Abstract
Background
Many publications report the prevalence of chronic kidney disease (CKD) in the general population. Comparisons across studies are hampered as CKD prevalence estimations are influenced by study population characteristics and laboratory methods.
Methods
For this systematic review, two researchers independently searched PubMed, MEDLINE and EMBASE to identify all original research articles that were published between 1 January 2003 and 1 November 2014 reporting the prevalence of CKD in the European adult general population. Data on study methodology and reporting of CKD prevalence results were independently extracted by two researchers.
Results
We identified 82 eligible publications and included 48 publications of individual studies for the data extraction. There was considerable variation in population sample selection. The majority of studies did not report the sampling frame used, and the response ranged from 10 to 87%. With regard to the assessment of kidney function, 67% used a Jaffe assay, whereas 13% used the enzymatic assay for creatinine determination. Isotope dilution mass spectrometry calibration was used in 29%. The CKD-EPI (52%) and MDRD (75%) equations were most often used to estimate glomerular filtration rate (GFR). CKD was defined as estimated GFR (eGFR) <60 mL/min/1.73 m2 in 92% of studies. Urinary markers of CKD were assessed in 60% of the studies. CKD prevalence was reported by sex and age strata in 54 and 50% of the studies, respectively. In publications with a primary objective of reporting CKD prevalence, 39% reported a 95% confidence interval.
Conclusions
The findings from this systematic review showed considerable variation in methods for sampling the general population and assessment of kidney function across studies reporting CKD prevalence. These results are utilized to provide recommendations to help optimize both the design and the reporting of future CKD prevalence studies, which will enhance comparability of study results.
Keywords: CKD, CKD-EPI equation, epidemiology, MDRD, systematic review
INTRODUCTION
Chronic kidney disease (CKD) is considered to be a major public health problem [1]. CKD has an important impact both at the patient level, by decreasing the quality of life and life expectancy, and at the population level, by increasing health-care costs and the demand for health-care services.
Since CKD prevalence estimation is central to CKD management and prevention planning at the population level [2], it is not surprising that many publications report CKD prevalence in the general population. It is common research practice to put study results into context by comparing them with previous publications to identify the regional CKD burden, assessing the impact on regional health-care systems and for tailoring preventive strategies to communities. In the case of CKD prevalence, such comparisons are likely hampered as CKD prevalence estimations are influenced by study population characteristics and by the methods used to assess kidney function [3, 4]. To realistically compare CKD prevalence across different population-based studies, methodological factors should be taken into account.
The purpose of this systematic literature review was to (i) identify all studies reporting on CKD prevalence in the European adult general population and (ii) to describe the methodology used in these studies. The findings from this review are utilized to provide recommendations that may help investigators to optimize both the design and the reporting of future CKD prevalence studies, which will enhance comparability of results across studies.
METHODS
Search strategy
A systematic literature search was performed in PubMed, MEDLINE and EMBASE to identify all original research articles reporting the prevalence of CKD in the adult general population. As Kidney Disease Outcomes Quality Initiative (KDOQI) published a guideline on CKD definition [5] in 2002, we included articles published between 1 January 2003, which is one year after the publication of the KDOQI guideline, and 1 November 2014, when our search was last updated. The database-specific search queries are presented in the Supplementary data, Appendix S1. Additionally, the representatives of national kidney foundations, renal registries and expert nephrologists in 39 European countries were asked to provide information on any relevant studies.
Study selection
Publications that presented original research, were designed to select a representative sample of a European adult general population and reported a CKD prevalence estimate were included. We excluded studies that ended subject recruitment prior to 1996 and studies lacking glomerular filtration rate (GFR) estimation based on serum creatinine. Cystatin C-based estimated GFR (eGFR) will lead to higher CKD prevalence estimates than creatinine-based eGFR [6]. For the sake of comparability, we chose not to include publications that solely reported cystatin C-based prevalence estimates. No language restrictions were applied. The literature search was done by two investigators (KB, ED). Any study that was judged relevant on the basis of its title was retrieved in abstract form, and if relevant, in full-text form. Any doubt about eligibility was resolved by discussion with another investigator (VS).
Data extraction
All publications were initially seen by one investigator (KB) and then independently reassessed by two additional investigators (ED for the first half and AK for the second half). For studies with multiple eligible publications, we selected the publication with a primary objective of reporting CKD prevalence or the most recent publication. Publications were assessed on method of population selection, which included the sampling frame (i.e. source used to identify subjects) and the sample design (i.e. the method of sample selection). Additionally, we extracted information on the assessment of kidney function. The extracted data were categorized as follows:
Creatinine assay was categorized as enzymatic, Jaffe, modified Jaffe, compensated Jaffe or unclear. The Jaffe method is known to suffer from interference by other substances [7], and multiple adaptations have been implemented to improve method specificity [7]. The compensated and modified Jaffe assays were developed to improve method specificity and minimize susceptibility of interfering substances [7]. The compensated Jaffe method is the use of a manufacturer-specific mathematical compensation [8]. The modified Jaffe assays are modifications of the method such as deproteinization of the sample prior to analysis or the addition of potassium ferricyanide [9].
Calibration was categorized as calibrated to the standardized isotope dilution mass spectrometry (IDMS) or calibrated by another method or calibrator.
Urinary albumin assay was categorized as dipstick, immunoassay (including both nephelometric and turbidometric immunoassays) or other.
The CKD definition was categorized as use of the KDOQI 2002 definitions [5] or use of other definitions. Use of chronicity criterion, i.e. persistence of albuminuria or decreased eGFR for at least 3 months, was assessed.
Ethnicity reporting was categorized as ‘yes’ if publication reported collection of ethnicity data and as ‘no’ if ‘ethnicity’ data were not collected or if those were not reported.
Finally, we extracted the following data on presentation of CKD prevalence results: the use of 95% confidence intervals (95%CI), the use of standardization of the prevalence estimate to a reference population and the presentation of results by age group and sex. If CKD prevalence was not the main focus of the publication, the use of 95%CI was rated as not applicable (n/a). The data extraction form is shown in the Supplementary data, Appendix S2.
RESULTS
Study selection
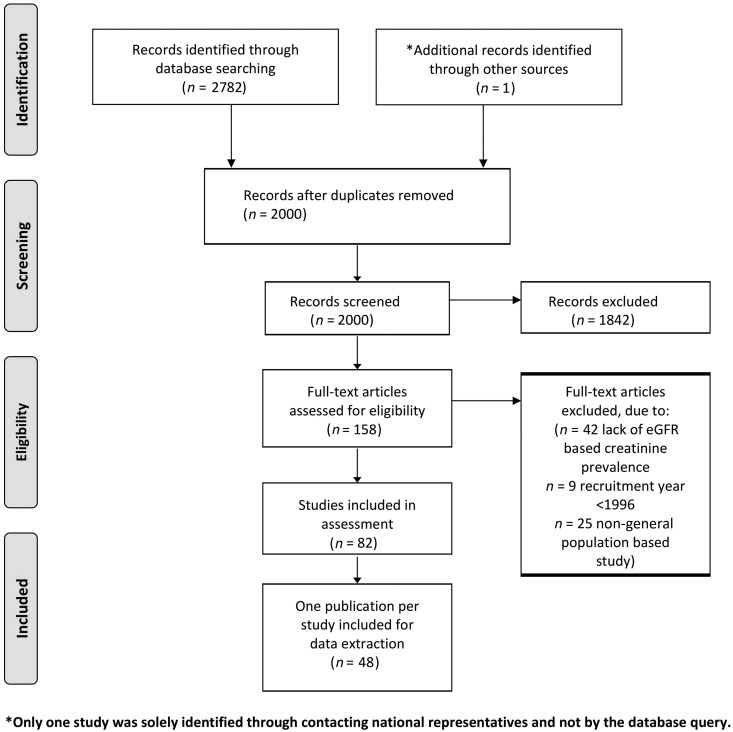
Figure 1 shows the selection process of inclusion and exclusion of publications in a flow chart. We retrieved 2000 individual publications of which only one study was solely identified through contacting national representatives. A total of 1842 publications were excluded based on title or abstract. Twenty-five publications were excluded as the study was not designed to select a representative sample of the general population, 9 studies were excluded as they ended recruitment prior to 1996 and 42 publications were excluded for not presenting a CKD prevalence estimate. Eighty-two publications fulfilled the inclusion criteria. Eighteen studies had multiple publications, highlighting various aspects of CKD (overall 34 publications). Finally, we included 48 publications of individual studies for the data extraction.
FIGURE 1:
Flow chart of publication selection.
Data extraction
Table 1 describes the method of general population sample selection including the response per study. Details on the laboratory assessment of kidney function, the CKD definition used and on the reporting of CKD prevalence are presented in Table 2.
Table 1.
Description of the method of general population sample selection per study
| Author (Ref.) | Study name | Country | Time period | Number of subjects, N | Age range | Sampling frame | Sample design | Response, % |
|---|---|---|---|---|---|---|---|---|
| Aumann et al. [10] | SHIP | Germany | 2001–6 | 2830 | 25–88 | Not specifieda | Multistage sampling | 69 |
| Bongard et al. [11] | MONA LISA | France | 2006–7 | 4727 | 35–75 | Electoral rolls | Age and sex stratified | Not given |
| Browne et al. [12] | SLAN | Ireland | 2007 | 1098 | 45+ | Other (Geo directory) | Multistage random sampling: by area and region | 66 |
| Capuano et al. [13] | VIP | Italy | 1998–99 and 2008–9 | 2400 | 25–74 | Electoral rolls | Age and sex stratified | Not given |
| Christensson et al. [14] | GAS | Sweden | 2001–4 | 2815 | 60–93 | Census | Stratified, age, sex and urban/rural location | 60 |
| Chudek et al. [15] | PolSenior | Poland | 2007–11 | 3793 | 65+ | Not specifieda | Not specifieda | 32 |
| Cirillo et al. [16] | Gubbio Population Study | Italy | Not specified | 4574 | 18–95 | Not specifieda | Not specifieda | Not givena |
| Codreanu et al. [17] | Early Detection and Intervention Program for Chronic Renal and Cardiovascular Disease in the Rep Moldova | Moldova | 2006–7 | 973 | 18–77 | Not specified | Not specified | Not given |
| De Nicola et al. [18] | CARHES | Italy | 2008 | 4077 | 35–79 | Electoral rolls | Age and sex stratified | 45 |
| Delanaye et al. [19] | Belgium | 2008–9 | 1992 | 45–75 | Not specified | Voluntary nature | Not given | |
| Donfrancesco et al. [20] | MATISS | Italy | 1993–96 | 2924 | 20–79 | Electoral rolls | Age- and sex-stratified random sample | 60 |
| Formiga et al. [21] | Octabaix | Spain | 2009 | 328 | 85 | Not specifieda | Not specifieda | Not given |
| Fraser et al. [22] | HSE | England | 2009–10 | 5799 | 16+ | Other (address list) | Random two-stage sample | Not givena |
| Gambaro et al. [23] | INCIPE | Italy | 2006 | 3629 | 40+ | General practitioner list | Random sample | 62 |
| Gianelli et al. [24] | InChianti | Italy | 1998–2000 | 676 | 65+ | Not specified | Multistage stratified random sample | Not given |
| Goek et al. [25] | KORA | Germany | 1999–1 | 1104 | 54–75 | Not specified | Not specified | Not given |
| Gu et al. [26] | FLEMENGHO | Belgium | 2005–10 | 797 | 18–89 | Not specified | Not specified | 78 |
| Guessous et al. [27] | Swiss Study on Salt intake | Switzerland | 2010–11 | 1145 | 15+ | Other (phone directory) | Age- and sex-stratified random sample | 10 |
| Hallan et al. [28] | HUNT 2 | Norway | 1995–97 | 65 181 | 20+ | Not specified | All inhabitants | 70 |
| Hernandez et al. [29] | IMAP | Spain | 2007 | 2270 | 18–80 | Not specifieda | Random sample | Not given |
| Juutilainen et al. [30] | FINRISK | Finland | 2002 and 2007 | 11 277 | 25–74 | Census | Age- and sex-stratified random sample | 71 in men 74 in women |
| Lieb et al. [31] | MONICA/KORA | Germany | Not specified | 1187 | 25–74 | Not specified | Age- and sex-stratified random sample | 71 |
| Meuwese et al. [32] | Leiden 85 + study | Netherlands | 1997–99 | 558 | 85 | Not specified | All in birth cohort | 87 |
| Nitsch et al. [33] | BWHHS | UK | 1999–2001 | 3851 | 60–79 | Not specifieda | Random sample | 60 |
| Nitsch et al. [34] | SAPALDIA 2 | Switzerland | 1991 and 2002 | 6317 | 18+ | Not specifieda | Random sample | 73 |
| Otero et al. [35] | EPIRCE | Spain | 2004–8 | 2746 | 20+ | Census | Age-, sex- and region-stratified random sample | 43 |
| Pani et al. [36] | SardiNIA study | Italy | 2001– | 4471 | 14–102 | Not specifieda | Not specifieda | 56 |
| Pattaro et al. [37] | MICROS | Italy | 2002–3 | 1199 | 18+ | Not specifieda | Not specifieda | Not given |
| Ponte et al. [38] | CoLaus | Switzerland | 2003–6 | 5921 | 35–75 | Population registry | Random sample | 41 |
| Redon et al. [39] | PREV-ICTUS | Spain | 2005 | 6419 | 60+ | General practitioner lists | Random sample | 72 |
| Robles et al. [40] | HERMEX | Spain | Not specified | 2813 | 25–79 | Other (health-care system database) | Age- and sex-stratified random sample | 83 |
| Roderick et al. [41] | MRC Older Age Study | UK | 1994–99 | 13 179 | 75+ | General practitioner list | Practices stratified by mortality score and deprivation score | 73 |
| Rothenbacher et al. [42] | ActiFE Ulm | Germany | 2009–10 | 1471 | 65+ | Census | Random sample | 20 |
| Rutkowski et al. [43] | PolNef | Poland | 2004–5 | 2476 | n/a | Other (address list) | Random sample | 26 |
| Sahin et al. [44] | Turkey | 2005 | 1079 | 18–95 | Not specified | Age, sex and region stratified | Not given | |
| Schaeffner et al. [45] | BIS | Germany | 2011 | 570 | 70+ | Not specifieda | Not specifieda | Not given |
| Scheven et al. [46] | PREVEND | The Netherlands | 1997–98 | 8121 | 28–75 | Not specified | All inhabitants | 48 |
| Stasevic et al. [47] | Kosovo + Metohia | 2006 | 423 | 18+ | Not specified | All inhabitants | 43 | |
| Stengel et al. [48] | 3C | France | 1991–2001 | 8705 | 65+ | Electoral rolls | Random sample | 37 |
| Suleymanlar et al. [49] | CREDIT | Turkey | Not specified | 10 056 | 18+ | Not specified | Age, sex and region stratified | Not given |
| Tavira et al. [50] | RENASTUR | Spain | 2010–12 | 592 | 55–85 | Not specified | Random sample | Not given |
| Van Pottelbergh et al. [51] | Crystal | Russia | 2009 | 611 | 65–91 | General practitioner list | All registered on list | 66 |
| Viktorsdottir et al. [52] | RHS | Iceland | 1967–96 | 19 256 | 33–85 | Not specified | All in birth cohort | Not given |
| Vinhas et al. [53] | PREVADIAB | Portugal | 2008–9 | 5167 | 20–79 | Other (universal health card) | Age, sex and region stratified | 84 |
| Wasen et al. [54] | Finland | 1998–99 | 1246 | 64–100 | Not specified | All residents born ≤1933 | 83 | |
| Wetmore et al. [55] | Iceland | 2001–3 | 1630 | 18+ | Not specified | Random sample | 71 | |
| Zambon et al. [56] | ProV.A. | Italy | 1995–97 | 3063 | 65+ | Other (health district registries) | Age- and sex-stratified random sample | 77 in men 64 in women |
| Zhang et al. [57] | ESTHER | Germany | 2000–2 | 9806 | 50–74 | General practitioners | All participants who underwent a general health check-up | Not given |
N, Number of subjects with creatinine measurement; n/a, not applicable.
aAuthors refer to previous publication.
Table 2.
Laboratory assessment of kidney function, CKD definition used and details on the reporting of CKD prevalence per study
| Author (Ref.) | Creatinine assay | IDMS | Albuminuria | CKD definition | eGFR equation | Ethnicity | CI | Age and sex standardized | Stratified prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Aumann et al. [10] | Jaffe | Other | n/a | 2 | CKD-EPI + other | Yes | n/a | No | Yes: other |
| Bongard et al. [11] | Jaffe | No | n/a | 2 | MDRD (old) | No | Yes | Yes to national pop. | No |
| Browne et al. [12] | Modified Jaffe | Yes | Other | 1 + 2 | CKD-EPI + new MDRD | No | Yes | Yes to national pop. | Yes: age, sex and other |
| Capuano et al. [13] | Modified Jaffe | No | n/a | 2 | CG | No | No | Yes to national pop. | Yes: age, sex and other |
| Christensson et al. [14] | Unclear | Other | n/a | Other | CKD-EPI, MDRD (old) + CG | Yes | No | No | Yes: age and sex |
| Chudek et al. [15] | Jaffe | Unclear | If dipstick − → immunoassay | 1 + 2 + 3 | CKD-EPI | No | No | No | Yes: age, sex and other |
| Cirillo et al. [16] | Modified Jaffe | No | Immunoassay | 2 | MDRD (old) | Yes | Yes for N Not for % |
Yes to national pop. | Yes: age and sex |
| Codreanu et al. [17] | Unclear | No | Other | 2 + 3 | MDRD (old) | No | No | No | Yes: age, sex and other |
| De Nicola et al. [18] | Enzymatic | Yes | Immunoassay | 1 + 2 + 3 | CKD-EPI | No | Yes | No | No |
| Delanaye et al. [19] | Compensated Jaffe | Yes | n/a | 2 | CKD-EPI + new MDRD | No | No | No | Yes: sex |
| Donfrancesco et al. [20] | Enzymatic | Yes | n/a | 2 | CKD-EPI | No | No | No | Yes: sex |
| Formiga et al. [21] | Compensated Jaffe | No | n/a | 2 | MDRD (old) | No | No | No | No |
| Fraser et al. [22] | Enzymatic | Yes | Not specified | 1 + 2 + 3 + other | CKD-EPI + new MDRD | Yes | No | Unclear | Yes: other |
| Gambaro et al. [23] | Modified Jaffe | Other | If dipstick + → immunoassay | 1 + 2 + 3 | CKD-EPI | Yes | Yes | Yes to US pop. | Yes: age, sex and other |
| Gianelli et al. [24] | Modified Jaffe | No | n/a | 2 | MDRD (old) and CG | No | No | No | No |
| Goek et al. [25] | Compensated Jaffe | Unclear | n/a | 2 | CKD-EPI | No | n/a | No | No |
| Gu et al. [26] | Modified Jaffe | Unclear | Not specified | 2 | CKD-EPI + MDRD (old) | No | No | No | No |
| Guessous et al. [27] | Compensated Jaffe | Unclear | Unclear | 1 | CKD-EPI | Yes | n/a | No | No |
| Hallan et al. [28] | Jaffe | Other | Immunoassay | 1 + 2 + 3 | New MDRD | Yes | Yes | Yes to national + US pop. | Yes: age, sex and other |
| Hernandez et al. [29] | Not specified | Unclear | Not specified | 1 + other | CKD-EPI | Yes | n/a | No | Yes: other |
| Juutilainen et al. [30] | Enzymatic | Yes | n/a | 2 + other | CKD-EPI + new MDRD | No | no | No | Yes: age and sex |
| Lieb et al. [31] | Enzymatic | No | Immunoassay | 3 + other | MDRD (old) | No | n/a | No | No |
| Meuwese et al. [32] | Jaffe | No | n/a | 2 | CKD-EPI + MDRD (old) | No | n/a | No | No |
| Nitsch et al. [33] | Modified Jaffe | Other | n/a | 2 | MDRD (old) | Yes | n/a | No | Yes: other |
| Nitsch et al. [34] | Jaffe | Other | n/a | 2 | MDRD (old) and CG | Yes | Yes | No | Yes: age and sex |
| Otero et al. [35] | Unclear | Unclear | Unclear | 1 + 2 | MDRD (old) | Yes | Yes | Yes to national pop. | Yes: age, sex and other |
| Pani et al. [36] | Not specified | Other | Not specified | 1 + 2 + 3 | CKD-EPI + new MDRD | No | Yes | No | Yes: age and sex |
| Pattaro et al. [37] | Enzymatic | Yes | n/a | 2 | CKD-EPI, new MDRD + other | No | Yes | No | Yes: age |
| Ponte et al. [38] | Compensated Jaffe | Yes | Immunoassay | 1 + 2 + 3 | CKD-EPI + new MDRD | Yes | Yes | No | Yes: age and sex |
| Redon et al. [39] | Jaffe | Yes | Immunoassay | 2 | CG | No | n/a | No | No |
| Robles et al. [40] | Modified Jaffe + enzymatic | No | Dipstick | 2 + other | CKD-EPI + new MDRD | Yes | Yes | Yes to EU pop. | Yes: age and sex |
| Roderick et al. [41] | Modified Jaffe | Yes | Immunoassay | 2 + other | MDRD (old) | No | Yes | No | Yes: age and sex |
| Rothenbacher et al. [42] | Modified Jaffe | No | If dipstick + → immunoassay | 1 + 2 + 3 | CKD-EPI + new MDRD | No | No | No | Yes: age and sex |
| Rutkowski et al. [43] | Modified Jaffe | Unclear | n/a | 1 + 2 + 3 | MDRD (old) | No | No | No | No |
| Sahin et al. [44] | Enzymatic | Yes | Not specified | 2 | New MDRD | No | No | No | Yes: age, sex and other |
| Schaeffner et al. [45] | Unclear | Unclear | Immunoassay | 2 | CKD-EPI + other | Yes | n/a | No | No |
| Scheven et al. [46] | Modified Jaffe | Unclear | If dipstick + → immunoassay | 1 + 2 + 3 | CKD-EPI | No | n/a | No* | No |
| Stasevic et al. [47] | Jaffe | Yes | Unclear | 2 + 3 + other | MDRD (old) | No | No | No | No |
| Stengel et al. [48] | Jaffe | Yesa | Immunoassay | 1 + 2 | CKD-EPI + new MDRD | No | No | No | Yes: age and sex |
| Suleymanlar et al. [49] | Not specified | No | Not specified | 1 + 2 + 3 | MDRD (old) | Yes | No | Yes to national pop. | Yes: age and sex |
| Tavira et al. [50] | Modified Jaffe | No | n/a | 2 | MDRD (old) | Yes | n/a | No | No |
| Van Pottelbergh et al. [51] | Modified Jaffe | No | Dipstick | 2 | MDRD (old) and CG | No | No | No | Yes: age and sex |
| Viktorsdottir et al. [52] | Modified Jaffe | No | n/a | 1 + 2 + 3 | MDRD (old) and CG | Yes | No | Yes to global pop. | Yes: age and sex |
| Vinhas et al. [53] | Jaffe | Unclear | Immunoassay | 2 | MDRD (old) | No | Yes | Yes to national pop. | Yes: age, sex and other |
| Wasen et al. [54] | Unclear | Yes | n/a | 2 + other | New MDRD and CG | No | No | No | Yes per sex |
| Wetmore et al. [55] | Jaffe | Other | Dipstick | 2 | New MDRD and CG | Yes | No | No | No |
| Zambon et al. [56] | Modified Jaffe | No | Immunoassay | 2 + other | CKD-EPI and MDRD (old) | Yes | n/a | Yes to national pop. | No |
| Zhang et al. [57] | Modified Jaffe | Other | n/a | 2 + other | MDRD (old) | No | No | No | Yes: age, sex and other |
Albuminuria = method of albuminuria measurement; CKD definition 1 = eGFR below 60 mL/min/1.73 m2 and or the presence of albuminuria >30 mg/g (i.e. CKD Stages 1–5); 2 = eGFR below 60 mL/min/1.73 m2 (i.e. CKD Stages 3–5); 3 = albuminuria >30 mg/g. Ethnicity = ‘yes’ if collection is reported; ‘no’ if not reported or not collected. CI, confidence interval given for prevalence estimate; CG, Cockcroft and Gault equation; n/a, not applicable.
aIn order to standardize creatinine values, 1720 frozen serum samples were remeasured in a single laboratory with an IDMS-traceable enzymatic assay. Hereafter, equations relating the Jaffe and IDMS-traceable creatinine were developed to standardize all baseline values as follows: ScrIDMS = 0.86 × ScrJaffe + 4.40. *Population corrected for sampling design (i.e. oversampling of albuminuria).
Population selection
All studies combined described a total of 247 342 subjects. The size of the study population ranged from 328 to 65 181 subjects. Twenty-three studies (48%) included virtually the entire age range of the adult population. The remaining (n = 25; 52%) studies restricted the recruitment of subjects to a higher age range.
Four studies (8%) used census data as the sampling frame to identify eligible study subjects. More than half of the studies (n = 26; 54%) did not report the sampling frame used. Fourteen studies (29%) were designed to select their population by age and sex stratification, and 12 studies (25%) selected a random sample. Ten studies (21%) did not provide details on the sample design, six of which referred to previous publications for more details.
The response was given in 31 studies (65%) and ranged from 10 to 87%. Of the 17 studies that did not report a response, 2 studies referred to a previous publication for details regarding responders and non-responders.
Assessment of kidney function
Serum creatinine was determined by Jaffe assay in the majority of studies (n = 32; 67%) and by enzymatic assay in six (13%) studies. Only few creatinine assays were calibrated to IDMS (n = 14; 29%). Urinary markers for kidney disease were assessed in 29 studies (60%), 15 of which (31%) used immunoassay to detect albuminuria. Seven studies (15%) used dipsticks to identify proteinuria, with confirmation of albuminuria by immunoassay in four studies (8%).
CKD definition
Almost all studies (n = 44; 92%) defined CKD as eGFR below 60 mL/min/1.73 m2. Eighteen studies (38%) reported CKD prevalence defined as eGFR below 60 mL/min/1.73 m2 and/or the presence of albuminuria >30 mg/g, and 15 studies (32%) reported CKD prevalence defined as albuminuria >30 mg/g. Although 10 studies (21%) additionally reported CKD according to another definition, only one study exclusively reported a CKD prevalence not defined by KDOQI.
The Modification of Diet in Renal Disease (MDRD) equation for unstandardized creatinine was used to estimate GFR in 22 studies (46%), and the MDRD equation for standardized creatinine was used in 14 studies (29%). Twenty-five studies (52%) used the CKD Epidemiology Collaboration (CKD-EPI) equation, and nine studies (19%) used the Cockcroft and Gault equation. Even though both the CKD-EPI and MDRD equations include an ethnicity variable, only 18 studies (38%) reported collecting ethnicity data. Eleven studies (23%) did not indicate whether ethnicity data were collected.
Reporting results
CKD prevalence reporting was the main objective in 36 publications, of which 39% reported a 95%CI. An age- and sex-standardized prevalence was reported in 12 studies (25%), of which 9 standardized to their national population. Although two studies standardized their population to the US population, only one study standardized to the European population. The presentation of CKD prevalence by strata was done by 31 studies, and these studies presented the CKD prevalence stratified per risk factor, mostly by age (n = 24; 50%) and by sex (n = 26; 54%).
DISCUSSION
We assessed 48 publications, published between 1 January 2003 and 1 November 2014, reporting CKD prevalence for the adult general population in 20 European countries. The results of this systematic literature review revealed considerable variation in general population sample selection methods and assessment of kidney function across studies. Moreover, often a clear description of the methods used was lacking, and the reporting of CKD prevalence was heterogeneous. These factors may have considerable influence on the prevalence estimates of CKD and need to be taken into account to allow comparison of CKD prevalence across studies.
Population sample selection
Although we restricted our search to studies that were designed to be representative of the general population, we observed great heterogeneity in population sample selection methods. Part of this variation was found in the sampling frame used to identify contact details of eligible subjects. The sampling frame should ideally include the entire target population [58], which in this case is the entire general population. National census or population registry data are ideal for sampling the general population; in principle, these should include all inhabitants of a country or region. However, general population surveys are typically limited to community-dwelling subjects who are physically and mentally capable to participate in such studies. At old age, a substantial proportion of those with age-related chronic diseases such as CKD may no longer fulfill these inclusion criteria, which may lead to substantial underestimation of the true prevalence of such diseases. In such circumstances, depending on the health system or country, general practitioner list- or registry-based approaches might be required to provide more valid estimates of true prevalence.
Additionally, there existed great variation in sample design. For example, some studies first performed stratification of population by age and sex, whereas others invited all inhabitants in the selected region. Both the sampling frame and sample design influence the response and non-response bias [58], which in turn may influence the representativeness of the resulting sample for the general population and consequently of the CKD prevalence estimate. Collecting information on non-responders may help to assess the possibility and likely direction of non-response bias [58].
Assessment of kidney function
Serum creatinine and albuminuria measurements
There was great variation in the laboratory methods used in studies that reported details of those methods, especially in the calibration of serum creatinine. Differences in creatinine assays are important to take into account in CKD prevalence comparisons, as Jaffe methods overestimate serum creatinine and therefore overestimate CKD prevalence [59]. In 2006, IDMS standardization has been implemented to reduce the systematic bias in creatinine determination and to increase inter laboratory comparability [7]. The publications that clearly reported the use of IDMS standardization were only published in 2010 or later.
Ethnicity
In equations used to estimate GFR, like MDRD and CKD-EPI, the variable ‘ethnicity’ is included to adjust for ethnicity-specific differences. Ethnicity may, therefore, influence CKD prevalence estimates; even so, less than half of the publications reported collection of ethnicity data. Since in most European countries the vast majority of the European population is Caucasian, the lack of ethnicity data is unlikely to influence the CKD prevalence of most countries. In the future, however, the proportion of Caucasian subjects in the European population may change, making the collection of ethnicity data more important.
CKD definition
Despite the KDOQI guideline on CKD that was published in 2002 [5] and updated by Kidney Disease Improving Global Outcomes (KDIGO) in 2012 [60], we observed great variation in the definition of CKD, both in eGFR equations used and in cut-off values for both eGFR and albuminuria. For future studies, it is advisable to report CKD as recommended in the updated KDIGO guideline, including six eGFR categories and three albuminuria categories, as this classification allows presentation by mortality and progression risk [61]. The chronicity criterion was never used, mainly because follow-up data on serum creatinine were not collected. In more recent studies, CKD was most commonly defined using the CKD-EPI equation, as recommended by KDOQI [5].
Reporting methods
A clear description of the population sample selection methods and assessment of kidney function may facilitate a more fair comparison of CKD prevalence across studies. Studies should, therefore, preferably report this in detail in the method section of their publication. Unfortunately, many studies did not report the sampling frame used. In addition, information about biological sample collection (e.g. nature of collecting procedure, participants conditions, time between sampling and further processing) and sample storage conditions (duration of storage, thawing cycles, etc.) should also be reported [62].
Reporting results
Another observed difference was the presentation of the results on CKD prevalence estimates. Part of this variation is likely explained by the fact that CKD prevalence was not the main focus of 12 publications. However, even in publications with the main focus on CKD prevalence, there was great variation in reporting. All studies did report unadjusted prevalence estimates, yet they were mostly reported without a 95%CI. The reporting of the 95%CI is necessary as it provides an indication of how much uncertainty there is in the prevalence estimate.
Future studies should preferably report CKD prevalence standardized to the European population to enable international comparison, at least across Europe. In the case of regional prevalence estimates, additional standardization to the national population is required for within-country comparison. This standardization is essential when comparing CKD prevalence estimates from different countries or regions to avoid the influence of differences in national or regional age and sex distributions.
European CKD Burden Consortium
In 2012, the European CKD Burden Consortium was established, including both nephrologists and epidemiologists, to enhance comparability of CKD prevalence across European regions and countries.
Box 1 provides an overview of the methodology used by the European CKD Burden Consortium to compare CKD prevalence results across different general population-based studies in Europe. This methodology facilitates comparability by providing a detailed description of the population selection method and the response of each study to help assess representativeness of the study population sample. Additionally, the figures and tables clearly show the serum creatinine method used (i.e. Jaffe versus enzymatic) and whether IDMS calibration standardization was used.
Box 1: Recommended methodology for comparison of CKD prevalence results across general population-based studies as used by European CKD Burden Consortium.
| Recommended tools | Details |
|---|---|
| 1. General population sampling | |
| Sampling methods | Describe:
|
| Response | Report the response in percentages |
| 2. Assessment of kidney function | |
| Serum creatinine assay | Describe assay used, i.e. Jaffe or enzymatic |
| Albuminuria assay | Describe assay used, e.g. immunoassay and dipstick |
| IDMS calibration standardization | Describe if IDMS calibration standardization was used (yes/no) |
| CKD definition | Use of the same definition of CKD:
|
| 3. Presentation of results | |
| CKD prevalence estimate |
|
| CKD prevalence estimate by strata |
|
| Serum creatinine determination |
|
ACR, urinary albumin to urinary creatinine ratio; IDMS, isotope dilution mass spectrometry.
Furthermore, a uniform definition of CKD based on the KDIGO guideline was established [60]. CKD was defined as the presence of albuminuria >30 mg/g and/or an eGFR of <60 mL/min/1.73 m2 as calculated by the CKD-EPI equation. The chronicity criterion was not applied, for none of the assessed general population-based studies had this available.
The Consortium will additionally harmonize reporting of results in their publications. All CKD prevalence estimates will be presented as unadjusted rates and standardized to the EU27 population of 2005 [63] and include a 95%CI. As the occurrence of CKD is associated with age and not all study populations cover the entire range of the adult population, the CKD prevalence will also be presented for different age ranges, i.e. 20–44, 45–64, 65–74 and 75–84 years. Additionally, the prevalence estimates will be presented with stratification for the presence of the following risk factors: diabetes, hypertension and obesity. This stratification is useful to determine if differences in CKD prevalence are caused by differences in risk factor presence or differences in overall health status of the general population. Whether disparities in CKD prevalence are explained by important risk factors for CKD will guide policy makers to focus on secondary or primary prevention.
Implications
This systematic literature review revealed considerable variation in general population sample selection methods and assessment of kidney function across studies. In addition, a clear description of the methods used was often lacking, and the reporting of CKD prevalence was heterogeneous. The approach of The European CKD Burden Consortium will not eliminate the differences in population selection methods and laboratory assessment of kidney function. However, the recommendations regarding the reporting of both methods and results of CKD prevalence studies may enhance comparability of CKD prevalence results across Europe and even worldwide [64]. Our recommendations may be used by investigators to optimize both the design and the reporting of future CKD prevalence studies.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
The authors hereby declare that the results presented in this article have not been published previously in whole or part, except in abstract format.
This article was written by K. B., K. J. J. and V. S. S. on behalf of the ERA-EDTA Registry which is an official body of the ERA-EDTA (European Renal Association – European Dialysis and Transplant Association). Dorothea Nitsch has received funding from BMJ informatica to carry out analyses for the Health Quality Improvement Partnership funded National CKD Audit in primary care.
NON-AUTHOR CONTRIBUTORS
FINRISK: Pekka Jousilahti; The Three City (3C) Study: Catherine Helmer, Marie Metzger; MONALISA: Jean Bernard Ruidavets, Vanina Bongard; ActiFE: Wolfgang Koenig, Michael D. Denkinger; ESTHER: Hermann Brenner, Kai-Uwe Saum; SHIP: Matthias Nauck, Sylvia Stracke; SLAN: Ivan Perry, Joseph Eustace; INCIPE: Antonio Lupo; MATISS: Chiara Donfrancesco, Simonetta Palleschi; VIP: Norman Lamaida, Ernesto Capuano; LifeLines: Steef Sinkeler, B.H.R. Wolffenbuttel; PREVEND: Stephan J.L. Bakker; HUNT: Knut Aasarød, Jostein Holmen; PolSenior: Jerzy Chudek, Mossakowska Malgorzata; PREVADIAB: Luis Gardete-Correia, João F. Raposo; EPIRCE: A.L. Martin de Francisco, P. Gayoso Diz; PIVUS: Elisabet Nerpin, Lars Lind; Bus Santé: Murielle Bochud, Jean-Michel Gaspoz; MRC: Astrid Fletcher, Paul Roderick; BELFRAIL + Intego Project: Gijs Van Pottelbergh; URIS: Arjan Van Der Tol; SURDIAGENE: Samy Hadjadj; SKROBB: Olivera Stojceva-Taneva.
Supplementary Material
Contributor Information
Collaborators: on behalf of the European CKD Burden Consortium, Pekka Jousilahti, Catherine Helmer, Marie Metzger, Jean Bernard Ruidavets, Vanina Bongard, Wolfgang Koenig, Michael D. Denkinger, Hermann Brenner, Kai-Uwe Saum, Matthias Nauck, Sylvia Stracke, Ivan Perry, Joseph Eustace, Antonio Lupo, Chiara Donfrancesco, Simonetta Palleschi, Norman Lamaida, Ernesto Capuano, Steef Sinkeler, B.H.R. Wolffenbuttel, Stephan J.L. Bakker, Knut Aasarød, Jostein Holmen, Jerzy Chudek, Mossakowska Malgorzata, Luis Gardete-Correia, João F. Raposo, A.L. Martin de Francisco, P. Gayoso Diz, Elisabet Nerpin, Lars Lind, Murielle Bochud, Jean-Michel Gaspoz, Astrid Fletcher, Paul Roderick, Gijs Van Pottelbergh, Arjan Van Der Tol, Samy Hadjadj, and Olivera Stojceva-Taneva
REFERENCES
- 1.Levey AS, Atkins R, Coresh J et al. . Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007; 72: 247–259 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA et al. . Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 3.Boutten A, Bargnoux AS, Carlier MC et al. . Enzymatic but not compensated Jaffe methods reach the desirable specifications of NKDEP at normal levels of creatinine. Results of the French multicentric evaluation. ClinChimActa 2013; 419: 132–135 [DOI] [PubMed] [Google Scholar]
- 4.Van Biesen W, Vanholder R, Veys N et al. . The importance of standardization of creatinine in the implementation of guidelines and recommendations for CKD: implications for CKD management programmes. Nephrol Dial Transplant 2006; 21: 77–83 [DOI] [PubMed] [Google Scholar]
- 5.K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation. 2002; 39(February Supplement 1) [PubMed]
- 6.Shlipak MG, Matsushita K, Arnlov J et al. . Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013; 369: 932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers GL, Miller WG, Coresh J et al. . Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. ClinChem 2006; 52: 5–18 [DOI] [PubMed] [Google Scholar]
- 8.Delanaye P, Cavalier E, Cristol JP et al. . Calibration and precision of serum creatinine and plasma cystatin C measurement: impact on the estimation of glomerular filtration rate. JNephrol 2014; 27: 467–475 [DOI] [PubMed] [Google Scholar]
- 9.Cholongitas E, Marelli L, Kerry A et al. . Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl Sur 2007; 13: 523–529 [DOI] [PubMed] [Google Scholar]
- 10.Aumann N, Baumeister SE, Werner A et al. . Inverse association of estimated cystatin C- and creatinine-based glomerular filtration rate with left ventricular mass: Results from the Study of Health in Pomerania. Int J Cardiol 2013; 167: 2786–2791 [DOI] [PubMed] [Google Scholar]
- 11.Bongard V, Dallongeville J, Arveiler D et al. . Assessment and characteristics of chronic kidney disease in France. [French] Ann Cardiol Angeiol (Paris) 2012; 61: 239–244 [DOI] [PubMed] [Google Scholar]
- 12.Browne GM, Eustace JA, Fitzgerald AP et al. . Prevalence of diminished kidney function in a representative sample of middle and older age adults in the Irish population. BMC Nephrol 2012; 13: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capuano V, Lamaida N, Borrelli MI et al. . [Chronic kidney disease prevalence and trends (1998–2008) in an area of southern Italy. The data of the VIP project]. G Ital Nefrol 2012; 29: 445–451 [PubMed] [Google Scholar]
- 14.Christensson A, Elmstahl S. Estimation of the age-dependent decline of glomerular filtration rate from formulas based on creatinine and cystatin C in the general elderly population. Nephron Clin Prac 2011; 117: c40–c50 [DOI] [PubMed] [Google Scholar]
- 15.Chudek J, Wieczorowska-Tobis K, Zejda J et al. . The prevalence of chronic kidney disease and its relation to socioeconomic conditions in an elderly Polish population: results from the national population-based study PolSenior. Nephrol Dial Transplant 2014; 29: 1073–1082 [DOI] [PubMed] [Google Scholar]
- 16.Cirillo M, Laurenzi M, Mancini M et al. . Low glomerular filtration in the population: Prevalence, associated disorders, and awareness. Kidney Int 2006; 70: 800–806 [DOI] [PubMed] [Google Scholar]
- 17.Codreanu I, Sali V, Gaibu S et al. . Prevalence of hypertension and diabetes and coexistence of chronic kidney disease and cardiovascular risk in the population of the Republic of Moldova. Int J Hypertens 2012; 2012: Article ID 951734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Nicola L, Donfrancesco C, Minutolo R et al. . Epidemiology of chronic kidney disease in Italy: current state and contribution of the CARHES study. Giornale Italiano di Nefrologia: Organo Ufficiale Della Societa Italiana di Nefrologia 2011; 28: 401–407 [PubMed] [Google Scholar]
- 19.Delanaye P, Cavalier E, Mariat C et al. . MDRD or CKD-EPI study equations for estimating prevalence of stage 3 CKD in epidemiological studies: which difference? Is this difference relevant? BMC Nephrol 2010; 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donfrancesco C, Palleschi S, Palmieri L et al. . Estimated glomerular filtration rate, all-cause mortality and cardiovascular diseases incidence in a low risk population: the MATISS study. [Erratum appears in PLoS One. 2014; 9(1). doi:10.1371/annotation/1f5e18af-4a68–4419-9f3f-7e8bff410b48] PLoS ONE 2013; 8: e78475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Formiga F, Ferrer A, Cruzado JM et al. . Geriatric assessment and chronic kidney disease in the oldest old: The Octabaix study. Eur J Intern Med 2012; 23: 534–538 [DOI] [PubMed] [Google Scholar]
- 22.Fraser SD, Roderick PJ, Aitken G et al. . Chronic kidney disease, albuminuria and socioeconomic status in the Health Surveys for England 2009 and 2010. J Public Health (Oxf) 2014; 36: 577–586 [DOI] [PubMed] [Google Scholar]
- 23.Gambaro G, Yabarek T, Graziani MS et al. . Prevalence of CKD in northeastern Italy: results of the INCIPE study and comparison with NHANES. Clin J Am Soc Nephrol 2010; 5: 1946–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannelli SV, Graf CE, Herrmann FR et al. . Natural history of older adults with impaired kidney function: The InCHIANTI study. Rejuvenation Res 2011; 14: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goek ON, Prehn C, Sekula P et al. . Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol Dial Transplant 2013; 28: 2131–2138 [DOI] [PubMed] [Google Scholar]
- 26.Gu YM, Thijs L, Liu YP et al. . The urinary proteome as correlate and predictor of renal function in a population study. Nephrol Dial Transplant 2014; 29: 2260–2268 [DOI] [PubMed] [Google Scholar]
- 27.Guessous I, McClellan W, Kleinbaum D et al. . Comparisons of serum vitamin D levels, status, and determinants in populations with and without chronic kidney disease not requiring renal dialysis: a 24-hour urine collection population-based study. J Ren Nutr 2014; 24: 303–312 [DOI] [PubMed] [Google Scholar]
- 28.Hallan SI, Coresh J, Astor BC et al. . International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 2006; 17: 2275–2284 [DOI] [PubMed] [Google Scholar]
- 29.Hernandez D, Espejo-Gil A, Bernal-Lopez MR et al. . Association of HbA1c and cardiovascular and renal disease in an adult Mediterranean population. BMC Nephrol 2013; 14: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juutilainen A, Kastarinen H, Antikainen R et al. . Comparison of the MDRD Study and the CKD-EPI Study equations in evaluating trends of estimated kidney function at population level: findings from the National FINRISK study. Nephrol Dial Transplant 2012; 27: 3210–3217 [DOI] [PubMed] [Google Scholar]
- 31.Lieb W, Mayer B, Stritzke J et al. . Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: the MONICA/KORA Augsburg echocardiographic substudy. Nephrol Dial Transplant 2006; 21: 2780–2787 [DOI] [PubMed] [Google Scholar]
- 32.Meuwese CL, Gussekloo J, de Craen AJ et al. . Thyroid status and renal function in older persons in the general population. J Clin Endocrinol Metab 2014; 99: 2689–2696 [DOI] [PubMed] [Google Scholar]
- 33.Nitsch D, Lawlor DA, Patel R et al. . The association of renal impairment with all-cause and cardiovascular disease mortality. Nephrol Dial Transplant 2010; 25: 1191–1199 [DOI] [PubMed] [Google Scholar]
- 34.Nitsch D, Felber Dietrich D, von Eckardstein A et al. . Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant 2006; 21: 935–944 [DOI] [PubMed] [Google Scholar]
- 35.Otero A, de Francisco A, Gayoso P et al. . Prevalence of chronic renal disease in Spain: results of the EPIRCE study. Nefrologia 2010; 30: 78–86 [DOI] [PubMed] [Google Scholar]
- 36.Pani A, Bragg-Gresham J, Masala M et al. . Prevalence of CKD and its relationship to eGFR-related genetic loci and clinical risk factors in the SardiNIA study cohort. J Am Soc Nephrol 2014; 25: 1533–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pattaro C, Riegler P, Stifter G et al. . Estimating the glomerular filtration rate in the general population using different equations: effects on classification and association. Nephron 2013; 123: 102–111 [DOI] [PubMed] [Google Scholar]
- 38.Ponte B, Pruijm M, Marques-Vidal P et al. . Determinants and burden of chronic kidney disease in the population-based CoLaus study: A cross-sectional analysis. Nephrol Dial Transplant 2013; 28: 2329–2339 [DOI] [PubMed] [Google Scholar]
- 39.Redon J, Gil V, Cea-Calvo L et al. . The impact of occult renal failure on the cardiovascular risk stratification in an elderly population: the PREV-ICTUS study. Blood Press 2008; 17: 212–219 [DOI] [PubMed] [Google Scholar]
- 40.Robles NR, Felix FJ, Fernandez-Berges D et al. . Cross-sectional survey of the prevalence of reduced estimated glomerular filtration rate, albuminuria and cardiovascular risk in a native Spanish population. J Nephrol 2013; 26: 675–682 [DOI] [PubMed] [Google Scholar]
- 41.Roderick PJ, Atkins RJ, Smeeth L et al. . Detecting chronic kidney disease in older people; what are the implications? [Erratum appears in Age Ageing. 2009; 38: 638 Note: Flectcher, Astrid E (corrected to Fletcher, Astrid E )] Age Ageing 2008; 37: 179–186 [DOI] [PubMed] [Google Scholar]
- 42.Rothenbacher D, Klenk J, Denkinger M et al. . Prevalence and determinants of chronic kidney disease in community-dwelling elderly by various estimating equations. BMC Public Health 2012; 12: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutkowski B, Krol E. Epidemiology of chronic kidney disease in Central and Eastern Europe. Blood Purif 2008; 26: 381–385 [DOI] [PubMed] [Google Scholar]
- 44.Sahin I, Yildirim B, Cetin I et al. . Prevalence of chronic kidney disease in the black sea region, Turkey, and investigation of the related factors with chronic kidney disease. Ren Fail 2009; 31: 920–927 [DOI] [PubMed] [Google Scholar]
- 45.Schaeffner ES, Ebert N, Delanaye P et al. . Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012; 157: 471–481 [DOI] [PubMed] [Google Scholar]
- 46.Scheven L, de Jong PE, Hillege HL et al. . High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J 2012; 33: 2272–2281 [DOI] [PubMed] [Google Scholar]
- 47.Stasevic Z, Gorgieva GS, Vasic S et al. . High prevalence of kidney disease in two rural communities in Kosovo and Metohia. Ren Fail 2010; 32: 541–546 [DOI] [PubMed] [Google Scholar]
- 48.Stengel B, Metzger M, Froissart M et al. . Epidemiology and prognostic significance of chronic kidney disease in the elderly—the Three-City prospective cohort study. Nephrol Dial Transplant 2011; 26: 3286–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suleymanlar G, Uta C, Arinsoy T et al. . A population-based survey of Chronic REnal Disease in Turkey—the CREDIT study. Nephrol Dial Transplant 2011; 26: 1862–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tavira B, Coto E, Gomez J et al. . Association between a MYH9 polymorphism (rs3752462) and renal function in the Spanish RENASTUR cohort. Gene 2013; 520: 73–76 [DOI] [PubMed] [Google Scholar]
- 51.Van Pottelbergh C, Gurina N, Degryse J et al. . Prevalence of impaired renal function in the elderly in the St. Petersburg District: results of the Crystal study. Adv Gerontol 2011; 24: 108–113 [PubMed] [Google Scholar]
- 52.Viktorsdottir O, Palsson R, Andresdottir MB et al. . Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant 2005; 20: 1799–1807 [DOI] [PubMed] [Google Scholar]
- 53.Vinhas J, Gardete-Correia L, Boavida JM et al. . Prevalence of chronic kidney disease and associated risk factors, and risk of end-stage renal disease: data from the PREVADIAB study. Nephron Clin Prac 2011; 119: c35–c40 [DOI] [PubMed] [Google Scholar]
- 54.Wasen E, Isoaho R, Mattila K et al. . Estimation of glomerular filtration rate in the elderly: a comparison of creatinine-based formulae with serum cystatin C. J Intern Med 2004; 256: 70–78 [DOI] [PubMed] [Google Scholar]
- 55.Wetmore JB, Palsson R, Belmont JM et al. . Discrepancies between creatinine- and cystatin C-based equations: implications for identification of chronic kidney disease in the general population. Scand J Urol Nephrol 2010; 44: 242–250 [DOI] [PubMed] [Google Scholar]
- 56.Zambon S, Maggi S, Zanoni S et al. . Association of single measurement of estimated glomerular filtration rate and non-quantitative dipstick proteinuria with all-cause and cardiovascular mortality in the elderly. Results From the Progetto Veneto Anziani (Pro.V.A.) Study. Atherosclerosis 2012; 220: 201–207 [DOI] [PubMed] [Google Scholar]
- 57.Zhang QL, Koenig W, Raum E et al. . Epidemiology of chronic kidney disease: results from a population of older adults in Germany. Prev Med 2009; 48: 122–127 [DOI] [PubMed] [Google Scholar]
- 58.Groves R, Fowler F, Couper M et al. . Survey Methodology. 2nd edn Hoboken, New Jersey: John Wiley & Sons, Inc., 2009 [Google Scholar]
- 59.Drion I, Cobbaert C, Groenier KH et al. . Clinical evaluation of analytical variations in serum creatinine measurements: why laboratories should abandon Jaffe techniques. BMC Nephrol 2012; 13: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney IntSuppl 2012; 3: 1–163 [Google Scholar]
- 61.Levey AS, de Jong PE, Coresh J et al. . The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011; 80: 17–28 [DOI] [PubMed] [Google Scholar]
- 62.Gallo V, Egger M, McCormack V et al. . STrengthening the Reporting of OBservational studies in Epidemiology—Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement. PLoS Medicine 2011; 8: e1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eurostat: http://epp.eurostat.ec.europa.eu/portal/page/portal/population/data/database Table: Average population by sex and five-year age groups. [Internet]. (15 August 2013, date last accessed)
- 64.Bikbov B, Perico N, Remuzzi G. Mortality landscape in the global burden of diseases, injuries and risk factors study. Eur J Intern Med 2014; 25: 1–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.