Abstract
A number of Japanese encephalitis (JE) vaccines have been used for preventing Japanese encephalitis around the world. We here reviewed the immunogenicity and safety of the currently available Japanese encephalitis vaccines. We searched Pubmed, Embase, Web of Science, the Cochrane Library and other online databases up to March 25, 2014 for studies focusing on currently used JE vaccines in any language. The primary outcomes were the seroconversion rate against JEV and adverse events. Meta-analysis was performed for the primary outcome when available. A total of 51 articles were included. Studies were grouped on the basic types of vaccines. This systematic review led to 2 aspects of the conclusions. On one hand, all the currently available JE vaccines are safe and effective. On the other hand, the overall of JE vaccine evaluation is disorganized, the large variation in study designs, vaccine types, schedules, doses, population and few hand-to-hand trails, make direct comparisons difficult. In order to make a more evidence-based decision on optimizing the JE vaccine, it is warranted to standardize the JE vaccine evaluation research.
Keywords: immunogenicity, Japanese encephalitis vaccine, meta-analysis, safety, systematic review
Abbreviations
- AEs
Adverse events
- JE-CV
Chimeric live-attenuated JE vaccine
- CENTRAL
Cochrane Central Register of Controlled Trials
- CIs
Confidence intervals
- DARE
Database of Abstracts of Reviews of Effects
- GMTs
Geometric mean titers
- HAART
Highly active antiretroviral therapy
- JEV
Japanese encephalitis virus
- JE
Japanese encephalitis
- LILACS
Latin American and Caribbean Health Sciences Literature
- MMR
Measles mumps rubella vaccine
- MBJEV
Mouse brain–derived inactivated vaccines
- NIP
National Immunization Program
- NOS
Newcastle–Ottawa scale
- ORs
Odd ratios
- PRNT50
Plaque-reduction neutralization tests
- PHK
Primary hamster kidney cells
- RCTs
Randomized controlled trials
- TGPO
Thai Governmental Pharmaceutical Organization
- ACIP
The Advisory Committee on Immunization Practices
- TBE
Tick-borne encephalitis vaccine
- WHO
World Health Organization
- YF-VAX
Yellow fever vaccine
- YFV17D
Yellow fever virus 17D vaccine strain
- YFV
Yellow fever virus
Introduction
Japanese encephalitis (JE) is a severe mosquito-borne zoonostic disease that caused by Japanese encephalitis virus(JEV).
Approximately 67900 JE cases occur worldwide annually (overall incidence: 1.8 per 100000), of which only about 10% are reported to the World Health Organization (WHO).2 JE usually is severe, which there are 10000–15000 fatal cases annually and 30–50% of the survivors develop long-term or permanent disabilities such as physical and mental impairments.
In the absence of specific antiviral treatments, human vaccination against JE is considered as the key component of JE control and prevention.
The first-generation of JEV vaccines have been available since the 1950s and were in routine use for decades: (1) Mouse brain–derived inactivated vaccines (MBJEVs). MBJEVs were made from either Nakayama or Beijing-1 virus strains propagated in mouse brain tissue. Due to the strong protective efficacy, MBJEVs (e.g. JE-VAX) were extensively employed worldwide especially in Asia, Europe and the USA. It resulted in a significant reduction of JE incidence rate in Japan and Taiwan.8 Due to the occurrence of a single case of acute disseminated encephalomyelitis temporally associated with JE-VAX prompted the major manufacturer (BIKEN®, distributed as JE-VAX by Sanofi Pasteur, Lyon, France) to discontinue production and all remaining doses expired in May 2011.9 However, MBJEV produced by the Thai Governmental Pharmaceutical Organization (TGPO) is currently available in Thailand, which has been introduced into the National Immunization Program (NIP) since 1990.10 (2) Inactivated JE vaccine cultivated on primary hamster kidney cells (PHK). The PHK cell culture inactivated JE vaccine (Beijing-3, P-3 strain) was the principal JE vaccine in routine use of nationwide campaigns in China until the mid-2000s. Approximately 70 million doses were administered in China annually before 2005. (3) Live attenuated SA14-14-2 virus vaccine. Live attenuated SA14-14-2 virus vaccine has been developed in China, 1988. The vaccine virus, SA14-14-2 was based on its wild-type SA14 strain of JEV originating from primary hamster kidney cells and animal species, followed by plaque purification on primary chick embryo cells.11 Following administration of 1300 million doses, it is replacing the PHK inactivated JE vaccine progressively.12-14 It has been available for use in China, Nepal, India, Sri Lanka and South Korea.2
Regarding the safety of MBJEVs, the development of new JE vaccine candidates has continued. At present several promising second generation JE vaccines are available. (1) Chimeric live-attenuated JE vaccine (ChimeriVax, JE-CV). JE-CV is a recombinant vaccine based on a chimeric of JEV and yellow fever virus 17D vaccine strain (YFV17D).8 The vaccine was constructed by replacing the prM and E-encoding cDNA of yellow fever virus (YFV) with that of the live-attenuated SA14-14-2 strain of JEV.15,16 It was approved in Australia and Thailand in 2010. (2) IXIARO (IC51; also named JESPECT in Australia and New Zealand). As a purified, formalin-inactivated, whole-virus JE vaccine, IXIARO is based on the SA14-14-2 strain that has been adapted to grow on Vero cells. The purified production is then formulated with 0.1% aluminum hydroxide.17 IXIARO is licensed in the USA (for people aged ≥ 17 years), Europe, Canada, Switzerland and Australia.18 (3) Inactivated Vero cell-derived vaccines. An inactive Vero cell-derived Japanese encephalitis vaccine19 was licensed in China. In addition, 2 inactivated Vero cell culture-derived JE vaccines (JEBIK®V by Biken; ENCEVAC® by Kaketsuken) were approved in Japan as a substitute for MBJEVs in 2009 and 2011, respectively.20
Recently, the JE vaccine landscape has changed. As several new JE vaccines received license for use, questions concerning the use of new vaccines in specific populations (e.g., previous JE-VAX immunized individuals, infants or children infected with HIV) have been raised. New data have become available since licensure of vaccines, which may offer new insight into these concerns. In addition, the Advisory Committee on Immunization Practices (ACIP) JE Vaccines Workgroup approved new JE vaccine recommendation in 2013.17 Thus, a careful immunogenicity and safety assessment of JE vaccines currently available should be established. Under systematic review, this article provides updates on the status of JE vaccines that are presently in use, with a particular emphasis on the development of the second-generation JE vaccines.
Methods
Inclusion criteria
Eligible study designs were randomized controlled trials (RCTs), quasi-RCTs, controlled clinical trials, uncontrolled clinical trials, cohort studies, case control studies and cross-sectional studies. To assess the safety of JEV vaccines, we also considered case reports because rare adverse events (AEs) could be identified.
Individuals were included irrespective of age, sex and ethnic origin. The intervention was any licensed, currently used JE vaccine alone or combined with other vaccines regardless of administration route, dosage and schedule. Seroconversion rate, seroprotection rate, vaccination response rate and post-vaccination geometric mean titers (GMTs) were used as the primary outcomes of the immunogenicity. Among them, seroprotection was defined as a JEV plaque-reduction neutralization tests (PRNT50) neutralizing antibody titer ≥ 10; seroconversion was defined as a JEV PRNT50 neutralizing antibody titer ≥ 10 in those who were seronegative at baseline (PRNT50 < 10), or a ≥ 4-fold titer rise in those who were seropositive ≥ 10 at baseline. Response to vaccination was defined as JEV PRNT50 neutralizing antibody titer ≥ 10 in those who were seronegative at baseline (PRNT50 < 10), or a ≥ 2-fold titer rise in those who were seropositive ≥ 10 at baseline. Safety and reactogenicity outcomes were numbers and types of local or systemic reactions and serious AEs, including deaths.
Literature search
We initially conducted broad searches identifying all published literature and meeting abstracts in Pubmed (−2014), Embase (−2014) and Web of Science (−2014). Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), Latin American and Caribbean Health Sciences Literature (LILACS), National Institutes of Health database (clinicaltrials.gov), Current Controlled Trials, Vaccine Adverse Event Reporting System Website, Opengrey and GreyNet website were also searched. There was no restriction on language.
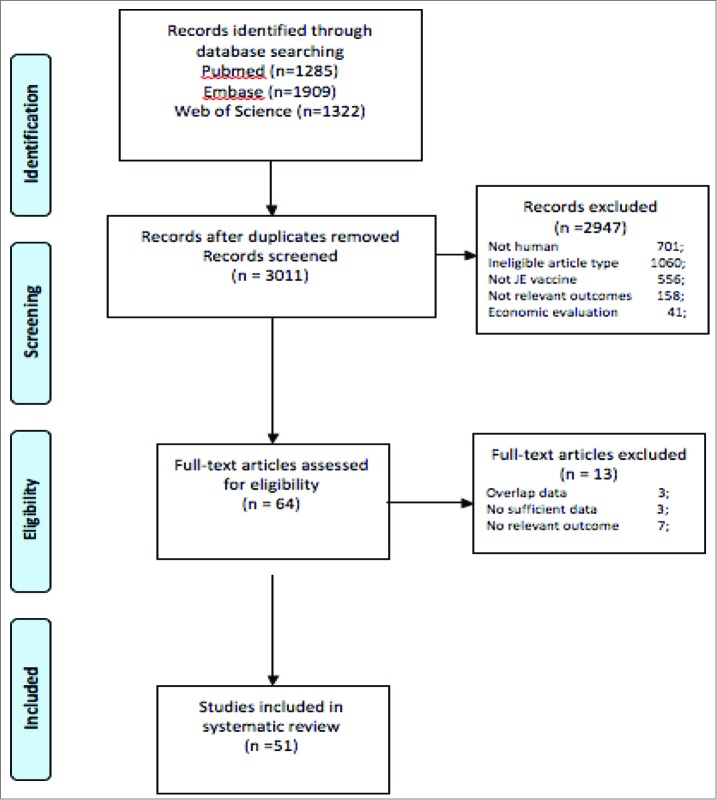
Articles were sought for the period up to March 25, 2014 by using combinations of the following search terms: Japanese encephalitis, JE, vaccine. The references of all identified publications, including previous review articles were hand-searched for other relevant publications. Citations were merged together in Endnote, version X7, which contributed to the retrieval of references (1285 from Pubmed, 1909 from Embase and 1322 from Web of Science). Duplicates were identified, inducing 3011 inclusions. Two review authors (Shu-juan Ma; Xie Liu) independently applied the inclusion criteria to all identified and retrieved articles. A third review author (LI Xing) arbitrated in the case of disagreement. Screening of these 3011 references leaded to 2960 exclusions. Xing Li provided an independent review of remaining potentially eligible references, resulting in 51 references for which the full-text article was retrieved and considered. In case of overlapping samples, we examined the most recently described and/or the largest sample. The corresponding authors of studies with missing data were contacted to obtain information. No reply was obtained from authors contacted. Flow diagram is shown in Figure 1.
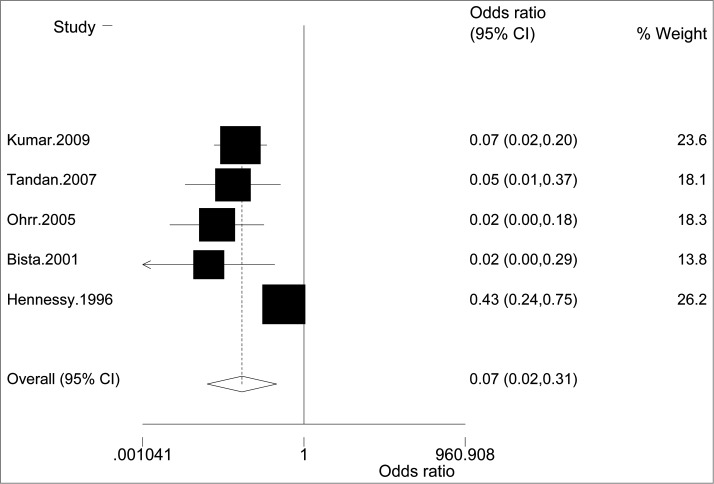
Figure 2.
Forest plot for case-control studies evaluating the effectiveness of Live attenuated SA14-14-2 virus vaccine.
Figure 1.
Flow diagram.
Data collection
For each of the eligible studies the following data elements were extracted:
(1) General study data: First author, journal, publication year, language, year and location of study. (2) Trial population: Number of participants, age, gender, health status. (3) Vaccines: vaccine type, number of doses, schedule, co-administered vaccine, length of follow up. (4) Immunogenicity: Seroconversion rate, seroprotection rate, vaccination response rate and GMTs. (5) Safety and reactogenicity: Number or percentage of subjects reporting local or systemic reactions, or AEs.
Validity assessment
To evaluate the methodological quality of the studies, we used the validated Newcastle–Ottawa scale (NOS) for case-control and cohort studies as mentioned by the Cochrane Non-Randomized Studies Methods Working Group (Information available from http://www.mrc-bsu.cam.ac.uk/cochrane/handbook/chapter_13/13_5_2_3_tools_for_assessing_methodological_quality_or_risk_of.htm). The score of overall quality ranges from 0 to 9.22 We divided NOS score into 3 levels (high quality, score≥ 7; moderate quality, 4 ≤ score < 7; low quality, score < 4).
We assessed the quality of RCTs, quasi-RCTs, controlled trials and uncontrolled trials using the criteria adapted from the Cochrane Handbook for Systematic Reviews of Interventions.18
Statistical analysis
We carried out data synthesis separately for different vaccines. We also classified and discussed included studies according to the type of outcomes they provided (immunogenicity or safety).
Based on the raw data of vaccination frequencies in cases and controls, crude odd ratios (ORs) as well as confidence intervals (CIs) were recalculated. Pooled estimates of ORs were calculated. Between-study heterogeneity and between-study inconsistency were assessed by using Cochrane Q statistic and by estimating I2, respectively.19 A fixed-effects model was used to calculate the pooled ORs when the test for heterogeneity was not statistically significant (P > 0.10), otherwise the random-effects model was employed.25 Evidence of publication bias was determined using funnel plot and Egger's statistical test.26 Statistical analyses were performed using STATA 12.0 (STATA Corporation, College Station, Texas).
Results
Numbers and characteristics of selected articles
Our search strategy identified 3011 articles, including 51 potentially eligible studies (53 samples) included 27 randomized controlled trials,7 controlled trials, 5 uncontrolled trials, 7 case-control studies, one follow up study, 3 observational study and one case report. The majority of studies were conducted among healthy adults, while a smaller number were conducted among infants and/or children. Details of study designs and the methodological quality are shown in Tables 1 and 2.1-2.3. In RCTs, masking of investigators, and laboratory staff were rarely reported. In non-randomized studies, confounders were often not taken into account in the analysis, and the numbers of withdrawals from analysis were rarely stated. The immunogenicity and safety of all included JE vaccines are summarized in Table 3.
Table 2.2.
Study quality of controlled trials and uncontrolled trials
| Description of selection of study participants | Baseline comparison (for controlled studies) | Control for confounding | |
|---|---|---|---|
| Woolpert, T.2012 | Y | Y | Y |
| Erra, E. O.2012 | Y | Y | Y |
| Eder, S.2010 | Y | — | Y |
| Puthanakit, T.2010 | Y | — | N |
| Dubischar-Kastner, K.2010 | Y | — | Y |
| Sohn, Y. M.2008 | Y | Y | Y |
| Puthanakit, T.2007 | Y | — | Y |
| Zhou, B.1999 | N | — | Y |
| Sohn, Y. M.1999 | N | — | N |
| Rojanasuphot, S.1998 | Y | N | N |
| Guo, W.1998 | Y | Y | N |
| Choi, U Y.2013 | Y | — | N |
| Kikukawa, A. 2012 | Y | Y | N |
| Chanthavanich P.2012 | Y | — | N |
| Yu,YX.1988 | Y | N | N |
N: no; Y: yes; -: no clear information.
Table 1.
Basic information of included articles
| First author, year | Design | Location | Population | Vaccine studied | Dosage of JE vaccine | Outcome reported | Number of participants | Time points |
|---|---|---|---|---|---|---|---|---|
| Monath.T.P.2002 | RCT | USA | Adult | JE-CV; YF-VAX | 4log10PFU; 5log10PFU | Immunogenicity; safety | 36 | Day 1, 6, 11 and 31 |
| Monath.T.P.(study2) | RCT | USA | Adult | JE-CV; JE-VAX | 1.8–5.8log10 | Immunogenicity; safety | 20 | Day 3,7,14 and 30 |
| Torresi,J.2010(study1) | RCT | USA and Australia | Adult | JE-CV; MBJEV | — | Immunogenicity; safety | 820 | Day 0, 44 and 60 |
| Torresi,J.2010 (study2) |
RCT | USA and Australia | Adult | JE-CV; saline placebo | 1.3 × 106PFU/mL (3.8log10PFU) |
Safety | 2004 | Day 0, 44 and 60 |
| Nasveld E.P.2010 | RCT | Australia | Adult | JE-CV; YF-17D | — | Immunogenicity; safety | 108 | Day 0, 30 and 60 |
| Nasveld E.P.2010.b | RCT | Australia | Adult | JE-CV; placebo | — | Immunogenicity; safety | 202 | Day 0, 14, 42 and 56. |
| Huang. L.M.2014 | RCT | Taiwan, China | Child | JE-CV; MMR | 4.0–5.8 log10 PFU/0.5 ml | Immunogenicity; safety | 550 | Day 0, 42, 84, month 6 and month12 |
| Liu, Z. L.1997 | RCT | China | Child | Live attenuated SA14-14-2 virus vaccine | log10 6.8 PFU/ml; log10 6.4 PFU/ml | Safety | 26239 | Day30 |
| Kuzuhara, S.2003 | RCT | Japan | Adult | inactivated Vero cell-derived | — | Immunogenicity; safety | 60 | Day 0, 2-4 weeks after the third injection |
| Schuller, E.2008 | RCT | USA, Germany and Austria | Adult | IXIARO; JE-VAX | 6 μg of purified virus adsorbed to 0.1% aluminum hydroxide | Immunogenicity; safety | 867 | Day 0, 28 and 56 |
| Tauber, E.2008 | RCT | Australia, Austria, Germany, Israel, New Zealand, Romania, and USA | Adult | IXIARO; placebo | 6 μg virus adsorbed to 0.1% aluminum hydroxide | Immunogenicity; safety | 2650 | — |
| Kaltenbock, A.2009 | RCT | Austria and Germany | Adult | IXIARO; HAVRIX®1440 | — | Immunogenicity; safety | 192 | A follow-up was done 28 days and 6 months after Day 0/D56 |
| Kaltenbock, A.2010 | RCT | India | Child | IXIARO; JeneVac | — | Immunogenicity; safety | 70 | D28, D56 |
| Li,W.2009 | RCT | China | Child | INACTIVATED JE VACCINE(PHK);JEV(vero) | — | Immunogenicity; safety | 943 | 30 min, 6/24/48 h |
| Sanofi-Aventis.2012 | RCT | India | Child<=18 years | JE-CV; JE Inactivated Mouse Brain Vaccine | — | Immunogenicity; safety | 96 | Day 14 up to Day 42 Post-vaccination |
| Sanofi-Aventis.2012.15 | RCT | USA | Adult | JE-CV; JE-VAX | — | Immunogenicity; safety | 60 | Day 0 (pre-vaccination) and up to Day 56 post-vaccination |
| Lyons, A.2007 | RCT | USA | Adult | IXIARO; JE-VAX | 6 mcg/dose | Immunogenicity; safety | 94 | D0, D28, D56 |
| Okada, K.2012 | RCT | Japan | Child<=18 years | JEBIK | — | Immunogenicity; safety | 370 | Month12 |
| Kikukawa, A. 2012 | Controlled trial | Japan | Child | JEBIK | — | Immunogenicity | 125 | not mentioned |
| Miyazaki, C. 2014 | RCT | Japan | Child | JEBIK; Encevac | 17 μg/0.5 ml; 4 μg/0.5 ml; 8 μg/0.5 ml | Immunogenicity; safety | 468/484 | 2-6 weeks |
| Takeshita, N. 2014 | Observatioal | Japan | Adult | Vero cell-derived JE vaccine | — | Immunogenicity | 79 | 3-5 weeks |
| Chanthavanich P.2012 | Uncontrolled trial | Thailand | Child | Inactivated purified vero cell-derived JE vaccine | Immunogenicity; safety | 152 | Day 0, 1 month after the second vaccination, 1 year, and 1 month after the third vaccination | |
| Schuller, E.2009 | RCT | Germany and Northern Ireland | Adult | IXIARO | 6 μg of purified virus adsorbed to 0.1% aluminum hydroxide | Immunogenicity; safety | 490 | A screening visit up to 28 days prior to the start of treatment was followed by 5 study visits on Days 0 (first vaccination), 10, 28 (second vaccination), 35, and 56. |
| Chokephaibulkit, K.2010 | RCT | Thailand | Child | JE-CV; HepatitisA | — | Immunogenicity; safety | 300 | Children attended safety and immunogenicity follow-up visits on Days 4, 15, 43, and 56. A follow-up visit occurred at |
| Feroldi. E.2012 | RCT | Thailand; the Philippines | Toddlers aged 12-18 months | JE-CV; Hepatitis A | — | Immunogenicity; safety | 1199 | four visits (screening, day0, 14, 28) and 2 phone calls or home visits (2 days and 6 months after vaccination) |
| Feroldi. E.2013 | RCT | Thailand; the Philippines | Child aged 36–42 months | JE-CV | — | Immunogenicity; safety | 450 | Day 7 and 28 |
| Tauber, E.2007 | RCT | USA; Austria and Germany | Adult | IXIARO; | 6 μg of purified virus adsorbed to 0.1% aluminum hydroxide | Immunogenicity; safety | 867 | Day 0, 28 and 56 |
| Woolpert, T.2012 | controlled trial | USA | ≥ 17 years | IXIARO; JE-VAX | — | Immunogenicity; safety | 123 | Day 0, 28 and 56 |
| Muangchana, C.2012 | case control | Thailand | 1–5 years | MBJEV | — | Immunogenicity | 129 | — |
| Erra, E. O.2012 | controlled trial | Finland; Sweden | 18–72 years | IXIARO; MBJEV | — | Immunogenicity | 120 | Day 0,4-8 weeks |
| Jia, N.2011 | case report | China | Child | Live attenuated SA14-14-2 virus vaccine | — | Safety | 4 | 7-14 days after vaccination |
| Eder, S.2010 | uncontrolled trial | Austria and Germany | Adult | IXIARO | 6 μg of purified virus adsorbed to 0.1% aluminum hydroxide | Immunogenicity; safety | 204 | Day 28 and month 6 |
| Puthanakit, T.2010 | follow up | Thailand | Child >5 years | MBJEV (Beijing) | — | Immunogenicity; safety | 43 | 3 years |
| Dubischar-Kastner, K.2010 | uncontrolled trial | North Ireland and Germany | Adult | IXIARO | 6 mcg of purified virus adsorbed to 0.1% aluminum hydroxide | Immunogenicity; safety | 349 | Mouth 6, 12 and 24 |
| Zhou, Li-bao.2009 | RCT | China | 8 months- 10 years | vero inattenuated JEV | — | Immunogenicity, safety | 307 | Day 0,28-30 days after the second vaccination |
| Kumar, R.2009 | case control | India | Child | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity | 449 | — |
| Sohn, Y. M.2008 | controlled trial | Nepal | Child | Live attenuated SA14-14-2 virus vaccine | 6.77 PFU/dose | Long term immunogenicity | 138 | Day 0,7 and 30 |
| Choi, U Y.2013 | Uncontrolled trial | Korea | Child | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity; safety | 68 | 4 -6 weeks |
| Gatchalian, S.2008 | RCT | the Philippine | Infant | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity; safety | 600 | Day 0 and 28 |
| Tandan, J. B.2007 | case control | Nepal | Child, adolescents and young Adults | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity | 239 | — |
| Puthanakit, T.2007 | uncontrolled trial | Thailand | Child | MBJEV (Beijing) |
— | Immunogenicity; safety | 50 | Two months after first dose, prior to second dose and at one month after second dose. |
| Ohrr, H.2005 | case control | Nepal | 2–16 years | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity | 465 | — |
| Bista, M. B.2001 | case control | Nepal | 1–15 years | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity | 577 | — |
| Zhou, B.1999 | observational study | China | Child | Live attenuated SA14-14-2 virus vaccine | — | immunogenicity; safety | 335941 | — |
| uncontrolled trial | Korea | 1–3 years | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity; safety | 68 | Day 0 and day28 | |
| Tsai, T. F.1998 | RCT | China | Middle school- aged child | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity | 231 | the first and second immunizations and 30 days after the second dose |
| Rojanasuphot, S.1998 | controlled trial | Thailand | Infant | MBJEV (Nak ) | — | Immunogenicity; safety | 46 | 1, 2, 4, 6, 9, 12, 15 and 18 months |
| Guo, W.1998 | controlled trial | China | Infant | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity; safety | 319 | 1,6,12 months |
| Liu, Y. 2014 | Observational | China | Child | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity; Safety | 1426 | — |
| Yu, YX.1988 | controlled trial | China | Child | Live attenuated SA14-14-2 virus vaccine | 106.7TCID50/0.5 ml | Immunogenicity; safety | 1026 | two weeks |
| Hennessy, S.1996 | case control | China | Child | Live attenuated SA14-14-2 virus vaccine | — | Immunogenicity | 1355 | — |
| Florian Marks.2012 | case control | Vietnam | Child <15 years | MBJEV | — | Immunogenicity | 150 | — |
HAVRIX®1440: Hepatitis A vaccine; JE-CV: Chimeric live-attenuated JE vaccine; MMR: measles, mumps, rubella vaccine;JE-VAX: An inactivated mouse brain-derived JE vaccine; MBJEV: Mouse brain–derived inactivated vaccines; RCT: randomized controlled trial; YF-17D: yellow fever virus 17D vaccine strain; YF-VAX: yellow fever vaccine;—: no clear information.
Table 2.1.
Study quality of RCTs
| Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | ||
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias | |
| Monath.T.P.2002 | Y | Y | Y | N | Y | Y | Y |
| Monath.T.P.(study2) | N | N | Y | Y | Y | Y | Y |
| Torresi,J.2010(Study1) | Y | N | Y | N | Y | Y | Y |
| Torresi,J.2010(Study2) | Y | N | Y | N | Y | Y | Y |
| Nasveld E.P.2010 | Y | Y | Y | N | Y | Y | Y |
| Nasveld E.P.2010.b | Y | Y | N | N | Y | Y | Y |
| Liu, Z. L.1997 | Y | N | N | N | Y | Y | Y |
| Kuzuhara, S.2003 | N | N | N | N | Y | Y | Y |
| Schuller, E.2008 | Y | N | N | N | Y | Y | Y |
| Tauber, E.2008 | Y | Y | Y | N | Y | Y | Y |
| Kaltenbock, A.2009 | Y | Y | Y | N | Y | Y | Y |
| Kaltenbock, A.2010 | Y | N | N | N | Y | Y | Y |
| Li,W.2009 | N | N | N | N | Y | Y | Y |
| Sanofi-Aventis.2012 | Y | N | Y | Y | Y | Y | Y |
| Sanofi-Aventis.2012.2 | Y | N | Y | Y | Y | Y | Y |
| Lyons, A.2007 | Y | Y | N | N | Y | Y | Y |
| Defraites, R F.1999 | Y | Y | N | N | Y | Y | Y |
| Okada, K.2012 | Y | Y | Y | N | Y | Y | Y |
| Schuller, E.2009 | N | N | Y | Y | Y | Y | Y |
| Chokephaibulkit, K.2010 | Y | N | N | N | Y | Y | Y |
| Feroldi.E.2012 | Y | Y | Y | Y | Y | Y | Y |
| Tauber, E.2007 | Y | Y | Y | N | Y | Y | Y |
| Zhou, Li-bao.2009 | N | N | N | Y | Y | Y | Y |
| Gatchalian, S.2008 | Y | N | N | N | Y | Y | Y |
| Tsai, T. F.1998 | N | N | N | N | Y | Y | Y |
| Feroldi. E.2013 | Y | Y | N | N | Y | Y | Y |
| Huang, L. 2014 | Y | Y | N | N | Y | Y | Y |
| Miyazaki, C. 2014 | Y | Y | Y | N | Y | Y | Y |
N: no; Y: yes.
Table 2.3.
NOS score of included studies (case control)
| Author | Selection | Comparability | Exposure | Total score |
|---|---|---|---|---|
| Hennessy, S.1996 | 4 | 2 | 2 | 8 |
| Bista, M. B.2001 | 4 | 2 | 2 | 8 |
| Ohrr, H.2005 | 4 | 2 | 2 | 8 |
| Tandan, J. B.2007 | 4 | 2 | 2 | 8 |
| Kumar, R.2009 | 4 | 2 | 2 | 8 |
| Muangchana, C.2012 | 4 | 1 | 2 | 7 |
| Florian Marks.2012 | 4 | 2 | 2 | 8 |
Table 3.
Summary of immunogenicity and safety results of all included JE vaccines
| Vaccine type | Immunogenicity | Safety | |||
|---|---|---|---|---|---|
| Number of studies | GMT | Seroconversion rate | Number of studies | AEs | |
| JE-CV | 12 | 128–2634 | 84–100% | 13 | Diarrhea; fatigue; feeling hot and cold; headache; injection site erythema; injection site pain; malaise; myalgia; nasal congestion; nausea; pyalgia |
| IXIARO | 10 | 182–2496 | 83–100% | 10 | Fever; hardening; headache; itching; myalgia; pain; rash; redness; swelling; tenderness; vomiting or diarrhea |
| Live attenuated SA14-14-2 virus vaccine | 8 | 115–517 | 83–100% | 8 | Anaphylaxis; angioedema; arthus reaction; elevated temperature; generalized rash; henoch-schonlein purpura; idiopathic thrombocytopenic purpura; irritability; loss of appetite; measles-like or scarlet fever rash; skin rash; urticarial; vomiting |
| Inactivated, Vero cell-derived JE vaccines | 6 | 70–622 | 87–100% | 1 | Tenderness; redness; ecchymosis; fever; vomiting; loss of appetite |
| MBJEV | 5 | 15–306 | 85–93% | 1 | Low-grade fever; injection site pain, rash or swelling |
| Inactivated JE vaccine (PHK) | 1 | 1:328 | 94.90% | 2 | Erythema; fever |
AEs: Adverse events; GMT: Geometric mean titer.
Immunogenicity
JE-CV
12 clinical trials have been completed in different geographical regions for JE-CV. These studies evaluated various issues related to immunogenicity of JE-CV and correlated the findings with those obtained in preclinical studies to answer some of the concerns over the genetically modified nature of JE-CV.
Seven out of 12 studies assessing the immunogenicity of JE-CV were conducted among adults from non-endemic countries, while the rest 4 were conducted among children from endemic countries.
A Phase I RCT trial27 including yellow fever (YF)-immune (n = 18) and non-YF-immune (n = 18) subjects showed the immunogenicity of a single dose of JE-CV at 2 different dosages (5.0 log10 PFU vs. 4.0 log10 PFU) was comparable to YF-VAX (1 × 5.0 log10 PFU). 100% of YF-immune and non-YF-immune subjects were seroconvert to the JE-CV strain. GMTs in 5.0 log10 PFU JE-CV groups (YF-immune: 327 vs. non-immune: 254) were higher than 4.0 log10 PFU JE-CV groups (YF-immune: 270 vs. non-immune: 128).
In a Phase II trial28 with 99 adults in the USA, the immunogenicity of graded doses of JE-CV (1.8–5.8 log10 PFU) was evaluated. 82 (94%) of 87 subjects administered JE-CV developed neutralizing antibodies. Administered 30 days later, the second dose of JE-CV had no booster effect. Previous inoculation with YF did not alter responses to JE-CV, but there was a suggestion that administration of JE-CV interfered with YF-VAX administered 30 days later.
Another phase II RCT29 was conducted to evaluate the immunogenicity and safety of JE-CV co-administered with live-attenuated YF vaccine (YF17D strain; Stamaril®, Sanofi Pasteur) or administered successively. Participants (n = 108) were randomized to receive: YF then JE-CV 30 days later, JE then YF 30 days later, or the coadministration of JE and YF followed or preceded by placebo 30 days later or earlier. 100% and 91% participants in the JE/YF and YF/JE sequential vaccination groups seroconverted to JE-CV, respectively, compared with 96% in the coadministration group. Neutralizing antibodies against JE vaccine were detected in 82–100% of participants at 6 months.
In the phase III immunogenicity study,30 seroconversion rates against JE-CV on 60 days post-vaccination were 99.1% (95%CI: 97.5-99.8) in JE-CV recipients compared with 95.1% (95%CI: 92.3-97.1) in JE-VAX group. Similarly, the proportion of participants in JE-CV group (80.9%; 95%CI: 76.4-84.9) that seroconverted to Nakayama strain was higher than JE-VAX group (74.8%; 70.0-79.2).
In the study evaluating the long-term immunogenicity of JE-CV booster,31 202 healthy adults received JE-CV and placebo 28 days apart in a crossover design, then a subgroup of 98 participants received a JE-CV booster 6 months later. At 60 months, seroconversion rate in JE-CV booster recipients remained at 96% (95%CI: 89-100), comparing with 87%(78-86) in non-booster group.
In a phase II trial,32 100 children (2–5 year-old) with a history of 2 doses of MBJEV vaccine and 200 JE vaccination-naive toddlers (12–24 month-old) were randomized to receive JE-CV one month before or after hepatitis A vaccine. Using the JE-CV PRNT50, GMTs 28 days after vaccination in the 2–5 year-old (2634, 95%CI: 1928–3600) were higher than the 12–24 month-old (281, 95%CI: 219–362). One year later, seropositive rates were 97% and 84% and GMTs were 454 and 62.3, in 2–5 year olds and 12–24 month-old, respectively.
A trial33 which included 1200 JE-CV vaccination naive children (12–18 month-old) in Thailand and the Philippines demonstrated that a single dose of JE-CV is well tolerated and elicits a high protective immune response. Children received JE-CV (from one of 3 successive industrial scale lots produced in Thailand (n = 899) or from a fourth development lot produced in the US (n = 199) or hepatitis A control vaccine (n = 102). Seroconversion rate on 28th day after JE-CV vaccination (Thai lots) was 95.0% (95% CI; 93.3–96.3), while the seroconversion rate was 96.3% (95% CI: 92.6–98.5) in the JE-CV US lot group. The corresponding GMTs were 214 (95% CI: 168–271) and 187 (95% CI: 168–208), respectively. Immune responses were generally comparable across countries and centers.
A randomized,open-label, multicenter trial was conducted to evaluate the concomitant administration of JE-CV and measles, mumps, rubella vaccine (MMR) in 550 children aged 12–18 months in Taiwan. Seroconversion rate for JE was 96.9%, 97.9% and 100% in concomitant, JE-CV and MMR sequential groups. But non-inferiority was demonstrated for all the groups.34
Afterwards, another phase III RCT35 involving children aged 36–42 months was conducted to evaluate the memory immune response and safety of a booster dose. 105 healthy children were randomized to receive JE-CV (46 children) or varicella vaccine (safety control group; 59 children). Immunological memory was observed in children who had received the primary dose of JE-CV before. Twenty-eight days after the JE-CV booster dose, seroprotection and seroconversion were achieved in 100% and 95.3% of children, respectively, and the GMT was 2242 1/dil. One year after receiving the JE-CV booster dose, 99.4% of children remained seroprotected.
A pediatric clinical trial of JE-CV36 was conducted in India aiming to compare the efficacy of JE-CV and MBJEV. Ninety-six children aged 3.7 ± 2.52 years old were randomized to receive JE-CV or MBJEV. The GMTs were 313.5 (95%CI: 40 – 5120) and 49.7 (95%CI: 5 – 320) for JE-CV and MBJEV group, respectively.
Recently, the results of a Phase III trial for assessing safety and efficacy of JE-CV have been announced,37 although these have not yet been described in peer-reviewed literature. The proportion of participants that seroconverted to the JE-CV with a single dose of JE-CV (100%) was higher than 3 doses of JE-VAX (80.8%) on the 28th day after the last dose. The efficacy studies suggested a single dose of JE-CV induced antibodies to JEV at levels greater than the 3-dose vaccination by JE-VAX during 12 months.
IXIARO
Studies meeting the inclusion criteria did not only compare GMT over time but also included comparisons with a different vaccine, different vaccine schedules and different dosages.
Two (6.0 or 12.0 μg) or 3 (6.0 μg) doses of IXIARO administered on days 0 and 28 or days 0, 14 and 28 were compared with 3 doses of JE-VAX (days 0, 7 and 28).38 In the IXIARO groups the percentage of seroconverters varied between 83% and 100%, while only half of the JE-VAX subjects showed maintenance of serum neutralization antibody over the long term up to 720 days following vaccination.
Additional study38 demonstrated high seroconversion rates (83%) 1 year following vaccination; the superiority of the standard dosing regimen (2 × 6.0 μg), compared with using low or high-dose strategies.
A multinational Phase III trial39 demonstrated that IXIARO was more immunogenic compared with control (JE-VAX) among adults aged ≥ 18 years in Austria, Germany and USA. Two doses of 6.0 μg IXIARO were given to 428 adults on days 0 and 28, while 3 doses of JE-VAX were given to 435 adults on days 0, 7 and 28. A seroconversion rate of 98% was recorded in subjects who received IXIARO, compared with 95% in those who received JE-VAX. IXIARO group showed a substantially higher GMT than JE-VAX group (IXIARO, 244; JE-VAX, 102) on day 56.
A phase III trial40 investigated the neutralizing antibody response to IXIARO in subjects with and without pre-existing tick-borne encephalitis vaccine (TBE) induced antibodies. In this trial, 430 and 437 subjects received IXIARO (2 × 6.0 μg on days 0 and 28) or JE-VAX (3 × 1.0 ml on days 0, 7 and 28), respectively, with 54 TBE ELISA-positive subjects in each group. Subjects who had received TBE vaccination in the IXIARO group had higher seroconversion rates (98% vs. 92%) and higher GMTs (28.4 vs. 16.0). There is significant difference in seroconversion rates between TBE ELISA-positive and negative subjects after single dose (ELISA positive, 91%; negative, 48%), whereas the seroconversion rates (ELISA positive, 96%; negative, 91%) and GMTs (ELISA positive, 230 ± 469.5; negative, 182.7 ± 1135.3) were comparable in both groups on day 56 after 2 doses of IXIARO.
A phase III trial41 investigated the immunogenicity of IXIARO and Havrix®1440 (Hepatitis A vaccine) when administered alone or concomitantly to healthy adults. Seroconversion rates and GMTs were comparable for IXIARO and Havrix®1440 whether administered concomitantly or separately on day 56 (IXIARO + placebo group, 98%; IXIARO + Havrix® group, 100%), which demonstrated that when IXIARO was administered concomitantly with Havrix®1440 no adverse influence on immunogenicity of either vaccine.
Another phase III trial42 aimed to investigate the immunogenicity and safety of administering IXIARO to 1–3-year-old children in India (n = 60). 2 × 6 μg and 2 × 3 μg doses IXIARO delivered on days 0 and 28 were compared with 3 doses of JenceVacTM (inactivated mouse-brain derived vaccine). On day 56 the seroconversion rates and GMTs for 6 μg IXIARO, 3 μg IXIARO and JenceVacTM groups were 95% (GMT: 218; 95% CI: 121–395), 96% (GMT: 201; 95% CI: 106–380) and 91% (GMT: 230; 95% CI: 68–784), respectively. These early immunogenicity data in children are promising and support the use of a 3 μg dose in children below the age of 3.
Three dosing schedules43 were evaluated by a phase III RCT: 2 × 6.0 μg (given on day 0 and 28); 1× 12.0 μg (given on day 0) and 1 × 6.0 μg (given on day 0). The 1 × 12.0 μg group resulted in almost 60% seroconversion on 10 days after administration, while the 2 × 6.0 μg group conferred essentially 100% seroconversion 7 days after the second immunization. On 56 days after receiving the first dose of vaccine, the 2 × 6.0 μg group produced superior seroconversion and GMT (97%, n = 218) compared with the other 2 regimens (1× 12.0 μg, 41%, n = 11; 1 × 6.0 μg, 26%, n = 8).
A follow-up study44 was designed to assess the long-term immunogenicity of primary IXIARO vaccination and the immune response to an IXIARO booster dose in subjects without protective neutralizing antibody titers. After primary immunization with a day 0/28 dose schedule, seroprotection rates declined gradually from 83% (99/116) in month 6 to 58% (67/115) in month 12 and 50% (56/113) in month 24. A booster dose in month 11 and/or month 23 in PRNT-negative subjects led to 100% seroconversion. GMTs increased from below the limit of detection to 676.2 (95%CI, 365.0 – 1252.5; n = 16) and to 2496.1 (95%CI, 1407.5 – 4426.7; n = 24) following the month11 and 23 boosters, respectively.
Eder et al.45 assessed the immunogenicity of a booster dose of IXIARO that was administered 15 months after the primary immunization. The protective rate of subjects reached 100% in 1 month after the booster while it remained at 98.5% in 6 and 12 months. GMTs were 22.5 before the booster, and maintained at 900, 487 and 361 in 1, 6 and 12 months after the booster, respectively.
A controlled trial that evaluated the interchangeability of IXIARO in military personnel who previously received MBJEV determined that a single dose of IXIARO provide adequate boosting and at least short-term protection.46 Seropositive rates among previously vaccinated participants post-dose one (44/44,100%) were noninferior to those achieved in previously naïve participants post dose-2 (53/57, 93%). The GMT was significantly higher in previously vaccinated participants post-dose one (315; 95%CI, 191-520) compared to previously naïve participants post-dose 2 (79; 95%CI, 54-114).
Erra et al.47 conducted a study among travelers to estimate whether IXIARO can be used to boost immunity after a primary series of MBJEV. In travelers primed with MBJEV, vaccination response rates after a booster dose of MBJEV or IXIARO were 91% and 98% for the Nakayama strain, respectively, while 91% and 95% for SA14-14-2 strain, respectively.
Live attenuated SA14-14-2 virus vaccine
The efficacy of live attenuated SA14-14-2 virus vaccine was first reported by YU et al.48 The seroconversion rates of live attenuated SA14-14-2 virus vaccine were 100% (GMT, 35.3; n = 11), 100% (GMT, 31.7; n = 12) and 83.3% (GMT, 23; n = 10) in seronegative children receiving vaccine diluted 1:3, 1:5 and 1:50, respectively.
Tsai et al.49 compared neutralizing antibody responses in children immunized with 2 vaccine doses of 1 or 2.5-month immunization schedules with 2 live attenuated SA14-14-2 virus vaccine lots (lot1,941010-1; lot2, 941010-2). In one-month schedule, GMTs rose from 46 to 89, 39 to 65 for lot 1 and 2, respectively. With a 2.5-month schedule, GMTs rose from 25 to 158 for lot 1 while from 19 to 115 for lot 2. But a 2.5 -month schedule did not increase the response rate.
Guo et al.50 compared the immunogenicity of live attenuated SA14-14-2 virus vaccine with inactivated vaccine in 319 infants aged 5-18 months. After the primary immunization, the serum positive rates were 56.08% and 59.56% respectively, and further study showed after the booster one year later, the serum positive rates reached 98.21% and 94.62%, respectively. Six months after the booster, it declined to 72.34% in live attenuated SA14-14-2 virus vaccine group while remained at 94.74% in the live attenuated group.
Live attenuated SA14-14-2 virus vaccine provided 83.87% to 94.74% protection after a single dose administration in a 5-year field trial among 33594 vaccinated children.51
Live attenuated SA14-14-2 virus vaccine was first used outside of China among Korean children, Sohn et al.52 evaluated the immunogenicity in 68 infants aged 1-3 years old. 65 (96%) of the subjects were seroprotected after the primary vaccination, and the GMT was 188. The 16 children who had either a history of previous JE vaccination or detectable JE viral antibody before the immunization, were all seroconverted after a single dose of live attenuated SA14-14-2 virus vaccine.
In a 5-year follow up study among Korean children,53 the persistence of neutralizing antibody of single dose live attenuated SA14-14-2 virus vaccine was observed (89.9% after 4 years and 63.8% after 5 years). Twenty-four subjects (Group1) who were seronegative in 2005 were revaccinated in 2006, while forty-nine seronegative subjects (Group2) were given one dose of primary vaccine in 2006. On the 7th day after vaccination, the seropositive rate raised to 76.5% (13/17) with GMT 168.52 in Group 1, whereas no subject was seroprotected in Group 2. On thirty days after vaccination, the seropositive rate and GMT of Group 1 were higher compared to those of Group 2 (82.4% vs. 75.7%; 392.01 vs. 45.72).
Sixty-eight Korea children aged 5 to 7 -year-old who had received one or 2 doses of live attenuated SA14-14-2 virus vaccine in 1-2 years old were administrated with a booster dose.54 After vaccination of the booster dose, the titer of the neutralizing antibody was found to be positive in all subjects. The GMT showed an increase of 4.44-fold, from 116.46 to 517.11 IU/ml.
Gatchalian et al.55 conducted a RCT to evaluate the immunogenicity of live attenuated SA14-14-2 virus vaccine and measles vaccine (MV) administrated concomitantly. Healthy infants were randomized into 3 groups to receive live attenuated SA14-14-2 virus vaccine at age of 8 months and MV at 9 months (Group 1; n = 100); MV and live attenuated SA14-14-2 virus vaccine together at 9 months (Group 2; n = 236) or MV at 9 months and live attenuated SA14-14-2 virus vaccine one month later (Group 3; n = 235). JE seroprotection rates in 4 weeks after vaccination in Group 1, 2, 3 were 92.1%, 90.5% and 90.6%, respectively. The GMTs for JE antibodies were 279.3 (Group 1), 221.9 (Group 2) and 197.3 (Group3), respectively.
In addition, we identified 5 case-control studies evaluating the effectiveness of live attenuated SA14-14-2 virus vaccine.13,14,56-58 The pooled OR was 0.07 (95%CI, 0.02-0.31) with a moderate heterogeneity (I2 = 21.84%) (Figure 2). The funnel plot was not symmetric (data not shown) and the Egger's test was significant (P = 0.02), which indicated a high probability of publication bias. Exclusion of the earliest sample from the meta-analysis altered the overall results (OR, 0.05; 95%CI, 0.02- 0.11; I2 = 0%). Hennessy et al.13 estimated the effectiveness of one dose live attenuated SA14-14-2 virus vaccine was 80% (95%CI, 44-93%) in China, while the efficacy was 94.5% (95%CI, 81.5-98.9%) observed by Kumar et al.14 Efficacy of 99.3% (95%CI, 94.9-100%) was observed in a median of 2 weeks after vaccination by Bista et al.58 in Nepal. The protective effect of vaccine after 12-15 months was 98.5% (95%CI, 90.1-99.2%),57 and maintained at 96.2% (95%CI, 73.1-99.9%) 5 years after administration of a single dose.56
Inactivated, Vero cell-derived JE vaccines
In a phase I trial, the seroconversion rate after the first dose of inactivated, Vero cell-derived JE vaccine and MBJEV groups were 96.7% and 92.9%, respectively, while the seroconversion rate was 100% after the second dose of both vaccines.59 Even though there were no significant differences of GMTs after the first and second injection between the 2 groups, the GMTs after the third injection was significantly higher in the inactivated Vero cell-derived JE vaccine group than in the MBJEV group (102.35 vs. 102.03).
In a field trial among 551 children (aged 8 months to 10 years old), Zhou et al.19 observed similar immunogenicity of liquid and freeze-dried inactivated Vero cells derived JE vaccine (seroconversion rates, 93.3% vs. 90.4%; GMTs, 1:24.0 vs. 1:21.8.). One hundred and fifty-two healthy Thai children aged 1-3 years were administered 3 doses of inactivated Vero cell-derived JE vaccine on Day 0, 7-28, and 365.60 One month after the second vaccination, all subjects (100%) had anti-JE level higher than the protective level (GMT, 150). Seroprotection rate one year after the second vaccination and one month after the third vaccination were 89.2% (GMT, 49.3) and 100% (GMT, 621.7), respectively.
A multicenter, double–blinded RCT61 was conducted to evaluate the immunogenicity of the JEBIK®V. The seroconversion rates were 100%, 99.2% and 95.0% after 2 doses in the 5 μg, 2.5 μg and 1.25 μg groups, respectively, and 100.0% after 3 doses in all groups. The GMTs were high for 3 groups after the second and third doses, with a dose-response relationship. The immune response of JEBIK®V and MBJEV was compared,62 the results showed the GMT in the MBJEV group was lower than that in the JEBIK H group for both 2 and 3 vaccinations (lot TJE01) (p = 0.04 and 0.002).
The immunogenicity and safety of Encevac were compared with MBJEV in 2 Phase III multicenter trials among Japanese children. Trial 1 (conducted among 468 healthy children from February 2003 to August 2004) found the inactivated, Vero cell-derived JE vaccine was more immunogenic and reactive than MBJEV at the same dose. Trial 2 (conducted in 480 children from June 2008 to May 2009) found that seroconversion rates and GMTs of different dose of Encevac were non-inferior compared to MBJEV.63
The neutralizing antibodies of 79 Japanese adults who received Vero cell-derived JE vaccine (BIKEN, Japan) were measured pre- and post-JE vaccination. Seroconversion rate was 86.8%; GMT was 14.7 before vaccination and 70.1 after vaccination. More participants aged ≥ 50 years were seroconverted compared with those aged 25-39 years.64
MBJEV
A retrospective study65 was conducted to evaluate the immunogenicity of MBJEV among 46 HIV-infected or uninfected children who received 2 doses of JE vaccine at 12 months of age. The GMT of HIV-infected children with positive titer was lower than that of controls (15.1 vs. 23.8; P = 0.17), but the difference was not significant.
Puthanakit et al.66 assessed MBJEV effectiveness in HIV-infected children with immune recovery after highly active antiretroviral therapy (HAART). Forty-four children (88%) developed protective antibody. A follow-up study67 showed that the JE neutralizing antibody was detected among 35 (81%) children 3 years after revaccination. However, the GMT dropped from 306 (range, 13-163, 617) in one month after revaccination to 106 (range 11- 4645) 3 year later.
In 2012, Charung et al.68 conducted a case-control study to assess the effectiveness of MBJEV among Thai children aged 1 to 6 years old. The risk of infecting with JE was decreased to 15% (overall OR = 0.15) among vaccinated children, and the vaccine effectiveness was 84.8% (95% CI, 57.7-94.7%). In addition, Marks et al.69 evaluated the local-produced MBJEV in Vietnam by a matched case-control study. The effectiveness was 92.9% (95% CI, 66.6-98.5%).
Inactivated JE vaccine (PHK)
In phase II and III trials, 903 healthy volunteers were inoculated inactivated JE vaccine (PHK) and 101 healthy volunteers were inoculated with the Vero cell-derived JEV vaccine.70 For those participants whose antibodies were negative before vaccination, the seroconversion rates were 96.9% (GMT, 1: 32.8) and 84.0% (GMT, 1:43.9) in inactivated JE vaccine (PHK) and Vero cell-derived JEV vaccine group, respectively.
Safety and reactogenicity
JE-CV
In thirteen studies AEs of JE-CV vaccination were assessed. Few events requiring major medical attention or hospitalization were described. Eight studies reported that diary cards were used to collect data.
Vaccine groups were generally comparable for all baseline characteristics. Seven studies included adults while 6 were conducted in children under 18 years old. The percentage of women was comparable with men in the pooled analysis. In all studies the majority of subjects had no relevant medical history, and there were no relevant differences between individual studies or treatment groups. The most common classes of prior medication were similar between studies and included in all studies for example analgesics/antipyretics or hormonal contraceptives. The observation period was at least 28 days for the majority of subjects.
A total of 5190 subjects who received at least one dose of JE-CV vaccination were included in the pooled safety analysis. These seven studies included 2517 (48.5%) adults and 2673 children. A total of 4404 subjects (84.9%) received the standard primary vaccination schedule of one JE-CV dose; while 487 subjects (9.4%) received 2 doses. 1645 subjects (31.7%) received at least one concomitant vaccination after enrolment. Overall, headache (14.5%) was the most frequently reported AE in JE-CV groups of these studies, followed by injection site erythema (11.3%), fatigue (10.7%) and feeling cold or hot (10.7%). AEs were more frequent after the first dose than after the second dose.
In adult group with JE-CV, the most frequent AEs were headache (26.5%), fatigue (21.7%), malaise (16.6%) and myalgia (15.5%). No difference was found in the incidence rate of fatigue or malaise between JE-CV compared with placebo. Headache was significantly more common among JE-CV vaccine recipients when compared with placebo, while myalgia was more common in placebo group. In MBJEV group, AEs were most commonly reported as injection site pain (63.9%), headache (37.8%), injection site erythema (30.0%), fatigue (26.6%) and injection site pruritus (26.1%). The reporting rates of headache, fatigue, malaise (22.4%) and myalgia(21.5%) was higher than JE-CV adults group. There was no significant difference between the treatment groups in the total number of subjects with AEs.
Six studies explicitly studied AEs in children under 18 years old. Numbers were highest for feeling hot and cold (19.6%), followed by abnormal crying (19.2%), drowsiness (17.7%), injection site erythema (16.6%) and vomiting (13.9%).
51.7% of adults in JE-CV group reported at least one treatment-emergent AE. Most AEs were mild or moderate. Fifty six (1.1%) subjects experienced at least one serious AE, among which 11 (0.21%) were considered possibly related to vaccination. The severe treatment-related AEs varied from severe injection site erythema to acute viral illness. No subjects with AEs leaded to withdrawal of study medication. There were no AEs leading to death considered related to the vaccination during the observation period.
IXIARO
The safety profile of IXIARO was overviewed by Schuller et al.,71 including post marketing data. No serious AEs after IXIARO vaccination revealed causal relationship with vaccination. Vaccine reactions, both local and systemic, were consistently reported to be mild and transient, and thus of little clinical relevance.
Live attenuated SA14-14-2 virus vaccine
No serious hypersensitivity reactions or neurologic AEs were identified among live attenuated SA14-14-2 virus vaccine recipients in publications.
In a safety study of live attenuated SA14-14-2 virus vaccine,48 no local reactions or systemic symptoms were reported among 991 children during a 14-day observation after vaccination.
In another large-scale study51 on the safety and efficacy of live attenuated SA14-14-2 virus vaccine compromising 335941 children, no allergic response or encephalitis was observed. Several subjects reported local swelling or low-grade fever.
Sohn et al.52 reported no immediate local or systemic reactions among 86 children received live attenuated SA14-14-2 virus vaccine. The proportions of subjects reporting mild and self-limited symptoms were as followed: elevated temperature (37.5-38℃) 7% (6/86), vomiting 1%(1/86), skin rash 1%(1/86), loss of appetite 1% (1/86), and irritability 1%(1/86). For a booster dose, Choi et al.54 observed solicited injection site adverse reactions in 6.45% (4/62), solicited systemic adverse reactions in 3.22% (2/68) and unsolicited adverse reactions in 11.29% (7/62) of subjects.
In a study50 compared immunogenicity in 150 infants who received live attenuated SA14-14-2 virus vaccine with 169 infants who received inactivated JE vaccine, similar reactogenicity and AEs were indicated (low grade fever, 3.3%, 5/150; nausea, 2%, 3/150). No serious adverse event was reported in live attenuated SA14-14-2 virus vaccine group.
A clinical trial in which live attenuated SA14-14-2 virus vaccine was administered concomitantly with MV55 indicated no Severe AEs related to live attenuated SA14-14-2 virus vaccine. Live attenuated SA14-14-2 virus vaccine was well tolerated when given alone or concomitantly with MV. The majority of the local reactions among all groups were mild and transient.
Four cases of encephalitis temporally associated with live attenuated SA14-14-2 virus vaccine should be noted.72 All cases presented with acute encephalitis that all received a first dose of live attenuated JE vaccine within 2 weeks prior to the onset of illness.
During the period of 2005-2012, 23.29 million doses of live attenuated SA14-14-2 virus vaccine were used and 1426 cases of AEs were reported in Guangdong Province, China.73 The overall rate of reported AEs was 61.2 per million doses. Thirty-six cases (the overall rate was 1.55 per million vaccine doses) were diagnosed as severe AEs. For both serious and non-serious categories, most of the cases were observed in children younger than 2 years. There were 706 cases with fever. A total of 570 cases (37.17%; 22.8 per million doses) had allergic reactions, including 509 with generalized rash, 28 with urticaria, 7 with angioedema, 6 with anaphylaxis, 5 with measles-like or scarlet fever rash, 4 with Arthus reaction, 3 with Idiopathic Thrombocytopenic Purpura and 2 with Henoch-Schonlein Purpura. Two deaths (0.14%; 0.086 per million doses) were reported after the vaccination.
Inactivated, Vero cell-derived JE vaccines
Chanthavanich et al.60 reported local AEs including tenderness (0.5%), redness (0.5%), ecchymosis (0.2%), and systemic reactions including fever (17.6%), vomiting (8%), poor appetite (5.3%) but no vaccine-related Severe AEs were noted.
MBJEV
In Puthanakit's study,66 pain at the injection site was the most commonly reported adverse reaction that was reported in 29 (58%) and 14 (28%) of participants after the first and second vaccine doses, respectively. The pain was mild and resolved spontaneously within 1–3 days without any treatment. One participant (2%) had swelling at the injection site, 2 (4%) had low-grade fever and 3 (6%) had rash after the first dose of vaccine. Two participants (4%) had swelling at the injection site after the second dose of vaccine. None of the participants had hypersensitivity reactions or neurological complications after vaccination and none withdrew from the study because of vaccine-related AEs.
Inactivated JE vaccine (PHK)
Li et al.70 found that the total adverse reaction rates were 6.2% and 14.2% in inactivated JE vaccine group and Vero cell-derived JE vaccine group, respectively. No serious vaccine-related adverse events were identified in inactivated JE vaccine.
Miyazaki et al.63 observed that the most common vaccine-related AEs were erythema (15.8% vs. 11.7%) and fever (17.5% vs. 11.7%). But there were no statistically significant difference between Inactivated JE vaccine group and MBJEV group.
Discussions
The current systematic review was based on data about immunogenicity and safety of currently used JE vaccine including inactivated and live attenuated vaccines. The results indicated that all the evaluated vaccines was as immunogenic as JE-VAX, with no significant vaccine-related AEs been detected.
For live attenuated vaccines, JE-CV is recommended for children aged 2 months through 16 years as for people aged ≥ 17 years.21 Live attenuated SA14-14-2 virus vaccine has a simple schedule and good safety profile.74 The vaccine's manufacturer, Chengdu Institute of Biological Products has made a submission to WHO for prequalification for JE vaccine.
For inactivated JE vaccines, IXIARO is highly immunogenic, showing significantly higher GMT compared with MBJEVs. Post marketing studies have confirmed the excellent safety profile. Studies on children aged 2 months to 18 years have been published. Based on these data, positive opinions from the EMA for vaccination of children have recently been given. The Vero cell-derived JE vaccine in Japan has shown a safety profile and good immunogenicity data, but post marketing surveillance for long-term immunity and follow-up on rare severe AEs in clinical safety evaluation is warranted.
All the evaluated vaccines were claimed to be immunogenic and generally safe. Unfortunately, we did not have sufficient head-to-head data to specify effects and safeties of different vaccines formally.
There were several strengths of our review. Firstly, the systematic strategy and broad search terms used in multiple databases could identify as many studies as possible. Secondly, we used the rigorous methods to extract and appraise the data. In addition, we included case control studies, uncontrolled studies as well as randomized and non-randomized controlled trials to obtain as much information about the effects of JE vaccines as possible. Last but not least, the methodological quality of the studies was adequate.
However, this result should be interpreted with caution due to several potential limitations. First of all, unlike measles and influenza vaccines, few studies compared the currently used JE vaccines head-to-head. Reviewing massive literature throughout the world, we found that the overall of JE vaccine evaluation is disorganized. The large variation in study designs, vaccine types, schedules, doses and population makes direct comparisons difficult. It is hard to draw direct conclusion on the relative efficacy and safety of these JE vaccines. Therefore we conducted an overall qualitative systematic review. When it is appropriate a quantitative approach was applied on the evaluation of efficiency of live attenuated SA14-14-2 virus vaccine and the safety of JE-CV.75 The second limitation was the heterogeneity between studies. Study designs varied with respect to the vaccine types, schedules, evaluation time points and population. Nasveld29 used JE vaccine with YF-vaccine in the intervention group and control group, but in other studies, volunteers did not receive YF-vaccine in advance. This may overestimate the effect of safe effects and immunogenicity in that it is easy to attribute vaccine effects to JV-vaccine. The heterogeneity may introduce confounding and bias in the comprehensive and comparative analysis. In addition, lack of RCTs may restrict the power of evidence (e.g. live attenuated SA14-14-2 virus vaccine). Furthermore, safety evaluation is particularly difficult, because of the lack of access to surveillance data (e.g., live attenuated SA14-14-2 virus vaccine) and inconsistence in documenting outcomes across studies. Even though JE-CV and MBJEV showed excellent safety profile with few AEs reported, we should be careful because the total sample size was not large enough to exclude the possibility of rare AEs.
Challenges remain that may require future studies in the areas of the safety of live attenuated SA14-14-2 virus vaccine, inactivated Vero-derived JE vaccine, JE-CV and IXIARO; the duration of protective immunity in infants; and the immunogenicity and safety of JE vaccines for children living in JE-endemic countries.
In conclusion, we found the currently available JE vaccine to be safe, well tolerated and similarly effective, suitable for the control of Japanese encephalitis according to existing policy recommendations. However, the overall of JE vaccine evaluation currently is disorganized. The large variation in study designs, vaccine types, schedules, doses, population and few hand-to-hand trails, make direct comparisons difficult. From a public health viewpoint, standardized methods should be required to assess the efficiency, safety and cost-effectiveness of health technology such as vaccines against infectious diseases. In order to make a more evidence-based decision on optimizing the JE vaccine, it is warranted to standardize the JE vaccine evaluation research.
Acknowlegdments
The authors would like to thank the statistics expert, CHEN Qingshan from Jinan University for reviewing and confirming the statistical result of this work. The authors are also grateful to the following for their contributions to this work: Xue-Yan Zheng, Xiao Zhang, Jin-Yan Lin, Xue-Shan Zhong and Shao-Wei Chen from Department of Epidemiology, School of Public Health and Tropical Medicine, Southern Medical University, China.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Reference
- 1. Solomon T. Control of Japanese encephalitis–within our grasp? N Engl J Med 2006; 355:869-71; PMID: 16943399; http://dx.doi.org/ 10.1056/NEJMp058263 [DOI] [PubMed] [Google Scholar]
- 2. Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, et al. . Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 2011; 89:766-74, 74A-74E; PMID:22084515; http://dx.doi.org/ 10.2471/BLT.10.085233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO position paper Japanese encephalitis vaccines. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations=Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2006; 81:331-40 [Google Scholar]
- 4. Appaiahgari MB, Vrati S. IMOJEV((R)): a Yellow fever virus-based novel Japanese encephalitis vaccine. Expert Rev Vaccines 2010; 9:1371-84; PMID:21105774; http://dx.doi.org/ 10.1586/erv.10.139 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and PreventionUpdate on Japanese encephalitis vaccine for children: United States, May 2011. MMWR Morbidity and mortality weekly report 2011; 60:664-5 [Google Scholar]
- 6. Thailand GPOGo Japanese encephalitis cell culture-derived vaccine development project. Proposal: Govermental Pharmaceutical Organization (GPO) of Thailand [Google Scholar]
- 7. Chen BQ, Beaty BJ. Japanese encephalitis vaccine (2-8 strain) and parent (SA 14 strain) viruses in Culex tritaeniorhynchus mosquitoes. Am J Trop Med Hyg 1982; 31:403-7; PMID:7072902 [DOI] [PubMed] [Google Scholar]
- 8. Liu ZL, Hennessy S, Strom BL, Tsai TF, Wan CM, Tang SC, Xiang CF, Bilker WB, Pan XP, Yao YJ, et al. . Short-term safety of live attenuated Japanese encephalitis vaccine (SA14-14-2): results of a randomized trial with 26,239 subjects. J Infect Dis 1997; 176:1366-9; PMID:9359740; http://dx.doi.org/ 10.1086/517323 [DOI] [PubMed] [Google Scholar]
- 9. Hennessy S, Liu Z, Tsai TF, Strom BL, Wan CM, Liu HL, Wu TX, Yu HJ, Liu QM, Karabatsos N, et al. . Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case-control study. Lancet 1996; 347:1583-6; PMID:8667866; http://dx.doi.org/ 10.1016/S0140-6736(96)91075-2 [DOI] [PubMed] [Google Scholar]
- 10. Kumar R, Tripathi P, Rizvi A. Effectiveness of one dose of SA 14-14-2 vaccine against Japanese encephalitis. N Engl J Med 2009; 360:1465-6; PMID:19339732; http://dx.doi.org/ 10.1056/NEJMc0808664 [DOI] [PubMed] [Google Scholar]
- 11. Guirakhoo F, Zhang ZX, Chambers TJ, Delagrave S, Arroyo J, Barrett AD, Monath TP. Immunogenicity, genetic stability, and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (ChimeriVax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology 1999; 257:363-72; PMID:10329547; http://dx.doi.org/ 10.1006/viro.1999.9695 [DOI] [PubMed] [Google Scholar]
- 12. Chambers TJ, Nestorowicz A, Mason PW, Rice CM. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol 1999; 73:3095-101; PMID:10074160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eckels KH, Yu YX, Dubois DR, Marchette NJ, Trent DW, Johnson AJ. Japanese encephalitis virus live-attenuated vaccine, Chinese strain SA14-14-2; adaptation to primary canine kidney cell cultures and preparation of a vaccine for human use. Vaccine 1988; 6:513-8; PMID:3149829; http://dx.doi.org/ 10.1016/0264-410X(88)90103-X [DOI] [PubMed] [Google Scholar]
- 14. Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines 2011; 10:355-64; PMID:21434803; http://dx.doi.org/ 10.1586/erv.11.7 [DOI] [PubMed] [Google Scholar]
- 15. Zhou L-b, Zhao X, Wu X-t. Adverse reaction and immune effect induced by inactivated Japanese encephalitis vaccine prepared with vero cells. Chin J Biol 2009; 22:809-11 [Google Scholar]
- 16. Kikukawa A, Gomi Y, Akechi M, Onishi T, Manabe S, Namazue J, Fuke I, Ishikawa T, Okuno Y, Ueda S. Superior immunogenicity of a freeze-dried, cell culture-derived Japanese encephalitis vaccine (inactivated). Vaccine 2012; 30:2329-35; PMID:22306856; http://dx.doi.org/ 10.1016/j.vaccine.2012.01.054 [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease C, Prevention Use of Japanese encephalitis vaccine in children: recommendations of the advisory committee on immunization practices, 2013. MMWR Morbidity and mortality weekly report 2013; 62:898-900 [Google Scholar]
- 18. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. . The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928; PMID:22008217; http://dx.doi.org/ 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557-60; PMID:12958120; http://dx.doi.org/ 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof 2001; 24:126-51; PMID:11523383; http://dx.doi.org/ 10.1177/016327870102400203 [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629-34; PMID:9310563; http://dx.doi.org/ 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, Marchesani R, Knauber M, Wells KH, Arroyo J, et al. . Clinical proof of principle for ChimeriVax(trademark): Recombinant live, attenuated vaccines against flavivirus infections. Vaccine 2002; 20:1004-18; PMID:11803060; http://dx.doi.org/ 10.1016/S0264-410X(01)00457-1 [DOI] [PubMed] [Google Scholar]
- 23. Monath TP, Guirakhoo F, Nichols R, Yoksan S, Schrader R, Murphy C, Blum P, Woodward S, McCarthy K, Mathis D, et al. . Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis 2003; 188:1213-30; PMID:14551893; http://dx.doi.org/ 10.1086/378356 [DOI] [PubMed] [Google Scholar]
- 24. Nasveld PE, Marjason J, Bennett S, Aaskov J, Elliott S, McCarthy K, Kanesa-Thasan N, Feroldi E, Reid M. Concomitant or sequential administration of live attenuated Japanese encephalitis chimeric virus vaccine and yellow fever 17D vaccine: randomized double-blind phase II evaluation of safety and immunogenicity. Hum Vaccines 2010; 6:906-14; PMID:20864814; http://dx.doi.org/ 10.4161/hv.6.11.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torresi J, McCarthy K, Feroldi E, Meric C. Immunogenicity, safety and tolerability in adults of a new single-dose, live-attenuated vaccine against Japanese encephalitis: Randomised controlled phase 3 trials. Vaccine 2010; 28:7993-8000; PMID:20934459; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.035 [DOI] [PubMed] [Google Scholar]
- 26. Nasveld PE, Ebringer A, Elmes N, Bennett S, Yoksan S, Aaskov J, McCarthy K, Kanesa-thasan N, Meric C, Reid M. Long term immunity to live attenuated Japanese encephalitis chimeric virus vaccine: randomized, double-blind, 5-year phase II study in healthy adults. Hum Vaccines 2010; 6:1038-46; PMID:21150279; http://dx.doi.org/ 10.4161/hv.6.12.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Sabchareon A, Pancharoen C, Bouckenooghe A, Gailhardou S, Boaz M, Feroldi E. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J 2010; 29:1111-7; PMID:20856164; http://dx.doi.org/ 10.1097/INF.0b013e3181f68e9c [DOI] [PubMed] [Google Scholar]
- 28. Feroldi E, Pancharoen C, Kosalaraksa P, Watanaveeradej V, Phirangkul K, Capeding MR, Boaz M, Gailhardou S, Bouckenooghe A. Single-dose, live-attenuated Japanese encephalitis vaccine in children aged 12-18 months: Randomized, controlled phase 3 immunogenicity and safety trial. Hum Vaccines Immunother 2012; 8:929-37; PMID:22777096; http://dx.doi.org/ 10.4161/hv.20071 [DOI] [PubMed] [Google Scholar]
- 29. Huang LM, Lin TY, Chiu CH, Chiu NC, Chen PY, Yeh SJ, Boaz M, Hutagalung Y, Bouckenooghe A, Feroldi E. Concomitant administration of live attenuated Japanese encephalitis chimeric virus vaccine (JE-CV) and measles, mumps, rubella (MMR) vaccine: Randomized study in toddlers in Taiwan. Vaccine 2014; 32(41):5363-9; PMID:24631095 [DOI] [PubMed] [Google Scholar]
- 30. Feroldi E, Capeding MR, Boaz M, Gailhardou S, Meric C, Bouckenooghe A. Memory immune response and safety of a booster dose of Japanese encephalitis chimeric virus vaccine (JE-CV) in JE-CV-primed children. Hum Vaccines Immunother 2013; 9:889-97; PMID:23442823; http://dx.doi.org/ 10.4161/hv.23087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanofi-Aventis Safety and efficacy study of chimeriVax™-JE and JE inactivated mouse brain vaccine in children of descending age. Clinicaltrials.gov, 2012. [Google Scholar]
- 32. Sanofi A safety and efficacy study of two Japanese encephalitis caccines chimeriVaxTM-JE and JE-VAX. Clinicaltrials.gov, 2012. [Google Scholar]
- 33. Lyons A, Kanesa-thasan N, Kuschner RA, Eckels KH, Putnak R, Sun W, Burge R, Towle AC, Wilson P, Tauber E, et al. . A phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis. Vaccine 2007; 25:3445-53; PMID:17241714; http://dx.doi.org/ 10.1016/j.vaccine.2006.12.046 [DOI] [PubMed] [Google Scholar]
- 34. Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, Schranz S, Jong E, Klingler A, Dewasthaly S, et al. . Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet 2007; 370:1847-53; PMID:18061060; http://dx.doi.org/ 10.1016/S0140-6736(07)61780-2 [DOI] [PubMed] [Google Scholar]
- 35. Schuller E, Jilma B, Voicu V, Golor G, Kollaritsch H, Kaltenbock A, Klade C, Tauber E. Long-term immunogenicity of the new Vero cell-derived, inactivated Japanese encephalitis virus vaccine IC51 Six and 12 month results of a multicenter follow-up phase 3 study. Vaccine 2008; 26:4382-6; PMID:18599165; http://dx.doi.org/ 10.1016/j.vaccine.2008.05.081 [DOI] [PubMed] [Google Scholar]
- 36. Kaltenbock A, Dubischar-Kastner K, Eder G, Jilg W, Klade C, Kollaritsch H, Paulke-Korinek M, von Sonnenburg F, Spruth M, Tauber E, et al. . Safety and immunogenicity of concomitant vaccination with the cell-culture based Japanese Encephalitis vaccine IC51 and the hepatitis a vaccine HAVRIX(registered trademark)1440 in healthy subjects: a single-blind, randomized, controlled phase 3 study. Vaccine 2009; 27:4483-9; PMID:19486955; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.034 [DOI] [PubMed] [Google Scholar]
- 37. Kaltenbock A, Dubischar-Kastner K, Schuller E, Datla M, Klade CS, Kishore TS. Immunogenicity and safety of IXIARO (IC51) in a Phase II study in healthy Indian children between 1 and 3 years of age. Vaccine 2010; 28:834-9; PMID:19857447; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 38. Schuller E, Klade CS, Wolfl G, Kaltenbock A, Dewasthaly S, Tauber E. Comparison of a single, high-dose vaccination regimen to the standard regimen for the investigational Japanese encephalitis vaccine, IC51: a randomized, observer-blind, controlled Phase 3 study. Vaccine 2009; 27:2188-93; PMID:19200452; http://dx.doi.org/ 10.1016/j.vaccine.2008.12.062 [DOI] [PubMed] [Google Scholar]
- 39. Dubischar-Kastner K, Eder S, Buerger V, Gartner-Woelfl G, Kaltenboeck A, Schuller E, Tauber E, Klade C. Long-term immunity and immune response to a booster dose following vaccination with the inactivated Japanese encephalitis vaccine IXIARO, IC51. Vaccine 2010; 28:5197-202; PMID:20541581; http://dx.doi.org/ 10.1016/j.vaccine.2010.05.069 [DOI] [PubMed] [Google Scholar]
- 40. Eder S, Dubischar-Kastner K, Firbas C, Jelinek T, Jilma B, Kaltenboeck A, Knappik M, Kollaritsch H, Kundi M, Paulke-Korinek M, et al. . Long term immunity following a booster dose of the inactivated Japanese Encephalitis vaccine IXIARO(registered trademark), IC51. Vaccine 2011; 29:2607-12; PMID:21288804; http://dx.doi.org/ 10.1016/j.vaccine.2011.01.058 [DOI] [PubMed] [Google Scholar]
- 41. Woolpert T, Staples JE, Faix DJ, Nett RJ, Kosoy OI, Biggerstaff BJ, Johnson BW, Sracic M, Fischer M. Immunogenicity of one dose of Vero cell culture-derived Japanese encephalitis (JE) vaccine in adults previously vaccinated with mouse brain-derived JE vaccine. Vaccine 2012; 30:3090-6; PMID:22406277; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.063 [DOI] [PubMed] [Google Scholar]
- 42. Erra EO, Askling HH, Rombo L, Riutta J, Vene S, Yoksan S, Lindquist L, Pakkanen SH, Huhtamo E, Vapalahti O, et al. . A single dose of vero cell-derived Japanese encephalitis (JE) vaccine (ixiaro) effectively boosts immunity in travelers primed with mouse brain-derived JE vaccines. Clin Infect Dis 2012; 55:825-34; PMID:22696017; http://dx.doi.org/ 10.1093/cid/cis542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xin YY, Ming ZG, Peng GY, Jian A, Min LH. Safety of a live-attenuated Japanese encephalitis virus vaccine (SA14-14-2) for children. The Am J Trop Med Hyg 1988; 39:214-7; PMID:2841880 [DOI] [PubMed] [Google Scholar]
- 44. Tsai TF, Yu YX, Jia LL, Putvatana R, Zhang R, Wang S, Halstead SB. Immunogenicity of live attenuated SA14-14-2 Japanese encephalitis vaccine – a comparison of 1- and 3-month immunization schedules. J Infect Dis 1998; 177:221-3; PMID:9419193; http://dx.doi.org/ 10.1086/517358 [DOI] [PubMed] [Google Scholar]
- 45. Guo W, Li L, Wu Z. [Analysis on the immuno-effects of two different Japanese encephalitis virus vaccines]. Zhonghua Liu Xing Bing Xue Xa Zhi 1998; 19:97-9; PMID:10322719 [PubMed] [Google Scholar]
- 46. Zhou B, Jia L, Xu X. [A large-scale study on the safety and epidemiological efficacy of Japanese encephalitis (JE) live vaccine (SA14-14-2) in the JE endemic areas]. Zhonghua Liu Xing Bing Xue Za Zhi 1999; 20:38-41; PMID:10682513 [PubMed] [Google Scholar]
- 47. Sohn YM, Park MS, Rho HO, Chandler LJ, Shope RE, Tsai TF. Primary and booster immune responses to SA14-14-2 Japanese encephalitis vaccine in Korean infants. Vaccine 1999; 17:2259-64; PMID:10403593; http://dx.doi.org/ 10.1016/S0264-410X(99)00006-7 [DOI] [PubMed] [Google Scholar]
- 48. Sohn YM, Tandan JB, Yoksan S, Ji M, Ohrr H. A 5-year follow-up of antibody response in children vaccinated with single dose of live attenuated SA14-14-2 Japanese encephalitis vaccine: immunogenicity and anamnestic responses. Vaccine 2008; 26:1638-43; PMID:18294743; http://dx.doi.org/ 10.1016/j.vaccine.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 49. Choi UY, Lee SY, Kim KH, Kim DS, Choi KM, Cha SH, Kang JH. Is a booster dose necessary in children after immunization with live attenuated Japanese encephalitis vaccine? J Trop Pediatr 2013; 59:423-5; PMID:23764540; http://dx.doi.org/ 10.1093/tropej/fmt043 [DOI] [PubMed] [Google Scholar]
- 50. Gatchalian S, Yao Y, Zhou B, Zhang L, Yoksan S, Kelly K, Neuzil KM, Yaïch M, Jacobson J. Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants. Vaccine 2008; 26:2234-41; PMID:18394765; http://dx.doi.org/ 10.1016/j.vaccine.2008.02.042 [DOI] [PubMed] [Google Scholar]
- 51. Tandan JB, Ohrr H, Sohn YM, Yoksan S, Ji M, Nam CM, Halstead SB. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: a case-control study in Nepalese children 5 years after immunization. Vaccine 2007; 25:5041-5; PMID:17521781; http://dx.doi.org/ 10.1016/j.vaccine.2007.04.052 [DOI] [PubMed] [Google Scholar]
- 52. Ohrr H, Tandan JB, Sohn YM, Shin SH, Pradhan DP, Halstead SB. Effect of single dose of SA 14-14-2 vaccine 1 year after immunisation in Nepalese children with Japanese encephalitis: a case-control study. Lancet 2005; 366:1375-8; PMID:16226615; http://dx.doi.org/ 10.1016/S0140-6736(05)67567-8 [DOI] [PubMed] [Google Scholar]
- 53. Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, Ohrr HC, Tang JL, Halstead SB. Efficacy of single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet 2001; 358:791-5; PMID:11564484; http://dx.doi.org/ 10.1016/S0140-6736(01)05967-0 [DOI] [PubMed] [Google Scholar]
- 54. Kuzuhara S, Nakamura H, Hayashida K, Obata J, Abe M, Sonoda K, Nishiyama K, Sugawara K, Takeda K, Honda T, et al. . Non-clinical and phase I clinical trials of a Vero cell-derived inactivated Japanese encephalitis vaccine. Vaccine 2003; 21:4519-26; PMID:14575762; http://dx.doi.org/ 10.1016/S0264-410X(03)00506-1 [DOI] [PubMed] [Google Scholar]
- 55. Chanthavanich P, Limkittikul K, Sirivichayakul C, Chokejindachai W, Hattasingh W, Surangsrirat S, Srisuwanporn T, Kaewma B, Pengsaa K, Jun G. Immunogenicity and safety of inactivated chromatographically purified vero cell-derived japanese encephalitis vaccine in children. American Journal of Tropical Medicine and Hygiene 2012; 87:335-6. [Google Scholar]
- 56. Okada K, Iwasa T, Namazue J, Akechi M, Ueda S. Safety and immunogenicity of a freeze-dried, cell culture-derived Japanese encephalitis vaccine (Inactivated) (JEBIK(registered trademark)V) in children. Vaccine 2012; 30:5967-72; PMID:22841478; http://dx.doi.org/ 10.1016/j.vaccine.2012.07.034 [DOI] [PubMed] [Google Scholar]
- 57. Kikukawa A, Gomi Y, Akechi M, Onishi T, Manabe S, Namazue J, Fuke I, Ishikawa T, Okuno Y, Ueda S. Superior immunogenicity of a freeze-dried, cell culture-derived Japanese encephalitis vaccine (inactivated). Vaccine 2012; 30:2329-35; PMID:22306856; http://dx.doi.org/ 10.1016/j.vaccine.2012.01.054 [DOI] [PubMed] [Google Scholar]
- 58. Miyazaki C, Okada K, Ozaki T, Hirose M, Iribe K, Yokote H, Ishikawa Y, Togashi T, Ueda K. Phase III clinical trials comparing the immunogenicity and safety of the vero cell-derived Japanese encephalitis vaccine Encevac with those of mouse brain-derived vaccine by using the Beijing-1 strain. Clin Vaccine Immunol 2014; 21:188-95; PMID:24334689; http://dx.doi.org/ 10.1128/CVI.00377-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takeshita N, Lim CK, Mizuno Y, Shimbo T, Kotaki A, Ujiie M, Hayakawa K, Kato Y, Kanagawa S, Kaku M, et al. . Immunogenicity of single-dose Vero cell-derived Japanese encephalitis vaccine in Japanese adults. J Infect Chemother 2014; 20(4):238-42; PMID:24485326 [DOI] [PubMed] [Google Scholar]
- 60. Rojanasuphot S, Shaffer N, Chotpitayasunondh T, Phumiamorn S, Mock P, Chearskul S, Waranawat N, Yuentrakul P, Mastro TD, et al. . Response to JE vaccine among HIV-infected children, Bangkok, Thailand. Southeast Asian J Trop Med Public Health 1998; 29:443-50; PMID:10437937 [PubMed] [Google Scholar]
- 61. Puthanakit T, Aurpibul L, Yoksan S, Sirisanthana T, Sirisanthana V. Japanese encephalitis vaccination in HIV-infected children with immune recovery after highly active antiretroviral therapy. Vaccine 2007; 25:8257-61; PMID:17964009; http://dx.doi.org/ 10.1016/j.vaccine.2007.09.052 [DOI] [PubMed] [Google Scholar]
- 62. Puthanakit T, Aurpibul L, Yoksan S, Sirisanthana T, Sirisanthana V. A 3-year follow-up of antibody response in HIV-infected children with immune recovery vaccinated with inactivated Japanese encephalitis vaccine. Vaccine 2010; 28:5900-2; PMID:20600498; http://dx.doi.org/ 10.1016/j.vaccine.2010.06.048 [DOI] [PubMed] [Google Scholar]
- 63. Muangchana C, Henprasertthae N, Nurach K, Theppang K, Yoocharoen P, Varinsathien P, Techathawat S, Sanohsieng S, Anantapreecha S. Effectiveness of mouse brain-derived inactivated Japanese encephalitis vaccine in Thai National Immunization Program: a case-control study. Vaccine 2012; 30:361-7; PMID:22075090; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.083 [DOI] [PubMed] [Google Scholar]
- 64. Marks F, Nguyen TT, Tran ND, Nguyen MH, Vu HH, Meyer CG, You YA, Konings F, Liu W, Wierzba TF, et al. . Effectiveness of the viet nam produced, mouse brain-derived, inactivated Japanese encephalitis vaccine in northern viet nam. PLoS Negl Trop Dis 2012; 6:e1952; PMID:23272262; http://dx.doi.org/ 10.1371/journal.pntd.0001952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li W, Jia LL, Zou Y. [Study on safety and effect of purified inactivited Japanese encephalitis vaccine (primary hamster kidney cell, PHK)]. Zhongguo Yi Miao He Mian Yi 2009; 15:333-6; PMID:20077733 [PubMed] [Google Scholar]
- 66. Schuller E, Klingler A, Dubischar-Kastner K, Dewasthaly S, Muller Z. Safety profile of the Vero cell-derived Japanese encephalitis virus (JEV) vaccine IXIARO((R)). Vaccine 2011; 29:8669-76; PMID:21907747; http://dx.doi.org/ 10.1016/j.vaccine.2011.08.117 [DOI] [PubMed] [Google Scholar]
- 67. Jia N, Zhao QM, Guo XF, Cheng JX, Wu C, Zuo SQ, Dai PF, Zhao JY, Zhang JS. Encephalitis temporally associated with live attenuated Japanese encephalitis vaccine: four case reports. BMC Infect Dis 2011; 11:344; PMID:22168358; http://dx.doi.org/ 10.1186/1471-2334-11-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu Y, Lin H, Zhu Q, Wu C, Zhao Z, Zheng H. Safety of Japanese encephalitis live attenuated vaccination in post-marketing surveillance in Guangdong, China, 2005-2012. Vaccine 2014; 32:1768-73; PMID:24503272; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.107 [DOI] [PubMed] [Google Scholar]
- 69. Trent DW, Minor P, Jivapaisarnpong T, Shin J. WHO working group on the quality, safety and efficacy of japanese encephalitis vaccines (live attenuated) for human use, Bangkok, Thailand, 21-23 February 2012. Biologicals 2013; 41:450-7; PMID:23891495; http://dx.doi.org/ 10.1016/j.biologicals.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 70. Jacobson RM. Promises and pitfalls of meta-analysis in vaccine research. Vaccine 1999; 17:1628-34; PMID:10194815; http://dx.doi.org/ 10.1016/S0264-410X(98)00425-3 [DOI] [PubMed] [Google Scholar]