Abstract
Surveillance of adverse events following immunisation (AEFI) is an essential component of vaccine safety monitoring. The most commonly utilized passive surveillance systems rely predominantly on reporting by health care providers (HCP). We reviewed adverse event reports received in Victoria, Australia since surveillance commencement in July 2007, to June 2013 (6 years) to ascertain the contribution of consumer (vaccinee or their parent/guardian) reporting to vaccine safety monitoring and to inform future surveillance system development directions. Categorical data included were: reporter type; serious and non-serious AEFI category; and, vaccinee age group. Chi-square test and 2-sample test of proportions were used to compare categories; trend changes were assessed using linear regression. Consumer reporting increased over the 6 years, reaching 21% of reports received in 2013 (P <0.001), most commonly for children aged less than 7 years. Consumer reports were 5% more likely to describe serious AEFI than HCP (P = 0.018) and 10% more likely to result in specialist clinic attendance (P <0.001). Although online reporting increased to 32% of all report since its introduction in 2010, 85% of consumers continued to report by phone. Consumer reporting of AEFI is a valuable component of vaccine safety surveillance in addition to HCP reporting. Changes are required to AEFI reporting systems to implement efficient consumer AEFI reporting, but may be justified for their potential impact on signal detection sensitivity.
Keywords: adverse event, consumer, immunisation, safety, surveillance, vaccine
Abbreviations
- AEFI
Adverse event following immunization
- HCP
Health care provider
Introduction
An adverse event following immunisation (AEFI) is defined as “any untoward medical occurrence which follows immunisation and which does not necessarily have a causal relationship with the usage of the vaccine.”1 AEFI surveillance forms part of post-licensure vaccine safety monitoring worldwide and is a critical component of every immunisation program.2 Surveillance systems aim to record rare AEFI, unexpected events, or changes in rates of expected events. By maximizing reporting and use of report data, the ability to rapidly detect and investigate potential vaccine safety ‘signals’ is enhanced.2-4
Passive surveillance systems, which rely upon spontaneous reporting of AEFI, are most frequently utilised for AEFI surveillance.1 They have demonstrated advantages of being useful, simple and timely systems to identify AEFI reports that can then be further investigated and/or described as part of a cohort or case series, however, overall sensitivity is low.5,6 Under-reporting is a known issue with estimates showing up to 95% under-reporting occurring even in systems specifically targeting serious AEFI reports.7 Reporting is predominantly by health care providers (HCP), a group which is more likely to report already known AEFI than unexpected events.8
In response to these limitations, there is growing advocacy to encourage self-reporting by vaccinees or their parents (consumers) to maximize data collection.8-11 This was highlighted in Australia following the suspension of the 2010 seasonal influenza program for children following an increase in febrile seizures.12 However, evaluations of the relative contribution and utility of consumer reporting to pharmacovigilance are rare.11
In Victoria, Australia, reporting of AEFI is voluntary, however HCP are requested to report to the state-based AEFI surveillance system, SAEFVIC, an enhanced passive surveillance system established in 2007. It aims to enhance reporting by combining passive surveillance of AEFI with clinical services for reporting HCP and individuals experiencing AEFI.13 To attend a clinical follow-up appointment a referral from a medical practitioner is required. Detection and validation of vaccine safety signals, either as occurrences of serious AEFI or increased frequency in minor/common expected AEFI, is a core objective. AEFI reports are collated and summary information forwarded by the next working day to the national regulatory agency, the Therapeutic Goods Administration (TGA), which collates AEFI reports from all Australian jurisdictions.
SAEFVIC targets reporting from HCP, but accepts consumer reports if they arise. A key question to determine whether to target consumer reporting as well is: Are consumers less likely than HCP to report “significant” (serious or targeted) AEFI? There are currently no published data comparing the proportion of consumer and HCP reports considered to be serious AEFI, although there is increasing evidence of consumer awareness of AEFI.14,15 This is important for informing if the additional demand on resources to receive, collate and respond to an increased number of AEFI reports via consumers is justified, and whether it is beneficial to surveillance system objectives and the immunisation program.
Reports to SAEFVIC can be made by telephone, facsimile, mail and, since 2010, online (www.saefvic.org.au/). The online reporting interface was developed specifically for HCP reporters; but can also be utilized for consumer reporting.
Reviewing the potential for consumer reports to contribute to vaccine safety surveillance objectives will help inform future directions of AEFI surveillance locally and internationally.
Results
A total of 5455 reports were included. Reporter type was known for 99% (n = 5394) of reports, of which 515 (10%) were from consumers and 4879 (90%) from providers (of which 0.2% originated from pharmaceutical companies).
The proportion of direct consumer reports increased from an average of 6% of all reports received from 2007–2011 to 15% in 2012 and 21% in the first 6 months of 2013 (linear trend P <0.001) (Fig. 1).
Figure 1.
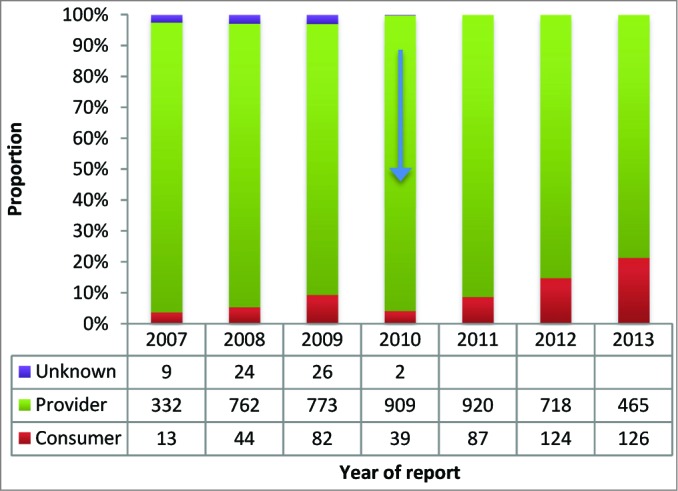
Proportion of AEFI reports received from consumers and providers, Victoria, 2007–2013*. * Arrow indicates commencement of online reporting facility1.
Age groups
Consumer reports were more likely to describe AEFI in a child than providers (P <0.001), but were less likely to describe adults (P <0.001) (Table 1).
Table 1.
Number and proportion of AEFI reports received by age group and reporter type
| Number of reports received (%) | Comparison of proportions | ||||
|---|---|---|---|---|---|
| Age at vaccination | Consumer | Provider | Total | P value | |
| Child | <7 | 379 (74) | 2912 (61) | 3291 | <0.001 |
| School | 7-<19 | 76 (15) | 807 (17) | 883 | 0.267 |
| Adult | 19-<60 | 38 (7) | 717 (15) | 755 | <0.001 |
| Elderly | 60+ | 16 (3) | 350 (7) | 366 | <0.001 |
| Total | 509 (10) | 4786 (90) | 5295 | ||
*Age not available for 99 reports
Serious AEFI
Overall 18% (969/5455) of SAEFVIC reports received in the first 6 years of operation (2007-2013) met the definition of ‘serious’. No trend over time was observed in the proportion of serious AEFI reported each year (P = 0.26).
Consumers were more likely to report a serious AEFI than providers; 22% compared with 17% (χ2 = 5.61, P = 0.02).
Time taken to report
Consumers were marginally slower to report an AEFI than providers with 44% compared to 50% reports received within 7 days of symptom onset respectively (P = 0.005) but by 100 days both reporter types were equal at approximately 80% of reports submitted (Fig. 2)
Figure 2.
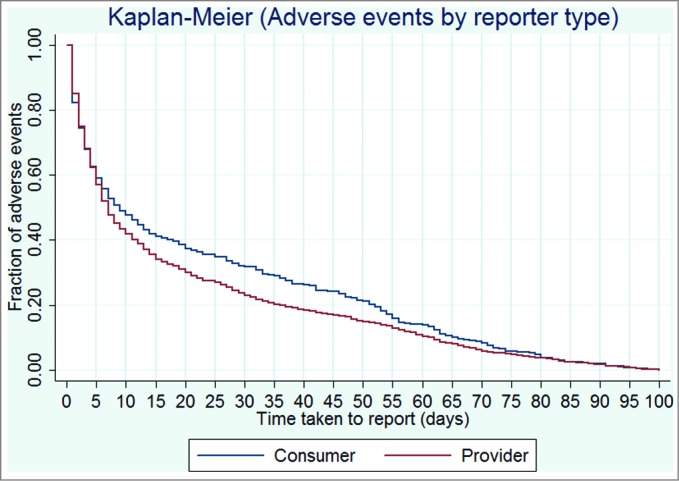
Kaplan-Meier comparison of consumer and provider time to report in days.
Reporting modalities
Online reporting now comprises approximately one third of all reports received, with a relative decrease in fax and postal reporting (Fig. 3).
Figure 3.
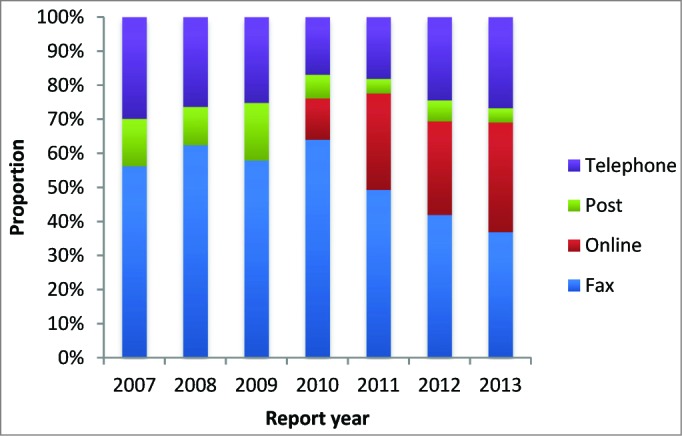
Proportion of AEFI reports received by reporting modality and year of report.
A marked decline in reporting by telephone was observed from 2007 to 2010 as alternative methods became more popular. However, a reverse of this trend was observed in 2012 and 2013 directly as a result of consumer reporting (P <0.001) (Fig. 4).
Figure 4.
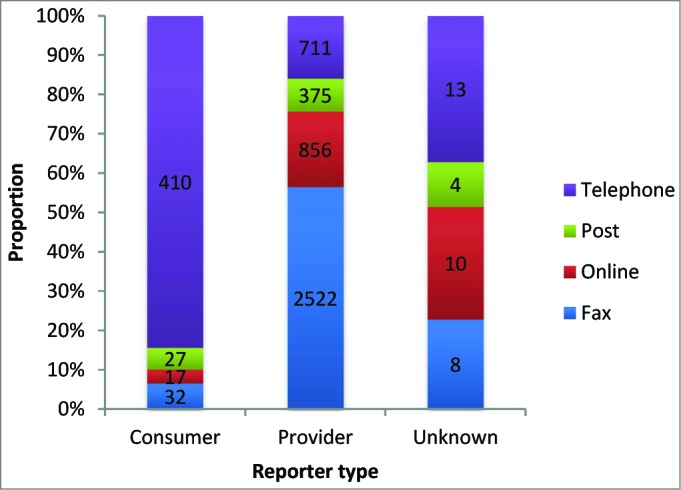
Proportion of AEFI reports received by reporter type and reporting modality.
Specialist vaccine safety clinic referral
Specialist vaccine safety clinic was attended by 30% (1629/5455) of persons for whom an AEFI was reported. A larger proportion of consumer reported AEFI 39% (203/515) required specialist clinical referral compared with 29% (1406/4879) of HCP reported AEFI (χ2 25.006, P <0.001).
Discussion
AEFI surveillance in Victoria has, in its first 6 years of operation, focused on health care provider reporting, however, unsolicited consumer reports have steadily increased and now comprise one-fifth of all AEFI reported.
Consumer AEFI reporting to the Victorian SAEFVIC vaccine safety service was not intentionally solicited, however, the increase over the study period was likely influenced by a number of contributing factors making reporting more accessible. These factors included: 1) a highly publicized vaccine safety scare and suspension relating to influenza vaccination of children in 2010 in Australia,12 which may have raised consumer awareness of AEFI in children generally; 2) the formation of a SAEFVIC website for online reporting (October 2010); 3) more prominent inclusion of SAEFVIC information on the Department of Health Immunisation website (December 2012), and; 4) the implementation of a SAEFVIC information sticker provided as a pilot to attendees of 2 tertiary hospital immunisation centers (March 2012) followed by general distribution together with national immunization program vaccine supplies to providers (September 2012). In 2013 the SAEFVIC Victorian vaccine safety service phone number was also included on an information sheet given to parents as part of the secondary school immunisation program.
Interestingly a 2011 survey conducted in South Australia found one-third of parents whose child had experienced an AEFI reported the symptoms to either an HCP or the department of health16 and their reporting was not associated with awareness of AEFI surveillance systems.15 With this finding, and our observation of increasing unsolicited consumer reporting, it would suggest consumer reporting is already entrenched in vaccine safety surveillance even in the absence of a direct decision to encourage consumer reporting and that AEFI surveillance systems need to be able to accommodate such reports and address issues arising.17
Consumer reporting was notably skewed toward reporting AEFI in younger children rather than in adults themselves. However, the presumption that consumers would be more likely to report a high proportion of minor or common AEFI was not upheld. Our retrospective review has described for the first time in vaccine pharmacovigilance, that consumers reported a higher proportion of serious AEFI than HCP (22% versus 17% (p = 0.018). This is in contrast to review of medicines consumer reporting to the Netherlands, Denmark's and UK's adverse drug reaction (ADR) systems, which found HCPs were as or more likely to report serious ADRs.9-11
Determining the contribution impact of consumer reporting upon the generation or earlier detection of safety signals is outside the scope of this study. Our study did not demonstrate any advantage in timeliness of reporting from consumers. Furthermore, international studies have suggested that it may be appropriate to analyze consumer and HCP AEFI reports separately, in addition to combined datasets, in order to maximize signal detection.10 Avery et al. found that while combining patient and HCP reports generated more potential signals than HCP reports alone; some potential signals in the ‘HCP-only’ data set were lost when combined with patient reports.9
Significant differences in the profiles of consumers who report AEFI have been described (e.g. ethnicity and perception of and experience with immunisation and AEFI).18 In Australia the only noted demographic difference was that consumer-reporters were more likely to be Australian-born than non-reporters.15 Understanding reporting profiles of both HCP and consumers is important for assessing potential biases in data collection and further studies will be required to assess the impact of increasing consumer reporting on signal detection.
If consumer reporting is to be routinely solicited, appropriate reporting modalities and increases in the resources for responsive management will also be required. Prior to the increase in consumer reporting, the predominant reporting modalities in Victoria were by fax, with replacement by online reporting as it became available in 2010. As consumer reporting increased, the trend reversed, with 85% of consumers reporting by phone. Consumer ability to report to SAEFVIC by modes other than telephone is restricted by the current online registration process, which implies, but is not actually limited, to being a provider. New reporting modalities taking advantage of modern communication technologies such as SMS 19 and /or a smart-phone applications may help with both accessibility and timeliness. These could be offered as an ‘on-demand’ reporting tool, integrated with other immunisation applications or provided pro-actively at the immunisation encounter. These strategies carry the potential to record a range of outcomes and integrate into active surveillance strategies.6
SAEFVIC services, unlike many passive surveillance systems, include the option for specialist review at its integrated clinical services for AEFI management and planning of subsequent vaccination. A significantly higher proportion of consumer reported vaccinees with AEFI attended for specialist clinical review compared to those reported by providers. Potential explanation for this may include serious AEFI being more likely to require an immunisation specialist consultation, as well as a possible preference by reporting consumers for a specialist opinion which may have prompted their decision to report. It is unknown if there was prior knowledge of the availability of these clinical services prior to reporting.
Methods
Data for AEFI reports received by SAEFVIC in the 6-year period July 2007–June 2013 were extracted (ethics approval - DA017-2013-04) and analyses conducted using Excel 2010 (Microsoft, Redmond, WA) and Stata 13 (Statacorp, Texas). Reports initially submitted to TGA and redirected to SAEFVIC were excluded from analyses as they primarily related to part of a specific investigation using non-standard surveillance reporting mechanism (e.g., Panvax H1N1TM vaccine in 2009) and reporter information was not provided.
Reporter type, as stated at the time of reporting by phone or entered on the AEFI report form, was defined as Consumer if submitted directly to SAEFVIC by a vaccinee, parent, guardian or family member; Provider if submitted by a health care professional, pharmaceutical company, or any representative of a health care provider institution or; Unknown where reporter type could not be ascertained. Reporter type is recorded as that of the first point of contact made (i.e either consumer or provider), with information from any subsequent reports merged into the initial record, therefore determination of duplicate reporting was not possible. Submission modality was recorded manually prior to commencement of the online database in 2010, after which it became a mandatory data field completed at the time of report receipt with online submission automatically recorded and option of post, fax or telephone selected manually.
Four age categories were defined as: child (birth to less than 7 years of age); school aged (7 to 18 years); adult (19 to less than 60); and elderly (60 years and older). Age at the time of vaccination was calculated by subtracting date of birth from date of vaccination. Reports with data fields of ‘date of birth’ or ‘date of vaccination’ missing - and therefore age at vaccination unknown (n = 160) - were excluded from analyses related to age.
Descriptions of reported AEFI are recorded in the database verbatim and categorized according to standard case definitions where available.15 Reports were review by specialist immunisation nurses or clinician for verification of the AEFI and determination of medical importance.12 Serious AEFI were those that resulted in death; were life-threatening; required in-patient hospitalization or prolongation of existing hospitalization; resulted in persistent or significant disability/incapacity, or; were a congenital anomaly/birth defect.16
Time taken to report was calculated as the difference in days between report submission and date of vaccination minus days to symptom onset where this information was available. Comparison of consumer and reporter time taken to report was demonstrated using Kaplan-Meier analysis and by proportion reported within 7 days.
Calculations to determine differences in proportions and proportional reporting ratios excluded reports with unknown variables (reporter type, reporting modality).
The Chi-square test (χ2) was used to compare the proportions of consumer and provider reporting designated as serious AEFI and requiring clinical referral. Comparison of consumer and provider reporting by vaccine age group were calculated as 2-sample test of proportions.
Chi-square statistic for trend (regression) was used to test trend of proportions between consumer and provider reporting by telephone. Tests were considered statistically significant at P <0.05.
Conclusion
This study identified that consumer reporting of AEFI is already an integral component of vaccine safety surveillance and needs to be accommodated in future development at both state and national level. Accepting or advocating consumer reporting may potentially benefit signal detection as well as improve consumer confidence in immunisation by improving vaccine safety communication between immunisation services and the community. A characterization of resources required to implement an efficient consumer AEFI reporting system is needed along with ongoing investigation of any demographic reporting biases or impact on signal detection sensitivity.
Acknowledgments
We thank the health care providers and consumers who made reports to our service and the SAEFVIC immunisation nurses and administration staff who receive and enter the reports. SAEFVIC is a Victorian Department of Health funded collaboration between the Murdoch Childrens Research Institute, Royal Children's Hospital, Monash Health and Melbourne Health.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Hazel Clothier is a recipient of an Australian Postgraduate Award scholarship.
References
- 1. World Health Organization Global Vaccine Safety Blueprint. Geneva, Switzerland: World Health Organization, 2012 [Google Scholar]
- 2. Brotherton JM, Gold MS. Monitoring vaccine safety: a critical component of every immunisation program. Med J Aust 2008; 189:243-4; PMID:18759715 [DOI] [PubMed] [Google Scholar]
- 3. Mahajan D, Cook J, Dey A, Macartney K, Menzies RI. Annual report: Surveillance of adverse events following immunisation in Australia, 2011. Commun Dis Intell Q Rep 2012; 36:E315-32; PMID:23330706 [PubMed] [Google Scholar]
- 4. Amarasinghe A, Black S, Bonhoeffer J, Carvalho SMD, Dodoo A, Eskola J, Larson H, Shin S, Olsson S, Balakrishnan MR, et al. . Effective vaccine safety systems in all countries: A challenge for more equitable access to immunization. Vaccine 2013; 31, Supplement 2:B108-B14; PMID:23598471; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.119 [DOI] [PubMed] [Google Scholar]
- 5. Monteiro SA, Takano OA, Waldman EA.; Evaluation of the Brazilian surveillance system for adverse events following vaccination. Rev Bras Epidemiol 2011; 14:361-71; PMID:22069005; http://dx.doi.org/ 10.1590/S1415-790X2011000300002 [DOI] [PubMed] [Google Scholar]
- 6. Crawford NW, Clothier H, Hodgson K, Selvaraj G, Easton ML, Buttery JP. Active surveillance for adverse events following immunization. Expert Rev Vaccines 2014; 13:265-76; PMID:24350637; http://dx.doi.org/ 10.1586/14760584.2014.866895 [DOI] [PubMed] [Google Scholar]
- 7. Hazell L, Shakir SA. Under-reporting of adverse drug reactions : a systematic review. Drug Saf 2006; 29:385-96; PMID:16689555; http://dx.doi.org/ 10.2165/00002018-200629050-00003 [DOI] [PubMed] [Google Scholar]
- 8. Parrella A, Braunack-Mayer A, Gold M, Marshall H, Baghurst P. Healthcare providers’ knowledge, experience and challenges of reporting adverse events following immunisation: a qualitative study. BMC Health Serv Res 2013; 13:313; PMID:23945045; http://dx.doi.org/ 10.1186/1472-6963-13-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Avery AJ, Anderson C, Bond CM, Fortnum H, Gifford A, Hannaford PC, Hazell L, Krska J, Lee AJ, McLernon DJ, et al. . Evaluation of patient reporting of adverse drug reactions to the UK ’Yellow Card Scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess 2011; 15:1-234, iii-iv; PMID:21545758; http://dx.doi.org/ 10.3310/hta15200 [DOI] [PubMed] [Google Scholar]
- 10. Hazell L, Cornelius V, Hannaford P, Shakir S, Avery AJ, Yellow Card Study Collaboration . How do patients contribute to signal detection?: a retrospective analysis of spontaneous reporting of adverse drug reactions in the UK's Yellow Card Scheme. Drug Saf 2013; 36:199-206; PMID:23444232; http://dx.doi.org/ 10.1007/s40264-013-0021-2 [DOI] [PubMed] [Google Scholar]
- 11. Inch J, Watson MC, Anakwe-Umeh S. Patient vs. healthcare professional spontaneous adverse drug reaction reporting: a systematic review. Drug Saf 2012; 35:807-18; PMID:22928729; http://dx.doi.org/ 10.1007/BF03261977 [DOI] [PubMed] [Google Scholar]
- 12. Armstrong PK, Dowse GK, Effler PV, Carcione D, Blyth CC, Richmond PC, Geelhoed GC, Mascaro F, Scully M, Weeramanthri TS. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ open 2011; May 30;1(1) 1:e000016; PMID:22021725; http://dx.doi.org/ 10.1136/bmjopen-2010-000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clothier HJ, Crawford NW, Kempe A, Buttery JP. Surveillance of adverse events following immunisation: the model of SAEFVIC, Victoria. Commun Dis Intell Q Rep 2011; 35:294-8; PMID:22624490 [PubMed] [Google Scholar]
- 14. Parrella A, Gold M, Marshall H, Braunack-Mayer A, Baghurst P. Parental perspectives of vaccine safety and experience of adverse events following immunisation. Vaccine 2013; 31:2067-74; PMID:23422146; http://dx.doi.org/ 10.1016/j.vaccine.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 15. Parrella A, Gold M, Braunack-Mayer A, Baghurst P, Marshall H. Consumer reporting of adverse events following immunization (AEFI): Identifying predictors of reporting an AEFI. Hum Vaccin Immunother 2014; 10:747-54; PMID:24406315; http://dx.doi.org/ 10.4161/hv.27459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parrella A, Gold M, Marshall H, Braunack-Mayer A, Baghurst P. Parental perspectives of vaccine safety and experience of adverse events following immunisation. Vaccine 2013; 31:2067-74; PMID:23422146; http://dx.doi.org/ 10.1016/j.vaccine.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 17. van Hunsel F, Harmark L, Pal S, Olsson S, van Grootheest K. Experiences with adverse drug reaction reporting by patients: an 11-country survey. Drug Saf 2012; 35:45-60; PMID:22149419; http://dx.doi.org/ 10.2165/11594320-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 18. Gust DA, Campbell S, Kennedy A, Shui I, Barker L, Schwartz B. Parental concerns and medical-seeking behavior after immunization. Am J Prev Med 2006; 31:32-5; PMID:16777540; http://dx.doi.org/ 10.1016/j.amepre.2006.03.017 [DOI] [PubMed] [Google Scholar]
- 19. Baron S, Goutard, Nguon K, Tarantola A, Eysenbach G. Use of a text message-based pharmacovigilance tool in Cambodia: pilot study. J M Int Res 2013; 15:1-; PMID:23591700; http://dx.doi.org/ 10.2196/jmir.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonhoeffer J, Bentsi-Enchill A, Chen RT, Fisher MC, Gold MS, Hartman K, Heininger U, Hoet B, Jefferson T, Khuri-Bulos N, et al. . Guidelines for collection, analysis and presentation of vaccine safety data in pre- and post-licensure clinical studies. Vaccine 2009; 27:2282-8; PMID:19056451; http://dx.doi.org/ 10.1016/j.vaccine.2008.11.036 [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization Definition and application of terms for vaccine pharmacovigilance. Report of CIOMS/WHO Working Group on Vaccine Pharmacovigilance. Geneva, Council for International Organizations of Medical Sciences, 2012. http://www.cioms.ch/index.php/component/booklibrary/?task=view&Itemid=&id=45&catid=58 (Accessed 2 July 2014)


