Abstract
The observer-blind, randomized, age-stratified, head-to-head study (NCT00423046) comparing immunogenicity and safety of HPV-16/18 and HPV-6/11/16/18 vaccines in healthy women aged 18-45 y was completed. Five y after vaccination, in subjects from the Month 60 according-to-protocol cohort (seronegative and DNA negative for HPV type analyzed at baseline), serum neutralizing antibody (nAb) responses induced by HPV-16/18 vaccine remained 7.8-fold (18-26-y stratum), 5.6-fold (27-35-y stratum) and 2.3-fold (36-45-y stratum) higher than those induced by HPV-6/11/16/18 vaccine for HPV-16. For HPV-18, the fold differences were 12.1, 13.0 and 7.8, respectively. At Month 60, all (100%) subjects in HPV-16/18 vaccine group and the majority (95.7%-97.5%) in HPV-6/11/16/18 vaccine group were seropositive for HPV-16. For HPV-18, the majority (98.1%-100%) of subjects in HPV-16/18 vaccine group were seropositive; however, seropositivity rates in HPV-6/11/16/18 vaccine group decreased considerably (61.1%-76.9%) across the 3 age strata. In the total vaccinated cohort (received ≥ 1 dose regardless of baseline HPV serostatus and DNA status), geometric mean titers for anti-HPV-16 and anti-HPV-18 nAb were higher in HPV-16/18 vaccine group than in HPV-6/11/16/18 vaccine group. Based on the 5-y data, piece-wise and modified power-law models predicted a longer durability of nAb response for HPV-16/18 vaccine compared to HPV-6/11/16/18 vaccine. Beyond the differences apparent between the vaccines in terms of immunogenicity and modeled persistence of antibody responses, comparative studies including clinical endpoints would be needed to determine whether differences exist in duration of vaccine-induced protection.
Keywords: antibodies, Cervarix®, Gardasil®, human papillomavirus, immunogenicity, models, neutralizing, statistical, safety
List of abbreviations
- AAHS
amorphous aluminum hydroxyphosphate sulfate
- ANOVA
analysis of variance
- AS04
Adjuvant System containing 3-O-desacyl-4’-monophosphoryl lipid A (MPL 50 μg) adsorbed on aluminum salt (500 μg Al3+)
- ATP
according-to-protocol
- CI
confidence interval
- ED50
effective dose producing 50% response
- ELISA
enzyme-linked immunosorbent assay
- GMT
geometric mean titer
- HPV
human papillomavirus
- MSC
medically significant condition
- nAb
neutralizing antibodies
- NOAD
new onset autoimmune disease
- NOCD
new onset chronic disease
- PBNA
pseudovirion-based neutralization assay
- SAE
serious adverse event
- SP
seropositivity
- TVC
total vaccinated cohort
Introduction
Two virus-like particle-based prophylactic vaccines against oncogenic, high-risk human papillomavirus (HPV) types 16 and 18 have been licensed in over 130 countries: the HPV-16/18 vaccine (Cervarix®, GlaxoSmithKline Vaccines) and the HPV-6/11/16/18 vaccine (Gardasil®, Merck). The HPV-16/18 vaccine is formulated with a proprietary immunostimulatory Adjuvant System 04 (AS04)1, containing 3-O-desacyl-4’-monophosphoryl lipid A (MPL, 50 μg) adsorbed on aluminum salt (Al3+), whereas the HPV-6/11/16/18 vaccine is formulated with a proprietary amorphous aluminum hydroxyphosphate sulfate (AAHS) adjuvant. A randomized, observer-blind, head-to-head study compared the immunogenicity and safety of the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine in healthy women aged 18-45 y (study HPV-010; NCT00423046). Analysis of the primary objective of the head-to-head study was performed at Month 7, where the serum neutralizing antibody (nAb) responses elicited by the HPV-16/18 vaccine were significantly higher than those elicited by the HPV-6/11/16/18 vaccine.2 The observed differences in the magnitude of immune responses between the vaccines were maintained at Month 243 and up to Month 48.4 This study is now complete and data on vaccine-induced antibody responses up to 5 y post-vaccination (Month 60) are presented.
Since the risk of acquiring an HPV infection persists throughout a woman's sexually active life, the duration of protection conferred by cervical cancer vaccination is critical. Modeling of anti-HPV-16 and -18 antibody dynamics following vaccination may be valuable in predicting duration of vaccine-induced HPV immunogenicity beyond the empirical follow-up.5,6 Predictive modeling has been applied to estimate the persistence of antibodies induced following hepatitis A and B vaccination.7-9
The aims of the end-of-study analysis reported here are: 1) to evaluate the serum antibody response of the HPV-16/18 and the HPV-6/11/16/18 vaccines measured by pseudovirion-based neutralization assay (PBNA) and enzyme-linked immunosorbent assay (ELISA) through Month 60 (i.e., 54 months after completion of the full vaccination series); 2) to predict the long-term persistence of vaccine-induced anti-HPV-16 and anti-HPV-18 antibody responses (PBNA, ELISA) in subjects receiving a full series of vaccination by applying statistical models to antibody levels measured during the 5-y study duration; and 3) to evaluate safety up to Month 60.
Results
Study population
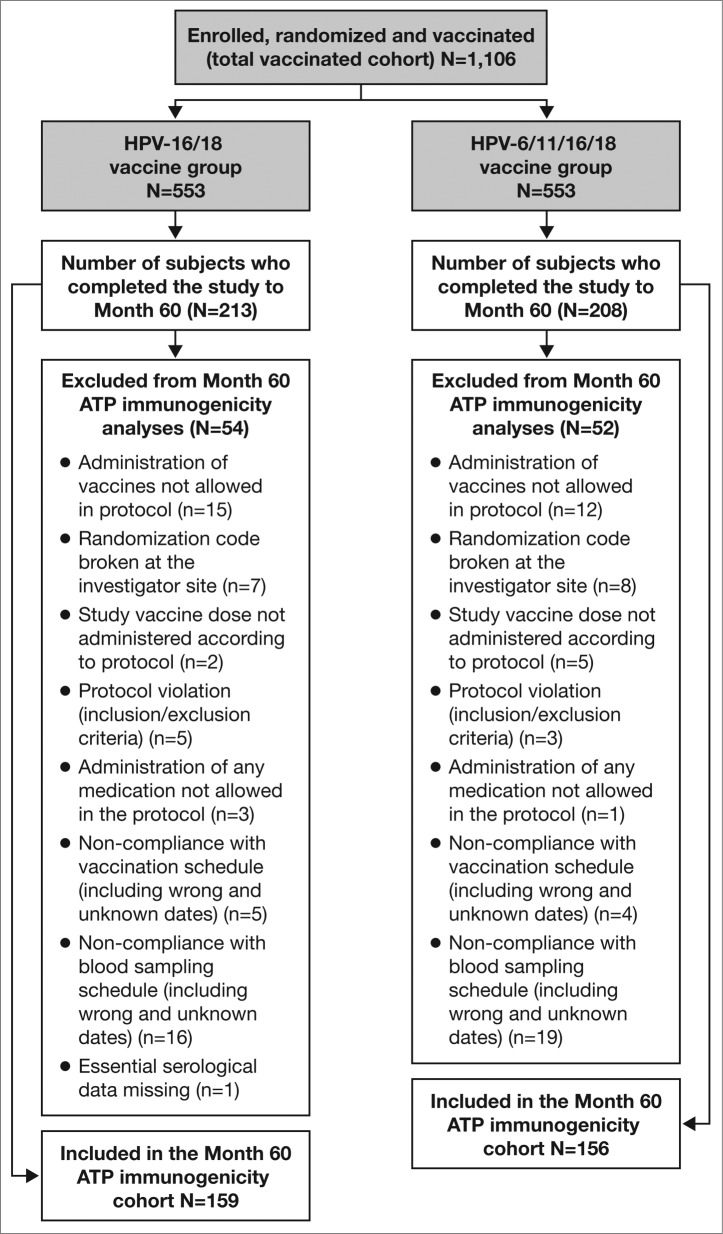
A total of 1,106 women were enrolled and vaccinated with at least one dose of HPV-16/18 vaccine (N = 553) or HPV-6/11/16/18 vaccine (N = 553); all of whom were included in the total vaccinated cohort (TVC). Among them, 421 women consented to participate in the extended Month 60 visit (213 in the HPV-16/18 vaccine group and 208 in the HPV-6/11/16/18 vaccine group); these subjects comprised the Month 60 TVC. The Month 60 according-to-protocol (ATP) cohort for immunogenicity included 315 women (159 women in the HPV-16/18 vaccine group and 156 in the HPV-6/11/16/18 vaccine group) who met the eligibility criteria, received a full series of 3-dose vaccination and complied with the procedures defined in the protocol. The number of women excluded from the Month 60 ATP cohort for immunogenicity analysis was similar between vaccine groups, as were the reasons for exclusion (Fig. 1).
Figure 1.
Subject disposition: CONSORT diagram ATP, according-to-protocol. The Month 60 ATP cohort for immunogenicity included all evaluable subjects who received 3 vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available. This included subjects for whom assay results were available for antibodies against at least one study vaccine antigen (HPV-16 or HPV-18) at the time point under analysis.
Antibody responses in serum
Table 1 shows the geometric mean titers (GMTs) and seropositivity rates of anti-HPV-16 and -18 nAbs measured by PBNA in the ATP cohort for immunogenicity in women who were seronegative and DNA-negative for the HPV type analyzed prior to vaccination. At Month 60, in women aged 18-26 y, anti-HPV-16 and -18 nAb GMTs in the HPV-16/18 vaccine group were 7.8 (95% confidence interval (CI) 4.3, 14.0) and 12.1 (6.6, 22.1) -fold higher, respectively, than those in the HPV-6/11/16/18 vaccine group. At the same time point, compared with the HPV-6/11/16/18 vaccine, anti-HPV-16 and -18 GMTs induced by the HPV-16/18 vaccine were 5.6 (3.0, 10.2) and 13.0 (7.6, 22.3) -fold higher, respectively, in women aged 27-35 y, and were 2.3 (1.3, 4.3) and 7.8 (4.5, 13.3) -fold higher, respectively, in women aged 36-45 y (Table 1). Exploratory analyses in the TVC (irrespective of serostatus and DNA status prior to vaccination) showed that anti-HPV-16 and -18 nAb levels induced by the HPV-16/18 vaccine were higher than those induced by the HPV‑6/11/16/18 vaccine in all age groups (Table 1).
Table 1.
Seropositivity rates and GMTs for serum anti-HPV-16 and anti-HPV-18 type-specific neutralizing antibodies measured by PBNA at Month 60 (ATP and TVC cohorts)
| ATP cohort for immunogenicity, seronegative and DNA-negative prior to vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | |||||||
| Age | Antigen | N | % SP [95% CI] | GMT [95% CI] | N | % SP [95% CI] | GMT [95% CI] | GMT Ratio [95% CI] |
| 18-26 y | ||||||||
| HPV-16 | 35 | 100 [90.0, 100] | 4118 [2742, 6184] | 40 | 97.5 [86.8, 99.9] | 530 [343, 818] | 7.8 [4.3, 14.0] | |
| HPV-18 | 39 | 100 [91.0, 100] | 1523 [968, 2395] | 52 | 76.9 [63.2, 87.5] | 126 [84.0, 190] | 12.1 [6.6, 22.1] | |
| 27-35 y | ||||||||
| HPV-16 | 43 | 100 [91.8, 100] | 1925 [1302, 2847] | 29 | 96.6 [82.2, 99.9] | 346 [215, 558] | 5.6 [3.0, 10.2] | |
| HPV-18 | 54 | 98.1 [90.1, 100] | 967 [701, 1334] | 36 | 61.1 [43.5, 76.9] | 74.4 [46.8, 118] | 13.0 [7.6, 22.3] | |
| 36-45 y | ||||||||
| HPV-16 | 46 | 100 [92.3, 100] | 1785 [1233, 2583] | 47 | 95.7 [85.5, 99.5] | 765 [468, 1249] | 2.3 [1.3, 4.3] | |
| HPV-18 | 55 | 100 [93.5, 100] | 817 [555, 1202] | 51 | 74.5 [60.4, 85.7] | 105 [71.8, 154] | 7.8 [4.5, 13.3] | |
| TVC, irrespective of serostatus and DNA status prior to vaccination | ||||||||
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | |||||||
| Age | Antigen | N | % SP [95% CI] | GMT [95% CI] | N | % SP [95% CI] | GMT [95% CI] | P value Kruskal Wallis* |
| 18-26 y | ||||||||
| HPV-16 | 62 | 100 [94.2, 100] | 4036 [2876, 5664] | 65 | 98.5 [91.7, 100] | 832 [570, 1214] | < 0.0001 | |
| HPV-18 | 62 | 100 [94.2, 100] | 1525 [1069, 2176] | 65 | 76.9 [64.8, 86.5] | 120 [84.0, 172] | < 0.0001 | |
| 27-35 y | ||||||||
| HPV-16 | 76 | 100 [95.3, 100] | 2550 [1866, 3485] | 60 | 98.3 [91.1, 100] | 859 [547, 1349] | < 0.0001 | |
| HPV-18 | 76 | 98.7 [92.9, 100] | 1094 [820, 1461] | 60 | 71.7 [58.6, 82.5] | 122 [80.2, 186] | < 0.0001 | |
| 36-45 y | ||||||||
| HPV-16 | 75 | 100 [95.2, 100] | 2321 [1629, 3306] | 82 | 97.6 [91.5, 99.7] | 1110 [741, 1661] | 0.00496 | |
| HPV-18 | 75 | 97.3 [90.7, 99.7] | 870 [611, 1239] | 82 | 80.5 [70.3, 88.4] | 190 [128, 281] | < 0.0001 | |
95% CI, exact 95% confidence interval; GMT, geometric mean titer; N, number of subjects with available results; PBNA, pseudovirion-based neutralization assay; ATP, according-to-protocol; TVC, total vaccinated cohort; SP, seropositivity (defined as neutralizing antibody titer ≥40 ED50 [effective dose producing 50% response]); y, year.
GMT ratio, GMT in the HPV-16/18 vaccine group over GMT in the HPV-6/11/16/18 vaccine group.
*For the comparison of GMT between vaccine groups, p-values were calculated using Kruskal Wallis test.
At Month 60 in the ATP cohort, seropositivity rates for HPV-16 nAbs (by PBNA) remained high in the HPV-16/18 vaccine group (18-26 y: 100% (95% CI: 90%, 100%); 27-35 y: 100% (91.8%, 100%); 36-45 y: 100% (92.3%, 100%)), as did the seropositivity rates for HPV‑18 (18-26 y: 100% (91%, 100%); 27-35 y: 98.1% (90.1%, 100%); 36-45 y: 100% (93.5%, 100%)). Seropositivity rates also remained high in the HPV-6/11/16/18 vaccine group for HPV-16 (18-26 y: 97.5% (86.8%, 99.9%); 27-35 y: 96.6% (82.2%, 99.9%); 36-45 y: 95.7% (85.5%, 99.5%)); however, there was a noticeable decrease for HPV-18 (18-26 y: 76.9% (63.2%, 87.5%); 27-35 y: 61.1% (43.5%, 76.9%); 36-45 y: 74.5% (60.4%, 85.7%)) (Table 1). In the Month 60 TVC cohort, the trend was similar to the Month 60 ATP cohort (Table 1). Data on serum anti-HPV-16 and -18 type-specific antibody responses as assessed by ELISA at Month 60 in the ATP and TVC cohorts (Supplementary Table 1) further substantiated the results obtained by PBNA.
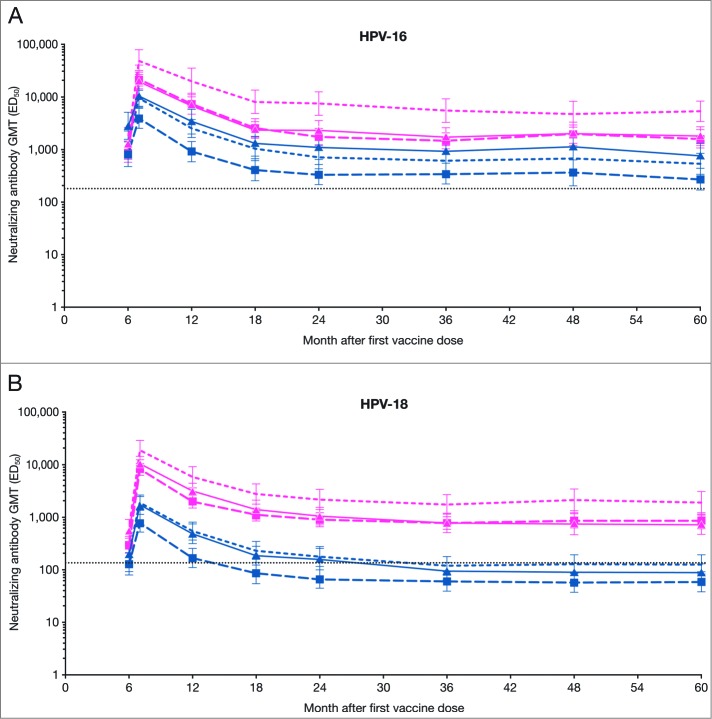
In subjects seronegative and DNA-negative for the HPV type analyzed at baseline with available and valid results at each time point, the kinetics of the antibody responses induced by both vaccines appeared similar in trend. Neutralizing antibody GMTs peaked at Month 7, declined thereafter and reached a plateau beyond 18-24 months post-vaccination. The plateau level of anti-HPV-16 and -18 nAbs from any age stratum in the HPV-16/18 vaccine group appeared higher than that from any age stratum in the HPV-6/11/16/18 vaccine group through Month 60. It is noteworthy that the plateau levels of anti-HPV-18 nAbs from all 3 age strata induced by the HPV-6/11/16/18 vaccine dropped to a level similar to or below that induced by natural infection, while the plateau levels induced by the HPV-16/18 vaccine remained several fold higher than the level associated with natural infection through to Month 60 (Fig. 2). The anti-HPV-16 and -18 nAb levels as measured by PBNA after natural infection within this study population (total vaccinated cohort) were determined in women of all age strata combined who were seropositive and cervical HPV-DNA negative for the type analyzed at baseline.2
Figure 2.
GMTs for (A) serum anti-HPV-16 and (B) anti-HPV-18 type-specific neutralizing antibodies at Months 6, 7, 12, 18, 24, 36, 48 and 60 (PBNA, ATP kinetic cohort; seronegative and DNA-negative for the HPV type analyzed prior to vaccination) ATP, according-to-protocol; ED50, effective dose producing 50% response; GMT, geometric mean titers; n, number of subjects with available results; PBNA, pseudovirion-based neutralization assay. Pink narrow dashed line, HPV-16/18 vaccine 18-26 y (HPV-16 n = 27, HPV-18, n = 31); pink wide dashed line with ▪, HPV-16/18 vaccine 27-35 y (HPV-16 n = 36, HPV-18 n = 47); solid pink line with ▴, HPV-16/18 vaccine 36-45 y (HPV-16 n = 37, HPV-18 n = 46); blue narrow dashed line, HPV-6/11/16/18 vaccine 18-26 y (HPV-16 n = 36, HPV-18 n = 46); blue wide dashed line with ▪, HPV-6/11/16/18 vaccine 27-35 y (HPV-16 n = 25, HPV-18 n = 30); solid blue line with ▴, HPV‑6/11/16/18 vaccine 36-45 y (HPV-16 n = 38, HPV-18 n = 43). Error bars denote 95% confidence intervals of GMTs. Dotted line, GMTs for natural infection neutralizing antibody levels as measured by PBNA in women in the total vaccinated cohort of the HPV-010 study who had cleared natural infection (prior to vaccination) [i.e., those who were seropositive and DNA-negative at Month 0]: 180.1 ED50 for HPV-16 and 137.3 ED50 for HPV-18.2
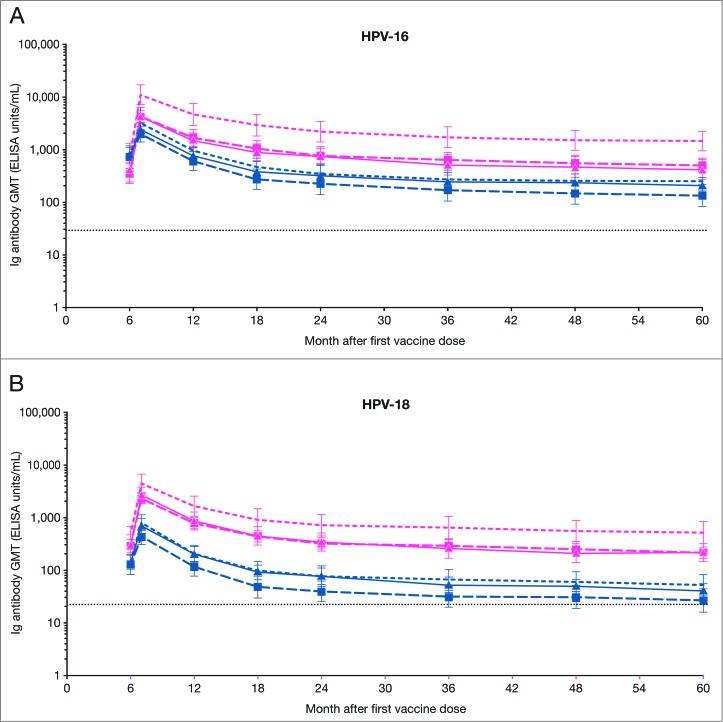
The kinetics of serum anti-HPV type-specific antibody responses assessed by ELISA followed a similar trend as those assessed by PBNA. Across the 3 age strata, the plateau levels for anti-HPV-16 and -18 antibodies in both the HPV-16/18 and HPV-6/11/16/18 vaccine groups remained above the levels induced by natural infection, as measured by ELISA in the HPV-008 study population.10 The antibody plateau levels induced by the HPV-16/18 vaccine appeared to be higher than those induced by the HPV-6/11/16/18 vaccine up to Month 60 across all 3 age strata (Fig. 3).
Figure 3.
GMTs for (A) serum anti-HPV-16 and (B) anti-HPV-18 type-specific antibodies at Months 6, 7, 12, 18, 24, 36, 48 and 60 (ELISA, ATP kinetic cohort; seronegative and DNA-negative for the HPV type analyzed prior to vaccination) ATP, according-to-protocol; ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titers; n, number of subjects with available results. Pink narrow dashed line, HPV-16/18 vaccine 18-26 y (HPV-16 n = 24, HPV-18, n = 25); pink wide dashed line with ▪, HPV-16/18 vaccine 27-35 y (HPV-16 n = 23, HPV-18 n = 34); solid pink line with ▴, HPV-16/18 vaccine 36-45 y (HPV-16 n = 25, HPV-18 n = 32); blue narrow dashed line, HPV-6/11/16/18 vaccine 18-26 y (HPV-16 n = 32, HPV-18 n = 36); blue wide dashed line with ▪, HPV-6/11/16/18 vaccine 27-35 y (HPV-16 n = 17, HPV-18 n = 23); solid blue line with ▴, HPV‑6/11/16/18 vaccine 36-45 y (HPV-16 n = 25, HPV-18 n = 36). Error bars denote 95% confidence intervals of GMTs. Dotted line, GMTs for natural infection antibody levels (measured by ELISA) in the HPV-008 study: 29.8 ELISA units/mL for HPV-16 and 22.6 ELISA units/mL for HPV-18.9
Predicted long-term persistence of antibody responses
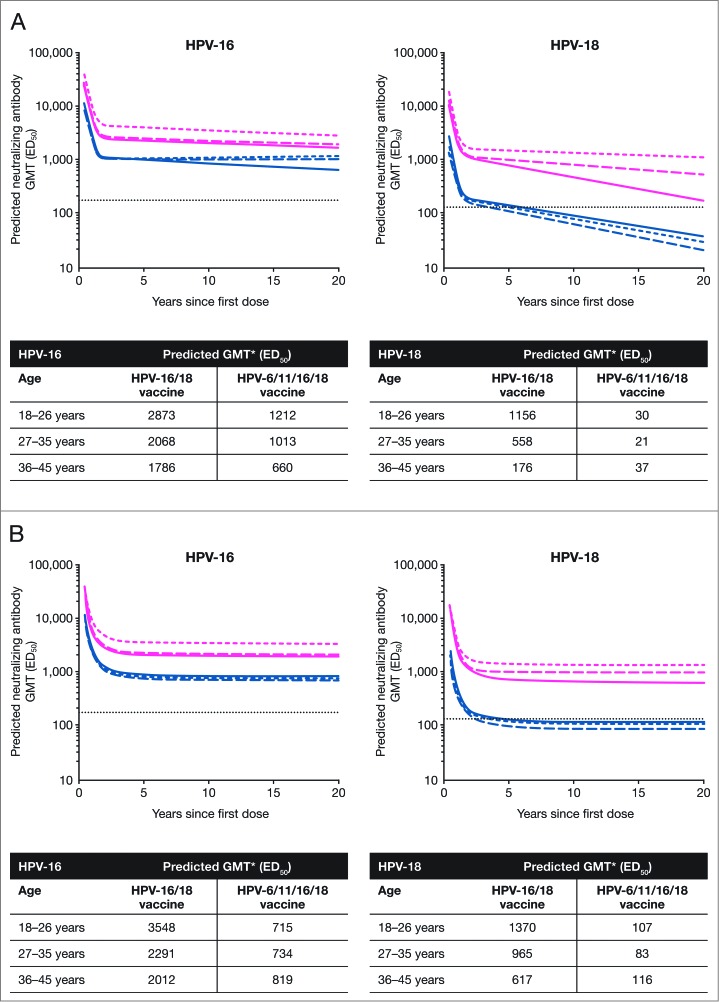
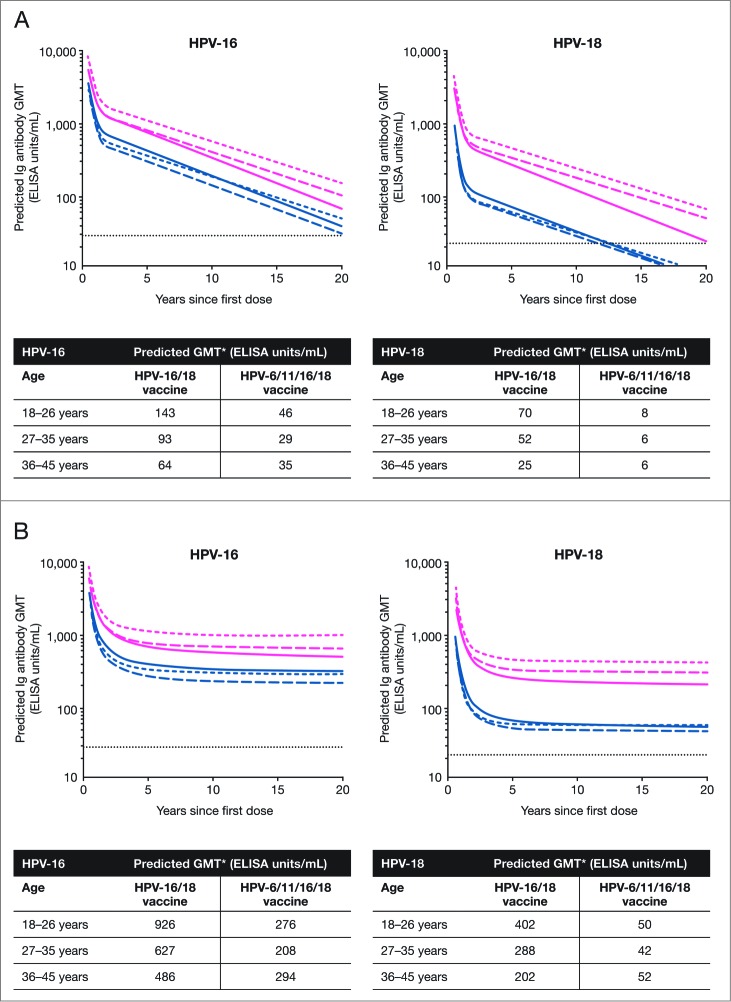
The individual data up to 60 months of follow-up from subjects in the TVC who received a full series of vaccination (i.e., 3 doses of either HPV-16/18 vaccine or HPV-6/11/16/18 vaccine) were used to fit 2 statistical models to predict the persistence of anti-HPV serum antibody response. Using the piece-wise model, for anti-HPV-16 and -18 nAbs measured by PBNA, GMTs 20 y after the first vaccine dose in the HPV-16/18 vaccine group were predicted to remain above the level induced by natural infection across the 3 age strata. In the HPV‑6/11/16/18 vaccine group, the predicted nAb GMTs 20 y after the first vaccine dose were also above the level induced by natural infection across the 3 age strata for anti-HPV-16 antibodies but below the natural infection level for anti-HPV-18 antibodies. The predicted anti-HPV-16 or -18 nAb GMTs at Year 20 from any age stratum in the HPV-16/18 vaccine group appeared to be higher than those from any age stratum in the HPV‑6/11/16/18 vaccine group, irrespective of age strata (Fig. 4A).
Figure 4.
Serum anti-HPV-16 and anti-HPV-18 type-specific neutralizing antibody responses (by PBNA analysis) over 20 y predicted by the (A) piece-wise linear model and (B) modified power-law model (total vaccinated cohort, 3 doses) ED50, effective dose producing 50% response; GMT, geometric mean titer; PBNA, pseudovirion-based neutralization assay. Pink narrow dashed line, HPV-16/18 vaccine 18-26 y; pink wide dashed line, HPV-16/18 vaccine 27-35 y; solid pink line, HPV-16/18 vaccine 36-45 y; blue narrow dashed line, HPV-6/11/16/18 vaccine 18-26 y; blue wide dashed line, HPV-6/11/16/18 vaccine 27-35 y; solid blue line, HPV‑6/11/16/18 vaccine 36-45 y; dotted line, neutralizing antibody GMTs measured by PBNA in women in the total vaccinated cohort of the HPV-010 study who had cleared natural infection (prior to vaccination) [i.e., those who were seropositive and DNA-negative at Month 0]: 180.1 ED50 for HPV-16 and 137.3 ED50 for HPV-18.2 *Predicted GMTs were calculated for 20 y after the first vaccine dose.
Applying the same model to anti-HPV-16 antibodies measured by ELISA, the predicted GMTs 20 y after the first vaccine dose were found to be above the level associated with natural infection in both HPV vaccine groups except the 27-35 y age stratum of the HPV-6/11/16/18 vaccine group. Across all 3 age strata, the predicted GMTs of anti-HPV-18 antibodies (ELISA) 20 y after the first vaccine dose were above the level induced by natural infection in the HPV-16/18 vaccine group but below the natural infection level in the HPV-6/11/16/18 vaccine group (Fig. 5A).
Figure 5.
Serum anti-HPV-16 and anti-HPV-18 type-specific antibody responses (by ELISA analysis) over 20 y predicted by the (A) piece-wise linear model and (B) modified power-law model (total vaccinated cohort, 3 doses) ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titer. Pink narrow dashed line, HPV-16/18 vaccine 18-26 y; pink wide dashed line, HPV-16/18 vaccine 27-35 y; solid pink line, HPV-16/18 vaccine 36-45 y; blue narrow dashed line, HPV-6/11/16/18 vaccine 18-26 y; blue wide dashed line, HPV-6/11/16/18 vaccine 27-35 y; solid blue line, HPV‑6/11/16/18 vaccine 36-45 y; dotted line, GMTs for natural infection antibody levels (measured by ELISA) in the HPV-008 study: 29.8 ELISA units/mL for HPV-16 and 22.6 ELISA units/mL for HPV-189. *Predicted GMTs were calculated for 20 y after the first vaccine dose.
By piece-wise modeling, the predicted duration over which at least 95% of women aged 18-26 y will remain with anti-HPV-16 and anti-HPV-18 nAb levels (PBNA) above the level induced by natural infection in the HPV-16/18 vaccine group was 68.2 and 40.6 y, respectively (Table 2A). In other age strata, respectively, the predicted durations for anti-HPV-16 and -18 nAb were 57.3 and 9.5 y (27-35 y age group) and 31.0 and 1.9 y (36-45 y age group) (Table 2A). The corresponding predicted durations in the HPV-6/11/16/18 vaccine group were: 1.7 y for HPV-16 and 9 months for HPV-18 in the 18-26 y age group; 1.3 y and <7 months (27-35 y age group); 1.3 y and 7 months (36-45 y age group) (Table 2A). The durations over which anti-HPV-16 and anti-HPV-18 antibody levels, as assessed by ELISA, were predicted to remain above the level induced by natural infection in at least 95% of women were also longer in the HPV-16/18 vaccine group than the HPV-6/11/16/18 vaccine group, supporting the results of the PBNA analysis (Table 2B).
Table 2.
Predicted duration (after the first vaccine dose) over which 95% of women will maintain antibody titers above natural infection titers for: (A) serum anti-HPV neutralizing antibody response (assessed by PBNA) and (B) serum anti-HPV type-specific antibody response (assessed by ELISA), by piece-wise and modified power-law models (total vaccinated cohort, 3 doses)
| (A) Serum neutralizing antibody responses (by PBNA analysis) | ||||
|---|---|---|---|---|
| Piece-wise model | Modified power-law model | |||
| Antigen† | HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | HPV-16/18 vaccine | HPV-6/11/16/18 vaccine |
| Predicted duration* | Predicted duration* | |||
| HPV-16 | ||||
| 18-26 y | 68.2 Y | 1.7 Y | ∞ | 1.8 Y |
| 27-35 y | 57.3 Y | 1.3 Y | ∞ | 1.1 Y |
| 36-45 y | 31.0 Y | 1.3 Y | ∞ | 1.2 Y |
| HPV-18 | ||||
| 18-26 y | 40.6 Y | 9 M | ∞ | 9 M |
| 27-35 y | 9.5 Y | < 7 M | ∞ | 7 M |
| 36-45 y | 1.9 Y | 7 M | 1.8 Y | 8 M |
| (B) Serum anti-HPV type-specific antibody responses (by ELISA analysis) | ||||
| Antigen†† | ||||
| HPV-16 | ||||
| 18-26 y | 21.0 Y | 11.3 Y | ∞ | ∞ |
| 27-35 y | 16.3 Y | 8.4 Y | ∞ | ∞ |
| 36-45 y | 13.3 Y | 9.3 Y | ∞ | ∞ |
| HPV-18 | ||||
| 18-26 y | 17.0 Y | 1.4 Y | ∞ | 1.3 Y |
| 27-35 y | 14.9 Y | 1.1 Y | ∞ | 1.1 Y |
| 36-45 y | 9.7 Y | 1.3 Y | ∞ | 1.3 Y |
∞, infinity; ELISA, enzyme-linked immunosorbent assay; M, month; PBNA, pseudovirion-based neutralization assay; Y, year.
*Predicted time after the first dose ensuring that 95% of women will still have levels above natural infection levels.
† Natural infection levels (ED50) for anti-HPV-16 and -18 neutralizing antibodies were 180.1 and 137.3, respectively.2 Anti-HPV-16 and -18 nAb levels as measured by PBNA after natural infection within this study population (total vaccinated cohort) were determined in women of all age strata combined who were seropositive and cervical HPV-DNA negative for the type analyzed at baseline.
†† Natural infection levels (ELISA units/mL) for anti-HPV-16 and -18 antibodies were 29.8 and 22.6, respectively.9 Natural infection Immunoglobulin G geometric mean titers (GMT) correspond to the GMT of ‘cleared’ natural infection (i.e., subjects DNA-negative and seropositive at the time of enrolment), obtained from the Phase III study (HPV-008), as benchmarks.
Applying the modified power-law model to anti-HPV-16 and -18 antibodies measured by PBNA, the predicted GMTs 20 y after the first vaccine dose were found to be above the natural infection level in all age strata in the HPV-16/18 vaccine group (Fig. 4B). The predicted GMTs of HPV-16 nAb at the same interval were also above the level induced by natural infection in the HPV-6/11/16/18 vaccine group but those for HPV-18 were below the natural infection level in all age strata (Fig. 4B). The corresponding results for predicted GMTs of anti-HPV-16 and ‑18 antibodies (ELISA) were all above the natural infection level in all age strata in both vaccine groups (Fig. 5B); however, the predicted anti-HPV-16 or -18 antibody GMTs at Year 20 from any age stratum in the HPV-16/18 vaccine group appeared to be higher than those from any age stratum in the HPV‑6/11/16/18 vaccine group (Fig. 5B).
Using the modified power-law model, the predicted duration over which at least 95% of women will remain with anti-HPV-16 and -18 antibodies (PBNA) above the level induced by natural infection was lifelong in all age strata in the HPV-16/18 vaccine group except in the 36-45 y age group where the anti-HPV‑18 nAb persistence above natural infection levels was 1.8 y (Table 2A). In the HPV-6/11/16/18 vaccine group, the predicted durations for anti-HPV-16 antibody persistence were 1.8 y (18-26 y age group), 1.1 y (27-35 y age group) and 1.2 y (36-45 y age group). In the same age strata, the predicted durations for HPV-18 persistence were 9 months, 7 months and 8 months, respectively (Table 2A). The modified power-law model predicts lifelong persistence of anti-HPV-16 and -18 antibodies (ELISA) in all age strata in the HPV-16/18 vaccine group, and lifelong persistence of anti-HPV-16 in all age strata in the HPV-6/11/16/18 vaccine group but persistence of anti-HPV-18 for 1.3 y, 1.1 y and 1.3 y in the 18-26, 27-35 and 36-45 y age groups, respectively (Table 2B).
Safety
From Month 0 to Month 60, the proportion of subjects reporting events in the HPV-16/18 and HPV-6/11/16/18 vaccine groups (TVC) were comparable (Table 3): serious adverse events (SAEs), 8% (n = 44) in HPV-16/18 vaccine group and 6.7% (n = 37) in HPV-6/11/16/18 vaccine group; medically significant conditions (MSCs), 46.8% (n = 259) in HPV-16/18 vaccine group and 40.9% (n = 226) in HPV-6/11/16/18 vaccine group; new onset chronic diseases (NOCDs), 7.1% (n = 39) in HPV-16/18 vaccine group and 7.8% (n = 43) in HPV-6/11/16/18 vaccine group; and new onset autoimmune diseases (NOADs), 1.3% (n = 7) in HPV-16/18 vaccine group and 2.4% (n = 13) in HPV-6/11/16/18 vaccine group.
Table 3.
Summary of safety and pregnancy outcomes from Month 0 to Month 60 (total vaccinated cohort; irrespective of serostatus and DNA status prior to vaccination)
| HPV-16/18 vaccine [N = 553] | HPV-6/11/16/18 vaccine [N = 553] | |
|---|---|---|
| Safety Outcomes | ||
| Number of subjects with safety outcomes | ||
| Serious adverse event, n (%) [95% CI] | 44 (8.0) [5.8, 10.5] | 37 (6.7) [4.8, 9.1] |
| Medically significant condition, n (%) [95% CI] | 259 (46.8) [42.6, 51.1] | 226 (40.9) [36.7, 45.1] |
| New onset chronic disease, n (%) [95% CI] | 39 (7.1) [5.1, 9.5] | 43 (7.8) [5.7, 10.3] |
| New onset autoimmune disease, n (%) [95% CI] | 7 (1.3) [0.5, 2.6] | 13 (2.4) [1.3, 4.0] |
| Pregnancy Outcomes | ||
| Number of subjects with pregnancies | 96 | 76 |
| Live infant NO apparent congenital anomaly, n (%) | 69 (71.9) | 56 (73.7) |
| Live infant congenital anomaly, n (%) | 0 | 2 (2.6) |
| Elective termination NO apparent congenital anomaly, n (%) | 6 (6.3) | 3 (3.9) |
| Ectopic pregnancy, n (%) | 1 (1.0) | 0 |
| Spontaneous abortion NO apparent congenital anomaly, n (%) | 15 (15.6) | 11 (14.5) |
| Stillbirth NO apparent congenital anomaly, n (%) | 1 (1.0) | 0 |
| Lost to follow-up, n (%) | 2 (2.1) | 3 (3.9) |
| Pregnancy ongoing, n (%) | 2 (2.1) | 1 (1.3) |
95% CI, exact 95% confidence interval; N, number of subjects with at least one administered dose; n (%), number (percentage) of subjects with event. Medically significant conditions were adverse events prompting an emergency room or physician visit that were not related to common diseases. As described previously2, all adverse events reported during the trial were compared with a pre-defined list of potential chronic diseases derived from the Medical Dictionary for Regulatory Activities. Determination of whether a chronic disease was of new onset was based on blinded review of the reported symptoms and the subject's pre-vaccination medical history by a physician from GlaxoSmithKline. A separate list, restricted to potential autoimmune events which excluded allergy-related events or isolated signs and symptoms and events not considered to be autoimmune in origin, was used to identify new onset autoimmune diseases among events identified as new onset chronic diseases.
In terms of SAEs, one subject in the HPV-16/18 vaccine group was diagnosed and died from metastatic renal cell carcinoma 287 d after the last dose of vaccine. The event was considered by the investigator to be unrelated to vaccination. Two SAEs were considered to be possibly related to vaccination: one was a grand mal convulsion in a subject in the HPV-16/18 vaccine group and one was a spontaneous abortion in a subject in the HPV-6/11/16/18 vaccine group.
The most commonly identified NOCD and NOAD was hypothyroidism, with the reporting frequencies similar between the groups, and all cases were considered to be unrelated to the vaccines.
A total of 172 pregnancies were reported up to Month 60, with similar outcomes between vaccine groups. There were 71.9% (n = 69) and 73.7% (n = 56) live infants, respectively, in the HPV-16/18 and HPV-6/11/16/18 vaccine groups, with no unanticipated anomalies or outcomes (Table 3). Fifteen (15.6%) and 11 cases (14.5%) of spontaneous abortion with no apparent congenital anomaly were reported in the HPV-16/18 and HPV-6/11/16/18 vaccine groups, respectively.
Discussion
In this end-of-study analysis, it was shown that the difference observed at Month 7 between HPV-16/18 and HPV-6/11/16/18 vaccines, in terms of serum nAb responses to HPV-16 and HPV-18, was sustained up to Month 60 in women of 18-45 y of age. Statistical models based on the measured data predict that nAb responses induced by the HPV-16/18 vaccine could persist longer than those induced by the HPV-6/11/16/18 vaccine.
The finding that the HPV-16/18 vaccine induced higher serum antibody response than did the HPV-6/11/16/18 vaccine reported here is consistent with observations from 2 independent head-to-head studies comparing the immunogenicity of the 2 vaccines.11,12 In the present report, both the HPV-16/18 and HPV-6/11/16/18 vaccines induced sustained nAb response against HPV-16; however, there were 2.3-7.8-fold differences in the magnitude of response between the 2 vaccines 5 y after the first vaccination, depending on the ages at which the vaccine was administered. As predicted by both the piece-wise and modified power-law models, even 20 y after the first vaccination, anti-HPV-16 nAb levels from women of any age stratum in the HPV-16/18 vaccine group appeared to remain higher than those from any age stratum in the HPV-6/11/16/18 vaccine group. The differences in anti-HPV-18 nAb response between the 2 vaccines (7.8-13.0 fold) were more pronounced. While the HPV-16/18 vaccine induced anti-HPV-18 nAb plateau levels across the 3 age strata that remained substantially above the level associated with natural infection, the HPV-6/11/16/18 vaccine induced anti-HPV-18 nAb plateau levels similar to or below the level associated with natural infection. Both the piece-wise and modified power-law models predicted an anti-HPV-18 nAb level lower than that associated with natural infection 20 y after vaccination with the HPV-6/11/16/18 vaccine. In a 5-y study of the HPV-6/11/16/18 vaccine in a younger age population (aged 16-23 y), while the vaccine-induced anti-HPV-16 nAb level (assessed by Merck competitive Luminex immunoassay) remained above the level associated with natural infection, the anti-HPV-18 nAb level decreased to a level close to that associated with natural infection.13
The decrease of anti-HPV-18 nAb levels within 5-y follow-up was further reflected in the loss of seropositivity among at least 20% of women in the HPV-6/11/16/18 vaccine group across all age strata. At Month 60, in women who received the HPV-6/11/16/18 vaccine, while the seropositivity rate for anti-HPV-16 nAb remained high (95.7-97.5%), the seropositivity rate for anti-HPV-18 nAbs decreased substantially (61.1-76.9%), across all age strata. In contrast, seropositivity rates for anti-HPV-16 and anti-HPV-18 nAbs remained high (100% and 98.1-100%, respectively) across all age groups in women who received the HPV-16/18 vaccine. An age-related decline in anti-HPV-18 antibodies was observed in a previously reported HPV-6/11/16/18 vaccine study.14 Although all subjects (aged 9-13 y and 16-26 y) remained seropositive for anti-HPV-16 antibodies until Month 36, at this time point, significantly more subjects in the younger age group remained seropositive for anti-HPV-18 antibodies compared with the older age group (95.3% vs. 79.4%; p < 0.01).14 However, these results cannot be directly compared with our study due to the different age populations and the different assays used.
The predicted durations over which 95% of women receiving a full 3-dose vaccine course would maintain an antibody response above that associated with natural infection were estimated by the piece-wise and modified power-law models. Both models showed that nAb responses induced by the HPV-16/18 vaccine could remain longer above the levels associated with natural infection than with the HPV-6/11/16/18 vaccine, across the 3 age strata. Actual measured ELISA results also compared well with the prediction models. Interestingly, the modified power-law model showed that the HPV-6/11/16/18 vaccine would induce a lifelong anti-HPV-16 immunoglobulin G antibody response (as determined by ELISA) above the level associated with natural infection; however, the anti-HPV-16 nAb response was predicted to remain above the level associated with natural infection for only 1-2 y. This needs to be considered with caution as protection is thought to be driven by nAb.
In a study of the HPV-16/18 vaccine, levels of anti-HPV-16 and anti-HPV-18 antibodies reached a plateau approximately 18 months after initial vaccination and remained several-fold above the levels induced by natural infection through 8.4 y of follow-up.15 Here, a similar trend was predicted for the HPV-16/18 vaccine by the modified power-law model; a plateau in antibody responses at approximately 18 months after initial vaccination and a long-term immune response with antibody titers predicted to be sustained above those associated with natural infection.
Limitations of this 5-y follow-up include the low number of subjects who attended the Month 60 visit. This may be explained by the fact that the study was initially planned for 4 y—only 38% of women consented for the one-y extension follow-up. Modeling programs have limitations as they process clinical data based on pre-established assumptions. The modified power-law model estimates the persistence of antibody levels assuming that 2 populations of B-cells (activated and memory B-cells) will remain stable over time.5 The assumed progressive decay of antibody and antibody producing B-cells, and that the proportion of memory B-cells remains stable and identical for all women, is biologically unlikely and introduces a bias toward plateau in predicting long-term antibody levels. Thus, this model imposes an asymptotic long-term antibody plateau. The piece-wise model is based solely upon antibody levels (fitted based on different non-overlapping time intervals) and makes the conservative assumption that antibody responses will follow a linear decrease from Month 21 onwards5. In reality, actual antibody levels at the subsequent time points (Months 24, 36, 48, and 60) would also influence the long-term persistence of the anti-HPV-16/18 antibody response. In a previous study, different data lock-points were used to build the models. Differences between the resulting predictive curves were minor, demonstrating the robustness of the models, though some improvements were evident when including an additional time point.5 It should be noted that the persistence of anti-HPV type-specific antibodies is not the only factor that contributes to long-term vaccine-induced immune response—T-cell-mediated immunity and B-cell immunologic memory also play important roles. Another limitation is that our study excluded adolescent girls, the primary target group for vaccination. However, a separate comparative study conducted in 12-15 y old girls reported a higher level of antibody response with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine,11 and another head-to-head immunogenicity study in 9-14 y old girls is ongoing (NCT01462357). An ongoing study has been measuring antibody response in preteen/adolescent girls up to 6 y after receiving the HPV-16/18 vaccine. This report will include modeling of the level of this response up to 20 y post-vaccination (NCT00877877).
Conclusions
Overall, data from this head-to-head clinical trial indicate that the HPV-16/18 vaccine is capable of inducing a higher immune response compared with the HPV-6/11/16/18 vaccine. Statistical modeling predicts that the immune response induced by the HPV-16/18 vaccine could persist for longer than those induced by the HPV-6/11/16/18 vaccine. These study results should be interpreted with caution as there is currently no defined immunological correlate of protection. Comparative studies including clinical endpoints would be needed to determine whether differences exist between the vaccines in terms of duration of vaccine-induced protection.
Patients and Methods
Study design, immunogenicity and safety assessments
Study participants, ethics, study design and vaccine composition have previously been reported.2,3 Briefly, this phase III, observer-blind, randomized, age-stratified, parallel group trial (ClinicalTrials.gov NCT00423046) was initiated in January 2007 and data for the Month 60 analysis were collected up to May 2012 in the USA. Women stratified by age (18-26, 27-35 and 36-45 y) were randomized (1:1 ratio) to receive 0.5 mL of HPV-16/18 vaccine or HPV-6/11/16/18 vaccine according to their recommended 3-dose regimens (Months 0, 1 and 6 or Months 0, 2 and 6, respectively). In order to maintain the blind, women received one dose of placebo (aluminum hydroxide) at either Month 1 or 2, as appropriate. The subjects, investigator, study personnel, and sponsor remained blinded until database freeze of the study. Of note, all data presented in this end-of-study analysis have been unblinded. Thirty centers participated in the Month 60 follow-up.
Anti-HPV-16 and -18 antibody levels were measured in all available blood samples. Methods for the PBNA and ELISA assays used in this investigation have been described elsewhere.16,17 Safety assessments in this end-of-study analysis were as described previously2, including SAEs, NOCDs, NOADs, MSCs, and pregnancy outcomes.
Statistical analysis
The objective of this end-of-study analysis was to determine the serological antibody responses to HPV-16 and -18 induced by the 2 vaccines by means of descriptive and/or exploratory analyses. For assessment of nAb responses, GMT ratios with 2-sided 95% CI (GMT in HPV-16/18 vaccine group over GMT in the HPV-6/11/16/18 vaccine group) were calculated in the ATP cohort for immunogenicity (all subjects who received 3 vaccine doses and for whom data concerning immunogenicity endpoint measurements were available at Month 60) for women who were seronegative and DNA-negative at baseline for the HPV type analyzed. Exploratory analyses (analysis of variance (ANOVA) model) were performed to compare the 2 vaccine groups in the TVC (all subjects who received ≥1 dose of vaccine; regardless of serostatus and DNA status at baseline), the cohort that is most representative of the general population.
To assess the persistence of anti-HPV-16 and anti-HPV-18 vaccine-induced antibody responses (PBNA, ELISA), individual antibody levels at each time point up to Month 60 (from TVC subjects who received all 3 vaccine doses) were fitted into 2 different statistical mixed-effects models 1) modified power-law, and 2) piece-wise, separately for both anti-HPV-16 and anti-HPV-18 antibodies as previously described.5,6 Results from modeling predictions are presented as 1) predicted GMTs for anti-HPV-16 and anti-HPV-18 antibodies at 20 y after the first vaccine dose, and 2) predicted time after first vaccination at which 95% of women will still have antibody titers above natural infection titers.
Notes
Cervarix is a registered trade mark of the GlaxoSmithKline group of companies.
Gardasil is a registered trade mark of Merck & Co., Inc.
Acknowledgments
The first author (M.E.) and the sponsor clinical team wrote the first draft of the manuscript with the support of medical writers Deborah Stanford and James Glossop (Meridian HealthComms Ltd, Plumley, UK) and publication managers Jérôme Leemans (Keyrus Biopharma, Belgium) and Bruno Baudoux (Business & Decision Life Sciences, Belgium) working on behalf of GlaxoSmithKline Vaccines. All authors contributed to the development of the subsequent drafts, with the writing and editorial assistance of the sponsor. All authors had full access to the data and gave final approval before submission. The authors received no financial support or other form of compensation for the development of the manuscript. GlaxoSmithKline Biologicals SA took in charge all the costs associated with the development and publishing of the present publication.
The authors thank the study participants and their families, and all investigators and their staff members for their contribution to the HPV-010 clinical study. The authors gratefully acknowledge the GlaxoSmithKline study group for the coordination of the HPV-010 study and for performing the laboratory assays.
Disclosure of Potential Conflicts of Interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf.
Institutions of A.C., M.B., R.S., N.C. and P.T. received grant from the GlaxoSmithKline group of companies to conduct this study. A.C.'s previous institution received funding for other clinical trials sponsored by Merck. M.B. also received research grants for conducting other clinical trials sponsored by Sanofi, Novartis and the GlaxoSmithKline group of companies. M.E. did not receive an honorarium from any companies. Montefiore Medical Center has received payment from Merck, Roche, Bristol-Myers Squibb, Hologic, Advaxis, Aura Biosciences, Inovio, Photocure, PDS Biotechnologies and the GlaxoSmithKline group of companies for M.E. time spent on educational speaking activities. If travel is required for meetings with any industry, the company paid for M.E.'s travel expenses. Also, Montefiore Medical Center has received grant funding from Merck, Roche, Advaxis, Photocure, Inovio, Endocyte, Fujiboro, Eli Lilly, PDS Biotechnologies, Becton-Dickinson, Cepheid, Hologic and the GlaxoSmithKline group of companies for research related costs of clinical trials where M.E. has been the overall Principal Investigator or Montefiore Principal Investigator. M.B. has received honoraria and served on advisory boards for Sanofi, Novartis and the GlaxoSmithKline group of companies. A.C. received fees from Merck and the GlaxoSmithKline group of companies for participating in advisory boards and for lectures including services on speaker bureaus. L.L. is a consultant outsourced from XPE Pharma & Science to the GlaxoSmithKline group of companies. J.L. has no conflict of interest to declare. G.D., M-P.D. and F.S. are employees of the GlaxoSmithKline group of companies and receive stock options/restricted shares from the GlaxoSmithKline group of companies. G.D. holds patents in the Human Papillomavirus and Herpes Simplex virus vaccine fields.
Funding
The study reported here (HPV-010; NCT00423046) was funded by GlaxoSmithKline Biologicals SA, which was involved in all stages of the study, from design to final report. A.C. was coordinating investigator and together with J.L., M.B., M.E., N.C., P.T. and R.S. participated in the recruitment and/or follow-up of subjects. M.E. was involved in the original study design in collaboration with G.D. (GlaxoSmithKline Vaccines, USA). M-P.D. (GlaxoSmithKline Vaccines, Belgium) contributed toward data analyses and interpretation, and prepared the statistical analysis report. F.S. and L.L. supervised the conduct of the study at GlaxoSmithKline Vaccines (Belgium) and, together with M.E., critically reviewed the study report.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Garçon N, Wettendorff M, Van Mechelen M. Role of AS04 in human papillomavirus vaccine: mode of action and clinical profile. Expert Opin Biol Ther 2011; 11:667-77; http://dx.doi.org/ 10.1517/14712598.2011.573624 [DOI] [PubMed] [Google Scholar]
- 2. Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. . Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5:705-19; PMID:19684472; http://dx.doi.org/ 10.4161/hv.5.10.9518 [DOI] [PubMed] [Google Scholar]
- 3. Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Meric D, Dessy FJ, et al. . Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin 2011; 7:1343-58; PMID:22048173; http://dx.doi.org/ 10.4161/hv.7.12.18281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Einstein MH, Levin MJ, Chatterjee A, Chakhtoura N, Takacs P, Catteau G, Dessy FJ, Moris P, Lin L, Struyf F, et al. . Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: follow-up through Month 48 in a Phase III randomized study. Human Vaccin Immunother (in submission) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. David MP, Van Herck K, Hardt K, Tibaldi F, Dubin G, Descamps D, Van Damme P. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol 2009; 115:S1-6; PMID:19217149; http://dx.doi.org/ 10.1016/j.ygyno.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 6. Fraser C, Tomassini JE, Xi L, Golm G, Watson M, Giuliano AR, Barr E, Ault KA. Modeling the long-term antibody response of a human papillomavirus (HPV) virus-like particle (VLP) type 16 prophylactic vaccine. Vaccine 2007; 25:4324-33; PMID:17445955; http://dx.doi.org/ 10.1016/j.vaccine.2007.02.069 [DOI] [PubMed] [Google Scholar]
- 7. Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine 2000; 19:877-85; PMID:11115711; http://dx.doi.org/ 10.1016/S0264-410X(00)00224-3 [DOI] [PubMed] [Google Scholar]
- 8. Van Damme P, Banatvala J, Fay O, Iwarson S, McMahon B, Van Herck K, Shouval D, Bonanni P, Connor B, Cooksley G, et al. . Hepatitis A booster vaccination: is there a need? Lancet 2003; 362:1065-71; PMID:14522539; http://dx.doi.org/ 10.1016/S0140-6736(03)14418-2 [DOI] [PubMed] [Google Scholar]
- 9. Wilson JN, Nokes DJ, Carman WF. Current status of HBV vaccine escape variants – a mathematical model of their epidemiology. J Viral Hepat 1998; 5 Suppl 2:25-30; PMID:9857357; http://dx.doi.org/ 10.1046/j.1365-2893.1998.0050s2025.x [DOI] [PubMed] [Google Scholar]
- 10. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al. . Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161-70; PMID:17602732; http://dx.doi.org/ 10.1016/S0140-6736(07)60946-5 [DOI] [PubMed] [Google Scholar]
- 11. Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, Andrews N, Miller E, Beddows S. A randomized, observer-blinded immunogenicity trial of Cervarix® and Gardasil® human papillomavirus vaccines in 12-15 year old girls. PloS One 2013; 8:e61825; PMID:23650505; http://dx.doi.org/ 10.1371/journal.pone.0061825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Squarzon L, Pacenti M, Masiero S, Marcati G, Gottardello L, Gabrielli L, Lazzarotto T, Pascucci MG, Palu G, Barzon L. Evaluation of neutralizing and cross-neutralizing antibodies induced by HPV prophylactic vaccines: an independent study. EUROGIN 2013. Florence, Italy: Abstract OC; 6-9 (page 204). Available at http://www.eurogin.com/2013/images/pdf/EUROGIN-2013-Abstracts-Part-2.pdf [Google Scholar]
- 13. Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Hoye J, Steinwall M, Riis-Johannessen G, et al. . High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459-66; PMID:17117182; http://dx.doi.org/ 10.1038/sj.bjc.6603469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krajden M, Cook D, Yu A, Chow R, Su Q, Mei W, McNeil S, Money D, Dionne M, Palefsky J, et al. . Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, Merck cLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine 2014; 32:624-30; PMID:24055350; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 15. Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B, Descamps D. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother 2012; 8:390-7; PMID:22327492; http://dx.doi.org/ 10.4161/hv.18865 [DOI] [PubMed] [Google Scholar]
- 16. Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425-34; PMID:18948732; http://dx.doi.org/ 10.4161/hv.4.6.6912 [DOI] [PubMed] [Google Scholar]
- 17. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. . Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757-65; PMID:15541448; http://dx.doi.org/ 10.1016/S0140-6736(04)17398-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.