Abstract
The therapeutic potential of dendritic cell (DC) cancer vaccines has gained momentum in recent years. However, clinical data indicate that antitumor immune responses generally fail to translate into measurable tumor regression. This has been ascribed to a variety of tolerance mechanisms, one of which is the expression of immunosuppressive factors by DCs and T cells. With respect to cancer immunotherapies, these factors antagonise the ability to induce robust and sustained immunity required for tumor cell eradication. Gene silencing of immunosuppressive factors in either DCs or adoptive transferred T cells enhanced anti-tumor immune responses and significantly inhibited tumor growth. Therefore, engineered next generation of DC vaccines or adoptive T-cell therapy should include immunomodulatory siRNAs to release the “brakes” imposed by the immune system. Moreover, the combination of gene silencing, antigen targeting to DCs and cytoplasmic cargo delivery will improve clinical benefits.
Keywords: cancer vaccine, gene silencing, immunotherapy, RNA interference, targeted therapies, T-cell therapy
Abbreviations
- AML
acute myeloid leukemia
- CMV
human cytomegalovirus
- CTLA4
T-lymphocyte-associated antigen 4
- DC
Dendritic cells
- Gal
galectin
- hTERT
human telomerase reverse transcriptase
- IDO
indoleamine 2,3-dioxygenase
- IL
interleukin
- INF
interferon
- NK
natural killer
- PD1
programmed cell death
- RNAi
RNA interference
- siRNA
small interfering RNA
- SOCS1
suppressor of cytokine signaling
- STAT
Signal transducer and activator of transcription
- TCR
T cell receptor
- TLR
toll like receptor
- Treg
Regulatory T
Introduction
Dendritic cells (DCs) are essential components of vaccination through their ability to capture, process, and present antigens to T cells.1 They display functional plasticity in that they can either mediate immune tolerance or induce immunity.1,2 Their capacity to respond to so many stimuli is reflected by the expression of a large panel of molecular sensors. Indeed, DCs not only express multiple pattern-recognition receptors including cell surface C-type lectins, and endosomal Toll-like receptors, but also a diversified array of cytokines/chemokines and other regulatory molecules.2 Activation of PRRs by pathogen-derived products induces DC maturation and serves as the critical switch from the maintenance of self-tolerance to the induction of immunity. Subsequent to antigen capture and activation, DCs induce the differentiation of antigen-specific T cells into effector T cells that display unique functions and cytokine profiles.2 DCs regulate T-cell differentiation through a variety of molecules that belong to 3 major families, interleukin 12, tumor necrosis factor, and B7. The B7 family includes members that can stimulate immune responses and others that can inhibit them. For instance, the T-lymphocyte-associated antigen 4 (CTLA4) and the co-stimulatory receptor CD28, although they bind to the same ligands (CD80/CD86), CD28 delivers stimulatory signals for T cells to become effector cells, whereas CTLA4 delivers inhibitory signals that suppress their function.3,4 The level of CTLA4 induction subsequent to T cell activation depends on the amplitude of the initial T cell receptor antigen (TCR) signals from contact with peptides bound to major histocompatibility complex (MHC) molecules. High-affinity TCR-binding peptides induce elevated levels of CTLA4, which dampens the amplitude of the initial response, both by out-competing CD28 for ligands (CD80/CD86) binding and through the recruitment of serine/threonine or tyrosine phosphatases, including the phosphatases Src homology (SH2) domain-containing phosphatase1 (SHP-1), SHP2, and protein phosphatase 2A (PP2A).3 The net result of CTLA4 engagement is decreased production of cytokines (e.g., IL2) and cell cycle arrest of T cell in G1.
Each step of T-cell activation by DCs is regulated by counterbalancing co-stimulatory and co-inhibitory signals that fine-tune the immune response and thus protect tissues from damage when the immune system is responding to pathogens (Fig. 1). The expression of these co-inhibitory molecules is part of the normal function of the immune system. However, in the case of cancer immunotherapies, such negative feedback mechanisms might suppress antitumor immune response.1 Several inhibitory signals such as those delivered by CTLA4 and programmed cell death protein 1 (PD1) are initiated through membrane receptors involving cell-cell interactions. When engaged by one of its ligands, expressed by DCs or tumor cells, PD1 induces the inhibition of T-cell activation and proliferation resulting in cell-cycle arrest.3 PD1 expression on T cells is induced subsequent to activation. As CTLA4, PD1 is also highly expressed on regulatory T (Treg) cells and it is involved in their immunosuppressive activity.5,6 In addition to cell-surface receptors, another class of inhibitory factors includes certain intracellular kinases, cytokines, and metabolic enzymes, which are expressed by immune cells and tumor cells.7,8 Notably, a number of the inhibitory effects can occur at the initiation of T cell responses in lymph nodes, supporting the necessity and importance of regulating the extent and duration of antigen presentation by mature DCs.
Figure 1.
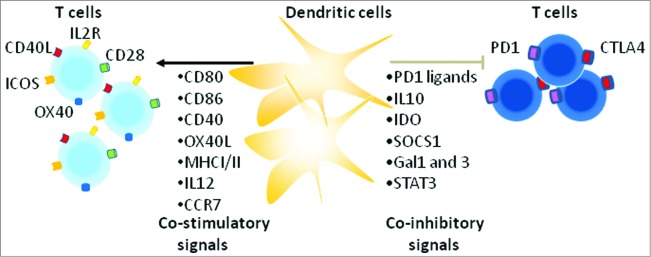
Various co-stimulatory and co-inhibitory molecules regulate T cell activation. Indicated are multiple molecules that are involved in the regulation of T-cell responses under physiological conditions. One important family of membrane-bound molecules that bind co-stimulatory and co-inhibitory receptors is the B7 family (e.g., CD86, DC80). Although B7-CD28-specific signaling is a critical component of T cell priming, signaling through others receptors, including OX40 and ICOS is often required to further enhance CD4 and CD8 T cell priming and generation of memory cells. Strong signaling through the TCR and CD28 upregulates both co-inhibitory (e,g. CTLA4 and PD1) and co-stimulatory molecules (e.g. OX40, ICOS) on T cells. Inhibition via CTLA4 and PD1 in the context TCR signaling is likely of central importance in controlling immunity (see main text).
To induce effective immune responses against tumors, there is a need of inhibiting the expression of factors that dampen the immune responses in patients. A promising strategy for reprogramming DC function is through the use of RNA interference (RNAi). This strategy was proven successful both in vitro and in vivo and holds promise for inclusion in immunotherapeutic strategies such as cancer vaccines and adjuvant therapies.9,10 Moreover, the combination of antigen targeting to DCs, endosome escape, and gene silencing might improve immune therapies. Hereunder, I present some examples how RNAi can improve cancer immunotherapies and highlight future directions.
Enhancing DC Immunogenic Function via RNAi
RNAi-based therapeutics promise to overcome the major limitation of existing medicine, which can currently only target a limited number of proteins involved in disease pathways.9,10 As compared to other nucleic acid-based strategies, small interfering (si) RNA benefits from harnessing endogenous RNAi pathways to trigger gene silencing.11 Virtually all genes involved in immune responses can be silenced by siRNAs (Table 1). To achieve effective immune responses against tumors, there is a need of blocking the signals that dampen the immune responses in patients. As indicated above, DCs and T cells are generated with inherent negative regulation mechanisms which attenuate their immune stimulatory activity. Among the inhibitory factors expressed by DCs are transforming growth factor-β, interleukin-10, PD1 ligand 1 and 2, suppressor of cytokine signaling (SOCS) 1, indoleamine 2,3-dioxygenase (IDO), and interleukin10 (IL10) (Fig. 1).12 The potential value of these inhibitors in suppressing immune responses is best exemplified by the significant enhanced immunity in mice lacking these factors.13-15
Table 1.
Preclinical and clinical development of siRNAs targeting inhibitory molecules
| Target | Function |
|---|---|
| Cbl-b CCR5 CD204 CTLA4 GAL1 GAL3 IDO IL10 PD1 PD-L1 PD-L2 SHP1 SOCS1 SOCS3 STAT3 |
E3 ubiquitin ligase involved in the degradation of TCR and signaling molecules Chemochine receptor Cell surface C-type lectin expressed by DCs involved in endocytosis Inhibitory receptor that blocks the activity of T cells Mutifunctional β-galactoside-binding lectin Mutifunctional β-galactoside-binding lectin Enzyme that degrades tryptophan within the kynurenine metabolic pathways Immunosuppressive cytokine Inhibitory receptor that blocks the activity of T cells Ligand for PD1 Ligand for PD1 Tyrosine phosphatase involved in TCR signaling Inhibitor of cytokine signaling Inhibitor of cytokine signaling Transcription factor that becomes activated in response to cytokines and growth factors |
Abbreviations: CTLA4, cytotoxic T-lymphocyte antigen 4; PD1, programmed death 1 receptor; IDO, indoleamine 2,3-dioxygenase; SOCS1, suppressor of cytokine signaling1; IL10, interleukin 10; Cbl-b, casitas b-linage lymphoma proto-oncogene b; Gal, galectin; STAT3, signal transducer and activator of transcription; SHP1, src homology region 2 domain-containing phosphatase-1; CCR5, human chemokine receptor 5; CD204/SRA, scavenger receptor A.
IDO is a cytosolic enzyme that catalyzes the limiting reaction in the degradation of tryptophan, an essential amino acid required for T-cell proliferation and survival.16-18 Depletion of tryptophan by IDO together with an increase in the production of active Trp metabolites (kynurenine) inhibit effector T cells and induces immune suppressive Treg cells (Fig. 2).16,18 These observations indicate that the regulation of tryptophan metabolism by IDO in DCs is a highly adaptable modulator of immunity. Indeed, injection of IDO-positive DCs into mice suppressed the activation of antigen-specific T cells in the lymph nodes draining the injection site.17 Effector T cells starved of tryptophan were unable to proliferate and enter into G1 cell cycle arrest. In addition, several studies indicated that IDO is essential for successful allogeneic pregnancy suggesting that it is important in suppressing immune responses under normal physiological conditions.16
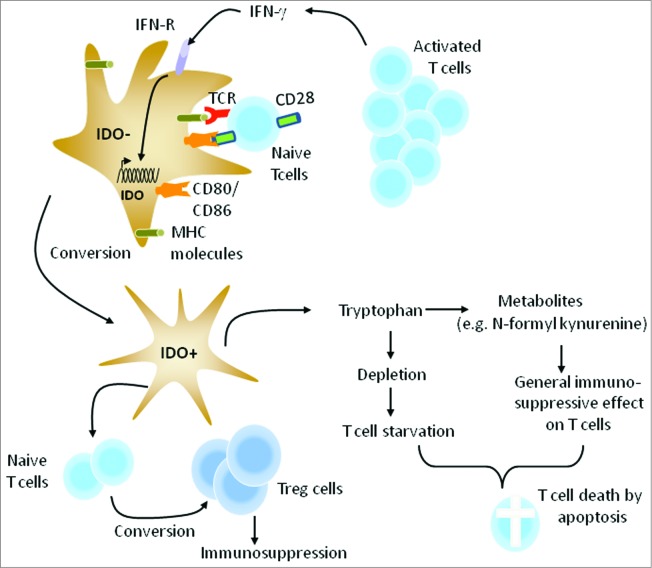
Figure 2.
Conversion of IDO negative DCs to IDO positive DCs. Subsequent to T-cell activation, IFN-γ produced by T cells induces the expression of IDO in DCs resulting in their conversion into tolerogenic DCs. This counter-regulatory mechanism is expected to control the magnitude and duration of adaptive immune responses. Activation of naïve T cells by IDO positive DCs leads to the generation of adaptive Treg cells, a population of CD4+ T cells that inhibit, rather than promotes, immune responses. Moreover, IDO positive DCs convert tryptophan into several metabolites with general immunosuppressive activity on lymphocytes.
In general, DCs control the quality of a T-cell response, particularly CD4+ T-cell differentiation. Once T cells are effectively primed, pro-inflammatory cytokines such as interferon (INF)-γ, and Treg cell signals such as CTLA4, induce IDO expression in DCs.16,19 This will lead to their conversion into tolerogenic DCs that can inhibit T-cell expansion as well as the induction of adaptive Treg cells, which suppress T-cell responses, including those against tumors (Fig. 2). Reverse signaling via B7 molecules (CD80/86) after interaction with CD28 on T cells can also induce IDO expression in DCs.16 In the case of cancer vaccines, IDO expression can occur during in vitro maturation of DCs as well as in vivo after T-cell activation.20,21 A promising strategy for enhancing the potency of DC cancer vaccines would be the blockade of IDO expression in these cells. In this respect, we have developed several super-active siRNAs that exhibited a silencing potency at nanomolar concentrations.21 Importantly, IDO gene silencing enhanced the ability of human DCs to stimulate T cells. Recently Zheng and colleagues showed IDO silencing in mouse DC vaccine can inhibit tumor growth in a syngeneic mouse breast cancer model.22 Moreover, the vaccine induced tumor antigen-specific cytotoxic T cells and decreased the number of Treg cells. More recently, we have assessed the feasibility, safety and immunogenicity of IDO-silenced DC cancer vaccine in patients with ovarian cancer.23 Fast DC preparations from patients were transfected ex vivo with IDO siRNA along with mRNA encoding tumor antigen human telomerase reverse transcriptase (hTERT) or survivin using square wave electroporation.24 Subsequent to overnight culture, the cells were cryo-preserved into separate vaccine doses until use. The clinical data revealed that IDO-silenced DC vaccine is safe, well tolerated and has therapeutic potential even in advanced stage ovarian cancer when compared to unmodified DCs. For example, a patient with metastatic ovarian cancer never achieved a complete tumor free interval on chemotherapy since 2007. However, when the patient was given the IDO-silenced DC vaccine she obtained a partial remission and continued to decline in tumor volume and markers and she is still alive. Fig. 3 illustrates the treatment schedule and clinical outcome of this patient. In conclusion, our developed IDO siRNA holds promise for inclusion with immunotherapeutic strategies such as cancer vaccines and adjuvant therapies. It should be noted that one of the major disappointments in the field of ovarian cancer is the failure of currently established therapies to induce a cure at diagnosis, even in chemosensitive tumors.25
Figure 3.
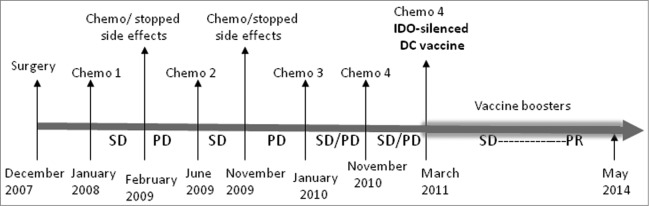
Timeline for the treatment and clinical development. The figure illustrates the treatment schedule for a patient with metastatic ovarian cancer. After surgery, the patient has received 4 combinations of chemotherapy prior to IDO-silenced DC vaccine. Chemo 1: Carboplatin, Taxol, Avastin; Chemo 2: Carboplatin, Caleyx; Chemo 3: Taxol; Chemo 4: Taxol, Avastin; SD, stable disease; PD, progressive disease; PR, partial remission.
In addition to IDO, suppressor of cytokine signaling (SOCS1), a member of the SOCS and cytokine-inducible SH2 family of intracellular proteins, has emerged as a critical inhibitory molecule for controlling the response to cytokines and antigen presentation by DCs.26 A number of studies in mice have documented the important role of SOCS1 in modulating the magnitude of immune stimulation by DCs suggesting that its inhibition may be a potentially useful strategy to enhance vaccine-induced immune responses.14 In this respect, SOCS1 silencing in DCs improved antigen-specific CD8+ T cell responses.27,28 A short stimulation with SOCS1-silenced DCs was enough to generate a strong primary response, suggesting that inhibition of SOCS1 expression in DCs could be exploited as a novel adjuvant strategy to boost the potency of vaccine-induced CD8+ T-cell responses.28 In addition to SOCS1, the induction of signal transducer and activator of transcription 3 (STAT3) in DCs by tumors-derived factors induced their conversion into tolerogenic DCs.12 Therefore, silencing STAT3 in DCs should also be beneficial for cancer immunotherapies. Indeed, STAT3 gene silencing restored DC maturation and enhanced CD8+ response to tumors.29 Likewise, silencing of the immunosuppressive scavenger receptor A (CD204) in DCs enhanced the therapeutic potency of local radiotherapy. In these experiments, CD204-silenced DCs were injected into the tumors followed by local radiotherapy.30
PD1 is a co-inhibitory receptor that is expressed by T cells and B cells upon activation. As opposed to CTLA4 signaling which occurs early, during T cell activation in lymphatic organs, PD1 signaling takes place during the effector phase of T-cell functions. PD1 receptor engagement with either of its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC) in the tumor microenvironment inhibit TCR signaling by recruiting the SHP1 and SHP2 phosphatases, which induce a TCR stop signal that limits T cell interactions with DCs. In addition to tumor cells and macrophages, PD-L1 is also expressed by DCs and was linked to their ability to induce tolerance.5 Notably, antibodies that bind to either CTLA4 or PD1 receptor, and thereby alleviate the immune inhibition, have generated promising clinical data in melanoma and other tumor types.31,32 However, the responses are only seen in a subset of patients and it is unclear why some tumors respond and others do not. Thus, additional strategies designed to block the expression of these 2 receptors are warranted. In this respect, Hobo et al. used RNAi to block the expression of PD1 ligands in DCs.33 PD-L1 and PD-L2-silenced DCs enhanced T-cell proliferation in-vitro. Since IDO and PD-L1 are expressed by DCs, their simultaneous inhibition might further enhance the efficacy of DC cancer vaccine potency. In line with this notion dual inhibition of IDO and PD-L1 or PD1 in T cells resulted in more enhanced T cell-response in-vitro when compared to mono-silencing using IDO, PD-L1, or PD1 siRNA (Fig. 4). As with previous standard-of-care therapies, it will be necessary to develop combination therapies to improve clinical benefits.
Figure 4.
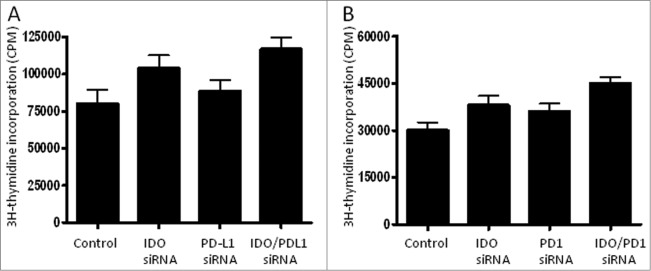
Simultaneous suppression of inhibitory signals enhanced T-cell proliferation. (A) Untransfected DCs or DCs transfected with IDO, PD-L1, or the combination IDO/PD-L1 siRNA for 24 h were co-cultured with allogeneic CD4+ T cells for 6 d at DC:T cell ratio 1:10. T cell proliferation was measured by thymidine incorporation as described previously.37 (B) As in A, except that allogeneic CD4+ T cells were transfected with PD1 siRNA. The results are represented as means of triplicate samples from the same experiment.
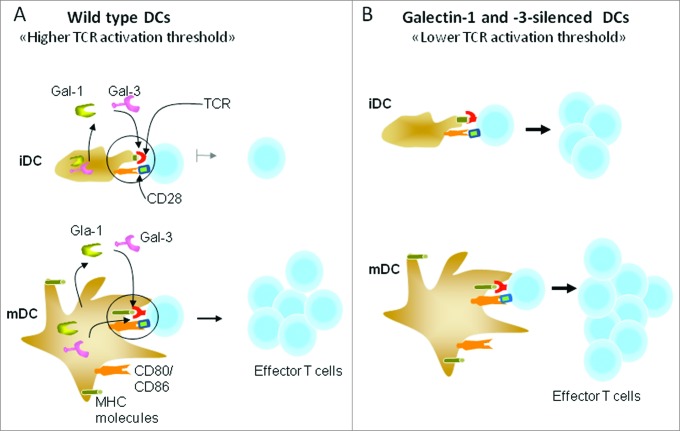
Several studies have demonstrated that members of the galectin (Gal) family of β-galactoside-binding proteins are directly involved in regulating leukocyte function and turnover.34 Gal1, one of the most widely studied members, has a broad range of immunomodulatory functions involving innate and adaptive immune cells. It controls the proliferation and survival of T cells.35 On the other hand, Gal3 influences adaptive immune responses but does not directly affect the development and maturation of lymphocytes.36 Specific gene silencing of Gal1 and Gal3 in DCs enhanced their capacity to stimulate T cell activation and IFN-γ production.37 Therefore, DC-expressed Gal-1 and Gal-3 may function as a negative regulator of T cell activation, more likely, by increasing the TCR activation threshold as illustrated in Fig. 5. The effects of Gal1 and Gal3 may happen at the level of immunological synapse formation, as both proteins have been shown to interact with CD45, CD7, and CD3 molecules.34 Notably, T cells undergo a process of TCR desensitization before entering the secondary lymphoid tissues.38 This TCR tuning modulates the intensity of TCR signaling and is thought to be especially important for cells with relatively high self-reactivity. Such immune regulatory mechanism is also prevalent for the regulation of T cell activation after antigen encounter in the periphery.
Figure 5.
Galectins 1 and 2 control T cell receptor (TCR) activation threshold. (A) Under steady-state conditions, DCs maintain an immature state (iDCs). Upon activation through inflammatory cytokines or pathogen-derived products, they mature (mDCs) and upregulate the expression of co-stimulatory molecules, such as CD80, CD86, and CD40. The lower expression of costimulatory molecules has been proposed to account for poor capacity of iDCs to stimulate T cells. (B) Gal1 and Gal3 gene silencing in iDCs enhanced T cell activation, despite low expression of costimulatory molecules. Also, gene silencing in mDCs enhanced T-cell activation. This enhancement is more likely due to a drop in TCR activation threshold.37 Endogenous or secreted Gal1 and Gal3 might interact with immunological synapses indicated by the circles. MHC = major histocompatibility complex.
Activation signals mediated by Toll-like receptor (TLR) ligands are necessary for DC activation and antigen presentation.2 Although TLR ligands such as CpG and RNA oligonucleotides induce DC maturation, they also induce the expression of immunosuppressive cytokines such as IL10, which can influence T cell-mediated immunity.39 IL10, whether produced by DCs themselves or present in the microenvironment, favors the capacity of DCs to stimulate a stronger Th2 response to the detriment of Th1 response that is essential for tumor cell eradication.40,41 Therefore, the development of agents that stimulate DC maturation through TLRs and simultaneously inhibit the expression of immunosuppressive cytokines may thus facilitate the design of effective vaccines. We and others have shown that chemically made siRNAs can activate innate immunity via TLR7/8 when delivered to the endosomes.42-44 While this off-target effect of siRNA is unwanted in many instances, cancer and infectious diseases may profit greatly from the activation of TLRs. For example, a combinatorial strategy that block the expression of immunosuppressive factors and simultaneously activate innate immunity could lead to more effective cancer treatments as illustrated in Fig. 6. In this respect, we have shown that targeting IL10 expression by a dual siRNA can block IL10 expression and activate TLR7/8 signaling in human monocytes and DCs.45 More recently, we applied a novel DC-based vaccine (i.e., DC loaded with leukemia antigens that have been transfected with a dual IL10 siRNA capable of co-ordinately activating DCs via TLR7/8 and silencing IL10 expression) in a syngeneic rat model of acute myeloid leukemia (AML).46 Leukemic rats treated with this new vaccine had less leukemic cell mass in their bone marrows and less extramedullar dissemination of the leukemic cells compared with rats given the control vaccine. The rat model that we have used harbours many characteristics of human AML and therefore is highly relevant to test immune stimulatory strategies before implementation in the clinic. More recently, Pradhan and colleagues showed that CpG-induced IL10 secretion in DCs can be inhibited by co-delivery of IL10 siRNAs.47 Interestingly, simultaneous immunotherapy with CpG oligonucleotide and IL10 siRNA enhanced immune protection of an idiotype DNA vaccine in a prophylactic murine model of B cell lymphoma when compared to control formulations. Thus, siRNA-mediated silencing of CpG induced IL10 production could be an attractive strategy to improve anti-tumor immune response of TLR9 driven immunotherapies. Similarly, it was shown that IL-10 gene silencing in DCs can generate a better cytotoxic T cell response against the human melanoma antigen MART-1 when compared to unmodified DCs.48
Figure 6.
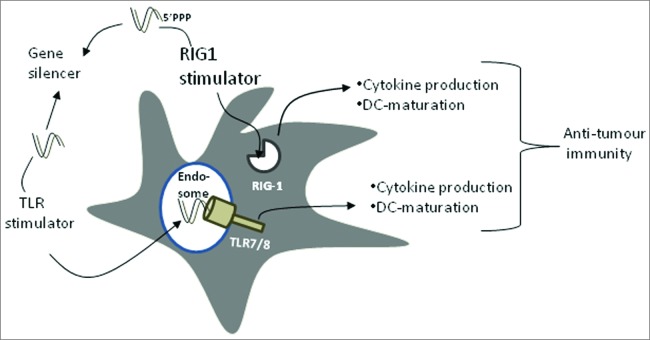
Dual siRNA can function as TLR activator and gene silencer. A dual siRNA can activate innate immunity via either endosomal TLRs or cytoplasmic RIG1 leading to cytokine production and eventually DC maturation. In addition, it is able to silence the expression of immunosuppressive factors (e.g., IL10, IDO, and SOCS1). Combination of gene silencing and induction of DC-maturation should enhance anti-tumor or anti-viral immunity.
In situ Manipulation of DC Function
Generation of DC with superior immunogenic potential is essential for developing effective immunotherapeutic strategies against infectious agents and cancer. In addition to the ex vivo siRNA-based approach that depletes suppressive factors in blood monocyte-derived DCs, strategies to activate natural DCs in situ may hold strong potential for cancer vaccines. Targeted activation of DCs in situ strategies should benefit from reaching multiple DC populations in their natural environment. In this respect, siRNA containing a 5′-triphosphate was used as activator of DCs in-situ leading to antitumoral efficacy through activation of the retinoic acid-inducible gene-1 (RIG-1) receptor (Fig. 6).49-51 In addition to antigen-specific immune responses mediated by cytotoxic CD8+ T cells, a major component of antitumoral immunity is the innate NK cell response. Cytokines produced by DCs in response to immunostimulatory siRNAs activated NK cells, which killed tumor cells.50 Overall, the concept of activating TLRs and/or RIG-1 with dual siRNAs is novel and can be broadly used to improve anti-tumor innate immunity and DC vaccination.
Today, the most used strategy for DC cancer vaccines is based on isolating monocytes from the blood of patients and exposing them to maturation stimuli. Subsequently, these monocyte-derived DCs are loaded with tumor antigens or mRNA and then re-infused to the patient.1 Although some clinical data were obtained with this standard protocol, the ex vivo generated DCs from blood monocytes migrate poorly in vivo and express immunosuppressive factors such as IDO and IL10 affecting the efficacy of the vaccine as discussed above.21 Moreover, the process used to create monocyte-derived DCs is a labor-intensive procedure for each individual patient and costly. Recent studies also indicated that various DC subsets are needed for the induction of potent cytotoxic T lymphocytes against tumor cells.52 Notably, most potent vaccines generated against, for example, yellow fever and smallpox activate multiple DC subsets.
Studies from Ralph Steinman and Michel Nussenzweig demonstrated the principle of targeting antigens to DCs in situ through the coupling of antigens to antibodies that target DC surface receptors involved in uptake such as members of the C-type lectin receptor family including CD205, Fc receptors, and the mannose receptor.53 Importantly, in the absence of adjuvants, targeting antigens to CD205 positive DCs in-vivo induces antigen-specific tolerance, which can be used as treatment against autoimmune diseases. Similarly, several studies have shown that the coupling of DC-receptor-specific antibody to the antigen of choice enhanced immunity when compared to that obtained with free antigen.54,55 Although immune responses have been achieved in most studies, antibody targeting may provide additional activation signals that may negatively affect DC function. Furthermore, large antigen-antibody conjugates may have disadvantages such as reduced tissue penetration. Also, the use of mouse antibodies in humans is expected to induce immunogenicity although some humanized antibodies were also developed.56
With the aim of developing small targeting molecules, we have biopanned peptide phage libraries on human DCs and selected new specific binders.57 In contrast to antibodies, small peptides are easy to conjugate to antigens and are invisible to the immune system. One of the selected peptides named NW peptide bound with high affinity to a single receptor expressed by several human DC subsets.57 Peptides that recognize their targets with high specificity and affinity such as the NW peptide should have potential for both clinical vaccine development and cancer immunotherapy. Targeting long peptides from human cytomegalovirus (CMV) pp65 protein to DCs via the NW peptide resulted in activation of T cells from CMV positive donors in the context of both MHC class I and II molecules. In addition to antigen targeting, the NW peptide mediated siRNA delivery to DCs and significant gene silencing was obtained relative to free siRNA molecules.57 Using streptavidin-biotin technology and an endosome-escaping peptide, we were able to co-deliver antigens and siRNA to DCs. The histidine-rich peptide was able to destabilize endosome membrane at acid pH, leading to cytoplasmic delivery. Notably, among the carriers that have been developed, complexes based on streptavidin-biotin interaction have attracted a lot of attention because of safety and ease of construction. The combination of antigen targeting, endosome escape, and the blockade of immune inhibitory factors in DCs are expected to further enhance anti-tumor immunity.
Although more developments are needed, systemic delivery of siRNA formulations can also be used to silence immunosuppressive factors in tumors and immune cells. In this respect, Kortylewski and colleagues developed a cell-specific delivery approach of siRNA based on the use of CpG oligonucleotides, a TLR9 ligand.58 TLR9 expression and STAT3 activation often involve both cancer cells and non-malignant immune cells such as DCs and macrophages. Using a syngeneic murine model of disseminated AML, the authors demonstrated that systemic administration of CpG-Stat3 siRNA can alleviate the immunosuppressive effects of persistent STAT3 signaling in both cancer cells and immune cells. The targeted blockade of STAT3 coupled to the activation of TLR9 resulted in systemic cancer-specific immune responses that led to tumor eradication in multiple organs and prolonged the survival of the majority of disease mice. Moreover, the treatment increased the circulating levels of interferon γ and interleukin 12, both critical mediators of Th-1 immune responses.58 Collectively, the data underscore the use of siRNA as modulator of DCs and T-cell functions in the tumor microenvironment.
Reprogramming T Cell Function
Similar to DCs, the activation of T cells are also under several immunosuppressive mechanisms, including T-cell intrinsic (e.g., CTLA4, PD1, SHP1, PP2A) and extrinsic (e.g., B7 family, IDO, IL10, T reg cells) regulators (Fig. 1).59 As for DCs, the expression of these inhibitory proteins by T cells is critical for the regulation of self-reactivity or exuberant responses to pathogens, but it may limit T-cell responses to tumors, particularly if the tumor antigens being targeted are self-proteins. Therefore, the next generation T cell therapies must confront and address these numerous inhibitory barriers that CD8+ cytotoxic T cells face.
Notably, manipulation of T cells ex vivo and their infusion back into patients is well characterized and may provide a potential therapeutic strategy for the treatment of immunological disorders such as cancer, and infectious diseases.60,61 However, ex vivo cultured T cells upregulate the expression of certain inhibitory factors such as CTLA4 and PD1, which dampen their in-vivo activation. It is therefore desirable to establish strategies that enhance the function of adoptively transferred CD8+ T cells. A versatile method that could potentially be used for siRNA delivery to T cells for therapeutic purposes is an ex vivo route, whereby T-cells could be isolated from a patient, transfected with siRNAs and then infused into the same patient. In our experience, the use of standard wave electroporation method can deliver siRNA to human blood T cells with close to 95% transfection efficiency and with a little effect on cell viability and function. With respect to immunotherapy, knockdown of SOCS1 in CD8+ T cells or STAT3 in CD4+ T cells using lentiviral vectors induced tumor regression following adoptive transfer to tumor-bearing mice.62,63 Moreover, silencing of SOCS3 expression in murine T cells via nucleofection attenuated allergic airway responses when the cells were adoptively transferred into recipient mice.64 Therefore, depending on the target, siRNAs can be used to either enhance or inhibit T-cell activation.
As mentioned above, certain molecules involved in TCR tuning that limit TCR responsiveness are certainly the most obvious potential targets for inhibition by siRNA. For example, the E3 ubiquitin ligase Cbl-b, the tyrosine phosphatase SHP-1, the serine/threonine PP2A phosphatase, all negatively regulate TCR activation.65 PP2A phosphatase has also been shown to interact with the cytoplasmic domains of CD28 and CTLA-4. Cbl-b is a member of the highly conserved family of Cbl (casitas b-lineage lymphoma) proteins and functions as a nonredundant negative regulator of T-cell activation.66 Accordingly, cblb-deficient mice are highly susceptible to spontaneous and antigen-induced experimental autoimmune diseases.66 Interestingly, silencing of Cbl-b gene expression in primary murine CD8+ T cells with siRNAs via nucleofection, followed by adoptive transfer of the cells into recipient mice potentiated the effects of a cancer vaccine in B16 melanoma model.67 Gene silencing increased effector functions and infiltration rates of adoptively transferred CD8+ T cells, resulting in substantial suppression of tumor growth and increased survival rates of tumor-bearing mice. Therefore, genetic modification of cbl-b expression in adoptively transfected T cells via siRNA should have clinical application. RNAi inhibition of SHP-1 in tumor-specific T cells also led to improved therapy of disseminated leukemia cells.68 SHP-1 adaptor protein functions as a rheostat for regulating TCR signaling after antigen encounter in part by diminishing T cell/DC interactions.69 The finding by Mantei et al. that siRNA chemical modifications can prolong gene silencing in T cells will facilitate the use of chemically made siRNAs in T cells where long-lasting gene silencing is needed.70 Transfected T cells remain functional and maintain siRNA-induced CD4 knockdown for up to 2 weeks after transfer into recipient mice. Overall, the encouraging data obtained with adoptively transferred T cells suggest that RNAi may be an effective and available therapeutic method for reprogramming T-cell function in cancer patients.
It should be noted that several investigators have successfully silenced gene expression in T cells. For example, silencing of viral and host cell factors in T cells using siRNA or shRNA technology has been shown to inhibit HIV infection and replication.71 Antibody-conjugated peptide delivery systems have been used to deliver siRNA into tumor T cells.72 Systemic delivery of CCR5 chemokine receptor siRNA to T cells via intravenous injection resulted in silencing of CCR5 in T cell population and blockade of HIV infection.73 Moreover, advances in lentiviral vector design have led to significant improvement for shRNA expression in T cells.74 Collectively, these developments in siRNA delivery to T cells would be clinically relevant in adoptive T cell therapy for cancer.
Conclusions and Perspectives
Since the immune responses induced by current DC cancer vaccines are not optimal, it is important to explore alternative DC formulations and novel adjuvants to generate protective immune response that is superior to the natural immunity against persistent infections or tumor cells. Among the most promising approaches for inhibiting gene expression in DCs and T cells is the use of siRNAs. Although neutralization of some inhibitory factors by antibody has improved cancer immunotherapy despite severe side effects,31 siRNA based knockdown of endogenous inhibitory factors is a viable method to make DCs more effective for therapeutic strategies such as active immunization. SiRNA-approach is also assumed to greatly improve the killing function of adoptively transferred T cells or NK cells.
One of the major challenges to the clinical development of gene silencing by siRNA has been in-vivo delivery in general, and particularly the ability to selectively target siRNA molecules to specific cell types. The use of siRNA in ex vivo setting as discussed in this review would overcome the delivery and targeting problems. Also, ex vivo delivery method of siRNA via electroporation would overcome the potential off-target effects due to systemic delivery of siRNAs. The experimental success of using short peptides to target antigens to DCs in situ in conjunction with gene silencing will have an interesting potential for the design of cancer vaccines. A greater understanding of the interplay between positive and negative signals regulating the function of the immune system as well as the interactions between tumor cells and immune cells will be crucial to guide the targeting choice; mono or combination therapies. With respect to cancer immunotherapy, inhibition of a single immunosuppressive factor will succeed when the inhibition is combined with a vaccine as demonstrated for IDO-silenced DCs. However, it is thought that combination therapies have the potential to establish synergistic anti-tumor immunity. As shown by Guo et al.,30 CD204-silenced DC vaccine showed preclinical synergistic effects when combined with radiotherapy. Also, we have found a synergistic effect when combining IDO-silenced DC vaccine with certain chemotherapeutic drugs. Moreover, the combination of CTLA4 blockade using Ipilimumab, a monoclonal antibody, and IDO-silenced DC vaccine enhanced tumor immunity in a patient with melanoma, thus our strategy holds promise for combination therapies (Sioud et al. in preparation). However, one should keep in mind that co-manipulation of various inhibitory signals could trigger autoimmunity, as a result of simultaneously loosing too many immunological “brakes." Therefore, it is important to select the correct therapeutic combinations. Comparable to monoclonal antibodies, siRNA provides a drug that transiently silences genes in specific cell types and may be particularly suited for blocking immunonosuppressive factors on a time-limited basis. In addition to the ex-vivo delivery route, continued research in diverse delivery technologies will help to facilitate the systemic delivery of siRNA therapeutics to patients.75
Funding
Work in the author's group is supported in part by the Gene Therapy Program and The Norwegian Cancer Society.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010; 33:464-78; PMID:21029958; http://dx.doi.org/ 10.1016/j.immuni.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev 2010; 234:45-54. [DOI] [PubMed] [Google Scholar]
- 3.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004; 4:336-47; PMID:15122199; http://dx.doi.org/ 10.1038/nri1349 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7BB1 in interleukin-2 production and immunotherapy. Cell 1992; 71:1065-8; PMID:1335362; http://dx.doi.org/ 10.1016/S0092-8674(05)80055-8 [DOI] [PubMed] [Google Scholar]
- 5.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interaction between PD-1 and PD-L-1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009; 10:1185-92; PMID:19783989; http://dx.doi.org/ 10.1038/ni.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Fox3+ regulatory T cell function. Science 2008; 322:271-5; PMID:18845758; http://dx.doi.org/ 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- 7.Munn DH, Mellor AL. Indolamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007; 17:1147-54; PMID:17476344; http://dx.doi.org/ 10.1172/JCI31178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corinti S, Albanesis C, la Salsa A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol 2001; 166:4312-20; PMID:11254683; http://dx.doi.org/ 10.4049/jimmunol.166.7.4312 [DOI] [PubMed] [Google Scholar]
- 9.Rettig GR, Behlke MA. Progress toward in vivo use of siRNAs-II. Mol Ther 2012; 20:483-512; PMID:22186795; http://dx.doi.org/ 10.1038/mt.2011.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnett JC, Rossi JJ, Tiemann K. Current progress of siRNAshRNA therapeutics in clinical trials. Biotechnol J 2011; 6:1130-46; PMID:21744502; http://dx.doi.org/ 10.1002/biot.201100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambros V. The functions of animal microRNAs. Nature 2004; 431:350-5; PMID:15372042; http://dx.doi.org/ 10.1038/nature02871 [DOI] [PubMed] [Google Scholar]
- 12.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003; 21:685-711; PMID:12615891; http://dx.doi.org/ 10.1146/annurev.immunol.21.120601.141040 [DOI] [PubMed] [Google Scholar]
- 13.Letterio JJ, Bottinger EP. TGF-beta knockout and dominant negative receptor transgenic mice. Miner Electrolyte Metab 1998; 24:161; PMID:9525700; http://dx.doi.org/ 10.1159/000057365 [DOI] [PubMed] [Google Scholar]
- 14.Marine JC. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 1999; 98:609-16; PMID:10490100; http://dx.doi.org/ 10.1016/S0092-8674(00)80048-3 [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdc1– mice as efficient animal model of type I diabetes. Proc Acad Sci USA 2005; 102:11823-8; PMID:16087865; http://dx.doi.org/ 10.1073/pnas.0505497102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munn DH, Mellor AL. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004; 4:762-74; PMID:15459668; http://dx.doi.org/ 10.1038/nri1457 [DOI] [PubMed] [Google Scholar]
- 17.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in draining-draining lymph noedes. J Clin Invest 2004; 114:280-90; PMID:15254595; http://dx.doi.org/ 10.1172/JCI200421583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosies by tryptophan catabolism. Cell Death Differ 2002; 9:1069-77; PMID:12232795; http://dx.doi.org/ 10.1038/sj.cdd.4401073 [DOI] [PubMed] [Google Scholar]
- 19.Furset G, Fløisand Y, Sioud M. Impaired expression of indoleamine 2, 3-dioxygenase in monocyte-derived dendritic cells in response to Toll-like receptor-78 ligands. Immunology 2007; 123:263-71; PMID:17725606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wobster M, Voigt H, Houben R, Eggert AO, Freiwald M, Kaemmerer U, Kaempgen E, Schrama D, Becker JC. Dendritic cell based antitumor vaccination: impact of functional indoleamine 2,3-dioxygenase expression. Cancer Immunol Immunother 2007; 56:1017-24; PMID:17195079; http://dx.doi.org/ 10.1007/s00262-006-0256-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flatekval GF, Sioud M. Modulation of dendritic cell maturation and function with mono- and bifunctional small interfering RNAs targeting indoleamine 2,3-dioxygenase. Immunology 2009; 128:e837-48; PMID:19740345; http://dx.doi.org/ 10.1111/j.1365-2567.2009.03093.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Koropatnick J, Chen D, Velenosi T, Ling H, Zhang X, Jiang N, Navarro B, Ichim TE, Urquhart B, Min W. Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model. Int J Cancer 2013; 132:967-77. [DOI] [PubMed] [Google Scholar]
- 23.Sioud M, Saebøe-Larssen S, Hetland TE, Kaern J, Mobergslien A, Kvalheim G. Silencing of indoleamine 2,3-dioxygenase in dendritic cell cancer vaccines: evaluation in vitro and in cancer patients. Int J Oncol 2013; 43:280-8; PMID:23620105 [DOI] [PubMed] [Google Scholar]
- 24.Sæbø-Larsen S, Fossberg E, Gaudernack G. mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT). J Immunol Methods 2002; 259:191-203; PMID:11730854; http://dx.doi.org/ 10.1016/S0022-1759(01)00506-3 [DOI] [PubMed] [Google Scholar]
- 25.Guppy AE, Nathan PD, Rustin GJ. Epithelial ovarian cancer: a review of current management. Clin Oncol (R Coll Radiol) 2005;17:399-411; PMID:16149282; http://dx.doi.org/ 10.1016/j.clon.2005.05.009 [DOI] [PubMed] [Google Scholar]
- 26.Kubo M, Handa T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol 2003; 4:1169-76; PMID:14639467; http://dx.doi.org/ 10.1038/ni1012 [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol 2004; 22:1546-52; PMID:15558048; http://dx.doi.org/ 10.1038/nbt1035 [DOI] [PubMed] [Google Scholar]
- 28.Song XT, Evel-Kabler K, Rollins L, Aldrich M, Gao F, Huang XF, Chen SY. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med 3:e11; PMID:16381597; http://dx.doi.org/ 10.1371/journal.pmed.0030011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alshamsan A, Haddadi A, Hamdy S, Samuel J, El-Kadi AO, Uludağ H, Lavasanifar A. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Molec Pharmaceut 2010; 7:1643-54; PMID:20804176; http://dx.doi.org/ 10.1021/mp100067u [DOI] [PubMed] [Google Scholar]
- 30.Guo C, Yi H, Yu X, Zuo D, Qian J, Yang G, Foster BA, Subjeck JR, Sun X, Mikkelsen RB, Fisher PB, Wang XY. In situ vaccination with CD204 gene-silenced dendritic cell, not unmodified dendritic cell, enhances radiation therapy. Mol Cancer Ther 2012; 11:2012; 2331-39; PMID:22896667; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Curr Opin Investig Drugs 2011; 11:1354-9; PMID:21154117 [PubMed] [Google Scholar]
- 33.Hobo W, Novobrantseva TI, Fredrix H, Wong J, Milstein S, Epstein-Barash H, Liu J, Schaap N, van der Voort R, Dolstra H. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol Immunother 2013; 62:285-97; PMID:22903385; http://dx.doi.org/ 10.1007/s00262-012-1334-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabinovich GA, Toscano MA. Turning “sweet” on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 2009; 9:338-52; PMID:19365409; http://dx.doi.org/ 10.1038/nri2536 [DOI] [PubMed] [Google Scholar]
- 35.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin 1: a small protein with major functions. Glycobiology 2006; 16:137-57; PMID:16840800; http://dx.doi.org/ 10.1093/glycob/cwl025 [DOI] [PubMed] [Google Scholar]
- 36.Hsu DK, Chen HY, Liu FT. Galectin-3 regulates T-cell functions. Immunol Rev 2009; 230, 114-27. [DOI] [PubMed] [Google Scholar]
- 37.Mobergslien A, Sioud M. Galectin-1 and -3 gene silencing in immature and mature dendritic cells enhances T cell activation and interferon-g production. J Leucocyte Biol 2012; 91:461-7; PMID:22167721Z; http://dx.doi.org/ 10.1189/jlb.0711361 [DOI] [PubMed] [Google Scholar]
- 38.Grossman Z, Singer A. Tuning of activation threshold explains flexibility in the selection and development of T cells in the thymus. Proc Acad Sci USA 1996; 93:14747-52; PMID:8962126; http://dx.doi.org/ 10.1073/pnas.93.25.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cekaite L, Furset G, Hvig E, Sioud M. Gene expression analysis in blood cells in response to umpdified and 2′-modified siRNAs reveals TLR-dependent and independent effects. J Mol Biol 2007; 365:90-108; PMID:17054988; http://dx.doi.org/ 10.1016/j.jmb.2006.09.034 [DOI] [PubMed] [Google Scholar]
- 40.Maldonado-Lopz R, Maliszewski C, Urbain J, Moser M. Cytokines regulates the capacity of CD8 alpha (+) and CD8 alpha (−) dendritic cells to prime Th1Th2 cells in vivo. J Immunol 2001; 167:4345-50; PMID:11591758; http://dx.doi.org/ 10.4049/jimmunol.167.8.4345 [DOI] [PubMed] [Google Scholar]
- 41.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the generation of dendritic cells from CD14+ blood monocytes, promotes the differentiation to mature macrophages and stimulates endocytosis of FITC-dextran. Avd Exp Med Biol 1997; 417:323-9; PMID:9286381; http://dx.doi.org/ 10.1007/978-1-4757-9966-8_53 [DOI] [PubMed] [Google Scholar]
- 42.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol 2005; 348:1079-10; PMID:15854645; http://dx.doi.org/ 10.1016/j.jmb.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 43.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G. Sequnece-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med 2005; 11:263-70; PMID:15723075; http://dx.doi.org/ 10.1038/nm1191 [DOI] [PubMed] [Google Scholar]
- 44.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 2005; 23:457-62; PMID:15778705; http://dx.doi.org/ 10.1038/nbt1081 [DOI] [PubMed] [Google Scholar]
- 45.Furset G, Sioud M. Design of bifunctional siRNAs: combining immunostimulation and gene-silencing in one single siRNA molecule. Biochem Biophys Res Comm 2007; 352:642-9; PMID:17150189; http://dx.doi.org/ 10.1016/j.bbrc.2006.11.059 [DOI] [PubMed] [Google Scholar]
- 46.Iversen PO, Semaeva E, Sørensen DR, Wiig H, Sioud M. Dendritic cells loaded with tumor antigens and a dual immunostimulatory and anti-interleukin 10-specific small interference RNA prime T lymphocytes against leukemic cells. Transl Oncol 2009; 2:242-6; PMID:19956385; http://dx.doi.org/ 10.1593/tlo.09154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pradhan P, Qin H, Leleux JA, Gwak D, Sakamaki I, Kwak LW, Roy K. The effect of combined IL10 siRNA and CpG ODN as pathogen-mimicking microparticles on Th1Th2 cytokine balance in dendritic cells and protective immunity against B cell lymphoma. Biomaterials 2014; 35:5491-504; PMID:24720881; http://dx.doi.org/ 10.1016/j.biomaterials.2014.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chhabra A, Chakraborty NG, Mukherji B. Scilencing of endogenous IL-10 in human dendritic cells leads to the generation of an improved CTL response against human melanoma associated antigenic epitope, MART-127-35. Clin Immunol 2008; 126:251-9; PMID:18249038; http://dx.doi.org/ 10.1016/j.clim.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glas M, Coch C, Trageser D, Dassler J, Simon M, Koch P. Targeting the cytosolic innate immune receptors RIG-1 and MDA5 effectively counteracts cancer cell heterogeneity in glioblastoma. Stem Cells 2013; 31:1064-74; PMID:23390110; http://dx.doi.org/ 10.1002/stem.1350 [DOI] [PubMed] [Google Scholar]
- 50.Kübler K, Gehrke N, Riemann S, Böhnert V, Zillinger T, Hartmann E, Pölcher M, Rudlowski C, Kuhn W, Hartmann G, Barchet W. Targeted activation of RNA helicase retinoic acid-inducible gene-1 induces proimmunogenic apoptosis of human ovarian cancer cells. Cancer Res 2010; 70:5293-304; PMID:20551064; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0825 [DOI] [PubMed] [Google Scholar]
- 51.Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, et al. 5′-triphosphate-siRNA: turning gene silencing and RIG-1 activation against melanoma. Nat Med 2008; 14:1256-63; PMID:18978796; http://dx.doi.org/ 10.1038/nm.1887 [DOI] [PubMed] [Google Scholar]
- 52.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediated tumor regression in mice. Sci Transl Med 2009; 8:8-19; PMID:20368186; http://dx.doi.org/ 10.1126/scitranslmed.3000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on manor histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 2002; 196:1627-38; PMID:12486105; http://dx.doi.org/ 10.1084/jem.20021598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 2004; 199:815-24; PMID:15024047; http://dx.doi.org/ 10.1084/jem.20032220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tacken PJ, Torensma R, Figdor CG. Targeting antigens to dendritic cells in vivo. Immunobiology 2006; 211:599-608; PMID:16920498; http://dx.doi.org/ 10.1016/j.imbio.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 56.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, Figdor CG. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood 2005; 106:1278-85; PMID:15878980; http://dx.doi.org/ 10.1182/blood-2005-01-0318 [DOI] [PubMed] [Google Scholar]
- 57.Sioud M, Skorstad G, Mobergslien A, Sæbøe-Larssen S. A novel peptide carrier for efficient targeting of antigens and nucleic acids to dendritic cells. FASEB J 2013; 27:3272-83; PMID:23671272; http://dx.doi.org/ 10.1096/fj.12-224758 [DOI] [PubMed] [Google Scholar]
- 58.Hossain DM, Dos Santos C, Zhang Q, Kozlowska A, Liu H, Gao C, Moreira D, Swiderski P, Jozwiak A, Kline J, et al. Leukemia cell-targeted STAT3 silencing and TLR9-triggering generate systemic antitumor immunity. Blood 2014; 123:15-25; PMID:24169824; http://dx.doi.org/ 10.1182/blood-2013-07-517987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nurieva R, Wang J, Sahoo A. T-cell tolerance in cancer. Immunotherapy 2013; 5:513-31; PMID:23638746; http://dx.doi.org/ 10.2217/imt.13.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.June CH. Adoptive T cell therapy for cancer in the clinic. J. Clin Invest 2007; 117:1466-76; PMID:17549249; http://dx.doi.org/ 10.1172/JCI32446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg SA., Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8:299-30; PMID:18354418; http://dx.doi.org/ 10.1038/nrc2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dudda JC, Salaun B, Ji Y, Palmer DC, Monnot GC, Merck E, Boudousquie C, Utzschneider DT, Escobar TM, Perret R, et al. microRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity 2013; 38:742-53; PMID:23601686; http://dx.doi.org/ 10.1016/j.immuni.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pallandre JR, Brillard E, Créhange G, Radlovic A, Remy-Martin JP, Saas P, Rohrlich PS, Pivot X, Ling X, et al. Role of STAT3 in CD4+ CD25 +FOXP3+ regulatory lymphocyte generation: implication in graft-versus-host disease and antitumor immunity. J Immunol 2007; 179:7593-604; PMID:18025205; http://dx.doi.org/ 10.4049/jimmunol.179.11.7593 [DOI] [PubMed] [Google Scholar]
- 64.Moriwaki A, Inoue H, Nakano T, Matsunaga Y, Matsuno Y, Matsumoto T, Fukuyama S, Kan-O K, Matsumoto K, Tsuda-Eguchi M, et al. T cell treatment with small interfering RNA for suppressor of cytokine signaling 3 modulates allergic airway responses in a murine model of asthma. Am J Respir Cell Mol Biol 2011; 44:448-55; PMID:20508071; http://dx.doi.org/ 10.1165/rcmb.2009-0051OC [DOI] [PubMed] [Google Scholar]
- 65.Pike KA, Tremblay ML. Regulating naive and memory CD8 T cell homeostasis –a role for protein tyrosine phosphatases. FEBS J 2013; 280:432-44; PMID:22458809; http://dx.doi.org/ 10.1111/j.1742-4658.2012.08587.x [DOI] [PubMed] [Google Scholar]
- 66.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 2004; 21:167-77; PMID:15308098; http://dx.doi.org/ 10.1016/j.immuni.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 67.Hinterleitner R, Gruber T, Pfeifhofer-Obermair C, Lutz-Nicoladoni C, Tzankov A, Schuster M, Penninger JM, Loibner H, Lametschwandtner G, Wolf D, et al. Adoptive transfer of siRNA Cblb-silenced CD8+ T lymphocytes augments tumour vaccine Efficacy in a B16 melanoma model. PLOS One 2012; 7:e44295; PMID:22962608; http://dx.doi.org/ 10.1371/journal.pone.0044295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stromnes IM, Fowler C, Casamina CC, Georgopolos CM, McAfee MS, Schmitt TM, Tan X, Kim TD, Choi I, Blattman JN, et al. Abrogation of Src homology region 2 domain-containing phosphatase 1 in tumor-specific T cells improves efficacy of adoptive immunotherapy by enhancing the effector function and accumulation of short-lived effector T cells in vivo. J Immunol 2012; 189:1812-25; PMID:22798667; http://dx.doi.org/ 10.4049/jimmunol.1200552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sathish JG, Dolton G, Leroy FG, Matthews RJ. Loss of Src homology region 2 domain-containing protein tyrosine phosphatase-1 increases CD8+ T cell-APC conjugate formation and is associated with enhanced in vivo CTL function. J Immunol 2007; 178:330-7; PMID:17182570; http://dx.doi.org/ 10.4049/jimmunol.178.1.330 [DOI] [PubMed] [Google Scholar]
- 70.Mantei A, Rutz S, Janke M, Kirchhoff D, Jung U, Patzel V, Vogel U, Rudel T, Andreou I, Weber M, Scheffold A. siRNA stabilization prolongs gene knockdown in primary T lymphocytes. Eur J Immunol 2008; 38:2616-25; PMID:18792414; http://dx.doi.org/ 10.1002/eji.200738075 [DOI] [PubMed] [Google Scholar]
- 71.Burnett JC., Zaia JA, Rossi JJ. Creating genetic resistance to HIV. Curr Opin Immunol 2012; 24, 625-32; PMID:22985479; http://dx.doi.org/ 10.1016/j.coi.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol 2005; 23:709-17; PMID:15908939; http://dx.doi.org/ 10.1038/nbt1101 [DOI] [PubMed] [Google Scholar]
- 73.Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 2008; 134:577-86; PMID:18691745; http://dx.doi.org/ 10.1016/j.cell.2008.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lebbink RJ, Lowe M, Chan T, Khine H, Wang X. and McManus MT. Polymerase II promoter strength determines efficacy of microRNA adapted shRNAs. PloS One 2011; 6:e26213-20; PMID:22031824; http://dx.doi.org/ 10.1371/journal.pone.0026213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mat 2013; 12:967-77; PMID:24150415; http://dx.doi.org/ 10.1038/nmat3765 [DOI] [PubMed] [Google Scholar]