Abstract
Though human papillomavirus (HPV) vaccines based on L1 virus-like particles (VLPs) have excellent protective effect against HPV-induced cervical cancer, they are too expensive to be afforded by the developing countries, where most cases of cervical cancer occur. A live bacterial-based vaccine could be an inexpensive alternative. The aim of this study was to evaluate the potential value of live attenuated Shigella. flexneri 2a sc602 strain-based HPV16L1 as a high-efficiency, low-cost HPV16L1 mucosal vaccine. Recombinant sc602/L1 vaccine induced high L1-specific systemic and mucosal immune responses as well as cell-mediated Th1 and Th2 immune responses in guinea pig model. Sc602/L1 vaccine induced higher L1-specific IgG and IgA antibodies as well as HPV16-neutralizing antibodies in genital region in sc602/L1 mucosal immunized animals than in L1 intramuscular immunized animals. Though both are via mucosal delivery, immunized sc602/L1 vaccine by rectum route induced higher L1-specific IgA and IgG titers in genital region than by conjunctiva route. In addition, sc602/L1 also strongly increased L1-specific IFN-γ and IL-4 expression, implying its effect on cell-mediated immune response. HPV16L1 was expressed in sc602 bacteria and their biologic characteristics were detected by immunoblot, electron microscope and HeLa cell invasion assay. Guinea pigs were immunized with sc602L1 through conjunctiva (i.c.) or rectum (i.r.). Mucosal and systemic immune responses were detected by ELISA, ELISPOT and Neutralization activity assays. Strong mucosal and systemic immune responses were induced by sc602/L1 vaccine. This study provides evidence that sc602/L1 vaccine may have protective effect on HPV infection.
Keywords: guinea pigs, human papillomavirus (HPV), immune response, sc602/L1, vaccine
Abbreviations
- HPV
human papillomavirus
- VLP
virus like particles
- S.flexneri
Shigella flexneri
- DMEM
Dulbecco's minimal essential medium
- ELISA
Enzyme linked immunosorbent assay
- ELISPOT
enzyme linked immunospot
- SEAP
secreted alkaline phosphatase
- IFN-γ
interferon γ
- IL-4
interleukin 4
Introduction
Cervical cancer is the most common genital cancer and is the major cancer-related cause of death among women in developing countries. Persistent infection with high-risk human papillomavirus (HPV) types, especially HPV-16 and HPV-18, is closely correlated with development of cervical cancer.1 The strong etiological relationship between high-risk HPVs infections and cervical cancer has prompted developing prophylactic and therapeutic vaccines against such agents.
In the last decade, 2 vaccines, Gardasil® (Merck Sharpe & Dohme) and Cervarix® (GlaxoSmithKline; GSK), that prevent infection with HPV-16 and HPV-18 have been introduced to the market with high effectiveness. These vaccines are based on in vitro expression of viral major structural protein L1, which can self-assemble into virus-like particles (VLP).2 These VLPs induce high titers of systemic neutralizing antibodies and successfully prevent initial virus infection and thus subsequent premalignant lesions (CIN) and ultimately cervical cancer.3-6 However, as these vaccines are expressed in and purified from eukaryotic cells (insect cells or yeast), they will likely too expensive to be affordable for resource-poor countries where, in fact, 83% of all cervical cancer cases occur.7,8 So, development of an affordable and easily distributable vaccine is the ultimate answer for preventing HPV-related cervical cancer worldwide.9 Moreover, the marketed HPV vaccines are given by intramuscular injection. Though HPV VLP intramuscular administration can induce a stronger antibody response, it still needs exploit a better immunized route. HPV L1 protein can be expressed and self-assembled into VLPs in bacteria. Taking this into account, generating recombinant attenuated pathogenic bacteria strain carrying HPV L1 is an attractive alternative, because it offers many potential advantages, such as the ability to elicit systemic and mucosal immunity, simplicity, convenience, and low cost.
Shigella bacilli are enteropathogenic bacteria that infect the ileum and colonic epithelium of humans and primates. A number of studies have shown that attenuated Shigella is an idea live vector because of its weak immunogenicity, unable to elicit permanent immunity, and its infection does not spread to the whole body.10-12 Moreover, attenuated Shigella also provides potential effect on Shigella infection. Therefore, in the present study, we explored the possibility of expressing the HPV16 L1 in an attenuated Shigella flexneri (S. flexneri) 2a sc602 strain to make recombinant strain and evaluated its immunogenicity and protective efficacy in guinea pig model.
Results
The characteristics of recombinant sc602/L1 vaccine
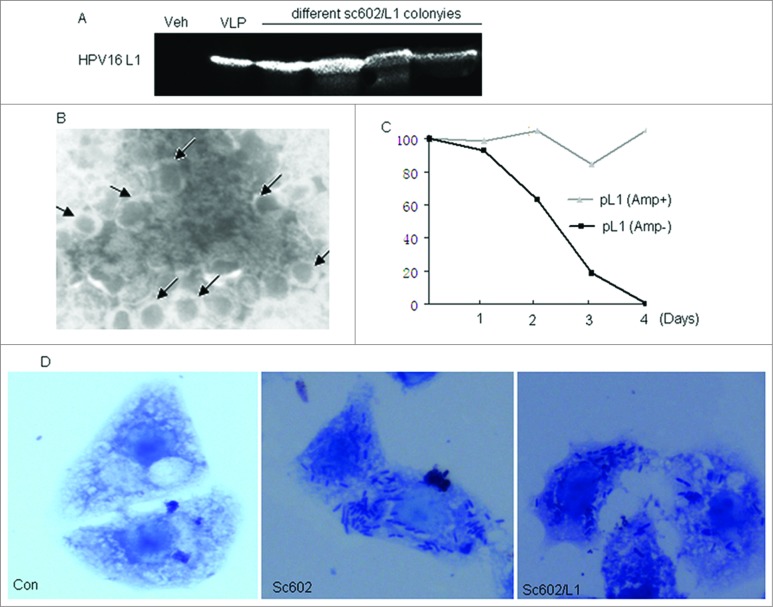
The expression of L1 protein in sc602/L1 strain was initially confirmed by immunoblot using HPV16 L1-specific monoclonal antibody (Fig. 1A). All sc602/L1 bacterial strains come from different colonies expressed HPV16 L1, but no HPV16 L1 protein expression was observed in control bacteria. The structure of the VLPs was examined by electron microscopy. Hollow spherical particles with 55 nm in diameter were seen in the recombinant sc602/L1 bacterial lysate (Fig. 1B). Xu et al has reported that plasmid vectors cloned into Shigella bacteria are unstable both in vitro and in vivo.15 We also observed the same result in sc602/L1 recombinant bacteria. Though sc602/L1 bacteria preserved L1 gene in the presence of ampicillin, about 40% of them lost L1 gene in the absence of antibiotic selection on the second day. On the fourth day, all of the bacteria lost L1 gene (Fig. 1C). The purpose of our experiments was to deliver the expressed L1 protein to the host by sc602/L1 mucosal infection. So in our experiment, L1 protein stability was more important than L1 gene stability. Sc602/L1 bacterial invasion ability was also detected by HeLa cell infection assay. Representative photomicrographs are shown in Fig. 1D. Both wild type sc602 and recombinant sc602/L1 strains induced bacterial accumulation in Hela cells, a phenomenon of Shigella bacterial invasion. The number of intracellular bacteria was 7362 ± 274/104 cells in wild type sc602 infection and 6976 ± 215/104 cells in recombinant sc602/L1 infection (P = 0.376).
Figure 1.
The characteristics of HPV16L1 expressed in recombinant sc602/L1. (A) HPV16L1 protein expression in recombinant sc602/L1 Shigella vector, monitored by Western Blot. Lane 1, wild type sc602; lane 2, HPV16 virus-like particles; lane 3–6, HPV16L1 protein expressed in recombinant sc602/L1, 4h after IPTG induction. (B) Electron micrographs of self-assembled HPV16 virus like particles negatively stained with phosphotungstic acid. Magnification, 50000×. (C) sc602/L1bacteria preserved L1 gene were detected in the present or absence of ampicillin. (D) Representative photomicrographs of Giemsa staining for intracellular bacteria in infected HeLa cells (Magnification, 1000×). Both wild type sc602 and recombinant sc602/L1 induced bacterial accumulation around nuclei, a phenomenon of Shigella bacterial invasion. The number of intracellular bacteria was higher in wild type sc602 infected cells than in sc602/L1 infected cells.
Humoral immune responses following administration of recombinant sc602/L1 vaccine
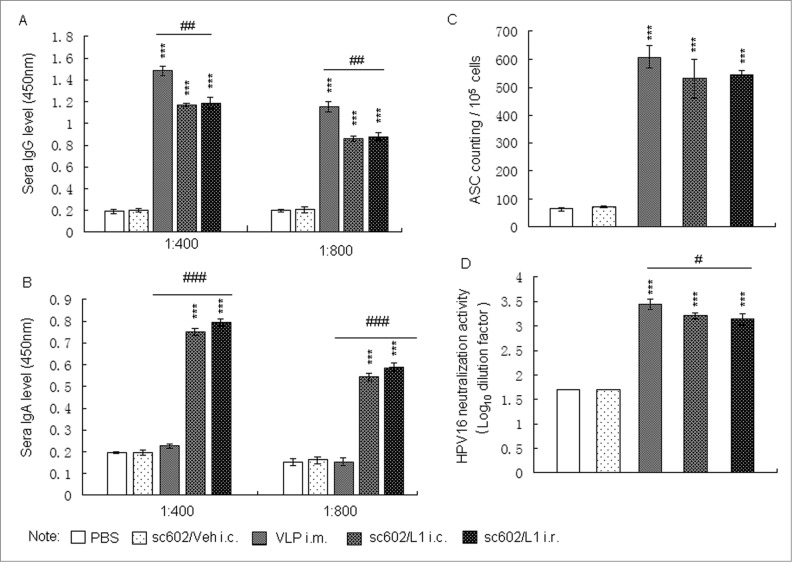
Serum IgG and IgA levels were determined by ELISA. Six weeks after last immunizations, the levels of serum IgG and IgA antibodies specific for HPV16 L1 were detected among groups treated with sc602/L1 and HPV16 L1 VLP, but not in PBS or sc602/Veh -immunized groups. IgG level was higher but IgA level was lower in HPV 16 L1 immunized animals than in sc602/L1 immunized animals. But there was no significant difference between sc602/L1 i.c. and i.r. groups (Fig.2A and B). ELISPOT results also showed that the HPV16 L1 specific IgG-ASCs significantly increased in the aforementioned spleen tissue (Fig. 2C). Consistent with HPVL1-specific serum IgG, neutralizing antibody did not differ among groups administered with sc602/L1 or HPV16 L1 VLP. Six weeks after the last immunization, the titers of HPV 16 neutralizing antibodies were higher in HP16 L1 i.m. groups, sc602/L1 i.c. groups, and sc602/L1 i.r. groups than control groups (Fig. 2D). All these results confirmed that the recombinant sc602/L1 live vector had good immunogenicity.
Figure 2.
Humoral immune responses following administration of recombinant sc602/L1.(A and B) Six weeks after last immunization, sera were collected. Serum IgG and IgA levels were determined by VLP capture ELISA. (C) ELISPOT assay for HPV16 L1 specific IgG-ASCs. The numbers of HPV16 L1 specific IgG-ASCs were count per 1 × 105 spleen cells. (D) Neutralization assays for HPV16 neutralization activity. Six weeks after the last immunization, the titers of HPV 16 neutralizing antibodies were higher in groups treated with HP16L1, sc602/L1 i.c. groups, and sc602/L1 i.r. groups than control groups. Means ± S.E.M., n = 6; *** P < 0.001 vs control group; # P < 0.05, ## P < 0.01, ### P < 0.001 vs HPV16L1 i.m. groups.
Vaginal immune responses following administration of recombinant sc602/L1 vaccine
Because genital antibodies are crucial in protecting against sexually transmitted infections and form the first line of defense against such infectious agents,20 mucosal immune responses following administration of recombinant sc602/L1 vaccine were also tested in vaginal secretions. The levels of vaginal IgG and IgA antibodies specific for HPV 16 L1 were detected among groups treated with sc602/L1 and HPV16 L1 VLP. Vaginal HPV16L1- specific IgG antibodies were observed among HPV16L1 i.m. groups, sc602/L1 i.c. groups, and sc602/L1 i.r. groups, with a slightly higher level in sc602/L1 i.r. groups (Fig. 3A). However, IgA antibodies specific for HPV 16 L1 were only detected in groups treated with sc602/L1, and sc602/L1 i.r. groups showed higher level of the vaginal IgA than sc602/L1 i.c. groups (Fig. 3B). The titers of HPV16L1-specific neutralizing antibodies were significantly higher among groups treated with HPV16L1 and sc602/L1, and sc602/L1 i.r. groups showed better results than sc602/L1 i.c. groups and HPV16L1 i.m. groups (Fig. 3C).
Figure 3.
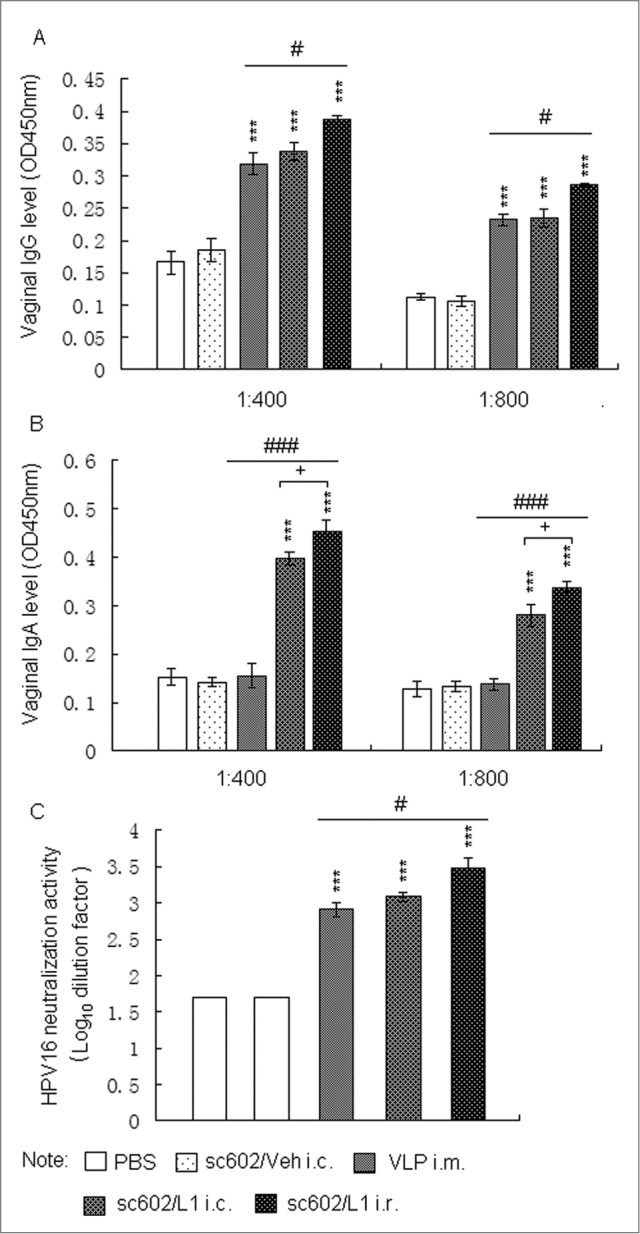
Vaginal immune responses following administration of recombinant sc602/L1. (A and B)Six weeks after last immunization, vaginal secretions were collected. Vaginal HPV16L1- specific IgG and IgA levels were determined by VLP capture ELISA. (C) Neutralization assays for HPV16 neutralization activity in vaginal secretions. Means ± S.E.M., n = 6; *** P < 0.001 vs control group; # P < 0.05, ### P < 0.001 vs HPV16L1 i.m. groups; + P < 0.05 vs sc602/L1 i.r. groups.
Cell-mediated immune responses induced by recombinant sc602/L1 vaccine
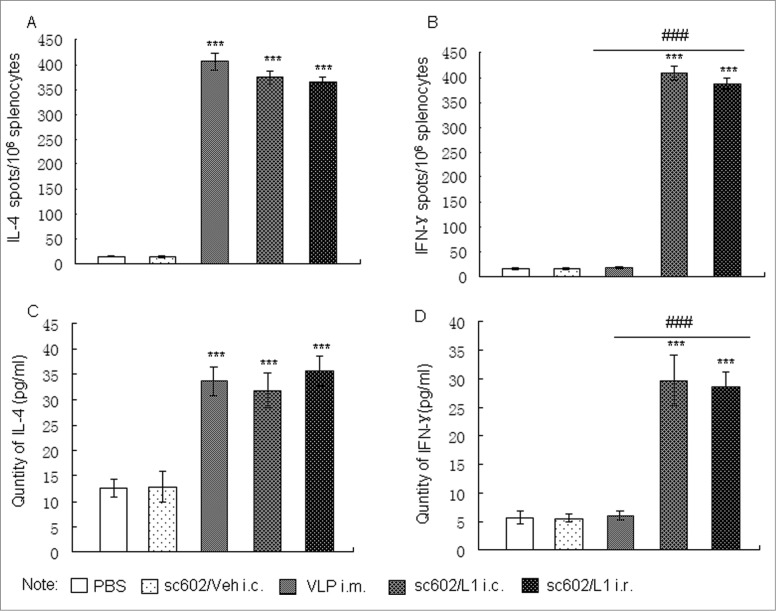
In addition to humoral immune responses, cell-mediated immune responses are also important in HPV prevention.21 Therefore, cell-mediated immune responses against HPV16 L1 were also examined by measuring IFN-γ (Th1) and IL-4 (Th2) levels and IFN-γ/IL-4-secreting cell population. The number of IFN-γ/IL-4-secreting cells after stimulation with HPV16L1 was analyzed using an ELISPOT assay. The mean number of IFN-γ/IL-4-secreting cells was significantly increased in the sc602/L1 vaccine immunized groups, either through conjunctiva immunization or rectum immunization, compared with those of control groups (P < 0.001). However, in HPV16 L1 immunized through intramuscular injection groups, only L1-specific IL-4 secreting cells increased (Fig. 4A and B). As expected, the IFN-γ and IL-4 levels were higher in these splenic cell media as analyzed by ELISA (Fig. 4C and D).
Figure 4.
Cell-mediated immune responses induced by recombinant sc602/L1. (A and B)The number of IL-4-secreting cells (A) and IFN-γ-secreting cells (B) was analyzed using an ELISPOT assay. (C and D) The IL-4 (C) and IFN-γ levels (D) were analyzed by ELISA. Means ± S.E.M., n = 6; *** P < 0.001 vs control group; ### P < 0.001 vs HPV16L1 i.m. groups.
Discussion
Development of a prophylactic HPV vaccine has been a long-sought strategy to prevent cervical cancer. Currently, 2 HPV L1-based virus-like particle (VLP) vaccines are available commercially. These VLP vaccines have shown excellent protective effect in preventing the development of HPV16/18-induced cervical intraepithelial neoplasia.22 However, these vaccines are expensive and administered in the form of intramuscular injections. These vaccines are not affordable in the developing or underdeveloped countries where most cases of cervical cancer occur.
To produce low-cost, convenient, HPV vaccine, constructing recombinant bacteria carrying HPV L1 gene to be used as live attenuated vaccine is an attractive strategy. Attenuated Salmonella has been demonstrated to express HPV16L1 protein and stimulate strong neutralizing antibodies in mucosal sites.23,24 Since Salmonella and Shigella have close genetic relationship, which means that Shigella may also work as promising antigen delivery vehicle for HPV. Furthermore, Shigella infection is restricted in the colon/rectum epithelium and does not cause carrier state. In contrast, other organisms, such as Salmonella spp., may disseminate into other tissues and cause carrier state, which are safety concerns in terms of vaccination.25 Additionally, attenuated sc602 Shigella strain can offer concomitant protection against Shigella infection.26 Therefore, the attenuated Shigella is a better vehicle. In this study we used sc602 strain as delivery system for HPV16 L1 and evaluated its immunogenicity in guinea pigs. We engineered a recombinant sc602/L1 starin, which stably expressed L1 protein as demonstrated by immunoblot. Since L1 protein assembled into capsid-like structure in bacteria, invasive ability is very important for delivery of the viral antigen, and it was found that Sc602/L1 had the same invasion ability like the wild type sc602.
Guinea pigs immunized with recombinant sc602/L1 induced humoral immune responses as well as mucosal immune responses by conjunctiva- or rectum- route. Previous studies showed that VLPs produce a higher titer of mucosal IgA antibodies only when VLPs are administered together with mucosal adjuvants; otherwise they cause immune tolerance.27,28 Our results also showed that HPV 16 L1 protein only induced high L1-specific IgG antibodies in sera and vaginal secretions, but no L1-specific IgA antibodies. However, recombinant sc602/L1 vaccine induced high levels of L1-specific IgG and IgA antibodies both in sera and vaginal secretions. Interestingly, though both are via mucosal delivery, immunized sc602/L1 vaccine by i.r. route induced higher L1-specific IgA and IgG titers in genital region than by i.c. route. HPV16-neutralizing antibodies were also detected in the sera and vaginal washes and showed the same trend like L1-specific IgG. Some previous studies have demonstrated that intrarectal immunization induces higher antigen-specific IgG and IgA in genital and rectal tissue than systemic immunization or distant mucosal immunization.29,30 We highly speculated that different immune responses between rectal and conjunctival immunization groups were due to the significant differences in the immune environments of the colon and conjunctiva. However, since we did not monitor the number and time of sc602/L1 vaccine invaded into the rectal epithelium and the conjunctiva in our experiment, we cannot exclude the possibility that different immune response between rectal and conjunctival immunization groups may be due to the number of sc602/L1 bacteria invaded into tissues. This is a shortcoming of our present study.
`In addition to humoral immune responses, cell-mediated immune responses are also important for HPV prevention. Mustafa W et al has reported that Listeria monocytogenes delivery of HPV-16 major capsid protein L1 induced systemic and mucosal cell-mediated CD4+ and CD8+ T-cell responses after oral immunization.31 Our results confirmed that living bacterial vectors delivery of HPV-16 major capsid protein L1 elicited strong cell-mediated immune response. IFN-γ and IL-4 are representative cytokines in Th1 and Th2 cell type immune responses. Our ELISPOT and ELISA results showed that IFN-γ/IL-4 cytokines and IFN-γ/IL-4 secreting cells were significantly increased in recombinant sc602/L1 immunized groups, compared with control groups and L1 protein immunized group. The cell mediated immune response elicited by recombinant sc602/L1 was against the HPV16L1 protein and there was no significant difference between i.c. or i.r. groups.
In conclusion, in this study, we demonstrated that recombinant sc602/L1vaccine induced high L1-specific systemic and mucosal immune responses as well as cell-mediated Th1 and Th2 immune responses in guinea pig model. Compared with L1 intramuscular injection, sc602/L1 vaccine induced higher L1-specific IgG and IgA antibody as well as HPV16-neutralizing antibodies in vaginal secretions in sc602/L1 mucosal immunized animals than in L1 intramuscular immunized animals. Immunized sc602/L1 vaccine by i.r. route induced strong antibodies in genital region than by i.c. route. In addition, sc602/L1 also strongly increased L1-specific IFN-γ and IL-4 expression and the corresponding IFN-γ/IL-4 secreting cell amount, implying its effect on cell-mediated immune response. Our results provide evidences that sc602/L1 vaccine may have protective effect against HPV infection, but its feasibility and efficacy needs to be characterized. Additionally, sc602/L1 is based on sc602, an attenuated Shigella bacteria, so the protection against Shigella also need to be further detected in the future study.
Materials and Methods
Chemicals and reagents
pMMB68/LTB plasmids and S. flexneri 2a sc602 (short as sc602) were kindly provided by Dr. Jun Yu, Strachclade University,UK; p16L1 plasmids was a gift by John T. Schiller, USA. Mouse anti-HPV16L1 was from NeoMarkers (Fremont, USA). Rabbit anti-guinea pig IgG was purchased from DAKO, USA and Rabbit anti-guinea pig IgA was purchased from Bethyl, Montgomery, USA. Goat anti-rabbit IgG conjugated to alkaline phosphatase was purchased from Sigma (St Louis, USA).
Animals
Female guinea pigs, 6–8 weeks of age, were housed in the animal experimental center in Medical College of Xi’an Jiaotong University. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Xi’an Jiaotong University.
Construction of recombinant sc602/L1 Shigella strain
The modified HPV-16 L1 fragment was amplified by PCR from p16L1 plasmids which containing codon-modified HPV-16 L1. Plasmid pMMB68/HPV16-L1 was constructed by exchanging the heat-labile enterotoxin B subunit gene in pMMB68/LTB plasmids32 with HPV16-L1 open reading frame (EcoRI –HindIII digestion). Recombinant pMMB68/HPV16-L1 plasmid was electroporated into sc602 and further confirmed by PCR, restriction enzyme digestion and DNA sequence analysis. The positive bacterial colony was named as sc602/L1.
Immunoblot
Recombinant sc602/L1 bacteria were inoculated into LB medium containing 100 μg/ml ampicillin and grown overnight. The overnight culture was inoculated into fresh 2×YT media (1:100) for exponential growth. When the OD600 value reached 0.3, 0.1 mM IPTG was added to the culture medium to induce protein expression and the cells were incubated for further 4 h at 30°C. The cells were collected and lysed by sonication in PBS buffer contains 1 mg/ml lysozyme. The cell lysate was separated on 4–12% SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked by incubation for 1 h at room temperature in 10% non-fat dry milk in Tris-buffered saline (pH 7.5), then incubated with mouse anti-HPV16L1 monoclonal antibodies (NeoMarkers, Fremont, USA) at 1:1000. Horseradish peroxidase conjugated goat anti-mouse IgG (DaKo, Carpinteria, USA) was used as the secondary antibody. Visualization was carried out by using an enhanced chemiluminescence kit (GE Healthcare) and Biorad ChemiDoc XRS+ instrument.
Cell infection assay
HeLa cell is a classical cell model for detecting Shigella bacterial invasion ability.13 Whether pMMB68/HPV16-L1 plasmids attenuated sc602 bacterial invasion ability was also investigated by using HeLa cell infection assay. Briefly, HeLa cells were incubated on 35 mm plates in 5% CO2 at 37ºC to 70% confluence in antibiotic free Dulbecco's minimal essential medium (DMEM) containing 10% fetal calf serum. Sc602/L1 bacteria from the mid-exponential phase were added to the cells at a ratio of 10 CFU/cell for CFU counting and 100 CFU/cell for Giemsa staining. Bacteria were centrifuged at 1500 r.p.m. for 10 min at room temperature to settle around the cells. After further 40 min incubation, extracellular bacteria were removed by washing the cells 6 times with DMEM, and treated with DMEM containing 50 μg/ml gentamicin for 90 min to kill the extracellular bacteria. After washing with PBS, cells were lysed for CFU counting or fixed for Giemsa staining. For CFU counting, the infected cells monolayer was washed with PBS and lysed with 0.1% Triton X-100 in water. Cell lysates were plated onto LB agar to determine the number of CFU. For Giemsa staining, cells were fixed with 4% paraformaldehyde and stained with Giemsa-solution. The sections with intracellular bacteria were observed under microscope (10 × 100).
Electron microscopy
The recombinant sc602-/L1 strain was cultured in 200 ml fresh 2×YT media. When the OD600 value reached 0.3, 0.1 mM IPTG was added and the cells were incubated for further 4 h at 30°C. The bacteria were harvested by centrifuge at 5000g for 20 min. The pellet was suspended in 5 ml of ice-cold PBS containing 0.5 M NaCl and a protease inhibitor cocktail (Complete). The bacteria were disrupted by sonication with 6 20-s pulses and centrifuged at 8000g for 20 min at 4°C. Supernatant was collected and applied slowly to the top of a 10 to 40% sucrose gradient (8 ml) prepared in PBS–0.5 M NaCl buffer. The gradient was centrifuged at 224,000g in a SW41Ti rotor (Beckman) at 4°C for 1.5 h and then fractionated into 23 aliquots from the bottom to the top of the centrifuge tube. Heavier L1-positive fractions (30–40% sucrose) were stained with phosphotungstic acid and viewed by H-600 transmission electron microscope.
Production of HPV16 L1 VLPs
HPV16 VLPs were produced in insect cells harboring HPV16 L1-containing recombinant baculovirus as described by Zheng et al.14 Briefly, Sf9 cells were infected with HPV16 L1-containing recombinant baculoviruses at a multiplicity of infection (MOI) of 20. Cells were harvested 72 h post-infection and fractionated by sonication with 3 15-s bursts at 60% maximal power (Vibra-cell) in ice-bath with the presence of protease inhibitor cocktails (Complete). His-tag was used to purify VLPs using Probond™ column (Invitrogen) under native condition following the manufacturer's instructions.
Immunization of guinea pigs with sc602/L1
In guinea pigs, shigella can invade into the corneal epithelium or rectal epithelium, causing keratoconjunctivitis or rectocolitis, closely mimics the invasion process in human intestinal epithelium.15,16 So, we chose guinea pigs as suitable animal model for our experiment. The red colony of sc602/L1 was picked and prepared as above described. Guinea pigs were immunized via conjunctival or intrarectal route as reported. The protocol has been approved by the Animal Research Ethical Committee of the Xi’an Jiaotong University. Briefly, female guinea pigs aged 6–8 weeks old, weighing 200–300 g, were divided into 5 groups (1) PBS; (2) sc602/Veh by conjunctival route; (3) HPV16 L1 VLP by intramuscular injection (i.m.); (4) sc602/L1 by conjunctival route (i.c.); (5) sc602/L1 by intrarectal immunization (i.r.), with 5 animals in each group. Recombinant sc602/L1 bacteria were administered at the rate of 1 × 109 CFU per animal. Animals of the intramuscular group were injected intramuscular with 10μg HPV16 VLPs in PBS. Immunization was boosted with the same dosages after 2 weeks. Guinea pigs were sacrificed 6 weeks after the second immunization. Blood and vaginal secretions were collected for antibodies detection, and lymphocytes from spleen were obtained for ELISPOT assay.
Enzyme linked immunosorbent assay (ELISA) and enzyme linked immunospot (ELISPOT) assay
ELISA was performed as previously reported to evaluate HPV16 VLP-specific antibodies in the serum and vaginal secretions.17,18 Briefly, 96-well plates were coated with insect cell-derived L1 at a concentration of 3 μg per well. Serum or vaginal secretions were incubated with coated proteins in 2-fold serial dilution at room temperature for 2 h. Bound antibody was probed with rabbit anti- guinea pigs IgG (1:1,000, DAKO) or IgA (1:1,000, Bethyl, Montgomery, USA) and HRP labeled goat anti-rabbit IgG (1:5000, DAKO), followed by incubation with TMB for 15–30 min and read at A450. ELISPOT assay was performed to evaluate HPV16L1 -specific antibody-secreting cells (ASCs) frequency in the spleens.17 Briefly, 96-well microtiter plates were coated with insect cell-derived L1 at a concentration of 3 μg per well. Then, lymphocyte suspension was dispensed into prepared 96 well plates, and the plates were incubated for 4 h at 37°C in a humidified CO2 incubator. Thereafter, the plates were washed with PBS-Tween, and 100 μl rabbit anti-guinea pigs IgG (1:1,000, DAKO) or IgA (1:1,000, Bethyl, Montgomery, USA) was added, respectively. The plates were then incubated at 4°C overnight. Later on, 100μl of goat anti- rabbit IgG conjugated to alkaline phosphatase (Sigma, St Louis, USA) at 1:1000 was added to each well, and the plates were incubated for 2 h at 37°C. Finally, after the plates were washed with PBS-Tween, and spots were developed with one-step NBT/BCIP reagent (Pierce). Antigen-specific lymphocytes were counted under a stereomicroscope, and recorded as ASC per 105 cells. The lowest serum dilutions tested were 1:100.
Neutralization assays
Neutralization assays (NA) were performed with secreted alkaline phosphatase (SEAP) HPV16 pseudoviruses as described by Buck et al and Fraillery et al.18,19 Briefly, purified SEAP HPV16 pseudoviruses diluted 1600-fold were incubated on ice for 1 h with two-fold serial dilutions of the guinea pig serum, and the pseudovirus-antibody mixtures were used to infect 293TT cells, which were then cultured at 37°C with 5% CO2 for 60 h. The SEAP content in 10 μl of clarified cell supernatant was determined using the chemiluminescence SEAP reporter gene assay following the manufacturer's instructions (Roche). Neutralization titers were defined as the reciprocal of the highest serum dilution that caused at least a 50% reduction in SEAP activity. The lowest serum dilutions tested were 1:100. At this dilution, guinea pig serum of the negative control group did not show significant neutralizing activity.
IFN-γ/IL-4 ELISA and ELISPOT assay
96-well plates were coated with 0.2 μg of anti- guinea pig IFN-γ or anti- guinea pigs IL-4 antibodies (Boster Biotechnology, Wuhan, China) per well. Plates were washed 3 times with PBS, and then blocked by incubating with 10% FBS at 37°C. Splenocytes were seeded at 1 × 106 cells per well in 100 μl of medium, and stimulated by adding 10μg HPV16L1 VLPs (purified from insect cells) and incubating for an additional 24 hours at 37°C. Supernatants were harvested, and IFN-γ and IL-4 level were measured by Guinea pig IFN-γ ELISA Kit and Guinea pig IL-4 ELISA Kit (Repidbio Biotechnology, USA) according to the manufacturer's recommendation. For ELISPOT, plates were then washed with PBS containing 0.05% Tween-20 and treated with 20 ng of biotinylated anti- guinea pigs IFN-γ and anti- guinea pigs IL-4 antibodies (Repidbio Biotechnology, USA). After 2 hours, streptavidin-alkaline phosphatase was added to the wells, and color was developed with one-step NBT/BCIP reagent (Pierce). The numbers of spots were counted under a stereomicroscope.
Statistical analysis
The data are expressed as means ± S.E.M. The difference between 2 groups was evaluated using Student's t test. Multiple group comparison was performed using one-way analysis of variance followed by Tukey's post hoc test. A probability level of 0.05 was used to establish significance.
Acknowledgments
We gratefully thank for Dr John T. Schiller for providing the modified HPV-16 L1 sequence. We also thank for Dr Yu Jun for providing pMMB68 plasmid and sc602 Shigella strain.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The project was supported by the Fundamental Research Funds for the Central Universities (No. XJTU-HRT-002).
References
- 1. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev 2003;16:1-17; PMID:12525422; http://dx.doi.org/ 10.1128/CMR.16.1.1-17.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. PNAS 1992; 89:12180-4; PMID:1334560; http://dx.doi.org/ 10.1073/pnas.89.24.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirnbauer R, Taub J, Greenstone H, Roden R, Durst M, Gissmann L, Lowy DR, Schiller JT. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol 1993; 67:6929-36; PMID:8230414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med 2002; 347:1645-51; PMID:12444178; http://dx.doi.org/ 10.1056/NEJMoa020586 [DOI] [PubMed] [Google Scholar]
- 5. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection withhuman papillomavirus types 16 and 18 in youngwomen: a randomised controlled trial. Lancet 2004; 364:1757-65; PMID:15541448 [DOI] [PubMed] [Google Scholar]
- 6. Villa LLCR, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Høye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459-66; PMID:17117182; http://dx.doi.org/ 10.1038/sj.bjc.6603469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006; 24:S11-25; http://dx.doi.org/ 10.1016/j.vaccine.2006.05.111 [DOI] [PubMed] [Google Scholar]
- 8. Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries - key challenges and issues. N Engl J Med 2007; 356:1908-10; PMID:17494923; http://dx.doi.org/ 10.1056/NEJMp078053 [DOI] [PubMed] [Google Scholar]
- 9. Schiller JT, Nardelli-Haefliger D. Chapter 17: Second generation HPV vaccines to prevent cervical cancer. Vaccine 2006; 24:S147-53; http://dx.doi.org/ 10.1016/j.vaccine.2006.05.123 [DOI] [PubMed] [Google Scholar]
- 10. Shata MT, Stevceva L, Agwale S, Lewis GK, Hone DM. Recent advances with recombinant bacterial vaccine vectors. Mol Med Today 2000; 6: 66-71; PMID:10652479; http://dx.doi.org/ 10.1016/S1357-4310(99)01633-0 [DOI] [PubMed] [Google Scholar]
- 11. Blumberg RS, Lancer WI, Zhu X, Kim HS, Claypool S, Balk SP, Saubermann LJ, Colgan SP. Antigen presentation by intestinal epithelial cells. Immunol Lett 1999; 69: 7-11; PMID:10436875; http://dx.doi.org/ 10.1016/S0165-2478(99)00093-0 [DOI] [PubMed] [Google Scholar]
- 12. Li W, Liu H, Yang X, Zheng J, Wang Y, Si L. Development of prophylactic recombinant HPV58-attenuated Shigella live vector vaccine and evaluation of its protective efficacy and immunogenicity in the guinea pig keratoconjunctivitis model. Acta Biochim Biophys Sin 2009; 41:137-45; PMID:19204831; http://dx.doi.org/ 10.1093/abbs/gmn016 [DOI] [PubMed] [Google Scholar]
- 13. Sansonetti PJ, Ryter A, Clerc P, Maurelli AT, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun 1986; 51:461-9; PMID:3510976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng J, Ma J, Yang XF, Liu HL, Cheng HW, Si LS, Wang YL. Highly efficient and economical baculovirus expression system for Escherichia coli: characterization of protein domains involved in preparing human papillomavirus type 16 virus-like particle. Acta Biochim Biophys Sin 2004, 36: 548-552; http://dx.doi.org/ 10.1093/abbs/36.8.548 [DOI] [PubMed] [Google Scholar]
- 15. Xu D, Wang D, Yang X, Cao M, Yu J, and Wang Y. Fusion of HPV L1 into Shigella surface IcsA: a new approach in developing live attenuated Shigella-HPV vaccine. Antiviral Res 2014; 102:61-9; PMID:24333518; http://dx.doi.org/ 10.1016/j.antiviral.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 16. Shim DH1, Suzuki T, Chang SY, Park SM, Sansonetti PJ, Sasakawa C, Kweon MN. New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J Immunol 2007; 178:2476-82; PMID:17277155; http://dx.doi.org/ 10.4049/jimmunol.178.4.2476 [DOI] [PubMed] [Google Scholar]
- 17. Yang XF, Qu XZ, Wang K, Zheng J, Si LS, Dong XP, Wang YL. Construction of prophylactic human papillomavirus type 16 L1 capsid protein vaccine delivered by live attenuated Shigella flexneri strain sh42. Acta Biochim Biophys Sin 2005; 37:743-50; PMID:16270153; http://dx.doi.org/ 10.1111/j.1745-7270.2005.00109.x [DOI] [PubMed] [Google Scholar]
- 18. Fraillery D, Baud D, Pang SY, Schiller J, Bobst M, Zosso N, Ponci F, Nardelli-Haefliger D. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin Vaccine Immunol 2007; 14:1285-95; PMID:17687110; http://dx.doi.org/ 10.1128/CVI.00164-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med 2005;119:445-62; PMID:16350417 [DOI] [PubMed] [Google Scholar]
- 20. Nieto K, Stahl-Hennig C, Leuchs B, Muller M, Gissmann L, et al. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Human gene therapy 2012; 23: 733-741; PMID:22401308; http://dx.doi.org/ 10.1089/hum.2011.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinto LA, Edwards J, Castle PE, Harro CD, Lowy DR, Schiller JT, Wallace D, Kopp W, Adelsberger JW, Baseler MW, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis 2003; 188:327-38; PMID:12854090; http://dx.doi.org/ 10.1086/376505 [DOI] [PubMed] [Google Scholar]
- 22. Haupt RM, Sattler C. HPV vaccine continues to be safe and effective, and its benefits continue to outweigh its risks. Expert Rev Vaccines 2010; 9: 697-701; PMID:20624041; http://dx.doi.org/ 10.1586/erv.10.56 [DOI] [PubMed] [Google Scholar]
- 23. Baud D, Benyacoub J, Revaz V, Kok M, Ponci F, Bobst M, Curtiss R, 3rd, De Grandi P, Nardelli-Haefliger D. Immunogenicity against human papillomavirus type 16 virus-like particles is strongly enhanced by the PhoPc phenotype in Salmonella enterica serovar Typhimurium. Infect Immun 2004 72:750.756; http://dx.doi.org/ 10.1128/IAI.72.2.750-756.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nardelli-Haefliger D, Roden RB, Benyacoub J, Sahli R, Kraehenbuhl JP, Schiller JT, Lachat P, Potts A, De Grandi P. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun 1997; 65:3328-36; PMID:9234794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shata MT, Stevceva L, Agwale S, Lewis GK, Hone DM. Recent advances with recombinant bacterial vaccine vectors. Mol Med Today 2000, 6: 66-71; http://dx.doi.org/ 10.1016/S1357-4310(99)01633-0 [DOI] [PubMed] [Google Scholar]
- 26. Rahman KM, Arifeen SE, Zaman K, Rahman M, Raqib R, Yunus M, Begum N, Islam MS, Sohel BM, Rahman M, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine. 2011; 29:1347-54; PMID:21040694; http://dx.doi.org/ 10.1016/j.vaccine.2010.10.035 [DOI] [PubMed] [Google Scholar]
- 27. Breitburd F, Coursaget P. Human papillomavirus vaccines. Semin Cancer Biol. 1999; 9:431-44;; PMID:10712890; http://dx.doi.org/ 10.1006/scbi.1999.0147 [DOI] [PubMed] [Google Scholar]
- 28. Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 2003; 21:S89-95; PMID:12763689; http://dx.doi.org/ 10.1016/S0264-410X(03)00206-8 [DOI] [PubMed] [Google Scholar]
- 29. Agnello D, Hervé CA, Lavaux A, Darniot M, Guillon P, Charpilienne A, Pothier P. Intrarectal immunization with rotavirus 2/6 virus-like particles induces an antirotavirus immune response localized in the intestinal mucosa and protects against rotavirus infection in mice. J Virol 2006; 80:3823-32; PMID:16571799; http://dx.doi.org/ 10.1128/JVI.80.8.3823-3832.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eriksson K, Quiding-Järbrink M, Osek J, Möller A, Björk S, Holmgren J, Czerkinsky C. Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect Immun 1998; 66:5889-96; PMID:9826370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mustafa W, Maciag PC, Pan ZK, Weaver JR, Xiao Y, Isaacs SN, Paterson Y. Listeria monocytogenes delivery of HPV-16 major capsid protein L1 induces systemic and mucosal cell-mediated CD4+ and CD8+ T-cell responses after oral immunization. Viral Immunol 2009; 22:195-204; PMID:19435416; http://dx.doi.org/ 10.1089/vim.2008.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramesh A, Panda AK, Maiti BR, Mukhopadhyay A. Studies on plasmid stability and LTB production by recombinant Vibrio cholerae in batch and chemostat cultures: a lesson for optimizing conditions for chemical induction. J Biotechnol 1995;43:45-51; PMID:8573322; http://dx.doi.org/ 10.1016/0168-1656(95)00121-0 [DOI] [PubMed] [Google Scholar]