Abstract
It has not been reported that administration of combining rabies vaccines and immunoglobulin resulted in acute disseminated encephalomyelitis (ADEM) yet. This report described that an old man acquired ADEM after being administrated with purified Vero cell rabies vaccine (PVRV) and Human Rabies Immunoglobulin (HRIG). Then he was given intravenous and oral glucocorticoids. Simultaneously, rabies vaccination was continued with purified Chick embryo cell vaccines (PCECV) instead of PVRV. Furthermore, we analyzed the rabies virus neutralizing antibodies (RVNA) levels in the patient's blood at different time points after rabies vaccination. Collectively, we observed that PCECV vaccination did not affect the prognosis of ADEM, and glucocorticoid was crucial and effective, which had no significant influence on efficacy of PCECV.
Keywords: ADEM, glucocorticoids, PVRV, HRIG, RVNA
Abbreviations
- ADEM
acute disseminated encephalomyelitis
- PVRV
purified Vero cell rabies vaccine
- HRIG
Human Rabies Immunoglobulin
- PCECV
purified Chick embryo cell vaccines
- RVNA
rabies virus neutralizing antibodies
- RABV
rabies virus
- PEP
post-exposure prophylaxis
- HDCV
human diploid cell vaccine
- PPRC
Pharmacopoeia of the People's Republic of China
- CT
Computed tomography
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- ASO
Anti-streptolycin O
- ANCA
Antineutrophil cytoplasmic antibodies
- EEG
electroencephalography
- CSF
Cerebrospinal fluid
- MRI
magnetic resonance image
- T2W
T2-weighted
- FLAIR
fluid-attenuated inversion recovery
- RFFIT
rapid fluorescent focus inhibition test
- MS
multiple sclerosis
Introduction
Rabies, also known as hydrophobia disease, is an acute zoonotic-infectious disease caused by the rabies virus (RABV). People may be infected after exposure to infected animal saliva, through bites, licks and scratches. It is reported that about 55,000 people per year globally die of rabies and there is a death per 10 minutes.1 Unfortunately, it has a case fatality rate of almost 100%. Consequently, post-exposure prophylaxis (PEP) is among the most important measures for prevention of rabies. PEP consists of wound cleaning, rabies vaccination and passive immunization with rabies immune globulin, if necessary.2 In the early period, rabies vaccines were made from rabies virus-infected animals' spinal cords or brains,3 which caused various and severe adverse events on account of high levels of myelin. In the modern times, cell culture derived vaccines including human diploid cell vaccine (HDCV),4,5 purified Chick embryo cell vaccines (PCECV),6 and purified Vero cell rabies vaccine (PVRV)7,8 are generally acknowledged to have high safety and immunogenicity. And PVRV is approved and widely used in our country. These vaccines cause rarely post-vaccination autoimmune response depending in part on the purity of the inactivated rabies viruses. Although, there are still some reports that delineate extensive neurological complications following rabies vaccination such as acute disseminated encephalomyelitis (ADEM),9 optical neuromyelitis, etc. Once these neurological complications occur, high-dose steroid therapy is very essential to the patients, whereas glucocorticoids are considered to suppress patients' immune system, thereby leading to the failure of rabies vaccination. So rabies vaccination (mainly for PVRV) should be delayed for the patients who receive corticosteroids or immunosuppressive agents in Pharmacopoeia of the People's Republic of China (PPRC, 2010 Edition). Therefore, in this study we intend to observe whether steroids therapy has a significant effect on rabies vaccination through analysis of RVNA of the patient with post-vaccination ADEM.
Case Report
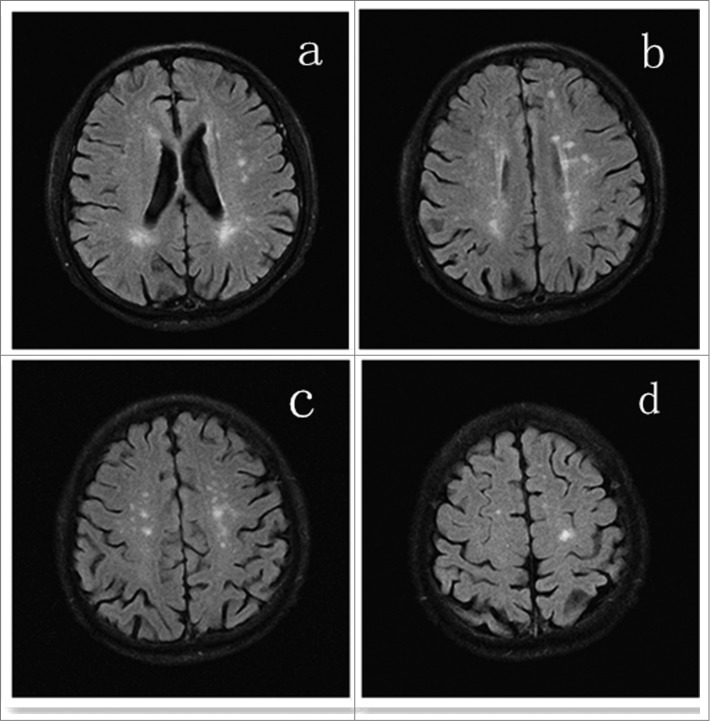
A 73-year-old male presented with complaint of seizures, characterized by 4 episodes of transient unconsciousness and generalized convulsion. Over the past decades, he never had been hospitalized because of any diseases, and he received no blood transfusion, surgery, or long-term medication. And there was no special medical history but occasional colds in the past. He had suffered a dog bite on the right lower extremity 1 day prior to admission. And the level of exposure was identified as Category III according to World Health Organization recommendations. Following the bite, the first 2 doses of PVRV (1.0ml) were injected in his left deltoid, and Human Rabies Immunoglobulin (HRIG, 20IU/kg, 1100IU totally) was injected around his wound and in the muscles of his right lower extremity within 3 hours. Four hours later, the patient underwent loss of consciousness and generalized convulsion. And no symptoms of fever, headache, vomiting and paralysis were complained. On admission, neurologic examination revealed nothing but spontaneous positive Babinski's sign of bilateral lower limbs. Computed tomography (CT) of head was taken one hour later in the local hospital, which showed no abnormality. Based on the symptoms, signs, history in the past and result of CT above, he was initially diagnosed as "Secondary epilepsy." Then the patient received series of blood tests. White cell count was normal in blood routine. C-reactive protein (CRP) was less than 3.34 mg/L (normal value< 8.00 mg/L). Erythrocyte sedimentation rate (ESR) was 2mm/h (normal value<15mm/h). Rheumatoid factor was less than 10.4 IU/ml (normal value<15.9 IU/ml). Anti-streptolycin O (ASO) was less than 54.1IU/ml (normal value<200.0 IU/ml). Antineutrophil cytoplasmic antibodies (ANCA) were within normal limits. Then electroencephalography (EEG) examination and lumbar puncture was respectively completed at 24 hours after admission. EEG demonstrated diffuse generalized slow background activity. And cerebrospinal fluid (CSF) examinations including conventional, biochemistry and cytology were completely normal. On the third day after admitted, he took the imaging examination. Brain magnetic resonance image (MRI) showed multifocal and patchy hyper-intense signals at white matter of bilateralfrontal, parietal lobes and centrum semiovale as well as around the lateral ventricle on T2-weighted (T2W) and fluid-attenuated inversion recovery (FLAIR) sequences (seen in Fig.1). Spinal cord MRI demonstrated no abnormal signals. Taken together, these features were suggestive of ADEM. In view of demyelinating disease the patient was treated with 15mg intravenous dexamethasone per day for 5 consecutive days, followed by 10mg for 3 days and then 5 mg for 3 days. Then oral tablet prednisolone was administrated instead and the doses of steroids were gradually tapered. Besides, he was given 20% mannitol and oral anti-epileptic drug. The patient was followed up for one year, when he was seizure-free. And he did not take any imaging and laboratory examinations during this one-year period.
Figure 1.
MRI brain showing multifocal and patchy hyper-tense lesions in white matter of bilateral frontal, parietal lobes and centrum semiovale as well as around the lateral ventricle in asymmetric pattern on T2W or FLAIR.
Immunogenicity Results
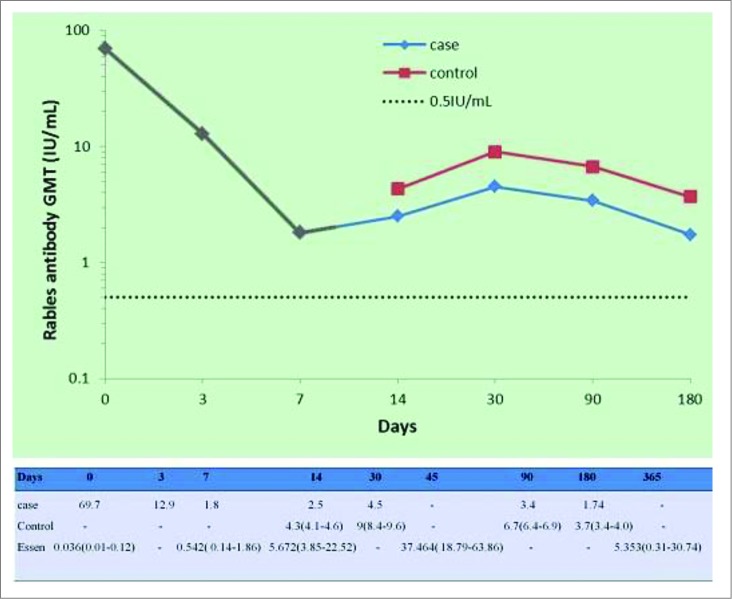
The immunogenicity analysis was completed using the homologous rapid fluorescent focus inhibition test (RFFIT) results. RVNA titer on 0d was 69.7 IU/ml, which may be attributed to the passive immune response of HRIG. Then the antibody level decreased dramatically from the maximum to 12.9 IU/ml (3d) and 1.8 IU/ml (7d). Following the third vaccine dose on day 7, there was a considerable increase in antibody titer. And the growing antibody titers on 14d (2.5 IU/ml) and 30d (4.5 IU/ml) indicated that positive immune started to work. While RVNA titers had begun to reduce again since 30d, reaching the minimum 1.74 IU/ml (187d). In addition, we observed that RVNA titers in our patient's sera were comparatively lower than that of control10 from 14d to 180d, but all of them were above 0.5 IU/ml. (seen in Fig.2)
Figure 2.
Gray part of the blue line meant that steroids were used in this period(dexamethasone, intravenously, 15mg/d for 5 days, followed by 10mg/d for 3 days and then 5 mg/d for 3 days). Besides, the data of the control came from a published paper and were put here just as a reference but not a real 'control'. In the table above, the data of a third group named “Essen” were from one of our previous studies.20 Here they were considered as reference to our case, because RVNA levels of both groups were detected by the same method and in the same laboratory.
Discussion
ADEM is an acute inflammatory demyelinating disorder of the central nervous system that often follows viral infection or vaccination.11 Its etiology mainly consists of infection and immunization. Cases of post vaccination account for about 5% of all ADEM ones and several vaccines have been described to be related to this condition.12 Clinical diagnosis of ADEM is based upon a combination of clinical, radiologic features and exclusion of other diseases that resemble the disease.13 In this report, our patient was clinically diagnosed with post-immunization ADEM based on the history, clinical findings, laboratory and neuroimaging findings.
At the moment of being admitted, the patient was considered as “secondary epilepsy.” Subsequently, the patient received series of blood tests, CSF, electrophysiological and imaging examination for etiologic diagnosis during hospitalization. Seizures, characterized by general convulsion with concomitant alteration of consciousness, were indicative of diffuse or global cortical dysfunction, which is the manifestation of encephalopathy.14 It is widely recognized that encephalopathy is proposed to be a required criterion for ADEM.. Also, MRI is the imaging modality of choice for demonstrating the lesions of demyelinating diseases. T2W and FLAIR images demonstrated bilateral asymmetric, patchy, increased signal intensities in the white matter of frontal-parietal and centrum semiovale, which was consistent with typical lesions in ADEM patients.15 In addition, patterns and waveforms demonstrated on EEG may be suggestive of subsequent seizure. The majority of ADEM cases are attributed to a post-infectious etiology, but our patient had no infecting sign before admission. And there was no positive outcome indicating inflammation in his body in laboratory investigation. Most importantly, his CSF examination was negative, indicating impossibility of encephalitis that was caused by pathogen infection. At the same time, all these findings also indicated no possibility of meningoencephalitis. And the patient's clinical manifestation and imaging finds were inconsistent with those of patients with acute cerebral infarction. The favorable outcome of our patient may be explained by the preservation of axons despite the occurrence of marked demyelination. Besides, in view of our patient's history, he did not be exposed to any drugs (immunosuppressive or chemotherapeutic agents, antiepileptic drugs, or heroin), metals (lead, manganese, or mercury), or industrial and environmental chemicals (solvent or carbon monoxide) lately. Hence, we speculated that demyelinating injury was induced by PVRV and HRIG. To our knowledge, this is the first case report about ADEM following administration of both PVRV and HRIG simultanously. Nevertheless, it was quite essential for the patient to continue the vaccination for the reason that the dog was highly doubted infection of rabies virus. As was mentioned above, injection of PVRV was advised to postpone when the patient was treated with corticosteroids in PPRC (2010 edition). So we replaced PVRV with PCECV. In the meanwhile, treatments for ADEM were started.
Based on the presumed autoimmune etiology of ADEM, the common treatment approach is administration of corticosteroid. High-dose corticosteroids like intravenous methylprednisolone, followed by an oral taper, are widely considered as first-line treatment for the acute phase of ADEM. Therefore, the patient was treated with intravenous dexamethasone, followed by oral prednisolone with subsequent tapering over several weeks. During the period of subsequent one-year follow up, there was no relapse in our patient, suggesting that PCECV had no impact on the prognosis of ADEM. In addition, the monophasic course of the disease was in favor of identification of ADEM and multiple sclerosis (MS).16
It is well known that glycoprotein is the only surface-exposed protein on the rabies virus particle,17,18 and a number of antigenic sites to which neutralizing monoclonal antibodies bind have been identified on this protein. Thus the key target for antibodies is virus glycoprotein. And the titer of RVNA, which reflects partly humoral and cell immunity in people after vaccination, is an important parameter for evaluating the efficacy of the immunization vaccine for either pre-exposure or post-exposure prophylaxis for rabies. Therefore, we detected RVNA titers of our patient at 7 different time points by RFFIT, the gold standard for testing the neutralizing antibodies currently.19 We found that our patient's antibody levels decreased indeed, compared with those of patients without treatment of glucorticoids as control and the findings in our previous study.20 Accordingly, we contributed the decrease of RVNA to negative effect of glucocorticoids on post-vaccinating immune response. They could suppress both cellular and humoral immunity through different pathways, thereby inhibiting antibody production. Nevertheless, 7 titers of vaccine-induced RVNA were all above the range of protective antibodies (≥0.5 IU/ml), showing that steroids had no significant effect on protection of these vaccines against rabies. Additionally, we observed that trend of our patient's RVNA levels was almost consistent with that of the subjects in control group, which might result from repeated administration of rabies vaccines (6 times/7 doses, together). Here we emphasized that the data of the control were cited here only as a reference but not a real control. However, we also observed that RVNA titer on 0d was 69.7 IU/ml. The antibody level was quite high and we attributed it to the following aspects. Actually, the '0d' was the third day after injection of HRIG and PVRV. These time points (0, 3, 7, 14, 30, 96 and 187d) began at administration of PCECV. Moreover, our way that most of HRIG was injected intramuscularly was in part different from other researchers' way of local infiltration around the wound. So we deemed that the RVNA level increased significantly due to absorption of HRIG into his blood but not induction of PVRV or PCECV.
To sum up, we reported that a man got ADEM following both PVRV and HRIG. Then intravenous and oral steroids therapy was rather essential and effective for him. And administration of PCECV did not affect prognosis of ADEM. Furthermore, we observed that steroids definitely had suppressed production of RVNA but the antibody levels had maintained within the range of protective ones. That was to say, rabies vaccines could induce the patient's adequate immune response for protection against rabies when steroids were used at the same time. However, this is just a preliminary observation of a single case. Therefore, more cases need to be collected and more work need to do for sufficient evidence of a general conclusion.
Materials
PCECV manufactured by factories of Novartis located in India, lot number (s20100074), with an antigen content of 7.0 IU per 1.0 ml ampoule; PVRV manufactured in China, with an antigen content of 7.5 IU per 1.0 ml ampoule; HRIG made in China, administered at 20 IU per kg of body weight (total dosages of 1100IU); reference serum, 30IU/ml, from the second International Standard(NIBSC/WHO); fluorescent antibody, from Millipore, Catalog Number 5100.
Methods
The patient's rabies vaccination had not yet been completed before he was admitted. Subsequently, he was vaccinated with PCECV in 1.0ml dose at one site on days 0, 3, 7, 14, 30 as Essen regimen21,22 instead of PVRV which was administrated 3 days ago. Then serum samples were collected respectively on days 0, 3, 7, 14, 30, 96 and 187 (Here it was worth noting that these time points started from the first injection of PCECV). Finally, RVNA in the isolated sera were measured by rapid fluorescent focus inhibition test (RFFIT).23 Briefly, a constant dose of previously titrated, cell culture adapted, challenge virus (CVS-11) was incubated with serial dilution (one of third dilution, from 1/3 to 1/6561) of the sera to be titrated. A reference serum of known titer was included in each test. After one hour of incubation at 37 °C, BSR cells (clone BHK21) were added in each well. After 24 h incubation, the estimation of the percentage of infected cells for each dilution of the sera allowed determination of the titer of the unknown sera by comparing with the reference serum. Meanwhile, one of reference sera that we bought was sent to National Centers for Disease Control (NCDC) in China for testing the antibody level to avoid deviation. Titers of sera were expressed as International Units per milliliter (IU/ml). Sera with titers ≥0.5 IU/ml, the WHO recommended protective level, were considered as positive.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. WHO Expert consultation on rabies. Second report. World Health Organ Tech Rep Ser 2013:1-139, back cover. [PubMed] [Google Scholar]
- 2. Rabies vaccines : WHO position paper–recommendations. Vaccine 2010; 28:7140-2; PMID:20831913; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 3. Fuenzalida E, Palacios R, Borgono JM. Antirabies antibody response in man to vaccine made from infected suckling-mouse brains. Bull World Health Organ 1964; 30:431-6; PMID:14163964 [PMC free article] [PubMed] [Google Scholar]
- 4. Kuwert EK, Marcus I, Hoher PG. Neutralizing and complement-fixing antibody responses in pre- and post-exposure vaccinees to a rabies vaccine produced in human diploid cells. J Biol Stand 1976; 4:249-62; PMID:825515; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 5. Fridell E, Grandien M, Johansson R. Pre-exposure prophylaxis against rabies in children by human diploid cell vaccine. Lancet 1984; 1:623; PMID:6142322; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 6. Koprowski H, Cox HR. Studies on chick embryo adapted rabies virus; culture characteristics and pathogenicity. J Immunol 1948; 60:533-54; PMID:18106204 [PubMed] [Google Scholar]
- 7. Wang LY, Sun MP, Zhang XC, Suo LD, Xu RH, Zou YJ, Zuo LB, Qi H. Safety and immunogenicity of two freeze-dried Vero cell rabies vaccines for human use in post-exposure prophylaxis. Vaccine 2011; 29:2679-81; PMID:21296694; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 8. Pichon S, Guinet-Morlot F, Minutello M, Donazzolo Y, Rouzier R, Chassard D, Fitoussi S, Hou V. A serum-free, purified vero cell rabies vaccine is safe and as immunogenic as the reference vaccine Verorab for pre-exposure use in healthy adults: results from a randomized controlled phase-II trial. Vaccine 2013; 31:2295-301; PMID:23510665; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 9. Kulkarni V, Nadgir D, Tapiawala S, Malabari A, Kalgikar A, Kela R, Nadkar M, Kamath S, Shah A. Biphasic demyelination of the nervous system following anti-rabies vaccination. Neurol India 2004; 52:106-8; PMID:15069254 [PubMed] [Google Scholar]
- 10. Madhusudana SN, Sanjay TV, Mahendra BJ, Sudarshan MK, Narayana DH, Giri A, Muhamuda K, Ravi V, Vakil HB, Malerczyk C. Comparison of saftey and immunogenicity of purified chick embryo cell rabies vaccine (PCECV) and purified vero cell rabies vaccine (PVRV) using the Thai Red Cross intradermal regimen at a dose of 0.1 ML. Hum Vaccin 2006; 2:200-4; PMID:17035734; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 11. Tselis A. Acute disseminated encephalomyelitis. Curr Treat Options Neurol 2001; 3:537-42; PMID:11581530; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 12. Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci 2008; 15:1315-22; PMID:18976924; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roos KL. Encephalitis. Neurol Clin 1999; 17:813-33; PMID:10517930; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 14. Bonhoeffer J, Menkes J, Gold MS, de Souza-Brito G, Fisher MC, Halsey N, Vermeer P. Generalized convulsive seizure as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. Vaccine 2004; 22:557-62; PMID:14741144; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 15. Singh S, Alexander M, Korah IP. Acute disseminated encephalomyelitis: MR imaging features. AJR Am J Roentgenol 1999; 173:1101-7; PMID:10511187; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 16. Ketelslegers IA, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ. A comparison of MRI criteria for diagnosing pediatric ADEM and MS. Neurology 2010; 74:1412-5; PMID:20335562; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 17. Wiktor TJ, Macfarlan RI, Reagan KJ, Dietzschold B, Curtis PJ, Wunner WH, Kieny MP, Lathe R, Lecocq JP, Mackett M, et al. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A 1984; 81:7194-8; PMID:6095272; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cadoz M, Strady A, Meignier B, Taylor J, Tartaglia J, Paoletti E, Plotkin S. Immunisation with canarypox virus expressing rabies glycoprotein. Lancet 1992; 339:1429-32; PMID:1351126; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 19. Yu PC, Noguchi A, Inoue S, Tang Q, Rayner S, Liang GD. Comparison of RFFIT tests with different standard sera and testing procedures. Virol Sin 2012; 27:187-93; PMID:22684473; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu Q, Liu MQ, Zhu ZG, Zhu ZR, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccin Immunother 2014; 10(6): 10.4161/hv.28420(DOI); http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plotkin SA, Wiktor TJ, Koprowski H, Rosanoff EI, Tint H. Immunization schedules for the new human diploid cell vaccine against rabies. Am J Epidemiol 1976; 103:75-80; PMID:942811 [DOI] [PubMed] [Google Scholar]
- 22. Pietropaolo V, Seganti L, Marchetti M, Sinibaldi L, Orsi N, Nicoletti R. Effect of natural and semisynthetic polymers on rabies virus infection in CER cells. Res Virol 1993; 144:151-8; PMID:8511399; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 23. Smith JS, Yager PA, Baer GM. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ 1973; 48:535-41; PMID:4544144 [PMC free article] [PubMed] [Google Scholar]