Abstract
The important biological roles of glycans and their implications in disease development and progression have created a demand for the development of sensitive quantitative glycomics methods. Quantitation of glycans existing at low abundance is still analytically challenging. In this study, an N-linked glycans quantitation method using multiple reaction monitoring (MRM) on a triple quadrupole instrument was developed. Optimum normalized collision energy (CE) for both sialylated and fucosylated N-glycan structures was determined to be 30% while it was found to be 35% for either fucosylated or sialylated structures The optimum CE for mannose and complex type N-glycan structures was determined to be 35%. Additionally, the use of three transitions was shown to facilitate reliable quantitation. A total of 88 N-glycan structures in human blood serum were quantified using this MRM approach. Reliable detection and quantitation of these structures was achieved when the equivalence of 0.005 μL of blood serum was analyzed. Accordingly, N-glycans down to the 100th of a μL level can be reliably quantified in pooled human blood serum, spanning a dynamic concentration range of three orders of magnitudes. MRM was also effectively utilized to quantitatively compare the expression of N-glycans derived from brain-targeting breast carcinoma cells (MDA-MB-231BR) and metastatic breast cancer cells (MDA-MB-231). Thus, the described MRM method of permethylated N-glycan structures enables a rapid and reliable identification and quantitation of glycans derived from glycoproteins purified or present in complex biological samples.
Keywords: Glycans, Permethylation, MRM-LC-MS, LC-MSMS, Quantitation
Introduction
Glycosylation is currently recognized as one of the most common posttranslational modifications (PTMs) of proteins. This PTM plays an important role in biological processes, such as cell recognition, cell-cell interaction, immune-surveillance, and cell division and adhesion [1–6]. Glycosylation changes may reflect the aberrant biological activities which indicate pathological alterations. Thus, glycans and glycoproteins can be utilized as potential therapeutic and diagnosis assets.
Accordingly, qualitative and quantitative glycomic information has been deemed necessary to understand the biological roles of glycans in biological systems. Observed differences could subsequently permit a better understanding of the biological attributes of glycan structures. Mass spectrometry (MS) is currently utilized in glycan quantitation because of its high sensitivity [7–9]. MS based quantitation methods can be classified into two main categories: label free or isotopic labeling approaches [10,11].
The label-free approaches have been widely used in glycan quantitation studies. The N-glycans relative intensities of several runs were compared for quantitation purpose. Several derivatization methods including reductive amination [12–14], permethylation [15–24] and peracetylation [25,26] have been employed to address the weak ionization efficiency of native glycans in MS sources.
Isotopic labeling of glycans is attained through chemical derivatization such as reductive amination [27–33], permethylation [34–36], metabolic incorporation of stable isotope tags [37,38] and introduction of isotope labels via enzymatic reactions [39]. These methods are facilitating the monitoring of glycan variations between different samples in a single mass spectrometric analysis.
The most reliable MS quantitation is selected reaction monitoring (SRM) and multiple reaction monitoring (MRM). These are MS modes with the first quadrupole (Q1) filters specific m/z values of the precursor ion, and the third quadrupole (Q3) filters the corresponding fragments of the precursor (ion transitions), while the second quadrupole (Q2) is a collision cell. Instead of quantifying ions using full MS scan, the quantitation of SRM and MRM is based on ion transitions, which can significantly enhance the signal-to-noise ratios for the detection of low abundant components. SRM is a targeted technique that selects a single transition for simultaneous identification and quantitation, which has been introduced for quantitation of proteins [40], glycopeptides [41] and glycans [42] in complex samples. In MRM mode, multiple transitions were selected thus allowing more precise analytical quantitation compared with SRM. MRM has been used for glycopeptide quantitation [43–47]. The first use of MRM for the analysis of glucose tetrasaccharide was reported in 2003 [48]. Recently another two MRM quantitation studies were published for the analysis of reduced native glycan [49] and 1-phenyl-3-methyl-5-pyrazolone (PMP) labeled glycan [50]. However, until now this method has not been exploited for permethylated N-glycan analysis.
Herein, we describe MRM for quantitation of permethylated N-glycans. The effectiveness and the analytical precision of MRM rely on transitions. Therefore, it is critical to choose representative transitions and optimize collision energy for efficient fragmentation. The optimization of transitions was performed using permethylated N-glycans derived from model glycoproteins, such as ribonuclease B, fetuin and porcine thyroglobulin. The number of transitions, precursor ions selection, and normalized collision energy were optimized to obtain effective and reliable MRM quantitation. Glycomic profiles of complex biological samples, such as human blood serum and cancer cell lines, were also acquired using the described MRM method to evaluate the ability to quantify glycans at low abundances. The linear concentration dynamic range and detection limits of N-glycans derived from model glycoproteins and different biological samples were also investigated.
Experimental
Materials and Reagents
Model glycoproteins, ribonuclease B (RNase B) from bovine pancreas, fetuin from bovine serum, porcine thyroglobulin (PTG), pooled human blood serum (HBS), borane-ammonia complex, sodium hydroxide beads, dimethyl sulfoxide (DMSO), iodomethane, trifluoroacetic acid and formic acid were purchased from Sigma-Aldrich (St. Louis, MO). Cell line MDA-MB-231 was purchased from American Type Culture Collection (ATCC, Manassas, VA), and MDA-MB-231BR was a generous gift from Dr. Paul Lockman (Texas Tech Health Sciences Centers, School of Pharmacy, Amarillo, TX). PNGase F, 10×G7 reaction buffer ((50 mM sodium phosphate buffer, pH 7.5), 10× glycoprotein denaturing buffer ((50 mM sodium phosphate buffer, pH 7.5, 5% SDS, 0.4 M dithiothreitol), and nonionic NP-40 detergent were acquired from New England Biolabs (Ipswich, MA). HPLC grade methanol, isopropanol, and acetic acid were procured from Fisher Scientific (Pittsburgh, PA). Acetonitrile (ACN) was obtained from JT Baker (Phillipsburg, NJ). HPLC grade water and sodium hydroxide were purchased from Mallinckrodt Chemicals (Phillipsburg, NJ).
Release, purification and reduction of N-glycans from model glycoproteins and pooled human blood serum
A 1-μg/μL aliquot of RNase B, and 10 μg/μL stock solutions of fetuin and PTG were prepared. A 25-μg aliquot of RNase B, 100-μg aliquot of fetuin, 100-μg aliquot of PTG and a 50-μL aliquot of HBS were taken from stock solutions. A 20-μL aliquot of phosphate-buffered saline (PBS) (50 mM sodium phosphate, 100 M sodium chloride, pH 7.5) was added to each sample. Samples were then incubated at a 60°C in a water bath (Thermo Scientific, Pittsburgh, PA) for one hour. A 2.4-μL aliquot of PNGase F diluted in 10×G7 reaction buffer was added to each sample. Samples were then incubated overnight at 37°C in a water bath. Next, released glycans were purified to remove salts and impurities using drop-dialysis for 18 hours on a 500–1000 Dalton cut-off molecular weight cellulose ester dialysis membrane (Spectrum Laboratories, Rancho Dominguez, CA). Reduction of N-glycans was achieved by adding a 10-μL aliquot of a 10 μg/μL ammonium-borane complex solution prepared water and. Samples were placed in 60°C water bath for one hour. A 10-μL aliquot of 5% acetic acid aqueous solution was added to each sample and dried under vacuum. Finally, a 300-μL aliquot of HPLC grade methanol was added to each sample and dried under vacuum. This was repeated until no more borate salt was visible.
Cell lysis, protein assay, denaturation, release, purification and reduction of N-glycans from cell lines
Breast cancer cell lines MDA-MB-231 and its brain targeting subline MDA-MB-231BR were initially suspended in 100-μL aliquots of PBS. The suspensions were then sonicated in iced water for 60 minutes. A 5-μL aliquot of the sonicated cells were subjected to BSA protein assay as suggested by the vendor (Fisher Scientific, Pittsburgh, PA) to determine protein concentrations. Next, the proteomes of lysates were denatured in 80°C water bath (Thermo Scientific, Pittsburgh, PA) for one hour. These samples were then cooled to room temperature prior to the addition of a 2.4-μL aliquot of a 10 times diluted PNGase F solution (50 U, New England Biolabs). Enzymatic digestion was allowed to proceed at 37°C in a water bath for 18 hours. Glycans released from the proteome of cell-line samples were purified by charcoal spin-column as previously described [51–53]. The columns were washed with a 400-μL aliquot of 100% ACN and a 400-μL aliquot of 85% ACN with 0.1% TFA 3-times. The spin-column was then conditioned with a 400-μL aliquot of 5% ACN with 0.1% TFA twice. Next, sample volumes were adjusted to 400μL using 5% ACN with 0.1% TFA and washed 4-times with a 400-μL aliquot of 5% ACN with 0.1% TFA. Glycans were then eluted using a 400-μL aliquot of 40% ACN with 0.1% TFA. Samples were dried under vacuum (Labconco, Kansas City, MO). Finally, glycans were reduced using borane-ammonium complex as described above.
Solid-phase permethylation of N-glycans released from model glycoproteins, pooled human blood serum, and cell lines
The N-glycans derived from model glycoproteins, human blood serum samples and cell lines were derivatized by solid-phase permethylation as previously described [17,18,54]. Briefly, spin columns (Harvard Apparatus, Holliston, MA) were filled to a 3-cm height with NaOH beads. The columns were then placed in eppendorf tubes prior to the addition of a 200-μL aliquot of DMSO. The samples were then centrifuged at 1.6k rpm. This was repeated twice.
Released N-glycans from RNase B, fetuin, PTG, and HBS were resuspended in 30 μL high purity DMSO while MDA-MB-231BR cell line and MDA-MB-231 cell line were resuspended in 15 μL high purity DMSO. A 1.2-μL aliquot of water was added to all samples and mixed prior to the addition of 20 μL methyl iodide. The conditioned spin columns were transferred to new 2 mL eppendorf tubes, and the sample mixture was applied to the columns and allowed to sit for 25 minutes then centrifuged at 1.6k rpm for 1 minute. Followed by another 15 minutes incubation after the addition of a second aliquot of 20-μL methyl iodide and the columns were centrifuged again at 1.6k rpm for 1 minute. Permethylated N-glycans were eluted using a 50-μL aliquot of ACN twice. Then the samples were dried under vacuum for online purification. The samples were resuspended in 20% aqueous ACN solution to obtain a series of dilutions from 1 ng/μL to 1000 ng/μL for each model glycoproteins, and a dilution ranged from 0.005 μg/μL to 0.5 μg/μL for blood serum. The final concentration of MDA-MB-231BR cell line and MDA-MB-231 after normalization was 0.47 μg of protein/μL of solution.
LC Separation Conditions
Reduced and permethylated glycans were separated by nano-LC system (Ultimate 3000, Dionex, Sunnyvale, CA) on a reverse phase Acclaim® PepMap capillary column (150 mm × 75 μm i.d) packed with 100 Å C18 bounded phase (Dionex). The mobile phases composed of solvent A (98% HPLC-grade water, 2% ACN, 0.1% FA) and solvent B (98% ACN, 2% HPLC-grade water 0.1% FA). Prior to injection into the column, the samples were trapped on an Acclaim® PepMap trapping column and washed for 10 min at a flow rate of 3 μL/min using solvent A. The separation of reduced and permethylated glycans was attained with a gradient of solvent B increasing from 20% to 38% over 11 min, from 38% to 45% over the next 22 min, then a fast ramp to 90% B in 3 min, stay at 90% for 4 min, then decreased from 90% to 20% in 1 min, and held at 20% B for the next 9 min. The separation was achieved at a 350-nL/min flow rate.
MS Data Acquisition
Thermo Scientific™ TSQ Vantage™ triple quadrupole mass spectrometer was employed in this study. Data dependent acquisition mode (DDA) with two scan events was performed in positive ion mode. The first event is a full MS scan at range of 300–1500 m/z in Q3 with a scan time of 0.7 s and peak width of 0.7 FWHM. Data dependent scan occurs for the second scan event where the five most intense ions from scan event one were subsequently selected and subjected to MS/MS. Precursor ion scan (PIS) has six scan events with each of them contains a product mass. The peak width was set to 0.7. MRM experiment was performed with a peak width of 0.7 FWHM, and scan time of 0.7 s for 500–1500 m/z scan range. The normalized CE values were ranged from 30%–45% in order to determine the most effective condition. Peak areas were determined by using Xcalibur software (Thermo Scientific, Pittsburgh, PA, USA).
Data evaluation
Extracted-ion chromatograms from the full MS scans were generated using Xcalibur Qual Browser (Thermo Fisher Scientific). Tandem mass spectra corresponding to glycan ions were manually annotated. The three most intense diagnostic ions assigned from MSMS spectrum were used as transitions. The reconstructed precursor ions from PIS were utilized to confirm diagnostic fragment ions (transitions). In MRM mode, three transitions were summed for each precursor ion to generate extracted ion chromatogram in Xcalibur Qual Browser. Peak areas were used for glycan quantitation.
Results and Discussion
The identification and quantitation of permethylated N-Glycans workflow are summarized in Figure 1. Data dependent acquisition (DDA) mode, which selects several ions of interest for subsequent MS/MS analysis was first used to obtain structural information of glycans within each sample: RNaseB, Fetuin, PTG, HBS, and cell lines (Figure 1a). The full MS scan under the retention time of 20 min was shown on Figure 1b. The MSMS scan (Figure 1c) provides the fragmentation information of the precursor ions. Three most intense fragments were selected as transitions. Precursor ion scan (PIS) (Figure 1d) was further applied to confirm the selected transitions are a true representation of the precursor ions. For example, the selected product ion at 344 m/z associated the recursor ion at an m/z value of 929.5 confirms that it is a fragment of the biantennary diasialylated glycan. The transitions that are confirmed through PIS were then used in MRM quantitation (Figure 1e).
Figure 1.
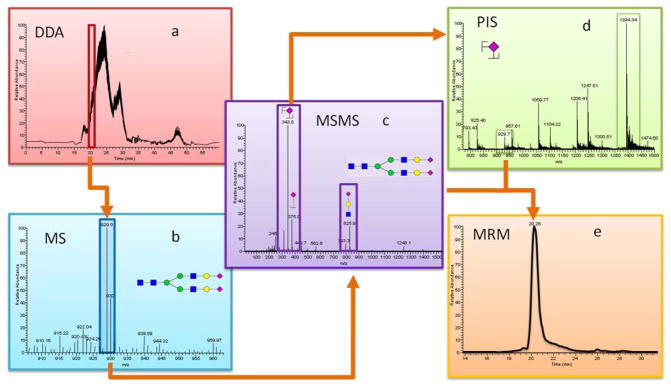
Workflow summarizing the analysis of permethylated N-glycans derived from RNase B, Fetuin, PTG, and pooled human blood serum on triple quadrupole mass spectrometer (a) DDA chromatogram, (b) precursor ion MS scan, (c) MSMS scan for selected precursor ion (PIS), (d) selected transitions are applied to PIS, (e) selected precursor ions and transitions are applied to MRM.
Selecting adequate ion transitions for each glycan structure is a critical component of this methodology. A series of tests were performed using one, two, or three transitions to determine the optimized transitions number. As shown in Figure S1, the intensity of N-glycans derived from RNase B increased with the increasing of the transition number. The optimal amount of transitions for quantitation was three, as shown by the highest intensity obtained for all analytes. Similar results were reported for the MRM quantitation of glycopeptides [47]. Also, increasing characteristic fragment for a specific glycan structure allows reducing the interference from other N-glycans and increase the selectivity of the method.
Representative transitions selection is another importance component of MRM quantitation. The N-glycan structures were divided into high mannose structure, complex structure hybrid structure (Table S1, supporting information). For each group, there are several characteristic fragments. For example, hexose fragments are commonly observed in the case of high mannose structure at m/z values of 110(0,2X), 230 (BZ0,2X), 187(BZ), 262(B0,2X) and 294 (C2,5 X). The ion transitions selected for complex structures with and without fucose were 260 (HexNac), 228(HexNAc-CH3OH) and 196 (HexNAc-2CH3OH). With the increase of molecular weight, more Hex-HexNAc (m/z=432 and 460) fragments are likely to be formed. In the case of sialic acid glycans, characteristic fragment ions containing sialic acid were observed at m/z values of 344, 376 and 825. Similar fragments pattern was also reported in previous studies involving permethylated N-glycans [55–58]. For glycans that have fucose and sialic acid, the sialic acid characteristic ions at m/z values of 312, 344 and 376 were applied as transitions. The fact that the presence of fucose does not affect the transition selection might be because the fucosyl linkage is much weaker than the other glycosidic linkages. Thus, the diagnostic ions with fucosylation are usually not observed [58,59]. The transitions of hybrid N-glycans also depend on the subtype of the structure. For example, hybrid N-glycans with sialic acid are likely to form fragment ions at m/z values of 344, 376 and 825 while the transitions of hybrid N-glycans without sialic acid are similar to those of complex N-glycans.
Precursor ion selection is another important factor for MRM quantitation. Glycans are likely to form multiple charge state ions and adducts. Also, the intensity of most abundance precursor ion was representative of the intensities of all adducts [60]. It is critical to choose the most intense ion adducts as precursor ions. For example, the high mannose glycans are more likely to form ammonium adducts instead of protonated adducts. The distribution of high mannose composition derived from RNase B will not be representative of the native form if the protonated ions were used as precursor ions. Therefore, defining the most intense ions as precursor ions is required.
Optimum CE for each structure was obtained by performing through a series of different normalized CE values spanning from 30% to 45%. At a CE value of 30%, the majority of N-glycans except complex glycans with fucose and sialic acid did not attain complete fragmentation and depicted low intensities. The optimal CE value was determined to be 35% for mannose, complex type and hybrid type glycans (Figure S2, supporting information). The phenomena observed for the fucosylated and sialylated glycans can be attributed to the weak glycosidic linkages. At a CE value of 45%, ion fragments were further fragmented which resulted in a loss of fragment integrity and caused an overall decreased in intensity.
N-glycans derived from model glycoproteins was subjected to triple quadrupole MS using MRM mode to evaluate the quantitation possibility. Different concentrations of permethylated RNase B glycans were obtained from a serial dilution. As the injection amount of reduced and permethylated RNase B reached as low as one ng, all five peaks associated with RNase B were detected. The sensitivity of RNase B is higher than our previous reported work of permethylated N-glycan analyzed by the data dependant mode of the Orbitrap mass spectometer [61]. The high sensitivity of this method might be attributed to the two stage mass filter of MRM, which decrease the chemical noise [62]. In another study, Zhang et al. [42] used LC-SRM with aniline labeled RNase B glycans and detected all 5 peaks at 50 μg. The sensitivity attained in the case of permethylated glycans is substantially higher than that described for aniline labeled N-glycans derived from RNase B. This might due to permethylation, which is known to enhance ionization efficiency of glycans as a result of increased hydrophobicity originating from the introduction of methyl groups. Moreover, increased sensitivity in our strategy can also be attributed to MRM methodology using representative fragment ions as transitions for a certain type of N-glycan. Table S2 depicts the linear dynamic range of N-glycans derived from RNase B. The R2 of five structures is higher than 0.99 at the range of one ng to 100 ng. The instrument ion detectors were saturated when the injection amount of Man 5 and Man 6 derived from RNase B exceeded 100 ng.
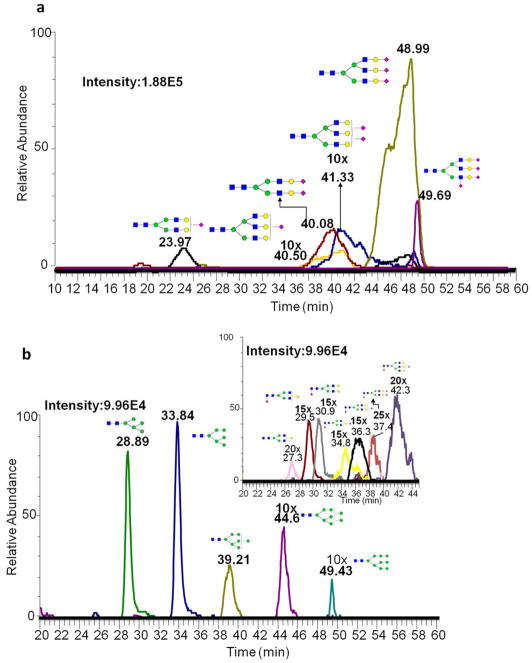
A serial dilution of N-glycans derived from fetuin and PTG glycoproteins were also used to investigate the quantitation ability of MRM. The linear dynamic range of fetuin was from 15 ng to 100 ng with an R2 value better than 0.95 (Table S2a). Six N-glycans derived from fetuin were all detected at sample injection amount as low as 15 ng of the glycoprotein (Figure 2a).
Figure 2.
Extracted ion chromatograms of permethylated N-glycans derived from (a) 15 ng fetuin (b) 25 ng PTG
Similar trend was also observed for permethylated N-glycans derived from PTG. Figure 2b depicts the detected N-glycans derived from PTG at an injection amount of 25 ng of the glycoprotein. The correlation coefficients R2 were better than 0.95 at a linear dynamic concentration range of 25 ng to 500 ng. A wide linear dynamic range for N-glycan quantitation in MRM allows the reliable analysis of disease and disease free biological samples, in which the intensities of certain glycan structures could vary within an order of magnitude.
The developed method was further validated using complex biological samples, such as HBS. Five different concentrations of HBS glycans were analyzed by MS. There are 88 N-glycans detected at an injection equivalent to permethylated glycans derived from a 0.1-μL aliquot of HBS. Even at the injection amount of 0.005 μL HBS, 55 glycan structures were detected (Table S1, supporting information). Previously, only 73 permethylated N-glycans were observed using LTQ Orbitrap velos mass spectometer [63]. The high sensitivity and a larger concentration dynamic range observed might be due to the two-stage mass filtering [62]. The MRM mode platform in a triple quadrupole MS provides targeted analysis that significantly decreases the chemical noise that is commonly associated with mass spectrometric detection. In another study, the MRM mode enables the detection of 42 aniline labeled N-glycans derived from rabbit serum [42]. The higher number of detected N-glycans in this study might be due to the different species used and higher sensitivity prompted by permethylation.
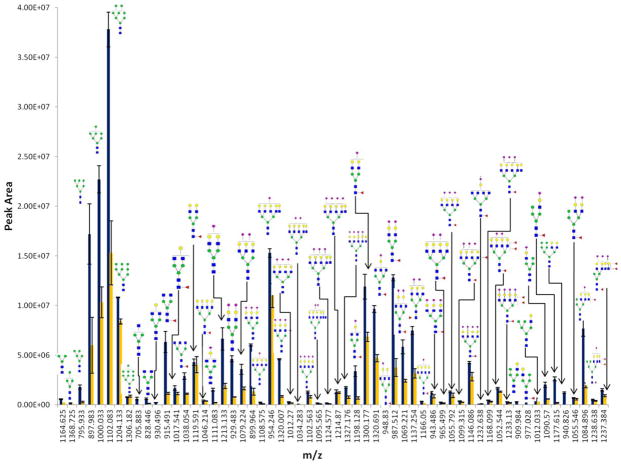
The aforementioned targeted methodology permits highly sensitive identification and quantitation of glycans from glycoproteins and HBS. This method is further applied to profiling N-glycan derived from two different cancer cells lines, MDA-MB-231 and MDA-MB-231BR. MDA-MB-231 is a breast cancer cell line capable of metastasizing anywhere, and MDA-MB-231BR is the brain seeking clone of MDA-MB-231. Since the mechanism of cancer cells crossing the blood-brain-barrier (BBB) is still not completely understood, establishing glycomic profiles of cancer cells could be of significant importance to better understand the role they play in breast cancer cell ability to cross BBB. The comprehensive transition list and optimized CE was applied in the quantitation of permethylated glycans derived from the glycoproteins associated with cell lines. The relative intensities of glycans derived from MDA-MB-231 and MDA-MB-231BR are shown in Figure 3. In total, 58 N-glycans were detected from MDA-MB-231BR and 53 N-glycans were detected for the MDA-MB-231 (Figure 3, Table S4, supporting information). The number of N-glycans detected using MRM is slightly higher compared to that attained using the LTQ Orbitrap Velos mass spectrometer(55 N-glycans were detected for MDA-MB-231BR and 50 N-glycans composition were observed from MDA-MB-231, data not shown). Thus, MRM provides greater quantitative information for the lower abundant N-glycans compared to the LTQ Orbitrap Velos. The average %CV of peak areas of N-glycans derived from MDA-MB-231BR and MDA-MB-231 was 10.3% and 17%, respectively. The low %CV indicates the reproducibility of this quantitation strategy. The average %CV of ESI is around 20%. The overall low %CV indicates high reproducibility of this strategy for N-glycan quantitation.
Figure 3.
Comparison of the extracted ion chromatograms of permethylated N-glycans derived from MDA-MB-231BR cell line (blue) MDA-MB-231 cell line (yellow) through MRM LC-MS.
Conclusions
The aforementioned method has been shown to be a viable strategy for quantitation of N-linked glycans in simple sand complex mixtures. The optimized CE conditions for MRM mode were set for at 30% for mannose and complex type, 35% for sialylated and fucosylated N-glycans. In addition, three high intensity and characteristic ions were chosen. These conditions were first validated using N-glycans derived from model glycoproteins then were applied to HBS and then the cell lines. Utilizing this newly developed method, linear dynamic range expanding over three orders of magnitude with high reproducibility was established. This approach allows for greater sensitivity and reproducibility in complex biological samples, including HBS and cell lines. A comprehensive list of permethylated glycans and their optimum ion transitions are compiled in this study that could be used in future studies to quantify glycomic changes associated with perturbed biological systems.
Supplementary Material
Acknowledgments
This work was fund by CPRIT-RP130624 and NIH-GM (1R01GM093322-01). We also express thanks to Dr. Paul Lockman and Dr. Quentin Smith from TTU/HSC, School of Pharmacy in Amarillo for providing cell line MDA-MB-231BR.
Abbreviations
- CID
Collision induced dissociation
- DDA
data dependent acquisition
- ESI
electrospray ionization
- nHPLC
nano-high performance liquid chromography
- MS
mass spectrometry
- MRM
multiple reaction monitoring
- PIS
precursor ion scan
References
- 1.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwek RA. Glycobiology—toward understanding the function of sugars. Chemical Reviews. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 3.Rudd PM, Wormald MR, Stanfield RL, Huang M, Mattson N, Speir JA, DiGennaro JA, Fetrow JS, Dwek RA, Wilson IA. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J Mol Biol. 1999;293:351–366. doi: 10.1006/jmbi.1999.3104. [DOI] [PubMed] [Google Scholar]
- 4.Dennis JW, Granovsky M, Warren CE. Protein glycosylation in development and disease. BioEssays. 1999;21:412–421. doi: 10.1002/(SICI)1521-1878(199905)21:5<412::AID-BIES8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Lowe JW, Marth M. A genetic approach to Mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 6.Rudd PM, Woods RJ, Wormald MR, Opdenakker G, Downing AK, Campbell ID, Dwek RA. The effects of variable glycosylation on the functional activities of ribonuclease, plasminogen and tissue plasminogen activator. Biochim Biophys Acta. 1995;1248:1–10. doi: 10.1016/0167-4838(94)00230-e. [DOI] [PubMed] [Google Scholar]
- 7.Dell A, Morris HR. Glycoprotein Structure Determination by Mass Spectrometry. Science. 2001;291:2351–2356. doi: 10.1126/science.1058890. [DOI] [PubMed] [Google Scholar]
- 8.Geiser H, Silvescu C, Reinhold V. Separation Methods. Proteomics. 2006:321–343. [Google Scholar]
- 9.Mechref Y, Novotny M. Structural investigations of glycoconjugates at high sensitivity. Chem Rev. 2002;102:321–369. doi: 10.1021/cr0103017. [DOI] [PubMed] [Google Scholar]
- 10.Ruhaak LR, Zauner G, Huhn C, Bruggink C, Deelder AM, Wuhrer M. Glycan labeling strategies and their use in identification and quantification. Anal Bioanal Chem. 2010:397. doi: 10.1007/s00216-010-3532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mechref Y, Hu Y, Desantos-Garcia JL, Hussein A, Tang H. Quantitative Glycomic Strategies. Mol Cell Proteomics. 2012;12:874–884. doi: 10.1074/mcp.R112.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruhaak LR, Huhn C, Waterreus WJ, de Boeer AR, Neususs C, Hokke CH, Deelder AM, Wuhrer M. Hydrophilic interaction chromatography-based high-throughput sample preparation method for N-glycan analysis from total human plasma glycoproteins. Anal Chem. 2008;80:6119–6126. doi: 10.1021/ac800630x. [DOI] [PubMed] [Google Scholar]
- 13.Gil GC, IIif B, Cerny R, Velander WH, Van Cott KE. High throughput quantification of N-glycans using one-pot sialic acid modification and matrix assisted laser desorption ionization time-of-flight mass spectrometry. Anal Chem. 2010;82:6613–6620. doi: 10.1021/ac1011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klapoetke S, Zhang J, Becht S, Xuelin G, Ding X. The evaluation of a novel approach for the profiling and identification of N-linked glycan with a procainamide tag by HPLC with fluorescent and mass spectrometric detection. J Pharm Biomed Anal. 2010;53:315–324. doi: 10.1016/j.jpba.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 15.Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1986;131:209–217. [Google Scholar]
- 16.Ciucanu I, Costello CE. Elimination of oxidative degradation during per-O-methylation of carbohydrates. J Am Chem Soc. 2003;125:16213–16219. doi: 10.1021/ja035660t. [DOI] [PubMed] [Google Scholar]
- 17.Kang P, Mechref Y, Klouckova I, Novotny MV. Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun Mass Spectrom. 2005;19:3421–3428. doi: 10.1002/rcm.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang P, Mechref Y, Novotny MV. High-throughput solid-phase permethylation of glycans prior to mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:721–734. doi: 10.1002/rcm.3395. [DOI] [PubMed] [Google Scholar]
- 19.Kyselova Z, Mechref Y, Al Bataineh MM, Dobrolecki LE, Hickey RJ, Vinson J, Sweeney CJ, Novotny MV. Alterations in the serum glycome due to metastatic prostate cancer. J Proteome Res. 2007;6:1822–1832. doi: 10.1021/pr060664t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman R, Ressom H, Varghese R, Goldman L, Bascug G, Loffredo C, Abdel-Hamid M, Gouda I, Abo-Elkhir S, Kyselova Z, Mechref Y, Novotny MV. Detection of hepatocellular carcinoma using glycomic analysis. Clin Cancer Res. 2009:15. doi: 10.1158/1078-0432.CCR-07-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyselova Z, Mechref Y, Kang P, Goetz JA, Dobrolecki LE, Sledge G, Schnaper L, Hickey RJ, Malkas LH, Novotny MV. Breast cancer diagnosis/prognosis through quantitative measurements of serum glycan profiles. Clin Chem. 2008;54:1166–1175. doi: 10.1373/clinchem.2007.087148. [DOI] [PubMed] [Google Scholar]
- 22.Mechref Y, Hussein A, Bekesova S, Pungpapong V, Zhang M, Dobrolecki LE, Hickey RJ, Hammoud ZT, Novotny MV. Quantitative serum glycomics of esophageal adenocarcinoma, and other esophageal disease onsets. J Protoeme Res. 2009;8:2656–2666. doi: 10.1021/pr8008385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alley WRJ, Vasseur JA, Goetz JA, Svoboda M, Mann BF, Matei DE, Menning N, Hussein A, Mechref Y, Novotny MV. N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients. J Proteome Res. 2012;11:2282–2300. doi: 10.1021/pr201070k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong HJ, Kim YG, Yang YH, Kim BG. High-throughput quantitative analysis of total N-glycans by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem. 2012;84:3453–3460. doi: 10.1021/ac203440c. [DOI] [PubMed] [Google Scholar]
- 25.Dell A. Preparation and desorption mass spectrometry of permethyl and peracetyl derivatives of oligosaccharides. Methods Enzymol. 1990;193:647–660. doi: 10.1016/0076-6879(90)93443-o. [DOI] [PubMed] [Google Scholar]
- 26.Mechref Y, Novotny MV. Structural Characterization of Oligosaccharides Using Maldi-TOF/TOF Tandem Mass Spectrometry. Anal Chem. 1998;70:455–463. doi: 10.1021/ac0341968. [DOI] [PubMed] [Google Scholar]
- 27.Xia B, Feasley CL, Sachdev GP, Smith DF, Cummings RD. Glycan reductive isotope labeling for quantitative glycomics. Anal Biochem. 2009;387:162–170. doi: 10.1016/j.ab.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowman MJ, Zaia J. Tags for the Stable Isotopic Labeling of Carbohydrates and Quantitative Analysis by Mass Spectrometry. Anal Chem. 2007;79:5777–5784. doi: 10.1021/ac070581b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowman MJ, Zaia J. Comparative Glycomics Using a Tetraplex Stable-Isotope Coded Tag. Anal Chem. 2010;82:3023–3031. doi: 10.1021/ac100108w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hitchcock AM, Costello CE, JZ Glycoform quantification of chondroitin/dermatan sulfate using an LC/MS/MS platform. Biochemistry. 2006;45:2350–2361. doi: 10.1021/bi052100t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hitchcock AM, Yates KE, Shortkroff S, Costello CE, Zaia J. Optimized extraction of glycosaminoglycans from normal and osteoarthritic cartilage for glycomics profiling. Glycobiology. 2006;17:25–35. doi: 10.1093/glycob/cwl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prien JM, Prater BD, Qin Q, Corckrill SL. Mass Spectrometric-Based Stable Isotopic 2-Aminobenzoic Acid Glycan Mapping for Rapid Glycan Screening of Biotherapeutics. Anal Chem. 2010;82:1498–1508. doi: 10.1021/ac902617t. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence R, Olson SK, Steele RE, Wang L, Warrior R, Cummings RD, Esko JD. Evolutionary Differences in Glycosaminoglycan Fine Structure Detected by Quantitative Glycan Reductive Isotope Labeling. J Biol Chem. 2008;283:33674–33684. doi: 10.1074/jbc.M804288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atwood JA, III, Cheng L, Alvarez-Manilla G, Warren NL, York WS, Orlando R. Quantitation by Isobaric Labeling: Applications to Glycomics. J Proteome Res. 2008;7:367–374. doi: 10.1021/pr070476i. [DOI] [PubMed] [Google Scholar]
- 35.Kang P, Mechref Y, Kyselova Z, Goetz JA, Novotny MV. Comparative Glycomic Mapping through Quantitative Permethylation and Stable-Isotope Labeling. Anal Chem. 2007;79:6064–6073. doi: 10.1021/ac062098r. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Desantos-Garcia JL, Mechref Y. Comparative glycomic profiling of isotopically permethylated N-glycans by LC-ESI-MS. Rapid Commun Mass Spectrom. 2013;27:865–877. doi: 10.1002/rcm.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orlando R, Lim JM, Atwood JA, III, Angel PM, Fang M, Aoki K, Alvarez-Manilla G, Moremen KW, York WS, Tiemeyer M, Pierce M, Dalton S, Wells L. IDAWG: Metabolic Incorporation of Stable Isotope Labels for Quantitative Glycomics of Cultured Cells. J Proteome Res. 2009;8:3816–3823. doi: 10.1021/pr8010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breidenbach MA, Gallagher JEG, King DS, Smart BP, Wu P, Bertozzi CR. Targeted metabolic labeling of yeast N-glycans with unnatural sugars. Proc Natl Acad Sci USA. 2010;107:3988–3993. doi: 10.1073/pnas.0911247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Wang H, Tang H, Yang P. Endoglycosidase-Mediated Incorporation of 18O into Glycans for Relative Glycan Quantitation. Anal Chem. 2011;83:4975–4981. doi: 10.1021/ac200753e. [DOI] [PubMed] [Google Scholar]
- 40.Kuzyk MA, Smith D, Yang J, Cross TJ, Jackson AM, Hardie DB, Anderson NL, Borchers CH. Multiple Reaction Monitoring-based, Multiplexed, Absolute Quantitation of 45 Proteins in Human Plasma. Mol Cell Proteomics. 2009;8:1860–1877. doi: 10.1074/mcp.M800540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YJ, Zaidi-Ainouch Z, Gallien S, BD Mass spectrometry–based detection and quantification of plasma glycoproteins using selective reaction monitoring. Nat Protoc. 2012;7:859–871. doi: 10.1038/nprot.2012.023. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Wang Z, Stupak J, Ghribi O, Geiger JD, Liu QY, Li J. Targeted glycomics by selected reaction monitoring for highly sensitive glycan compositional analysis. Proteomics. 2012;12:2510–2522. doi: 10.1002/pmic.201100567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gil GC, Velander WH, Cott KEV. N-glycosylation microheterogeneity and site occupancy of an Asn-X-Cys sequon in plasma-derived and recombinant Protein C. proteomics. 2009;9:2555–2567. doi: 10.1002/pmic.200800775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn YH, Lee JY, Lee JY, Kim Y-S, Ko JH, Yoo JS. Quantitative Analysis of an Aberrant Glycoform of TIMP1 from Colon Cancer Serum by L-PHA-Enrichment and SISCAPA with MRM Mass Spectrometry. J Proteome Res. 2009;8 doi: 10.1021/pr900269s. [DOI] [PubMed] [Google Scholar]
- 45.Kurogochi M, Matsushista T, Amano M, Furukawa J-i, Shinohara Y, Aoshima M, Nishimura S-I. Sialic Acid-focused Quantitative Mouse Serum Glycoproteomics by Multiple Reaction Monitoring Assay. Mol Cell Proteomics. 2010;9:2354–2368. doi: 10.1074/mcp.M110.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Wei J, Wang J, Ying W, Zhang Y, XQ Fragmentation and Site-Specific Quantification of Core Fucosylated Glycoprotein by Multiple Reaction Monitoring-Mass Spectrometry. Anal Chem. 2011;22:8802–8809. doi: 10.1021/ac201676a. [DOI] [PubMed] [Google Scholar]
- 47.Song E, Pyreddy S, Mechref Y. Quantification of glycopeptides by multiple reaction monitoring liquid chromatography/tandem mass spectrometry. Anal Chem. 2012;26:1941–1954. doi: 10.1002/rcm.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young SP, Stevens RD, An Y, Chen YT, Millington DS. Analysis of a glucose tetrasaccharide elevated in Pompe disease by stable isotope dilution-electrospray ionization tandem mass spectrometry. Analytical Biochemistry. 2003;316:175–180. doi: 10.1016/s0003-2697(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 49.Hong QT, Ruhaak LR, Totten SM, Smilowitz JT, German JB, Lebrilla CB. Label-Free Absolute Quantitation of Oligosaccharides Using Multiple Reaction Monitoring. Analytical Chemistry. 2014;86:2640–2647. doi: 10.1021/ac404006z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sowell J, Wood T. Towards a selected reaction monitoring mass spectrometry fingerprint approach for the screening of oligosaccharidoses. Analytica Chimica Acta. 2011;686:102–106. doi: 10.1016/j.aca.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isailovic D, Kurulugama RT, Plasencia MD, Stokes ST, Kyselova Z, Goldman R, Mechref Y, Novotny MV, Clemmer DE. Profiling of Human Serum Glycans Associated with Liver Cancer and Cirrhosis by IMS–MS. J Proteome Res. 2008;7:1109–1117. doi: 10.1021/pr700702r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyselova Z, Mechref Y, Al Bataineh MM, Dobrolecki LE, Hickey RJ, Vinson J, Sweeney CJ, Novotny MV. Alterations in the Serum Glycome Due to Metastatic Prostate Cancer. J Proteome Res. 2007;6:1822–1832. doi: 10.1021/pr060664t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mechref Y, Hussein A, Bekesova S, Pungpapong V, Zhang M, Dobrolecki LE, Hickey RJ, Hammoud ZT, Novotny MV. Quantitative serum glycomics of esophageal adenocarcinoma and other esophageal disease onsets. J Proteome Res. 2009;8:2656–2666. doi: 10.1021/pr8008385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mechref Y, Kang P, Novotny MV. Solid-phase permethylation for glycomic analysis. Methods in Molecular Biology. 2009;534:53–64. doi: 10.1007/978-1-59745-022-5_4. [DOI] [PubMed] [Google Scholar]
- 55.Viseux N, de Hoffmann E, Domon B. Structural Analysis of Permethylated Oligosaccharides by Electrospray Tandem Mass Spectrometry. Anal Chem. 1997;69:3193–3198. doi: 10.1021/ac961285u. [DOI] [PubMed] [Google Scholar]
- 56.Mechref Y, Kang P, Novotny MV. Differentiating structural isomers of sialylated glycans by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:1381–1389. doi: 10.1002/rcm.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costello CE, Contado-Miller JM, Cipollo JF. A Glycomics Platform for the Analysis of Permethylated Oligosaccharide Alditols. J Am Chem Soc. 2007;18:1799–1812. doi: 10.1016/j.jasms.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaia J. Mass Spectrometry and the Emerging Field of Glycomics. Chem Biol. 2008;15:881–892. doi: 10.1016/j.chembiol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu SY, Wu SW, Khoo KH. Distinctive characteristics of MALDI-Q/TOF and TOF/TOF tandem mass spectrometry for sequencing of permethylated complex type N-glycans. Glycoconj J. 2006;23:355–369. doi: 10.1007/s10719-006-8492-3. [DOI] [PubMed] [Google Scholar]
- 60.Hu Y, Mechref Y. Comparing MALDI-MS, RP-LC-MALDI-MS and RP-LC-ESI-MS glycomic profiles of permethylated N-glycans derived from model glycoproteins and human blood serum. Electrophoresis. 2012;33:1768–1777. doi: 10.1002/elps.201100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu Y, Zhou S, Khalil SI, Renteria CL, Mechref Y. Glycomic Profiling of Tissue Sections by LC-MS. Anal Chem. 2013 doi: 10.1021/ac400106x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Picotti P, Rinner O, Stallmach R, Dautel F, Farrah T, Domon B, Wenschuh H, Aebersold R. High-throughput generation of selected reaction-monitoring assays for proteins and proteomes. Nat Methods. 2010;7:43–46. doi: 10.1038/nmeth.1408. [DOI] [PubMed] [Google Scholar]
- 63.DeSantos-Garcia J, LK SI, Hussein A, Mechref Y. Enhanced sensitivity of LC-MS analysis of permethylated N-glycans through online purification. Electrophoresis. 2011:32. doi: 10.1002/elps.201100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.