Pinedo HM. Adjuvant active specific immunotherapy of stage II and stage III colon cancer with an autologous tumor cell vaccine: first randomized phase III trials show promise. Vaccine 2001; 19:2576-2582; PMID:11257395; http://dx.doi.org/ 10.1016/S0264-410X(00)00485-0 [DOI] [PubMed] [Google Scholar]
- 10.Baars A, Claessen AM, van den Eertwegh AJ, Gall HE, Stam AG, Meijer S, Giaccone G, Meijer CJ, Scheper RJ, Wagstaff J, et al. Skin tests predict survival after autologous tumor cell vaccination in metastatic melanoma: experience in 81 patients. Ann Oncol 2000; 11:965-970; PMID:11038032; http://dx.doi.org/ 10.1023/A:1008363601515 [DOI] [PubMed] [Google Scholar]
- 11.Berd D, Sato T, Maguire HC, Jr., Kairys J, Mastrangelo MJ. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J Clin Oncol 2004; 22:403-415; PMID:14691123; http://dx.doi.org/ 10.1200/JCO.2004.06.043 [DOI] [PubMed] [Google Scholar]
- 12.Mendez R, Ruiz-Cabello F, Rodriguez T, Del CA, Paschen A, Schadendorf D, Garrido F. Identification of different tumor escape mechanisms in several metastases from a melanoma patient undergoing immunotherapy. Cancer Immunol Immunother 2007; 56:88-94; PMID:16622680; http://dx.doi.org/ 10.1007/s00262-006-0166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonia SJ, Seigne J, Diaz J, Muro-Cacho C, Extermann M, Farmelo MJ, Friberg M, Alsarraj M, Mahany JJ, Pow-Sang J, et al. Phase I trial of a B7-1 (CD80) gene modified autologous tumor cell vaccine in combination with systemic interleukin-2 in patients with metastatic renal cell carcinoma. J Urol 2002; 167:1995-2000; PMID:11956426; http://dx.doi.org/ 10.1016/S0022-5347(05)65071-9 [DOI] [PubMed] [Google Scholar]
- 14.Fishman M, Hunter TB, Soliman H, Thompson P, Dunn M, Smilee R, Farmelo MJ, Noyes DR, Mahany JJ, Lee JH, et al. Phase II trial of B7-1 (CD-86) transduced, cultured autologous tumor cell vaccine plus subcutaneous interleukin-2 for treatment of stage IV renal cell carcinoma. J Immunother 2008; 31:72-80; PMID:18157014; http://dx.doi.org/ 10.1097/CJI.0b013e31815ba792 [DOI] [PubMed] [Google Scholar]
- 15.Berger M, Kreutz FT, Horst JL, Baldi AC, Koff WJ. Phase I study with an autologous tumor cell vaccine for locally advanced or metastatic prostate cancer. J Pharm Pharm Sci 2007; 10:144-152; PMID:17706173 [PubMed] [Google Scholar]
- 16.Morton DL, Foshag LJ, Hoon DS, Nizze JA, Famatiga E, Wanek LA, Chang C, Davtyan DG, Gupta RK, Elashoff R, et al. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine. Ann Surg 1992; 216:463-482; PMID:1417196; http://dx.doi.org/ 10.1097/00000658-199210000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton DL, Hsueh EC, Essner R, Foshag LJ, O'Day SJ, Bilchik A, Gupta RK, Hoon DS, Ravindranath M, Nizze JA, et al. Prolonged survival of patients receiving active immunotherapy with Canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes. Ann Surg 2002; 236:438-448; PMID:12368672; http://dx.doi.org/ 10.1097/00000658-200210000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsueh EC, Essner R, Foshag LJ, Ollila DW, Gammon G, O'Day SJ, Boasberg PD, Stern SL, Ye X, Morton DL. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol 2002; 20:4549-4554; PMID:12454111; http://dx.doi.org/ 10.1200/JCO.2002.01.151 [DOI] [PubMed] [Google Scholar]
- 19.Sondak VK, Sabel MS, Mule JJ. Allogeneic and autologous melanoma vaccines: where have we been and where are we going? Clin Cancer Res 2006; 12:2337s-2341s; PMID:16609055; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-2555 [DOI] [PubMed] [Google Scholar]
- 20.Ward S, Casey D, Labarthe MC, Whelan M, Dalgleish A, Pandha H, Todryk S. Immunotherapeutic potential of whole tumour cells. Cancer Immunol Immunother 2002; 51:351-357; PMID:12192534; http://dx.doi.org/ 10.1007/s00262-002-0286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang CL, Benencia F, Coukos G. Whole tumor antigen vaccines. Semin Immunol 2010;; PMID:20356763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emens LA. GM-CSF-secreting vaccines for solid tumors. Curr Opin Investig Drugs 2009; 10:1315-1324; PMID:19943203 [PubMed] [Google Scholar]
- 23.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev 2002; 188:147-154; PMID:12445288; http://dx.doi.org/ 10.1034/j.1600-065X.2002.18813.x [DOI] [PubMed] [Google Scholar]
- 24.Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev 2008; 222:287-298; PMID:18364009; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00618.x [DOI] [PubMed] [Google Scholar]
- 25.Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, Borellini F, Clift SM, Hege KM, Ando DG, Piantadosi S, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin Cancer Res 2006; 12:3394-3401; PMID:16740763; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0145 [DOI] [PubMed] [Google Scholar]
- 26.Small EJ, Sacks N, Nemunaitis J, Urba WJ, Dula E, Centeno AS, Nelson WG, Ando D, Howard C, Borellini F, et al. Granulocyte macrophage colony-stimulating factor–secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res 2007; 13:3883-3891; PMID:17606721; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2937 [DOI] [PubMed] [Google Scholar]
- 27.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol 2009; 27:5911-5918; PMID:19805669; http://dx.doi.org/ 10.1200/JCO.2009.23.3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011; 253:328-335; PMID:21217520; http://dx.doi.org/ 10.1097/SLA.0b013e3181fd271c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clive KS, Tyler JA, Clifton GT, Holmes JP, Mittendorf EA, Ponniah S, Peoples GE. Use of GM-CSF as an adjuvant with cancer vaccines: beneficial or detrimental? Expert Rev Vaccines 2010; 9:519-525; PMID:20450326; http://dx.doi.org/ 10.1586/erv.10.40 [DOI] [PubMed] [Google Scholar]
- 30.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol 2007; 18:226-232; PMID:17116643; http://dx.doi.org/ 10.1093/annonc/mdl158 [DOI] [PubMed] [Google Scholar]
- 31.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, Tong A, Kumar P, Pappen B, Hamilton C, et al. Phase II study of belagenpumatucel-L, a transforming growth factor β-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 2006; 24:4721-4730; PMID:16966690; http://dx.doi.org/ 10.1200/JCO.2005.05.5335 [DOI] [PubMed] [Google Scholar]
- 32.Nemunaitis J, Nemunaitis M, Senzer N, Snitz P, Bedell C, Kumar P, Pappen B, Maples PB, Shawler D, Fakhrai H. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther 2009; 16:620-624; PMID:19287371; http://dx.doi.org/ 10.1038/cgt.2009.15 [DOI] [PubMed] [Google Scholar]
- 33.Ranieri E, Kierstead LS, Zarour H, Kirkwood JM, Lotze MT, Whiteside T, Storkus WJ. Dendritic cell/peptide cancer vaccines: clinical responsiveness and epitope spreading. Immunol Invest 2000; 29:121-125; PMID:10854179; http://dx.doi.org/ 10.3109/08820130009062294 [DOI] [PubMed] [Google Scholar]
- 34.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, Wang HJ, Elashoff RM, McBride WH, Mukherji B, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res 2003; 9:998-1008; PMID:12631598 [PubMed] [Google Scholar]
- 35.van der BP, Traversari C, Chomez P, Lurquin C, De PE, Van den EB, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254:1643-1647; PMID:1840703; http://dx.doi.org/ 10.1126/science.1840703 [DOI] [PubMed] [Google Scholar]
- 36.Buonaguro L, Petrizzo A, Tornesello ML, Buonaguro FM. Translating tumor antigens into cancer vaccines. Clin Vaccine Immunol 2011; 18:23-34; PMID:21048000; http://dx.doi.org/ 10.1128/CVI.00286-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Smet C, Lurquin C, van der BP, De PE, Brasseur F, Boon T. Sequence and expression pattern of the human MAGE2 gene. Immunogenetics 1994; 39:121-129; PMID:8276455; http://dx.doi.org/ 10.1007/BF00188615 [DOI] [PubMed] [Google Scholar]
- 38.De Smet C, Lurquin C, Lethe B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol 1999; 19:7327-7335; PMID:10523621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaux P, Luiten R, Demotte N, Vantomme V, Stroobant V, Traversari C, Russo V, Schultz E, Cornelis GR, Boon T, et al. Identification of five MAGE-A1 epitopes recognized by cytolytic T lymphocytes obtained by in vitro stimulation with dendritic cells transduced with MAGE-A1. J Immunol 1999; 163:2928-2936; PMID:10453041; . [PubMed] [Google Scholar]
- 40.Traversari C, van der BP, Luescher IF, Lurquin C, Chomez P, Van PA, De PE, mar-Costesec A, Boon T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med 1992; 176:1453-1457; PMID:1402688; http://dx.doi.org/ 10.1084/jem.176.5.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med 1998; 187:265-270; PMID:9432985; http://dx.doi.org/ 10.1084/jem.187.2.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayyoub M, Stevanovic S, Sahin U, Guillaume P, Servis C, Rimoldi D, Valmori D, Romero P, Cerottini JC, Rammensee HG, et al. Proteasome-assisted identification of a SSX-2-derived epitope recognized by tumor-reactive CTL infiltrating metastatic melanoma. J Immunol 2002; 168:1717-1722; PMID:11823502; http://dx.doi.org/ 10.4049/jimmunol.168.4.1717 [DOI] [PubMed] [Google Scholar]
- 43.Bakker AB, Schreurs MW, de Boer AJ, Kawakami Y, Rosenberg SA, Adema GJ, Figdor CG. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med 1994; 179:1005-1009; PMID:8113668; http://dx.doi.org/ 10.1084/jem.179.3.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawakami Y, Robbins PF, Wang X, Tupesis JP, Parkhurst MR, Kang X, Sakaguchi K, Appella E, Rosenberg SA. Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1, -A2, and -A3 alleles. J Immunol 1998; 161:6985-6992; PMID:9862734 [PubMed] [Google Scholar]
- 45.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol 1995; 154:3961-3968; PMID:7706734 [PubMed] [Google Scholar]
- 46.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med 1994; 180:347-352; PMID:7516411; http://dx.doi.org/ 10.1084/jem.180.1.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2). Cancer Res 1998; 58:4895-4901; PMID:9809996 [PubMed] [Google Scholar]
- 48.Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med 1996; 184:2207-2216; PMID:8976176; http://dx.doi.org/ 10.1084/jem.184.6.2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corman JM, Sercarz EE, Nanda NK. Recognition of prostate-specific antigenic peptide determinants by human CD4 and CD8 T cells. Clin Exp Immunol 1998; 114:166-172; PMID:9822272; http://dx.doi.org/ 10.1046/j.1365-2249.1998.00678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst 1997; 89:293-300; PMID:9048833; http://dx.doi.org/ 10.1093/jnci/89.4.293 [DOI] [PubMed] [Google Scholar]
- 51.Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T. Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer 2002; 102:499-506; PMID:12432553; http://dx.doi.org/ 10.1002/ijc.10736 [DOI] [PubMed] [Google Scholar]
- 52.Schmollinger JC, Vonderheide RH, Hoar KM, Maecker B, Schultze JL, Hodi FS, Soiffer RJ, Jung K, Kuroda MJ, Letvin NL, et al. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sci U S A 2003; 100:3398-3403; PMID:12626761; http://dx.doi.org/ 10.1073/pnas.0530311100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt SM, Schag K, Muller MR, Weck MM, Appel S, Kanz L, Grunebach F, Brossart P. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood 2003; 102:571-576; PMID:12576330; http://dx.doi.org/ 10.1182/blood-2002-08-2554 [DOI] [PubMed] [Google Scholar]
- 54.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity 1999; 10:673-679; PMID:10403642; http://dx.doi.org/ 10.1016/S1074-7613(00)80066-7 [DOI] [PubMed] [Google Scholar]
- 55.Vonderheide RH, Anderson KS, Hahn WC, Butler MO, Schultze JL, Nadler LM. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin Cancer Res 2001; 7:3343-3348; PMID:11705846 [PubMed] [Google Scholar]
- 56.Minev B, Hipp J, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci U S A 2000; 97:4796-4801; PMID:10759561; http://dx.doi.org/ 10.1073/pnas.070560797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Umano Y, Tsunoda T, Tanaka H, Matsuda K, Yamaue H, Tanimura H. Generation of cytotoxic T cell responses to an HLA-A24 restricted epitope peptide derived from wild-type p53. Br J Cancer 2001; 84:1052-1057; PMID:11308253; http://dx.doi.org/ 10.1054/bjoc.2000.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azuma K, Shichijo S, Maeda Y, Nakatsura T, Nonaka Y, Fujii T, Koike K, Itoh K. Mutated p53 gene encodes a nonmutated epitope recognized by HLA-B*4601-restricted and tumor cell-reactive CTLs at tumor site. Cancer Res 2003; 63:854-858; PMID:12591737 [PubMed] [Google Scholar]
- 59.Muller S, Alving K, Peter-Katalinic J, Zachara N, Gooley AA, Hanisch FG. High density O-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells. J Biol Chem 1999; 274:18165-18172; PMID:10373415; http://dx.doi.org/ 10.1074/jbc.274.26.18165 [DOI] [PubMed] [Google Scholar]
- 60.Ragupathi G. Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol Immunother 1996; 43:152-157; PMID:9001568; http://dx.doi.org/ 10.1007/s002620050316 [DOI] [PubMed] [Google Scholar]
- 61.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1999; 1473:21-34; PMID:10580127; http://dx.doi.org/ 10.1016/S0304-4165(99)00167-1 [DOI] [PubMed] [Google Scholar]
- 62.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol 2001; 491:369-402; PMID:14533809; http://dx.doi.org/ 10.1007/978-1-4615-1267-7_24 [DOI] [PubMed] [Google Scholar]
- 63.Parmiani G, De FA, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol 2007; 178:1975-1979; PMID:17277099; http://dx.doi.org/ 10.4049/jimmunol.178.4.1975 [DOI] [PubMed] [Google Scholar]
- 64.Heemskerk B, Kvistborg P, Schumacher TN. The cancer antigenome. EMBO J 2013; 32:194-203; PMID:23258224; http://dx.doi.org/ 10.1038/emboj.2012.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stratton MR. Exploring the genomes of cancer cells: progress and promise. Science 2011; 331:1553-1558 [DOI] [PubMed] [Google Scholar]
- 66.Castle JC, Kreiter S, Diekmann J, Lower M, van RN, de GJ, Selmi A, Diken M, Boegel S, Paret C, et al. Exploiting the mutanome for tumor vaccination. Cancer Res 2012; 72:1081-1091; PMID:22237626; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3722 [DOI] [PubMed] [Google Scholar]
- 67.Jones S, Wang TL, Shih I, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Jr., Vogelstein B, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010; 330:228-231; PMID:20826764; http://dx.doi.org/ 10.1126/science.1196333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, Davies H, Jones D, Lin ML, Teague J, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 2011; 469:539-542; PMID:21248752; http://dx.doi.org/ 10.1038/nature09639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C, et al. The genetic landscape of the childhood cancer medulloblastoma. Science 2011; 331:435-439; PMID:21163964; http://dx.doi.org/ 10.1126/science.1198056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee W, Jiang Z, Liu J, Haverty PM, Guan Y, Stinson J, Yue P, Zhang Y, Pant KP, Bhatt D, et al. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature 2010; 465:473-477; PMID:20505728; http://dx.doi.org/ 10.1038/nature09004 [DOI] [PubMed] [Google Scholar]
- 71.Timmermann B, Kerick M, Roehr C, Fischer A, Isau M, Boerno ST, Wunderlich A, Barmeyer C, Seemann P, Koenig J, et al. Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PLoS ONE 2010; 5:e15661-; PMID:21203531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei X, Walia V, Lin JC, Teer JK, Prickett TD, Gartner J, Davis S, Stemke-Hale K, Davies MA, Gershenwald JE, et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat Genet 2011; 43:442-446; PMID:21499247; http://dx.doi.org/ 10.1038/ng.810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cerami E, Demir E, Schultz N, Taylor BS, Sander C. Automated network analysis identifies core pathways in glioblastoma. PLoS One 2010; 5:e8918-; PMID:20169195; http://dx.doi.org/ 10.1371/journal.pone.0008918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The genomic landscapes of human breast and colorectal cancers. Science 2007; 318:1108-1113; PMID:17932254; http://dx.doi.org/ 10.1126/science.1145720 [DOI] [PubMed] [Google Scholar]
- 75.Junnila S, Kokkola A, Karjalainen-Lindsberg ML, Puolakkainen P, Monni O. Genome-wide gene copy number and expression analysis of primary gastric tumors and gastric cancer cell lines. BMC Cancer 2010; 10:73-; PMID:20187983; http://dx.doi.org/ 10.1186/1471-2407-10-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radtke I, Mullighan CG, Ishii M, Su X, Cheng J, Ma J, Ganti R, Cai Z, Goorha S, Pounds SB, et al. Genomic analysis reveals few genetic alterations in pediatric acute myeloid leukemia. Proc Natl Acad Sci U S A 2009; 106:12944-12949; PMID:19651601; http://dx.doi.org/ 10.1073/pnas.0903142106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321:1801-1806; PMID:18772397; http://dx.doi.org/ 10.1126/science.1164368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fox EJ, Salk JJ, Loeb LA. Cancer genome sequencing–an interim analysis. Cancer Res 2009; 69:4948-4950; PMID:19509220; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bendandi M. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat Rev Cancer 2009; 9:675-681; PMID:19701243; http://dx.doi.org/ 10.1038/nrc2717 [DOI] [PubMed] [Google Scholar]
- 80.Stevenson GT, Elliott EV, Stevenson FK. Idiotypic determinants on the surface immunoglobulin of neoplastic lymphocytes: a therapeutic target. Fed Proc 1977; 36:2268-2271; PMID:69552 [PubMed] [Google Scholar]
- 81.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 1999; 50:213-219; PMID:10602881; http://dx.doi.org/ 10.1007/s002510050595 [DOI] [PubMed] [Google Scholar]
- 82.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics 2001; 17:1236-1237; PMID:11751237; http://dx.doi.org/ 10.1093/bioinformatics/17.12.1236 [DOI] [PubMed] [Google Scholar]
- 83.Reche PA, Glutting JP, Reinherz EL. Prediction of MHC class I binding peptides using profile motifs. Hum Immunol 2002; 63:701-709; PMID:12175724; http://dx.doi.org/ 10.1016/S0198-8859(02)00432-9 [DOI] [PubMed] [Google Scholar]
- 84.Guan P, Hattotuwagama CK, Doytchinova IA, Flower DR. MHCPred 2.0: an updated quantitative T-cell epitope prediction server. Appl Bioinformatics 2006; 5:55-61; PMID:16539539; http://dx.doi.org/ 10.2165/00822942-200605010-00008 [DOI] [PubMed] [Google Scholar]
- 85.Bachinsky MM, Guillen DE, Patel SR, Singleton J, Chen C, Soltis DA, Tussey LG. Mapping and binding analysis of peptides derived from the tumor-associated antigen survivin for eight HLA alleles. Cancer Immun 2005; 5:6-; PMID:15779886 [PubMed] [Google Scholar]
- 86.Singh-Jasuja H, Emmerich NP, Rammensee HG. The Tubingen approach: identification, selection, and validation of tumor-associated HLA peptides for cancer therapy. Cancer Immunol Immunother 2004; 53:187-195; PMID:14758508; http://dx.doi.org/ 10.1007/s00262-003-0480-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J, Dorsch K, Flohr S, Fritsche J, Lewandrowski P, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 2012; 135:1042-1054; PMID:22418738; http://dx.doi.org/ 10.1093/brain/aws042 [DOI] [PubMed] [Google Scholar]
- 88.Rammensee HG, Singh-Jasuja H. HLA ligandome tumor antigen discovery for personalized vaccine approach. Expert Rev Vaccines 2013; 12:1211-1217; PMID:24090147; http://dx.doi.org/ 10.1586/14760584.2013.836911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Overwijk WW, Wang E, Marincola FM, Rammensee HG, Restifo NP. Mining the mutanome: developing highly personalized Immunotherapies based on mutational analysis of tumors. J Immunother Cancer 2013; 1:11-; PMID:24829748; http://dx.doi.org/ 10.1186/2051-1426-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst 2002; 94:805-818; PMID:12048268; http://dx.doi.org/ 10.1093/jnci/94.11.805 [DOI] [PubMed] [Google Scholar]
- 91.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10:909-915; PMID:15340416; http://dx.doi.org/ 10.1038/nm1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol 2005; 175:6169-6176; PMID:16237114; http://dx.doi.org/ 10.4049/jimmunol.175.9.6169 [DOI] [PubMed] [Google Scholar]
- 93.Chaudhuri D, Suriano R, Mittelman A, Tiwari RK. Targeting the immune system in cancer. Curr Pharm Biotechnol 2009; 10:166-184; PMID:19199949; http://dx.doi.org/ 10.2174/138920109787315114 [DOI] [PubMed] [Google Scholar]
- 94.Perez SA, von HE, Kallinteris NL, Gritzapis AD, Peoples GE, Papamichail M, Baxevanis CN. A new era in anticancer peptide vaccines. Cancer 2010; 116:2071-2080; PMID:20187092; . [DOI] [PubMed] [Google Scholar]
- 95.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res 2013; 119:421-475; PMID:23870514; http://dx.doi.org/ 10.1016/B978-0-12-407190-2.00007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006; 24:3089-3094; PMID:16809734; http://dx.doi.org/ 10.1200/JCO.2005.04.5252 [DOI] [PubMed] [Google Scholar]
- 97.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-422; PMID:20818862; http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 98.Slingluff CL, Jr., Petroni GR, Yamshchikov GV, Hibbitts S, Grosh WW, Chianese-Bullock KA, Bissonette EA, Barnd DL, Deacon DH, Patterson JW, et al. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol 2004; 22:4474-4485; PMID:15542798; http://dx.doi.org/ 10.1200/JCO.2004.10.212 [DOI] [PubMed] [Google Scholar]
- 99.Slingluff CL, Jr., Petroni GR, Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, Johansen N, Grosh WW, Yamshchikov GV, Neese PY, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res 2007; 13:6386-6395; PMID:17975151; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-0486 [DOI] [PubMed] [Google Scholar]
- 100.Slingluff CL, Jr., Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, von MM, Grosh WW. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol 2011; 29:2924-2932; PMID:21690475; http://dx.doi.org/ 10.1200/JCO.2010.33.8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol 2005; 174:2591-2601; PMID:15728465; http://dx.doi.org/ 10.4049/jimmunol.174.5.2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 1998; 188:2357-2368; PMID:9858522; http://dx.doi.org/ 10.1084/jem.188.12.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol 1998; 10:588-594; PMID:9794842; http://dx.doi.org/ 10.1016/S0952-7915(98)80228-8 [DOI] [PubMed] [Google Scholar]
- 104.Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol 2005; 35:2518-2527; PMID:16144035; http://dx.doi.org/ 10.1002/eji.200535002 [DOI] [PubMed] [Google Scholar]
- 105.Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: agents of antigen cross-presentation. Expert Rev Vaccines 2008; 7:1019-1030; PMID:18767951; http://dx.doi.org/ 10.1586/14760584.7.7.1019 [DOI] [PubMed] [Google Scholar]
- 106.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 2002; 20:395-425; PMID:11861608; http://dx.doi.org/ 10.1146/annurev.immunol.20.100301.064801 [DOI] [PubMed] [Google Scholar]
- 107.Wang XY, Subjeck JR. High molecular weight stress proteins: Identification, cloning and utilisation in cancer immunotherapy. Int J Hyperthermia 2013; 29:364-375; PMID:23829534; http://dx.doi.org/ 10.3109/02656736.2013.803607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang XY, Sun X, Chen X, Facciponte J, Repasky EA, Kane J, Subjeck JR. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J Immunol 2010; 184:6309-6319; PMID:20439916; http://dx.doi.org/ 10.4049/jimmunol.0903891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol 2008; 26:955-962; PMID:18281670; http://dx.doi.org/ 10.1200/JCO.2007.11.9941 [DOI] [PubMed] [Google Scholar]
- 110.Buhrman JD, Slansky JE. Improving T cell responses to modified peptides in tumor vaccines. Immunol Res 2013; 55:34-47; PMID:22936035; http://dx.doi.org/ 10.1007/s12026-012-8348-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Borbulevych OY, Baxter TK, Yu Z, Restifo NP, Baker BM. Increased immunogenicity of an anchor-modified tumor-associated antigen is due to the enhanced stability of the peptide/MHC complex: implications for vaccine design. J Immunol 2005; 174:4812-4820; PMID:15814707; http://dx.doi.org/ 10.4049/jimmunol.174.8.4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hou Y, Kavanagh B, Fong L. Distinct CD8+ T cell repertoires primed with agonist and native peptides derived from a tumor-associated antigen. J Immunol 2008; 180:1526-1534; PMID:18209048; http://dx.doi.org/ 10.4049/jimmunol.180.3.1526 [DOI] [PubMed] [Google Scholar]
- 113.Pierce BG, Hellman LM, Hossain M, Singh NK, Vander Kooi CW, Weng Z, Baker BM. Computational design of the affinity and specificity of a therapeutic T cell receptor. PLoS Comput Biol 2014; 10:e1003478; PMID:24550723; http://dx.doi.org/ 10.1371/journal.pcbi.1003478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oh S, Terabe M, Pendleton CD, Bhattacharyya A, Bera TK, Epel M, Reiter Y, Phillips J, Linehan WM, Kasten-Sportes C, et al. Human CTLs to wild-type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Res 2004; 64:2610-2618; PMID:15059918; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-2183 [DOI] [PubMed] [Google Scholar]
- 115.Berzofsky JA, Wood LV, Terabe M. Cancer vaccines: 21st century approaches to harnessing an ancient modality to fight cancer. Expert Rev Vaccines 2013; 12:1115-1118; PMID:24124874; http://dx.doi.org/ 10.1586/14760584.2013.836906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998; 4:321-327; PMID:9500606; http://dx.doi.org/ 10.1038/nm0398-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8:351-360; PMID:18418403; http://dx.doi.org/ 10.1038/nrc2373 [DOI] [PubMed] [Google Scholar]
- 118.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol 2008; 38:1033-1042; PMID:18350546; http://dx.doi.org/ 10.1002/eji.200737995 [DOI] [PubMed] [Google Scholar]
- 119.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 1998; 393:478-480; PMID:9624004; http://dx.doi.org/ 10.1038/30996 [DOI] [PubMed] [Google Scholar]
- 120.Schoenberger SP, Toes RE, van d V, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998; 393:480-483; PMID:9624005; http://dx.doi.org/ 10.1038/31002 [DOI] [PubMed] [Google Scholar]
- 121.Toes RE, Offringa R, Blom RJ, Melief CJ, Kast WM. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci U S A 1996; 93:7855-7860; PMID:8755566; http://dx.doi.org/ 10.1073/pnas.93.15.7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toes RE, Blom RJ, Offringa R, Kast WM, Melief CJ. Enhanced tumor outgrowth after peptide vaccination. Functional deletion of tumor-specific CTL induced by peptide vaccination can lead to the inability to reject tumors. J Immunol 1996; 156:3911-3918; PMID:8621930; . [PubMed] [Google Scholar]
- 123.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008; 14:169-177; PMID:18172268; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1881 [DOI] [PubMed] [Google Scholar]
- 124.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 2008; 14:178-187; PMID:18172269; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1880 [DOI] [PubMed] [Google Scholar]
- 125.Zeng G, Li Y, El-Gamil M, Sidney J, Sette A, Wang RF, Rosenberg SA, Robbins PF. Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: a new strategy for vaccine design. Cancer Res 2002; 62:3630-3635; PMID:12097265; . [PMC free article] [PubMed] [Google Scholar]
- 126.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 2002; 20:2624-2632; PMID:12039923; http://dx.doi.org/ 10.1200/JCO.2002.06.171 [DOI] [PubMed] [Google Scholar]
- 127.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest 2001; 107:477-484; PMID:11181647; http://dx.doi.org/ 10.1172/JCI11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tomita Y, Yuno A, Tsukamoto H, Senju S, Yoshimura S, Osawa R, Kuroda Y, Hirayama M, Irie A, Hamada A, et al. Identification of CDCA1-derived long peptides bearing both CD4+ and CD8+ T-cell epitopes: CDCA1-specific CD4+ T-cell immunity in cancer patients. Int J Cancer 2014; 134:352-366; PMID:24734272; http://dx.doi.org/ 10.1002/ijc.28376 [DOI] [PubMed] [Google Scholar]
- 129.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-264; PMID:22437870; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-723; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med 2011; 269:64-73; PMID:21158979; http://dx.doi.org/ 10.1111/j.1365-2796.2010.02317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bracci L, Capone I, Moschella F, Proietti E, Belardelli F. Exploiting dendritic cells in the development of cancer vaccines. Expert Rev Vaccines 2013; 12:1195-1210; PMID:24090117; http://dx.doi.org/ 10.1586/14760584.2013.836905 [DOI] [PubMed] [Google Scholar]
- 133.Cools N, Petrizzo A, Smits E, Buonaguro FM, Tornesello ML, Berneman Z, Buonaguro L. Dendritic cells in the pathogenesis and treatment of human diseases: a Janus Bifrons? Immunotherapy 2011; 3:1203-1222; PMID:21995572; http://dx.doi.org/ 10.2217/imt.11.110 [DOI] [PubMed] [Google Scholar]
- 134.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-252; PMID:9521319; http://dx.doi.org/ 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 135.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18:767-811; PMID:10837075; http://dx.doi.org/ 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- 136.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992; 176:1693-1702; PMID:1460426; http://dx.doi.org/ 10.1084/jem.176.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 2005; 5:296-306; PMID:15803149; http://dx.doi.org/ 10.1038/nri1592 [DOI] [PubMed] [Google Scholar]
- 138.Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med 2000; 192:1535-1544; PMID:11104796; http://dx.doi.org/ 10.1084/jem.192.11.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mule JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res 2001; 61:8513-8519; PMID:11731436 [PubMed] [Google Scholar]
- 140.Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother 2006; 29:545-557; PMID:16971810; http://dx.doi.org/ 10.1097/01.cji.0000211309.90621.8b [DOI] [PubMed] [Google Scholar]
- 141.Salcedo M, Bercovici N, Taylor R, Vereecken P, Massicard S, Duriau D, Vernel-Pauillac F, Boyer A, Baron-Bodo V, Mallard E, et al. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother 2006; 55:819-829; PMID:16187085; http://dx.doi.org/ 10.1007/s00262-005-0078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 2001; 61:6451-6458; PMID:11522640 [PubMed] [Google Scholar]
- 143.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate 1996; 29:371-380; PMID:8977634; http://dx.doi.org/ 10.1002/(SICI)1097-0045(199612)29:6%3c371::AID-PROS5%3e3.0.CO;2-B [DOI] [PubMed] [Google Scholar]
- 144.Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med 2002; 195:1279-1288; PMID:12021308; http://dx.doi.org/ 10.1084/jem.20012100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lesterhuis WJ, Schreibelt G, Scharenborg NM, Brouwer HM, Gerritsen MJ, Croockewit S, Coulie PG, Torensma R, Adema GJ, Figdor CG, et al. Wild-type and modified gp100 peptide-pulsed dendritic cell vaccination of advanced melanoma patients can lead to long-term clinical responses independent of the peptide used. Cancer Immunol Immunother 2011; 60:249-260; PMID:21069321; http://dx.doi.org/ 10.1007/s00262-010-0942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4:328-332; PMID:9500607; http://dx.doi.org/ 10.1038/nm0398-328 [DOI] [PubMed] [Google Scholar]
- 147.Romano E, Rossi M, Ratzinger G, de Cos MA, Chung DJ, Panageas KS, Wolchok JD, Houghton AN, Chapman PB, Heller G, et al. Peptide-loaded Langerhans cells, despite increased IL15 secretion and T-cell activation in vitro, elicit antitumor T-cell responses comparable to peptide-loaded monocyte-derived dendritic cells in vivo. Clin Cancer Res 2011; 17:1984-1997; PMID:21355077; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holtl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol 1999; 161:777-782; PMID:10022683; http://dx.doi.org/ 10.1016/S0022-5347(01)61767-1 [DOI] [PubMed] [Google Scholar]
- 149.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {α}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 2011; 29:330-336; PMID:21149657; http://dx.doi.org/ 10.1200/JCO.2010.30.7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 2001; 61:842-847; PMID:11221866 [PubMed] [Google Scholar]
- 151.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med 2010; 363:479-481; PMID:20818868; http://dx.doi.org/ 10.1056/NEJMe1006300 [DOI] [PubMed] [Google Scholar]
- 152.Bonehill A, Van Nuffel AM, Corthals J, Tuyaerts S, Heirman C, Francois V, Colau D, van der BP, Neyns B, Thielemans K. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res 2009; 15:3366-3375; PMID:19417017; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-2982 [DOI] [PubMed] [Google Scholar]
- 153.Knippertz I, Hesse A, Schunder T, Kampgen E, Brenner MK, Schuler G, Steinkasserer A, Nettelbeck DM. Generation of human dendritic cells that simultaneously secrete IL-12 and have migratory capacity by adenoviral gene transfer of hCD40L in combination with IFN-gamma. J Immunother 2009; 32:524-538; PMID:19609245; http://dx.doi.org/ 10.1097/CJI.0b013e3181a28422 [DOI] [PubMed] [Google Scholar]
- 154.Bonehill A, Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, Neyns B, Thielemans K. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther 2008; 16:1170-1180; PMID:18431362; http://dx.doi.org/ 10.1038/mt.2008.77 [DOI] [PubMed] [Google Scholar]
- 155.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 2005; 115:3623-3633; PMID:16308572; http://dx.doi.org/ 10.1172/JCI25947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grunebach F, Kayser K, Weck MM, Muller MR, Appel S, Brossart P. Cotransfection of dendritic cells with RNA coding for HER-2/neu and 4-1BBL increases the induction of tumor antigen specific cytotoxic T lymphocytes. Cancer Gene Ther 2005; 12:749-756; PMID:15877082; http://dx.doi.org/ 10.1038/sj.cgt.7700842 [DOI] [PubMed] [Google Scholar]
- 157.Iinuma H, Okinaga K, Fukushima R, Inaba T, Iwasaki K, Okinaga A, Takahashi I, Kaneko M. Superior protective and therapeutic effects of IL-12 and IL-18 gene-transduced dendritic neuroblastoma fusion cells on liver metastasis of murine neuroblastoma. J Immunol 2006; 176:3461-3469; PMID:16517714; http://dx.doi.org/ 10.4049/jimmunol.176.6.3461 [DOI] [PubMed] [Google Scholar]
- 158.Kang TH, Bae HC, Kim SH, Seo SH, Son SW, Choi EY, Seong SY, Kim TW. Modification of dendritic cells with interferon-gamma-inducible protein-10 gene to enhance vaccine potency. J Gene Med 2009; 11:889-898; PMID:19618483; http://dx.doi.org/ 10.1002/jgm.1371 [DOI] [PubMed] [Google Scholar]
- 159.Minkis K, Kavanagh DG, Alter G, Bogunovic D, O'Neill D, Adams S, Pavlick A, Walker BD, Brockman MA, Gandhi RT, et al. Type 2 Bias of T cells expanded from the blood of melanoma patients switched to type 1 by IL-12p70 mRNA-transfected dendritic cells. Cancer Res 2008; 68:9441-9450; PMID:19010919; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Breckpot K, erts-Toegaert C, Heirman C, Peeters U, Beyaert R, Aerts JL, Thielemans K. Attenuated expression of A20 markedly increases the efficacy of double-stranded RNA-activated dendritic cells as an anti-cancer vaccine. J Immunol 2009; 182:860-870; PMID:19124729; http://dx.doi.org/ 10.4049/jimmunol.182.2.860 [DOI] [PubMed] [Google Scholar]
- 161.Guo C, Yi H, Yu X, Zuo D, Qian J, Yang G, Foster BA, Subjeck JR, Sun X, Mikkelsen RB, et al. In situ vaccination with CD204 gene-silenced dendritic cell, not unmodified dendritic cell, enhances radiation therapy of prostate cancer. Mol Cancer Ther 2012; 11:2331-2341; PMID:22896667; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo C, Yi H, Yu X, Hu F, Zuo D, Subjeck JR, Wang XY. Absence of scavenger receptor A promotes dendritic cell-mediated cross-presentation of cell-associated antigen and antitumor immune response. Immunol Cell Biol 2012; 90:101-108; PMID:21383767; http://dx.doi.org/ 10.1038/icb.2011.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yi H, Guo C, Yu X, Gao P, Qian J, Zuo D, Manjili MH, Fisher PB, Subjeck JR, Wang XY. Targeting the immunoregulator SRA/CD204 potentiates specific dendritic cell vaccine-induced T-cell response and antitumor immunity. Cancer Res 2011; 71:6611-6620; PMID:21914786; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aurisicchio L, Ciliberto G. Genetic cancer vaccines: current status and perspectives. Expert Opin Biol Ther 2012; 12:1043-1058; PMID:22577875; http://dx.doi.org/ 10.1517/14712598.2012.689279 [DOI] [PubMed] [Google Scholar]
- 165.Walsh SR, Dolin R. Vaccinia viruses: vaccines against smallpox and vectors against infectious diseases and tumors. Expert Rev Vaccines 2011; 10:1221-1240; PMID:21854314; http://dx.doi.org/ 10.1586/erv.11.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xiang R, Luo Y, Niethammer AG, Reisfeld RA. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev 2008; 222:117-128; PMID:18363997; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00613.x [DOI] [PubMed] [Google Scholar]
- 167.Liu MA, Ulmer JB. Human clinical trials of plasmid DNA vaccines. Adv Genet 2005; 55:25-40; PMID:16291211; http://dx.doi.org/ 10.1016/S0065-2660(05)55002-8 [DOI] [PubMed] [Google Scholar]
- 168.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer 2008; 8:108-120; PMID:18219306; http://dx.doi.org/ 10.1038/nrc2326 [DOI] [PubMed] [Google Scholar]
- 169.Ribas A, Weber JS, Chmielowski B, Comin-Anduix B, Lu D, Douek M, Ragavendra N, Raman S, Seja E, Rosario D, et al. Intra-lymph node prime-boost vaccination against Melan A and tyrosinase for the treatment of metastatic melanoma: results of a phase 1 clinical trial. Clin Cancer Res 2011; 17:2987-2996; PMID:21385924; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-3272 [DOI] [PubMed] [Google Scholar]
- 170.Weber JS, Vogelzang NJ, Ernstoff MS, Goodman OB, Cranmer LD, Marshall JL, Miles S, Rosario D, Diamond DC, Qiu Z, et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J Immunother 2011; 34:556-567; PMID:21760528; http://dx.doi.org/ 10.1097/CJI.0b013e3182280db1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421-429; PMID:21530212; http://dx.doi.org/ 10.1016/j.coi.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aurisicchio L, Mancini R, Ciliberto G. Cancer vaccination by electro-gene-transfer. Expert Rev Vaccines 2013; 12:1127-1137; PMID:24066796; http://dx.doi.org/ 10.1586/14760584.2013.836903 [DOI] [PubMed] [Google Scholar]
- 173.Kreiter S, Selmi A, Diken M, Koslowski M, Britten CM, Huber C, Tureci O, Sahin U. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res 2010; 70:9031-9040; PMID:21045153; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0699 [DOI] [PubMed] [Google Scholar]
- 174.Ying H, Zaks TZ, Wang RF, Irvine KR, Kammula US, Marincola FM, Leitner WW, Restifo NP. Cancer therapy using a self-replicating RNA vaccine. Nat Med 1999; 5:823-827; PMID:10395329; http://dx.doi.org/ 10.1038/10548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou WZ, Hoon DS, Huang SK, Fujii S, Hashimoto K, Morishita R, Kaneda Y. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum Gene Ther 1999; 10:2719-2724; PMID:10566900; http://dx.doi.org/ 10.1089/10430349950016762 [DOI] [PubMed] [Google Scholar]
- 176.Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, Rammensee HG, Garbe C, Pascolo S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother 2008; 31:180-188; PMID:18481387; http://dx.doi.org/ 10.1097/CJI.0b013e31815ce501 [DOI] [PubMed] [Google Scholar]
- 177.Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I, Rammensee HG, Garbe C. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother 2009; 32:498-507; PMID:19609242; http://dx.doi.org/ 10.1097/CJI.0b013e3181a00068 [DOI] [PubMed] [Google Scholar]
- 178.Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, Horger MS, Maksimovic O, Stenzl A, Hoerr I, et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther 2011; 19:990-999; PMID:21189474; http://dx.doi.org/ 10.1038/mt.2010.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fotin-Mleczek M, Zanzinger K, Heidenreich R, Lorenz C, Thess A, Duchardt KM, Kallen KJ. Highly potent mRNA based cancer vaccines represent an attractive platform for combination therapies supporting an improved therapeutic effect. J Gene Med 2012; 14:428-439; PMID:22262664; http://dx.doi.org/ 10.1002/jgm.2605 [DOI] [PubMed] [Google Scholar]
- 180.Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, Pedicano JE, Gehan E, Peck RA, Arlen P, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol 2000; 18:3964-3973; PMID:11099326 [DOI] [PubMed] [Google Scholar]
- 181.Marshall JL, Hawkins MJ, Tsang KY, Richmond E, Pedicano JE, Zhu MZ, Schlom J. Phase I study in cancer patients of a replication-defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Clin Oncol 1999; 17:332-337; PMID:10458251 [DOI] [PubMed] [Google Scholar]
- 182.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol 2005; 174:5994-6004; PMID:15879092; http://dx.doi.org/ 10.4049/jimmunol.174.10.5994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:1099-1105; PMID:20100959; http://dx.doi.org/ 10.1200/JCO.2009.25.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010; 59:663-674; PMID:19890632; http://dx.doi.org/ 10.1007/s00262-009-0782-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oudard S, Rixe O, Beuselinck B, Linassier C, Banu E, Machiels JP, Baudard M, Ringeisen F, Velu T, Lefrere-Belda MA, et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol Immunother 2011; 60:261-271; PMID:21069322; http://dx.doi.org/ 10.1007/s00262-010-0935-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun D, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol 2011; 12:1125-1133; PMID:22019520; http://dx.doi.org/ 10.1016/S1470-2045(11)70259-5 [DOI] [PubMed] [Google Scholar]
- 187. http:// www.prnewswire.com /news-releases /therion -reports-results-of-phase-3-panvac-vf-trial-and-annou-nces-plans-for-company-sale-56997582.html [Google Scholar]