Abstract
High strain sequence variability, interference with innate immune mechanisms, and epitope deletion are all examples of strategies that pathogens have evolved to subvert host defenses. To this list we would add another strategy: immune camouflage. Pathogens whose epitope sequences are cross-conserved with multiple human proteins at the TCR-facing residues may be exploiting “ignorance and tolerance," which are mechanisms by which mature T cells avoid immune responses to self-antigens. By adopting amino acid configurations that may be recognized by autologous regulatory T cells, pathogens may be actively suppressing protective immunity. Using the new JanusMatrix TCR-homology-mapping tool, we have identified several such ‘camouflaged’ tolerizing epitopes that are present in the viral genomes of pathogens such as emerging H7N9 influenza. Thus in addition to the overall low number of T helper epitopes that is present in H7 hemaglutinin (as described previously, see http://dx.doi.org/10.4161/hv.24939), the presence of such tolerizing epitopes in H7N9 could explain why, in recent vaccine trials, whole H7N9-HA was poorly immunogenic and associated with low seroconversion rates (see http://dx.doi.org/10.4161/hv.28135). In this commentary, we provide an overview of the immunoinformatics process leading to the discovery of tolerizing epitopes in pathogen genomic sequences, provide a brief summary of laboratory data that validates the discovery, and point the way forward. Removal of viral, bacterial and parasite tolerizing epitopes may permit researchers to develop more effective vaccines and immunotherapeutics in the future.
Keywords: Biologic, Deimmunization, EpiMatrix, JanusMatrix, Treg, Tolerance, Tregitope, Vaccine
Abbreviations
- HA
hemagglutinin
- HLA
human leukocyte antigen
- HCV
Hepatitis C virus
- HIV
human immunodeficiency virus
- IAVs
influenza A viruses
- nTreg
natural regulatory T cells
- TCR
T cell receptor
- Td response
T cell-driven response
- Treg
regulatory T cell
- Tregitope
Treg epitope
Learning from Pathogens how to Make Better Vaccines and Biologics
Just as humans have evolved immune mechanisms to combat infection, viruses, bacteria, and parasites have found ways to fight back against human defenses. Immune evasion strategies contribute to pathogen persistence at the population and individual level, making it difficult to develop effective vaccines. Examples of pathogens for which effective vaccines are lacking include herpes simplex virus (HSV), human immuno-deficiency virus (HIV), respiratory syncytial virus (RSV), and cytomegalovirus (CMV) among viruses; M. tuberculosis, H. pylori, and S. aureus among bacteria; and Leishmania, Trypanosoma, and Filaria species among parasites. It appears that the emerging H7N9 influenza virus is one of several viruses that have learned to evade host defenses. Vaccines developed against H7N9 have also proven to be poorly immunogenic, as compared to those developed for the most recent pandemic (H1N1), perhaps due to immune evasion strategies described previously by our group.1,2
Fortunately, human beings can learn to fight back. Using advanced bioinformatics tools, we are now able to search pathogen sequences for elements that enable pathogen escape from the immune system and use this information to improve vaccines. The same approach may also be used to improve vaccines against cancer antigens (by identifying regions of those antigens that may be actively tolerizing) and to reduce the immunogenicity of biologic therapeutics (by introducing or conserving tolerogenic epitopes to promote drug-specific tolerance). Our group uses an integrated set of bioinformatics tools (iVAX) that have been extensively validated for antigen sequence analysis and vaccine design3 to identify immunogenic signals encoded in pathogen genomes; recent papers describing the use of the iVAX toolkit include applications to H. pylori4 and Hepatitis C.5
One weapon that we can use to fight back against human pathogens is to design better vaccines. JanusMatrix is a new tool for analyzing the genomes of pathogens that appears to be capable of identifying regulatory T cell (Treg) epitopes. We described the application of this tool to the emerging H7N9 avian influenza genome in this journal. Our initial immunoinformatics analysis revealed 2 potential means by which H7N9 might evade immune response: (1) reduced numbers of T helper epitopes in the H7N9 hemagglutinin (HA) protein, the primary antigen against which protective antibody response is focused1 and (2) the presence of T cell epitopes highly cross-conserved with the human genome.2 Subsequent reports revealed that vaccines developed using H7N9-HA were indeed poorly immunogenic, as was predicted. Extremely low seroconversion rates of 6% were observed.6,7 In contrast, the rate of seroconversion to unadjuvanted monovalent pandemic H1N1 is reported to be 89%.8-10 Perhaps more importantly, and related to the relative paucity of T helper epitopes, careful analysis of humoral immune responses to H7N9 revealed that human antibody response to the virus was diminished and delayed, and the resulting HA-specific antibodies had poor avidity compared to serological responses to other influenza subtypes.11
A new mechanism of immune escape: immune camouflage
More specifically, as will be reviewed here, the JanusMatrix tool has uncovered the ability of some pathogens to introduce HLA class II sequences (over the course of their co-evolution with human hosts) that are highly cross-conserved at the T cell receptor (TCR) face with the human genome12 (Fig. 1). This is a new mechanism of subterfuge, and it is deserving of intensive study, since it may explain why it has been difficult to develop effective vaccines for certain pathogens using subunit (whole antigen) vaccines. Furthermore, we can leverage this information by searching pathogen genomes for human homologs: these “human-like” epitopes may play an important role in the regulation of immune tolerance.
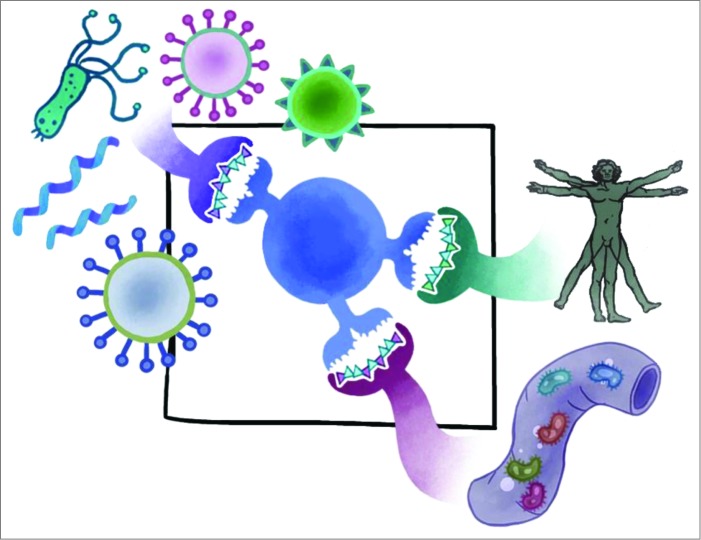
Figure 1.
Cross-conservation between T-cell receptor facing residues of Tcell epitopes may influence immune response. Pathogens may exploit cross-conservation with self to reduce immune recognition of pathogen epitopes. Cross-reactivity with the human microbiome has also been observed. The sum or ratio of these influences may determine the phenotype of the responding T cells. Understanding cross-conservation of the T cell epitope and its context is critically important for understanding human immune responses to infection and vaccination.
Maintaining tolerance is an active and constant process that involves regulatory T cells (Tregs), and reinforcement by the continued presence of antigen may be important.13 Circulating Tregs dampen immune responses to self-epitopes displayed on antigen-presenting cells, diminishing the chance of autoimmunity.
We believe that pathogens may use the same tolerance-inducing Tregs as a means of escaping immune response. Accordingly, this commentary expands on our previous observations related to emerging H7N9 in this journal by explaining how JanusMatrix can be used to identify HLA class II epitopes from pathogen sequences that have identical TCR-facing residues to multiple human genome epitopes.12 We are now using JanusMatrix for large-scale analyses of viral, bacterial and parasite sequences for such tolerizing, or Treg epitope (“Tregitope”) signals and validating this hypothesis in vitro and in vivo. While Tregitopes and tolerizing epitopes may improve biologics,14 they may hinder the effectiveness of subunit vaccines15 in the clinic.
Established immune escape mechanisms
Although novel, viral camouflage is not the first mechanism of immune escape to be discovered. There is an extensive literature on the many ways by which pathogens evade detection, some of which are described briefly in the following paragraphs.
-
Defense against innate immunity
Bacteria and viruses are known to interfere with innate immune responses by producing proteases that degrade host defense factors,16 secreting exact replicates of human cytokines that suppress immune response,17 cleaving or evading complement activation,18 impeding phagocyte recruitment,19 interfering with reactive oxygen species,20 escaping neutrophil extracellular traps,21 and generating pore-forming cytolysins,22 among other mechanisms.
-
Defense against adaptive immune response
Viruses also delete T cell epitopes to evade recognition by human T cells. This process, known as “deimmu-nization” in the biologics industry, has been observed in the course of infection by RNA viruses (HIV, HCV23-25). Evaluations of total T cell epitope content in bacterial genomes appear to confirm that deimmunization may also occur in selected bacteria.26 Since viruses have successfully demonstrated that T cell epitope deletion is a viable immune escape strategy, deimmunization has been applied to the development of less immunogenic protein therapeutics.27 Importantly, reduction of T cell epitopes is not limited to avoiding immune recognition; it also results in reduced antibody titers and diminished antibody affinity.11
-
Treg epitopes or tolerizing epitopes
In 2007, we made the surprising discovery that there were highly conserved, promiscuous T-cell epitopes located in the Fc region and framework of the Fab region of IgG.28 We hypothesized that these were regulatory T cell epitopes, which we nicknamed Tregitopes, and later determined that natural regulatory T cells (nTregs) upregulated FoxP3 following exposure to Tregitopes in vitro, suppressed bystander immune responses, modified antigen presenting cell phenotype toward a tolerogenic DC (DCreg29), and that Tregitope treatment in vivo induced adaptive tolerance.30
One question that frequently came up when we presented our new Tregitope discovery was whether similar peptides were also found in pathogen genomes. However, we were not able to search for pathogen Tregitopes until we developed JanusMatrix. This tool made it possible to begin to search viral, bacterial, and parasite genomes for human homology at the TCR face. For example, with Bailey-Kellogg and He of Dartmouth, we used JanusMatrix to scan viral genomes for Tregitopes, and found that chronic or commensal viruses (“hit-and-stay”) that establish persistent infection in humans are deimmunized and contain more viral Tregitopes than viruses that “hit-and-run” such as Ebola, Marburg and variola.12
Identification of tolerizing epitopes in pathogen sequences (and development of tools for defining them) has important ramifications for the design of vaccines against human pathogens, particularly those that persist in humans and appear to have adopted this immune defense. The evolution of this concept and a few case studies are presented in the following sections.
Defining pathogen “Tregitopes”?
Cross-reactivity is an intrinsic characteristic of the TCR that is widely recognized to be critically important for the development of thymus-derived T cells, autoimmunity, and heterologous immunity. To define cross-conserved T cell epitopes, we use JanusMatrix, which operates in conjunction with an existing T cell epitope-mapping platform (EpiMatrix)31 JanusMatrix harnesses EpiMatrix to define HLA-binding peptides while searching for cross-conservation at the TCR face in any protein sequence databases of interest (uploaded and selected by the user). We have examined TCR-facing residues for conservation against a variety of human sequence databases, including the complete human proteome, the plasma proteome and the human microbiome. 31
Preliminary studies appear to corroborate the immune camouflage hypothesis. In collaboration with Gregory and Losikoff, we discovered a tolerizing epitope in HCV using JanusMatrix.31 Subsequently, we discovered and described epitopes in H7N9 that have similar features32 (Fig. 2). In the next 3 sections, we summarize our recent experience with JanusMatrix and highlight the validation studies that have been performed. These newly defined viral Tregitopes will be described briefly below.
-
HCV
Gregory and Losikoff prospectively identified highly human-like and promiscuous HCV T cell epitopes that were subsequently shown to be Tregitopes in the context of chronic HCV infection.33 One particular sequence, which stimulated interferon-gamma production by T lymphocytes derived from non-HCV-infected patients, induced a significant increase in functional CD3+CD4+FoxP3+ Tregs among PBMCs derived from young adults who were recently infected (<5 years) and remained infected with HCV.34 Other, less human-like HCV peptides had no effect on Treg cell expansion. Importantly, a human peptide to which the HCV peptide had identical TCR-facing residues also stimulated a marked increase in Tregs among PBMCs from non-infected as well as infected individuals. This suggests that the HCV genome contains cross-reactive viral epitopes that can suppress Teffector cell function by promoting Treg cell activity. In fact, it is already well established that HCV-induced Treg activation is associated with extended chronicity of HCV infection,35 but until now it has been difficult to define which T cell epitopes engage human Tregs.
-
Influenza
We previously described H7N9 influenza 2013 as a “stealth” virus, capable of evading human immune response.1 In addition to the low T cell epitope content and limited conservation with circulating influenza strains, tolerizing epitopes may be present in H7-HA, contributing to the low immunogenicity and poor efficacy of the HA-based vaccine.2 Consistent with our previous analysis of viral genomes,12 we have identified a number of T cell epitopes in H7N9 that are cross-conserved with other influenza A viruses (IAVs) but also extensively cross-conserved with human genome. Peptides that are extensively cross-conserved with human genome may have an immuno-suppressive effect, which could explain why the development of antibodies to H7 HA appears to be delayed and the antibodies have poor affinity when compared to other antibodies to other HA proteins in H7N9-infected patients.11 Our in vitro studies of cross-reactive peptides in H7N9 appear to support the observations made in the case of HCV, described above.32
-
HIV
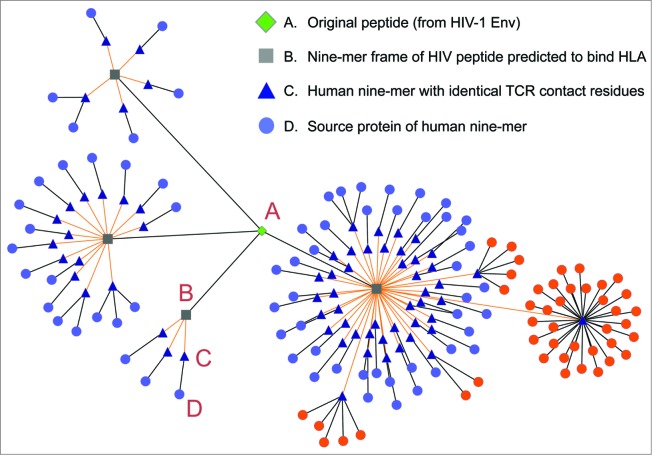
We recently applied JanusMatrix to analyzing the HIV genome, rationalizing that RNA viruses may be alike in their propensity to adopt immune camouflage as a means of escaping immune response. To our surprise, several epitopes contained in the Env protein appear to be highly cross-conserved with the human genome. One such epitope is depicted using Cytoscape to describe its connectedness with other epitopes in the human genome, Figure 3. We are currently evaluating this epitope, and other epitopes like it, to determine whether these epitopes may contribute to immune camouflage, in HIV infection.
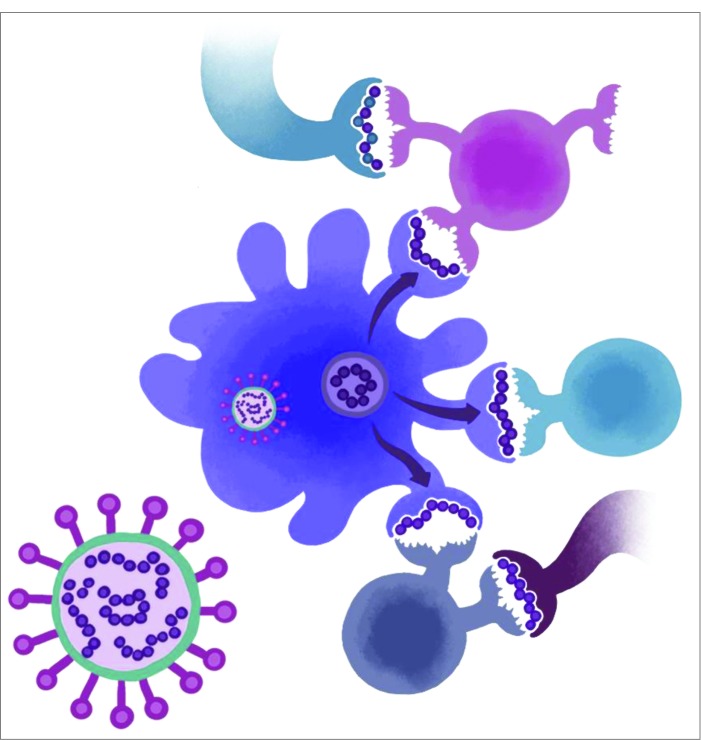
Figure 2.
Cross-conservation between T-cell receptor facing residues of Tcell epitopes and the human genome may influence the response to influenza H7N9. The virus may exploit cross-conservation between its own epitopes with self epitopes (top). Unique epitopes (middle) may be diminished, as has been demonstrated in our previous report. Cross-reactivity with other influenza A strains may also be present (bottom), further modifying the immune response. Each of these influences contributes to the final response to influenza infection or vaccination, including seroconversion rates and antibody maturation and affinity.
Figure 3.
Putative Tolerizing Tcell epitopes found in HIV. Cytoscape can be used to illustrate the relationship between a pathogen epitope and the human genome. In this figure the source HIV Env peptide is represented as a green diamond, its constituent 9-mer epitopes as gray squares, its cross-conserved partners in the human genome as blue triangles, and the source human proteins as light purple circles. As shown in this figure, not only is the Env peptide highly conserved in a number of different human proteins, but a single cross-conserved epitope can be found in 32 alleles of the same human protein (source protein highlighted in orange).
Relevance to vaccine design
Conservation with the human genome may be a means by which viruses escape human immune response. Better classification of viral epitopes as either effector or regulatory will improve the design of vaccines against pathogens that adopt immune camouflage. Modification of vaccine immunogens to epitopes that drive viral-specific T cell responses may also improve the efficacy of these vaccines. Alternatively, one might develop epitope-driven subunit vaccines, either as whole antigen vaccines, using a structure-based approach, or alternatively, as platform-neutral epitope-based vaccines that do not contain tolerizing epitopes or Tregitopes. Such vaccines may have major advantages over conventional approaches, as they simultaneously account for viral and human diversity for broad reactivity and drive protective viral-specific T cell responses. For instance, the low immunogenicity of H7N9 might be overcome by introducing into the vaccine T cell epitopes cross-conserved with seasonal influenza A strains and removing vaccine epitopes cross-conserved with human proteins, which may promote down-regulation of protective inflammatory responses.
Relevance to biologics design
Whereas immunogenicity is considered to be a positive attribute for vaccines, immunogenicity that occurs in the course of treatment with biologic therapies is a topic of some concern to biologics developers. While there are many factors that contribute to protein immunogenicity, T cell-driven (Td) responses appear to play a critical role in the development of antibody responses to biologic therapeutics. A range of methodologies to predict and mitigate Td immune responses to protein drugs has been developed. Perhaps integrating Tregitopes into protein therapeutics will reduce their immunogenicity.14 We also plan to scan protein therapeutics for pre-existing Tregitopes; our deimmunization programs would leave Tregitopes intact while removing other, more immunogenic T cell epitopes.
Conclusion
The implication of the studies emerging from the exploration of the “2-faced” T cell epitope with the JanusMatrix tool is that TCR cross-reactivity at the level of the human genome is potentially linked to a Treg phenotype of human T cell responses to pathogens. The implications for vaccine developers should be clear: remove such epitopes from vaccine antigens. The implications for biologics developers may be more nuanced but are equally important – prioritization of T cell epitopes for removal may depend on the degree of cross-conservation with self, even if the protein is autologous in origin.
In addition, TCR cross-reactivity may play a role in autoimmune disease through a concept known as molecular mimicry.36 Applications of JanusMatrix may be relevant, therefore, in the context of autoimmune diseases following vaccination, such as Guillain-Barre syndrome or Narcolepsy.37-39
Finally, pathogen subterfuge may teach us something about our own immune system. What is the role of human peptide epitopes that are themselves cross-reactive with other human sequences? Are these Tregitopes serving an important purpose, reinforcing tolerance to proteins that are critically important for human survival? Pathogens may have exploited an immune tolerance mechanism that we were completely unaware of until now.
Acknowledgments
The assistance of Genevieve S. De Groot with the figures for this manuscript and Sarah Beseme with the preparation of the manuscript and figures is sincerely appreciated.
Disclosure of Potential Conflicts of Interest
ADG and WM are founders and majority owners of EpiVax, Inc., a biotechnology company that provides access to immunoinformatics tools and to the Tregitope technology to commercial clients. Due to this relationship with EpiVax, the authors acknowledge that there is a potential conflict of interest inherent in the publication of this manuscript, and asserts that they made an effort to reduce or eliminate that conflict where possible. In addition to his role as a faculty member at Dartmouth, CBK is co-founder and CTO of Stealth Biologics, LLC, a therapeutic protein design company. Dartmouth has worked with him to manage all potential conflicts of interest arising from his commercial affiliation, and he likewise affirms that this paper presents work free of any bias.
References
- 1. De Groot AS, Ardito M, Terry F, Levitz L, Ross T, Moise L, Martin B. Low immunogenicity predicted for emerging avian-origin H7N9: Implication for influenza vaccine design. Hum Vaccin Immunother 2013; 9(5):950-6; PMID:23807079; http://dx.doi.org/ 10.4161/hv.24939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Groot AS, Moise L, Liu R, Gutierrez AH, Terry F, Koita OA, Ross TM, Martin W. Cross-conservation of T-cell epitopes: now even more relevant to [H7N9] influenza vaccine design. Hum Vaccin Immunother 2014; 10(2):256-62; PMID:24525618; http://dx.doi.org/ 10.4161/hv.28135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moise L, Cousens L, Fueyo J, De Groot AS. Harnessing the power of genomics and immunoinformatics to produce improved vaccines. Expert Opin Drug Discov 2011; 6(1):9-15; PMID:22646824; http://dx.doi.org/ 10.1517/17460441.2011.534454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang S, Desrosiers J, Aponte-Pieras JR, DaSilva K, Fast LD, Terry F, Martin WD, De Groot AS, Moise L, Moss S. Human immune responses to H. pylori HLA Class II epitopes identified by immunoinformatic methods. PLoS One 2014; 9(4):e94974; PMID:24740005; http://dx.doi.org/ 10.1371/journal.pone.0094974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mishra S, Losikoff PT, Self AA, Terry F, Ardito MT, Tassone R, Martin WD, De Groot AS, Gregory SH. Peptide-pulsed dendritic cells induce the hepatitis C viral epitope-specific responses of naïve human T cells. Vaccine 2014; 32(26):3285-92; PMID:24721533; http://dx.doi.org/ 10.1016/j.vaccine.2014.03.083 [DOI] [PubMed] [Google Scholar]
- 6. Novartis announces positive clinical trial results for novel H7N9 vaccine Media Release on Novartis Website [Internet] 2013 [cited 2014 Jul 28]; Available from: http://www.novartis.com/newsroom/media-releases/en/2013/1743124.shtml [Google Scholar]
- 7. Fries LF, Smith GE, Glenn GM. (NovaVax) A recombinant viruslike particle influenza A (H7N9) vaccine. N Engl J Med 2013; 369(26):2564-6; PMID:24224560; http://dx.doi.org/ 10.1056/NEJMc1313186 [DOI] [PubMed] [Google Scholar]
- 8. Griffin MR, Monto AS, Belongia EA, Treanor JJ, Chen Q, Chen J, Talbot HK, Ohmit SE, Coleman LA, Lofthus G, et al. Effectiveness of non-adjuvanted pandemic influenza A vaccines for preventing pandemic influenza acute respiratory illness visits in 4 US communities. PLoS One 2011; 6(8):e23085; PMID:21857999; http://dx.doi.org/ 10.1371/journal.pone.0023085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention (CDC) Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013-2014. MMWR Recomm Rep 2013; 62(RR-07):1-43 [PubMed] [Google Scholar]
- 10. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006; 24(8):1159-69; PMID:16213065; http://dx.doi.org/ 10.1016/j.vaccine.2005.08.105 [DOI] [PubMed] [Google Scholar]
- 11. Guo L, Zhang X, Ren L, Yu X, Chen L, Zhou H, Gao X, Teng Z, Li J, Hu J, et al. Human antibody responses to avian influenza A(H7N9) virus, 2013. Emerg Infect Dis 2014; 20(2):192-200; PMID:24447423; http://dx.doi.org/ 10.3201/eid2002.131094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He L, De Groot AS, Gutierrez AH, Martin WD, Moise L, Bailey-Kellogg C. Integrated assessment of predicted MHC binding and cross-conservation with self reveals patterns of viral camouflage. BMC Bioinformatics 2014; 15(Suppl 4):S1; PMID:25104221; http://dx.doi.org/ 10.1186/1471-2105-15-S4-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature 2011; 480(7378):538-42; PMID:22121024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Groot AS, Terry F, Cousens L, Martin W. Beyond humanization and de-immunization: tolerization as a method for reducing the immunogenicity of biologics. Expert Rev Clin Pharmacol 2013; 6(6):651-62; PMID:24164613; http://dx.doi.org/ 10.1586/17512433.2013.835698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity 2004; 20(1):107-18; PMID:14738769; http://dx.doi.org/ 10.1016/S1074-7613(03)00359-5 [DOI] [PubMed] [Google Scholar]
- 16. Dubin G, Koziel J, Pyrc K, Wladyka B, Potempa J. Bacterial proteases in disease - role in intracellular survival, evasion of coagulation/ fibrinolysis innate defenses, toxicoses and viral infections. Curr Pharm Des 2013; 19(6):1090-113; PMID:23016681; http://dx.doi.org/ 10.2174/1381612811319060011 [DOI] [PubMed] [Google Scholar]
- 17. Sin SH, Dittmer DP. Cytokine homologs of human gamma herpes viruses. J Interferon Cytokine Res 2012; 32(2):53-9; PMID:22142220; http://dx.doi.org/ 10.1089/jir.2011.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jongerius I, Ram S, Rooijakkers S. Bacterial complement escape. Adv Exp Med Biol 2009; 666:32-48; PMID:20054973; http://dx.doi.org/ 10.1007/978-1-4419-1601-3_3 [DOI] [PubMed] [Google Scholar]
- 19. de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, Poppelier MJ, Van Kessel KP, van Strijp JA. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 2004; 199(5):687-95; PMID:14993252; http://dx.doi.org/ 10.1084/jem.20031636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Genestet C, Le Gouellec A, Chaker H, Polack B, Guery B, Toussaint B, Stasia MJ. Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic Biol Med 2014; 73C:400-410; http://dx.doi.org/ 10.1016/j.freeradbiomed.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 21. Lappann M, Danhof S, Guenther F, Olivares-Florez S, Mordhorst IL, Vogel U. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol Microbiol 2013; 89(3):433-49; PMID:23750848; http://dx.doi.org/ 10.1111/mmi.12288 [DOI] [PubMed] [Google Scholar]
- 22. Malachowa N, Kobayashi SD, Freedman B, Dorward DW, DeLeo FR. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol 2013; 191(12):6022-9; PMID:24190656; http://dx.doi.org/ 10.4049/jimmunol.1301821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larrubia JR, Moreno-Cubero E, Lokhande MU, García-Garzón S, Lázaro A, Miquel J, Perna C, Sanz-de-Villalobos E. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol 2014; 20(13):3418-30; PMID:24707125; http://dx.doi.org/ 10.3748/wjg.v20.i13.3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song H, Pavlicek JW, Cai F, Bhattacharya T, Li H, Iyer SS, Bar KJ, Decker JM, Goonetilleke N, Liu MK, et al. Impact of immune escape mutations on HIV-1 fitness in the context of the cognate transmitted/founder genome. Retrovirology 2012; 9:89; PMID:23110705; http://dx.doi.org/ 10.1186/1742-4690-9-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vider-Shalit T, Sarid R, Maman K, Tsaban L, Levi R, Louzoun Y. Viruses selectively mutate their CD8+ T-cell epitopes–a large-scale immunomic analysis. Bioinformatics 2009; 25(12):i39-44; PMID:19478014; http://dx.doi.org/ 10.1093/bioinformatics/btp221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maman Y, Nir-Paz R, Louzoun Y. Bacteria modulate the CD8+ T cell epitope repertoire of host cytosol-exposed proteins to manipulate the host immune response. PLoS Comput Biol 2011; 7(10):e1002220; http://dx.doi.org/ 10.1371/journal.pcbi.1002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clin Immunol 2013; 149(3):534-55; PMID:24263283; http://dx.doi.org/ 10.1016/j.clim.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 28. De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, Scott DW, Martin W. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes." Blood 2008; 112(8):3303-11; PMID:18660382; http://dx.doi.org/ 10.1182/blood-2008-02-138073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cousens LA, De Groot AS, Najafian N, Martin WD. Tregitopes: An Immunomodulation Powerhouse, Hum Immunol. 2014 Dec;75(12):1139-46. doi: 10.1016/j.humimm.2014.10.012 PMID: 25454619 [DOI] [PubMed] [Google Scholar]
- 30. De Groot AS, Cousens L, Mingozzi F, Martin W. Tregitope peptides: the active pharmaceutical ingredient of IVIG? Clin Dev Immunol 2013; 2013:493138; PMID:24454476; http://dx.doi.org/ 10.1155/2013/493138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moise L, Gutierrez AH, Bailey-Kellogg C, Terry F, Leng Q, Abdel Hady KM, Verberkmoes N, Sztein MB, Losikoff P, Martin WD, et al. , Rothman A, De Groot AS. The two-faced T cell epitope: examining the host-microbe interface with JanusMatrix. Hum Vaccin Immunother 2013; 9(7) 1577-86; PMID:23584251; http://dx.doi.org/ 10.4161/hv.24615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu R, Moise L, Sangare K, Desrosiers J, Martin W, De Groot AS. Immunogenicity of Teffector and Treg epitopes from H7N9 avian-origin influenza: impact of cross-reactivity with the human genome. J Immunol 2014; 192(1) Suppl: 72.12; http://dx.doi.org/ 10.4049/jimmunol.1301513 [DOI] [Google Scholar]
- 33. Losikoff PT, Self AA, Gregory SH. Dendritic cells, regulatory T cells and the pathogenesis of chronic hepatitis C. Virulence 2012; 3(7):610-20; PMID:23076334; http://dx.doi.org/ 10.4161/viru.21823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Losikoff PT, Mishra S, Terry F, Gutierrez A, Ardito MT, Fast L, Nevola M, Martin WD, Bailey-Kellogg C, De Groot AS, et al. HCV Epitope, homologous to multiple human protein sequences, induces a regulatory T cell response in infected patients. J Hepatol 2014; pii: S0168-8278(14)00613-8. [DOI] [PubMed] [Google Scholar]
- 35. Li S, Floess S, Hamann A, Gaudieri S, Lucas A, Hellard M, Roberts S, Paukovic G, Plebanski M, Loveland BE, et al. Analysis of FOXP3+ regulatory T cells that display apparent viral antigen specificity during chronic hepatitis C virus infection. PLoS Pathog 2009; 5(12):e1000707 Erratum in: PLoS Pathog 2012; 8(6); http://dx.doi.org/ 10.1371/journal.ppat.1000707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oldstone MB. Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr Top Microbiol Immunol 2005; 296:1-17; PMID:16329189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prestel J, Volkers P, Mentzer D, Lehmann HC, Hartung HP, Keller-Stanislawski B; for the GBS Study Group Risk of Guillain-Barré syndrome following pandemic influenza A(H1N1) 2009 vaccination in Germany. Pharmacoepidemiol Drug Saf 2014. Nov;23(11):1192-204. PMID: 24817531 http://dx.doi.org/ 10.1002/pds.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li S, Jin T, Zhang HL, Yu H, Meng F, Concha Quezada H, Zhu J. Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barré syndrome and downregulated by IVIg treatments. Mediators Inflamm 2014; 2014:740947; PMID:24899787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, An P, Zhao L, Wang LH, Li QY, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol 2011; 70(3):410-7; PMID:21866560; http://dx.doi.org/ 10.1002/ana.22587 [DOI] [PubMed] [Google Scholar]