Abstract
In Germany, one dose of varicella vaccination has been recommended for children aged <24 months since 2004, and 2 doses have been recommended since 2009. Vaccination coverage (VC) is above 80% for one dose and 60% for 2 doses. In this study, data on varicella- and shingles-associated hospitalizations before and after vaccine introduction were assessed. Based on ICD-coded data of the main diagnosis of hospitalized cases from 1995–2012 in Germany, annual age-adjusted and age-specific hospitalization incidences (cases/100,000; HI) were calculated. HI means 1995–2003 (pre-vaccination-period) versus 2005–2012 (post-vaccination-period) were compared. Age-specific trends and annual percentage change rates (APC) were assessed by joinpoint regression. Overall age-adjusted varicella-HI decreased from 3.3/100,000 pre-vaccination to 1.9/100,000 post vaccination. The decline was greatest in regions with the highest VC. The post-vaccination decline was greatest in children aged <1, 1–4, and 5–9 y, who had APCs of –18.2,–27.2 and –15.2, respectively, and significant joinpoints. In all other age groups no post-vaccination joinpoints were detected or they did not lead to a consistent trend. Age-adjusted shingles-HI increased from 8.8/100,000 (1995) to 16.8/100,000 (2012). Shingles-HI increased in all age groups with no significant post-vaccination joinpoints, except in children <1 and 1–4 y, where APCs of –5.6 and –3.6 were detected.
Varicella vaccination significantly reduced varicella-HI in children below 10 y, but was not definitely related to varicella-HI in older age groups. A consistent increase of shingles-HI began before varicella vaccination was introduced and was not affected by vaccination.
Keywords: Germany, hospitalization, joinpoint regression, shingles, varicella vaccination, vaccination coverage, varicella
Abbreviations
- APC
annual percentage change rate
- HI
hospitalization incidence
- ICD
International Statistical Classification of Diseases and Related Health Problems
- MMRV
Measles, Mumps, Rubella, Varicella
- RR
reduction rate
- VC
vaccination coverage
- VZV
varicella zoster virus
Introduction
In Germany, one dose of varicella vaccination has been recommended for all children aged <24 months since 2004 and any licensed varicella vaccine may be used.1 In 2006 the recommendation was extended to 2 doses for children aged <24 months who were vaccinated with combined measles-mumps-rubella-varicella (MMRV) vaccine, and in 2009 2 doses were recommended for any varicella vaccine.2 Moreover, individual catch-up vaccinations have been recommended before children's 18th birthdays.
Vaccination coverage for children at age 24 months increased from 43% (1%) for one (two) vaccine doses for children born in 2004 to 87% (64%) for children born in 2009.3
A considerable amount of vaccine was also used for older children and adolescents: according to billing data regarding statutory health-insured persons, in 2011 about 9% of all 1st doses and 18% of all 2nd doses were administered to those aged 7–17 y.4
The aim of varicella vaccination is to reduce varicella-related disease burden. Despite being a generally mild disease, the estimated annual number of 735,000 varicella cases pre-vaccination in Germany is supposedly associated with a substantial amount of complications.5,6 Surveillance data were needed to evaluate the effect of varicella vaccination on a population level. However, varicella was not a notifiable disease in Germany until March 2013. Prior to this, sentinel surveillance in a convenience sample of physicians started in 2005 and focused on outpatient cases of varicella and herpes zoster (shingles), including varicella-related complications. Between 2005 and 2009 the total number of varicella cases per reporting practice dropped by 55%.7 At the same time, varicella-related complications were reduced by 81%, with the greatest decrease in age groups 0–4 and 5–9 y.8 When the number of complicated cases in the sentinel surveillance became very low, this part of the sentinel surveillance was no longer sensitive enough to generate valid data and was stopped in March 2011. Moreover, sentinel reporting was prone to underreporting of severe varicella cases that presented directly in the hospital and that were not retrospectively reported by sentinel physicians.
Varicella and shingles are caused by the same virus. After exogenous infection leading to varicella, the varicella-zoster-virus (VZV) retires into the nerve-ganglions, from where it may endogenously reactivate months or years later.9,10 Modeling studies on the transmission of VZV suggested an increase of shingles cases that would start 10 to 20 y following introduction of varicella vaccination because of a lack of opportunities for an external booster of immunity.11-13 One model found that the impact of varicella vaccination on shingles might depend on the country-specific amount of exogenous boosting.14 However, in contrast to varicella, sentinel surveillance of shingles showed no particular trend related to the introduction of varicella vaccination in Germany.15
Hospital diagnosis of varicella and herpes zoster may be regarded as an indicator of severe cases; therefore, it may serve as a further baseline for assumptions on the burden of disease. Data on the main diagnoses of hospital admissions coded according to the International Statistical Classification of Diseases and Related Health Problems (ICD) are available at the German Federal Statistical Office.16
We assessed data on varicella- and shingles-associated hospitalization before and after introduction of varicella vaccination to quantify the impact of vaccination on the incidence of severe cases at the country level, and calculated hospitalization incidences per 100,000 (HI) for varicella and shingles.
Results
Varicella hospitalizations
A total of 29,223 varicella cases were recorded at all hospitals in Germany from 1995–2012. Overall, age-adjusted varicella-HI showed no particular trend until 2003, peaked in 2004 and decreased thereafter (Fig. 1a). In each year of the observation period, varicella-HI was higher in males than females (Fig. 1a). Mean age-adjusted varicella-HI was significantly lower in the post-vaccination period compared with the pre-vaccination period (Table 1).
Figure 1.
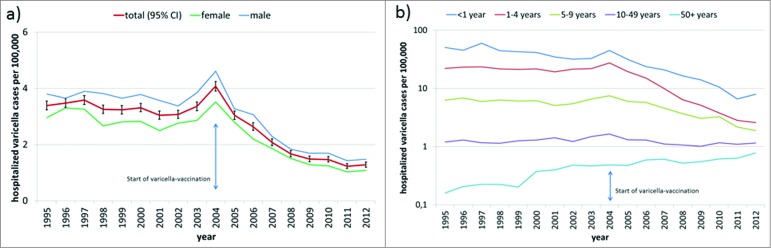
(a) Age-adjusted hospitalization incidence (HI) of varicella (hospitalized cases per 100,000 standard population) in total and by gender from 1995–2012 in Germany (b) Age-specific hospitalization incidence (HI) of varicella (hospitalized cases per 100,000) from 1995–2012 in Germany (y-axis: logarithmic scale).
Table 1.
Mean number and range of hospitalized varicella cases per year, mean age-specific incidence, and mean age-adjusted incidence of varicella hospitalizations per 100,000 (HI) before varicella vaccination (pre-vaccination, 1995–2003) and after the introduction of varicella vaccination (post-vaccination, 2005–2012) in Germany (*** P < 0.05 was regarded as significant)
| Age in years | pre-vaccination (1995–2003) | post-vaccination (2005–2012) | |||
|---|---|---|---|---|---|
| Mean number of cases per year (range: min; max) | Mean HI (cases per 100,000) | Mean number of cases per year (range: min; max) | Mean HI (cases per 100,000) | p-value for mean HI*** P < 0.05 | |
| <1 | 327.8 (232; 479) | 42.56 | 112.3 (44; 220) | 16.47 | *** |
| 1–4 | 691.9 (612; 747) | 21.76 | 231.1 (71; 569) | 8.15 | *** |
| 5–9 | 264.0 (206; 320) | 6.08 | 143.8 (66; 237) | 3.79 | *** |
| 10—14 | 64.2 (46; 84) | 1.40 | 47.3 (38; 56) | 1.18 | p = 0.06 |
| 15—19 | 58.0 (34; 69) | 1.27 | 50.8 (39; 65) | 1.13 | p = 0.15 |
| 20—49 | 450.4 (395; 520) | 1.26 | 392.4 (348; 469) | 1.15 | p = 0.09 |
| 50 and older | 88.7 (45; 143) | 0.30 | 193.4 (146; 265) | 0.60 | *** |
| total (age adjusted) | 1948.8 (1810; 2084) | 3.30 | 1171.0 (865; 1751) | 1.86 | *** |
Varicella-HI decreased with increasing age: it was up to 59.4 and 27.6/100,000 in children <1 y and 1–4 y, respectively, was between 2 and 7.5/100,000 in children 5–9 y, between 1 and 2/100,000 in persons aged 10–49 y, and was <1/100,000 in adults 50 y and older (Fig. 1b).
Mean age-specific varicella-HI was significantly lower post-vaccination compared with pre-vaccination for age groups <1, 1–4 and 5–9 y, but it was significantly higher post-vaccination for the age groups with the lowest incidence (50 y and older). No significant difference in mean varicella-HI was seen between the 2 periods in those aged 10–49 y (Table 1).
Trend analysis of age-specific varicella-HI identified trend changes (joinpoints) in the pre-vaccination time; specifically, in children aged 1–4 y in 2001, when the small but significant decrease since 1995 changed into a non-significant increase until 2004, and in age 50 y and older in 2002, when the continuous significant HI-increase since 1995 slowed down (Table 2).
Table 2.
Trends in age-specific hospitalization incidence of varicella (hospitalized cases per 100,000) with joinpoints (point with significant change in the slope of a trend) and annual percentage change rate (APC) from 1995–2012 in Germany
| Age in years | No. of joinpoints | Years range | APC | 95% CI | Significance |
|---|---|---|---|---|---|
| <1 | 1 | 1995–2004 | −3.9 | (−6.5; −1.2) | *** |
| 2004–2012 | −18.2 | (−22.8; −13.2) | *** | ||
| 1–4 | 2 | 1995–2001 | −2.6 | (−4.9; −0.4) | *** |
| 2001–2004 | 9.7 | (−4.9; 26.5) | — | ||
| 2004–2012 | −27.2 | (−29.3; −24.9) | *** | ||
| 5–9 | 1 | 1995–2005 | 0.2 | (−2.0; 2.5) | — |
| 2005–2012 | −15.2 | (−20.0; −10.1) | *** | ||
| 10–14 | 0 | 1995–2012 | −1.9 | (−3.4; −0.3) | *** |
| 15–19 | 0 | 1995–2012 | −1.4 | (−3.1; 0.4) | — |
| 20–49 | 2 | 1995–2004 | 4 | (2.4; 5.6) | *** |
| 2004–2008 | −9.7 | (−17.3; −1.4) | *** | ||
| 2008–2012 | 3.1 | (−2.3; 9.5) | — | ||
| 50 and older | 1 | 1995–2002 2002–2012 |
16.1 4.4 |
(9.0, 23.7) (2.1; 6.9) |
*** *** |
*** significant, – not significant.
In 2004, joinpoints were identified in 3 age groups (<1 y, 1–4 y, 20–49 y), when either the existing decreasing trend was amplified (<1 y) or an increasing trend changed into a decrease (Table 2).
Post-vaccination joinpoints were found for those aged 5–9 y in 2005, when the varicella-HI that was stable since 1995 started to decrease, and for those aged 20–49 y in 2008, when the previous decrease came to a halt.
Varicella vaccination coverage (VC) at school entry (age 4–6 y) in 2012 was 78.2% (67.6%) for one (2) doses in Germany. VC varied by Federal State and ranged from 55.2% to 94.0% for one and from 42.6% to 90.1% for 2 vaccine doses.17 The 2 Federal States with the highest VC showed the highest decrease in varicella-HI from pre- to post-vaccination time, whereas in the 2 Federal States with the lowest VC, reduction of varicella-HI was the lowest (Table 3).
Table 3.
Varicella vaccination coverage (VC) at school entry (children aged 4–6 y) in Germany 2012 overall and by Federal States with highest and lowest VC, post-vaccination reduction rate (RR) of mean varicella hospitalization incidence in 1–4-year-old children, and rank of Federal State sorted by VC and RR
| Federal State | 1 dose VC (%) | 2 doses VC (%) | RR (%) | VC-rank (1–16) | RR-rank (1–16) |
|---|---|---|---|---|---|
| Germany (16 Federal States) | 78.2 | 67.6 | 62.5 | ||
| Mecklenburg-Western Pomerania | 94.0 | 90.1 | 81.1 | 1 | 2 |
| Saxony-Anhalt | 93.3 | 85.8 | 85.8 | 2 | 1 |
| … | … | … | … | … | … |
| Bavaria | 62.0 | 48.6 | 42.1 | 15 | 15 |
| Bremen | 55.2 | 42.6 | 29.1 | 16 | 16 |
Shingles hospitalizations
A total of 249,905 shingles cases was recorded in German-wide hospital statistics from 1995–2012. Overall, age-adjusted shingles-HI increased continuously from 8.8 (1995) to 16.8 (2012). In each year it was higher in females than in males (Fig. 2a). The mean age-adjusted shingles-HI was significantly higher in the post-vaccination period compared with the pre-vaccination period (Table 4).
Figure 2.
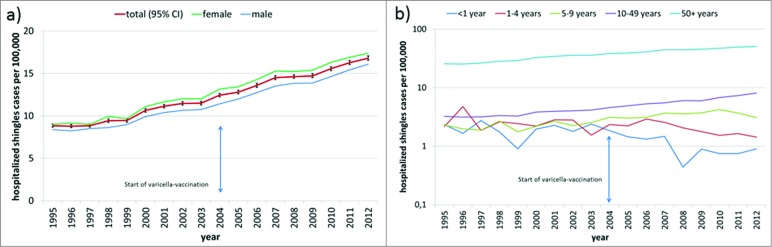
(a) Age-adjusted hospitalization incidence (HI) of shingles (hospitalized cases per 100,000 standard population) in total and by gender from 1995–2012 in Germany (b) Age-specific hospitalization incidence (HI) of shingles (hospitalized cases per 100,000) from 1995–2012 in Germany (y-axis: logarithmic scale).
Table 4.
Mean number and range of hospitalized shingles cases per year, mean age-specific incidence, and mean age-adjusted incidence of shingles hospitalizations per 100,000 (HI) before varicella vaccination (pre-vaccination, 1995–2003) and after the introduction of varicella vaccination (post-vaccination, 2005–2012) in Germany (*** p < 0.05 was regarded as significant)
| Age in years | pre-vaccination (1995–2003) | post-vaccination (2005–2012) | p-value for mean HI | ||
|---|---|---|---|---|---|
| Mean number of cases per year (range: min; max) | Mean HI (cases per 100,000) | Mean number of cases per year (range: min; max) | Mean HI (cases per 100,000) | ||
| <1 | 10.7 (7; 17) | 1.98 | 6.8 (3; 10) | 0.99 | *** |
| 1–4 | 81.9 (47; 154) | 2.57 | 56.5 (39; 83) | 2.02 | p = 0.145 |
| 5–9 | 97.0 (76; 120) | 2.25 | 130.5 (108; 152) | 3.50 | *** |
| 10–14 | 123.7 (103; 152) | 2.68 | 179.4 (147; 234) | 4.50 | *** |
| 15–19 | 126.2 (90; 151) | 2.76 | 195.9 (171; 239) | 4.43 | *** |
| 20–49 | 1352.0 (1197; 1558) | 3.78 | 2272.0 (1841; 2839) | 6.69 | *** |
| 50–59 | 1226.9 (1158; 1313) | 11.97 | 1873.4 (1466; 2240) | 16.60 | *** |
| 60–69 | 2212.7 (1693; 3028) | 23.29 | 3456.5 (3297; 3677) | 36.65 | *** |
| 70 and older | 5359.0 (4251; 6475) | 57.68 | 9319.5 (7317; 11509) | 79.74 | *** |
| total (age adjusted) | 10627.7 (8816; 12833) | 10.01 | 17531.0 (14529; 20613) | 14.86 | *** |
The mean age-specific shingles-HI was lower in the post- compared with the pre-vaccination period in children aged <1 y (significant) and 1–4 y (non-significant), but it was significantly higher in the post-vaccination period in all other age groups (Table 4).
Age-specific shingles-HI increased with increasing age; it was <5/100,000 in children <10 y of age, was <10/100,000 in persons aged 10–49 y, and was between 20 and 50 per 100,000 in persons aged 50 y and older (Fig. 2b).
From 1995 to 2012, no joinpoints were detected for age-specific shingles-HI below 20 y of age, for ages 50–59, or for those aged 70 y and older. In children below 5 y of age shingles-HI decreased from 1995–2012, with significant APCs of –5.6 (<1 y) and –3.6 (1–4 y), but in the other age groups an upward trend of shingles-HI was observed (Table 5). Pre-vaccination joinpoints were identified in those aged 20–49 y (in 1999) and 60–69 y (in 1997), each with a change from a stable situation to a significant upward trend. The only post-vaccination joinpoint was the cessation of this increasing trend in the 60–69-year-old age group in 2007, which is also reflected with a joinpoint in 2007 in the summarized age group of 50 y and older (Table 5).
Table 5.
Trends in age-specific hospitalization incidence of shingles (hospitalized cases per 100,000) with joinpoints (point with significant change in the slope of a trend), and annual percentage change rate (APC) from 1995–2012 in Germany
| Age group | No. of joinpoints | Years range | APC | 95% CI | Significance |
|---|---|---|---|---|---|
| <1 | 0 | 1995–2012 | −5.6 | (−8.6; −2.5) | *** |
| 1–4 | 0 | 1995–2012 | −3.6 | (−6.2; −0.9) | *** |
| 5–9 | 0 | 1995–2012 | 4.2 | (2.8; 5.5) | *** |
| 10–14 | 0 | 1995–2012 | 5.6 | (4.7; 6.5) | *** |
| 15–19 | 0 | 1995–2012 | 5.3 | (4.5; 6.2) | *** |
| 20–49 | 1 | 1995–1999 | 1.2 | (−2.7; 5.2) | — |
| 1999–2012 | 6.8 | (6.3; 7.4) | *** | ||
| 50 and older | 1 | 1995–2007 | 4.8 | (4.3; 5.3) | *** |
| 2007–2012 | 2.8 | (1.3; 4.3) | |||
| 50–59 | 0 | 1995–2012 | 3.5 | (3.2; 3.7) | *** |
| 60–69 | 2 | 1995–1997 | −0.5 | (−8.3; 8.1) | — |
| 1997–2007 | 6.4 | (5.8; 7.1) | *** | ||
| 2007–2012 | 0.7 | (−0.6; 2.0) | — | ||
| 70 and older | 0 | 1995–2012 | 3.5 | (3.1; 3.8) | *** |
***significant, – not significant.
Discussion
After the introduction of varicella vaccination into the childhood immunization program, an overall reduction in varicella-related hospitalizations was observed from an annual mean of about 1950 to 1170 varicella cases, while the pre-vaccination increasing trend in shingles-related hospitalization was sustained. However, there were age-specific distinctions within these overall results.
Regarding hospitalization as an indicator for severe disease, our analysis confirmed the reduction of complications and severe cases due to varicella vaccination in Germany that had been described previously.7,8,15 Our analysis showed that the burden of varicella-related disease has been reduced by about 40% overall, with an even greater decrease of about 60% in children below 5 y of age.
The reduction of varicella-HI was mainly attributed to the reduction in children below 10 y of age and was greatest in children below 5 y, including the age group in which vaccination is generally recommended. This effect seems plausible, because vaccination coverage in 2- and 3-year-old children has more than doubled since vaccination was recommended, and vaccine coverage data at school entry for 4–6 y olds has indicated overall acceptance of varicella vaccination in Germany.3,17
In support of causality between varicella vaccination and a reduction of varicella-HI, the reduction of mean varicella-HI in the post-vaccination period was strongly related to varicella-VC by Federal State.
Similar effects regarding varicella hospitalization following varicella vaccination have been described in countries with general childhood varicella vaccination programs like the US, Canada and Australia.18-21 However, the reduction of varicella-HI in our data is lower than in these countries and mainly restricted to children. Whereas in Germany mean varicella-HI in adults aged 20–49 y did not differ in the post- and pre-vaccination era, and in patients 50 y and older the hospitalization rates were even higher post-vaccination, in the US a decrease in varicella-related hospitalizations was observed post-vaccination also in adults aged 20 y and older after only one childhood vaccine dose.18 In Canada hospitalization rates across 10 provinces also indicated a strong effect of childhood varicella vaccination on the adult population, but this effect was not significant in all provinces for all adult age groups.21 However, it was suggested that the reduction of varicella-HI in adult groups not scheduled for varicella vaccination reflected herd protection.21 So far, our hospital data do not support herd protection for adults in Germany.
Further insight regarding the adult age groups was given by our joinpoint-analysis: i.e., the pre-vaccination increasing trend in varicella-HI in adults 20–49 y of age ceased in 2004 and turned into a decrease. This could be interpreted as an impact of varicella vaccination on varicella-HI in adults. Moreover, our analysis detected other secular trends and variations of varicella HI in the pre-vaccination period like the slight decreases in children <1 y (1995–2004) and 1–4 y (1995–2001), the latter followed by a stable situation until 2004. These pre-vaccination variations, together with the slight upward trend in varicella-HI in adults aged 50 y and older that started before vaccination, may explain the diminished effect of vaccination on varicella-HI in Germany in comparison to the US and Canada. Trends and variations from pre-vaccination time have also to be taken into account when evaluating an age-shift toward older ages, which has been postulated to happen, particularly when children are vaccinated against varicella with insufficient coverage rates.12
Hospital data are the only available population-based source on varicella and shingles cases in Germany that date back to the pre-vaccine era and that have been recorded systematically and continuously over time. At the time of analysis, data were available over a time-period of 18 y that was almost evenly divided into pre- and post-vaccination period. This makes it possible to compare data for both periods, as well as to distinguish between effects due to vaccination and secular variations or trends that have already started before and have probably not been affected by vaccination. Moreover, the long period allowed us to use joinpoints and APCs as robust markers for trends.
An example trend is the marked increase of shingles-HI in adults, which began before childhood varicella vaccination was introduced. The same trend has been reported in the US and Canada.18,19 From the available data it can be concluded that childhood varicella vaccination has not yet affected shingles in adults in countries where general varicella vaccination is in place.
Another trend in the pre-vaccination period is the decrease in shingles-HI in children of vaccination eligible age. Although no joinpoint was detected, the decrease seemed to have amplified post-vaccination; however, this was not significant. In analysis of outpatient data from German sentinel surveillance from 2005–2010, as well as in billing data from physicians for statutory health-insured patients from 2006–2008, the same post-vaccination trend was observed and was statistically significant.22 Interestingly, according to these outpatient data the downward trend of shingles was seen even in 5–9-year-old children. For children aged 5–9 y, shingles-HI in our analysis ceased increasing in 2010 and decreased in the following 2 years, but whether a new trend is developing can only be proven in a few years. However, a decrease of shingles in vaccinated children seems plausible if natural infection is prevented by varicella vaccination and if virus strains of vaccine-type are less likely to reactivate than wild-type strains. 23,24
In our analysis we did not rely only on the comparison of mean incidences between pre- and post-vaccination time, but described trends and changes over the entire observation period more precisely. We found joinpoint regression as an adequate tool for the analysis of time trends and possible changes. Its advantage was shown for segmented trends in pre- and post-vaccination periods that went in opposite directions and that were missed by the comparison of means from both periods (i.e., varicella-HI in those aged 20–49 y). Another example was the small but steady and significant decline of varicella-HI in children aged 10–14 y over the entire period from 1995–2012 by about 2% per annum, that was identified by joinpoint regression but not reflected by the comparison of mean pre- and post-vaccination varicella-HI, where the difference was not significant. The sensitivity of the joinpoint regression was also shown for shingles-HI in the 1–4-year age group, with a significant time trend from 1995–2012 but no significant difference in the mean values.
Unfortunately, the available hospitalization data did not allow for analysis of diagnosis in more detail and they are restricted to main diagnoses. Further cases of varicella and shingles might have been treated in hospital but escaped this analysis, if they had a varicella or shingles-associated complication as a main diagnosis (such as complication of the skin), leading to an underestimation of varicella- and shingles-related HI. In a prospective nationwide German study in 2003–2004 using 3 independent surveillance sources to capture varicella-related hospitalizations in children in Germany, an annual pre-vaccination varicella-related HI was estimated at 14.1/100,000 children ≤16 y.5 The pre-vaccination varicella-HI of 11.4/100,000 in children below 15 y in our data is only slightly lower, and the 2 German results are between the range of other European countries, which include a low of 6.8/100,000 in the Netherlands and high of 26/100,000 in France in children <16 y. 25 Our pre-vaccination age-adjusted varicella-HI of 3.3/100,000 is also within the range of 1.9 to 5.8/100,000 for the standardized annual varicella-HI from European countries without varicella vaccination programs.25
Limitations of an ecological analysis like ours include changes in coding, billing approaches, diagnostics, or other aspects that may have an influence on the case finding. Although within the study period the ICD codes have changed, we can assume that the ICD10-codes for varicella and for shingles represent the same diagnoses as the respective ICD9-codes. A modification of the legal base led to an extension of the survey field since 2003, when facilities with more than 100 beds had to give information on the diagnoses of patients. However, a respective joinpoint was not found in our analysis. General changes in the billing approach of hospitals were introduced in 2003 with the diagnosis-related groups (DRG) that were mandatorily used starting in 2004. This change might have led to the higher number of hospitalized varicella cases in 2004, observed in all ages and all German regions. Further clinical diagnostics may have been introduced over time (i.e., to confirm unspecific clinical pictures), but their effect on the hospitalization rates for varicella and shingles cannot be determined. The possible underestimation in our analysis by constraining the analysis to main diagnoses would affect both periods – pre- and post-vaccination – in the same way.
Despite these limitations, our analysis reflects the impact of childhood varicella vaccination on age-adjusted and age-specific trends of varicella- and shingles-HI. Using a robust statistical approach, we confirmed the impact of varicella vaccination in children, and also investigated detailed trends in age groups and time segments. Age-specific surveillance data on varicella and shingles from the pre-vaccine era are indispensable to compare with post-vaccination data to assess possible effects of vaccination.
Methods
Hospital statistics – diagnostic data
We used comprehensive national hospital discharge data available from the German Federal Statistical Office (Destatis).16 Data were annually reported from all German hospitals and include all patients that received full inpatient treatment in the year of reporting. The reports were mandatory for all hospitals and submitted to the Federal States Statistical Offices. Reports included the patient's gender, month and year of birth, residential postal code, date of hospital entry and exit, and main diagnosis during the hospital stay according to the International Statistical Classification of Diseases and Related Health Problems (ICD). The Federal States Offices submitted aggregated data to Destatis, where countrywide statistics were compiled and published.
Data were available according to ICD9-codes from 1995–1999 and ICD10-codes from 2000–2012. Analysis included all hospitalized cases with a main diagnosis of varicella according to the 3-digit codes ICD9-052 or ICD10-B01. Additionally, all hospitalized cases were identified as those with a main diagnosis of shingles (ICD9-053 or ICD10-B02).16 The main diagnosis refers to the disease or condition that mainly contributed to the hospital stay.
Statistical methods
To calculate hospitalization incidence per 100,000 population (HI), we obtained annual mid-year population estimates for Germany from 1995–2012 from Destatis.26
Annual age-adjusted HI was calculated per 100,000 of total European Standard population and by gender. Age-specific HI were estimated for each year and by age groups (<1 y, 1–4 y, 5–9 y, 10–14 y, 15–19 y, 20–49 y, 50 y and older; shingles-HI also for 50–59 y, 60–69 y, ≥ 70 y). We also calculated the mean values (arithmetic mean) for age-adjusted and age-specific HI during the pre- (1995–2003) and post-vaccination period (2005–2012). The year 2004 was excluded because nationwide immunization with varicella vaccine was implemented in mid-2004.
Welch's t-test was used to compare the mean values for age-specific HI between pre- and post-vaccination period. P-values < 0.05 were considered statistically significant.
Time trends for age-specific HI were estimated by joinpoint regression. A joinpoint was described as a statistically significant change in the slope of a trend that interrupts the continuous phase of a trend.27 Joinpoint regression is the non-linear model for such segmented trends.27 We identified segmented trends and joinpoints in the age-specific HI for varicella and shingles. Within each trend segment the annual percentage change rate (APC) was estimated to identify and quantify the direction and amplitude of the respective trend. Permutation tests were used to test whether the selected number of joinpoints and the change in trend were statistically significant.27
Joinpoint Regression Software (Version 4.0.4) was used to analyze time trends.28
All other statistical analysis was performed using R software (R version 3.0.2).
Vaccination coverage (VC) data by Federal State were available from school entry for children 4–6 y of age in 2012 and were compared with the mean pre- and post-vaccination varicella-HI in children 1–4 y of age by Federal State.17
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Koch-Institut Robert. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch- Institut, Stand: Juli 2004. Epidemiologisches Bulletin 2004:235–49. [Google Scholar]
- 2. Koch-Institut Robert. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut, Stand: Juli 2009. Epidemiologisches Bulletin 2009:279–98. [Google Scholar]
- 3. Rieck T, Feig M, Eckmanns T, Benzler J, Siedler A, Wichmann O. Vaccination coverage among children in Germany estimated by analysis of health insurance claims data. Human Vaccines & Immunotherapeutics 2013; 10:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koch-Institut Robert. Stellungnahme der Ständigen Impfkommission (STIKO): Evaluation der Varizellen-Impfempfehlung durch die STIKO. Epidemiologisches Bulletin 2013:1–5. [Google Scholar]
- 5. Liese JG, Grote V, Rosenfeld E, Fischer R, Belohradsky BH, v Kries R, et al. The burden of varicella complications before the introduction of routine varicella vaccination in Germany. Pediatr Infect Dis J 2008; 27:119–24. [DOI] [PubMed] [Google Scholar]
- 6. Wagenpfeil S, Neiss A, Banz K, Wutzler P. Empirical data on the varicella situation in Germany for vaccination decisions. Clinical Microbiology and Infection: 2004; 10:425–30. [DOI] [PubMed] [Google Scholar]
- 7. Siedler A, Arndt U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro Surveill 2010; 15 http://www.eurosurveillance.org/images/dynamic/EE/V15N13/V15N13.pdf [PubMed] [Google Scholar]
- 8. Spackova M, Muehlen M, Siedler A. Complications of varicella after implementation of routine childhood varicella vaccination in Germany. Pediatr Infect Dis J 2010; 29:884–6. [DOI] [PubMed] [Google Scholar]
- 9. Plotkin SA, Orenstein WA, Offit PA. Varicella Vaccine. Vaccines. Philadelphia: Elsevier Saunders, 2013:1550. [Google Scholar]
- 10. Chickenpox/Herpes Zoster. In: Heymann DL, ed. Control of Communicable Disease Manual. Washington: American Public Health Association, 2008:109–16. [Google Scholar]
- 11. Ogunjimi B, Van Damme P, Beutels P. Herpes Zoster Risk Reduction through Exposure to Chickenpox Patients: A Systematic Multidisciplinary Review. PloS one 2013; 8:e66485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Hoek AJ, Melegaro A, Zagheni E, Edmunds WJ, Gay N. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine 2011; 29:2411–20. [DOI] [PubMed] [Google Scholar]
- 13. Brisson M, Edmunds WJ, Gay NJ, Law B, De Serres G. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiology and infection 2000; 125:651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poletti P, Melegaro A, Ajelli M, Del Fava E, Guzzetta G, Faustini L, et al. Perspectives on the impact of varicella immunization on herpes zoster. A model-based evaluation from three European countries. PloS one 2013; 8:e60732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siedler A, Hecht J, Rieck T, Tolksdorf K, Hengel H. [Varicella vaccination in Germany. A provisional appraisal in the context of MMR vaccination]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2013; 56:1313–20. [DOI] [PubMed] [Google Scholar]
- 16. Diagnostic data of the hospitals by place of treatment, ICD-10: B01, B02, ICD-9: 052–053. https://www.gbe-bund.de/: Destatis, 2014. [Google Scholar]
- 17. Koch-Institut Robert. Impfquoten bei der Schuleingangsuntersuchung in Deutschland 2012. Epidemiologisches Bulletin 2014:137–41. [Google Scholar]
- 18. Lopez AS, Zhang J, Brown C, Bialek S. Varicella-related hospitalizations in the United States, 2000-2006: the 1-dose varicella vaccination era. Pediatrics 2011; 127:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan B, Bettinger J, McConnell A, Scheifele D, Halperin S, Vaudry W, et al. The effect of funded varicella immunization programs on varicella-related hospitalizations in IMPACT centers, Canada, 2000-2008. Pediatr Infect Dis J 2012; 31:956–63. [DOI] [PubMed] [Google Scholar]
- 20. Marshall HS, McIntyre P, Richmond P, Buttery JP, Royle JA, Gold MS, et al. Changes in patterns of hospitalized children with varicella and of associated varicella genotypes after introduction of varicella vaccine in Australia. Pediatr Infect Dis J 2013; 32:530–7. [DOI] [PubMed] [Google Scholar]
- 21. Waye A, Jacobs P, Tan B. The impact of the universal infant varicella immunization strategy on Canadian varicella-related hospitalization rates. Vaccine 2013; 31:4744–8. [DOI] [PubMed] [Google Scholar]
- 22. Siedler A, Ultsch B, Rieck T. Herpes zoster in children after introduction of universal childhood varicella vaccination in Germany. 29 Conference of the European Society for Pediatric Infectious Diseases (ESPID). The Hague, The Netherlands, 2011:1128 http://espid.kenes.com/Documents/ESPID2011%20ABSTRACTS.pdf
- 23. Civen R, Chaves SS, Jumaan A, Wu H, Mascola L, Gargiullo P, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J 2009; 28:954–9. [DOI] [PubMed] [Google Scholar]
- 24. Chun C, Weinmann S, Riedlinger K, Mullooly JP, Houston H, Schmid DS, et al. Laboratory characteristics of suspected herpes zoster in vaccinated children. Pediatr Infect Dis J 2011; 30:719–21. [DOI] [PubMed] [Google Scholar]
- 25. ECDC. Varicella vaccine in the European Union: Preliminary Guidance. http://www.ecdc.europa.eu/en/publications/Publications/Varicella-guidance-2014-consultation.pdf:ECDC, 2014. [Google Scholar]
- 26. The Information System of the Federal Health Monitoring. Population on an annual average. http://www.gbe-bund.de/: Destatis, 2014. [Google Scholar]
- 27. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine 2000; 19:335–51. [DOI] [PubMed] [Google Scholar]
- 28. National Cancer Institute. Joinpoint Regression Program. Version 4.0.4. https://surveillance.cancer.gov/joinpoint/, 2013. [Google Scholar]


