Summary
Cancer immunotherapy has come a long way. The hope that immunological approaches may help cancer patients has sparked many initiatives in research and development (R&D). For many years, progress was modest and disappointments were frequent. Today, the increasing scientific and medical knowledge has established a solid basis for improvements. Considerable clinical success was first achieved for patients with hematological cancers. More recently, immunotherapy has entered center stage in the development of novel therapies against solid cancers. Together with R&D in angiogenesis, the field of immunology has fundamentally extended the scientific scope, which has evolved from a cancer-cell-centered view to a comprehensive and integrated vision of tumor biology. Current R&D is focused on a large array of possible disease mechanisms, driven by cancer cells, and amplified by tumor stroma, inflammatory and immunological actors, blood and lymph vessels, and the “macroenvironment," i.e. systemic mechanisms of the host, particularly of the haematopoietic system. Contrasting to this large spectrum of pathophysiological events promoting tumor growth, only a small number of biological mechanisms, namely of the immune system, have the potential to counteract tumor growth. They are of prime interest because therapeutic enhancement may result in clinical benefit for patients. This special issue is dedicated to immunotherapeutics against cancer, with particular emphasis on vaccination and combination therapies, providing updates and extended insight in this booming field.
Central Roles of CD8 T Cells in Cancer
Clinical and histopathological assessments of tumors has long focused on tumor mass and tumor cells. The “TNM” system still plays a dominant role for the staging of patients, whereby tumors (T), metastatic lymph nodes (N) and metastases (M) are quantified and used to categorize patients for diagnostic and treatment decisions. Slowly but surely, additional criteria are being introduced. Particularly for the choice of treatments, driver mutations of tumors are determined, allowing the prediction of therapeutic usefulness of targeted therapies such as tyrosine kinase inhibitors capable of blocking oncogenic and angiogenic pathways. The diagnostic procedures for identifying oncogenic mutations is more advanced as compared to diagnostic assessments of the tumor microenvironment (TME), likely because the latter is heterogeneous and involves a large number of biological components. Research of the TME has revealed many new insights, as outlined in this special issue, but many are not yet exploited for diagnosis or treatment of cancer patients. Nevertheless, a leading role is played by tumor infiltrating lymphocytes (TILs), particularly the CD8 T cells in tumor cell nests. They are a major hallmark. The prognosis of the vast majority of cancer patients correlates with the pattern of TIL inflammation.1 Numerous research results revealed that TILs are beneficial not only for patients treated with immunotherapy but also with various other treatment modalities. TILs can directly destroy tumor cells, despite that they show features of functional impairment, referred to as T cell “exhaustion”.2 Furthermore, high numbers of TILs likely indicate a favorably high ratio of anti- versus pro-tumoral biological parameters constituting the TME and its dynamics. Thus, TILs are likely of broader diagnostic and prognostic value, justifying the current multicenter study efforts aiming at the establishment of diagnostic procedures based on TIL quantification by immunohistochemistry. Hopefully, biological features of the TME and the macroenvironment will increasingly help to identify disease mechanisms in individual patients, and allow better predictions of therapeutic outcomes.
The path to immune diagnosis and treatment of cancer patients is scattered with various pitfalls for which there are rational solutions.3 Innovative approaches have brought much progress and will likely continue to do so. In parallel, efforts must be coordinated, for more rapid insights and clarification which approaches are most useful. The large heterogeneity of patients and their diseases represent a remarkable challenge, requiring systematic clinical and laboratory approaches. Clinical trial designs should correspond to real-life situations, and data should be publicly accessible to optimize information exchange. Similarly, laboratory methods should be standardized and transparent. The program of “Minimum Information for Biological and Biomedical Investigations (MIBBI) 4 contains instructions for immunotherapy studies, for example, minimum information about a flow cytometry experiment,5 minimum information about a cellular assay (MIACA), and minimum information about a T cell assay.6 The Society for Immunotherapy of Cancer (SITC), the Food and Drug Administration (FDA) and the National Cancer Institute (NCI) provide recommendations.7,8 They deal with central laboratory immune monitoring of multi-institutional trials and standardized large scale banking of clinical specimens allowing future analysis of serum, viable cells, and DNA/RNA.
Immunotherapy
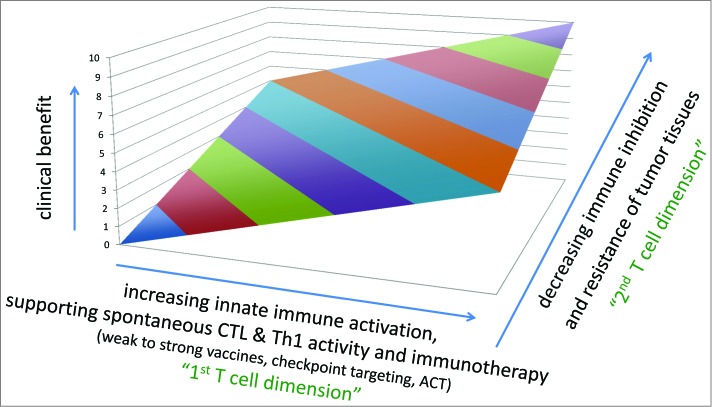
Since several years, antibodies are being used to treat patients with solid tumors, targeting e.g. growth or angiogenic factors. In parallel, various approaches were developed to target T cells, with the aim to increase numbers and activation of anti-cancer T cells (Fig. 1). Remarkable clinical responses are achieved with adoptive cell therapy (ACT), which is however only feasible in small fractions of selected cancer patients. More recently, more wide application of immunotherapy has become reality for patients with solid tumors. This has become possible thanks to the so-called “checkpoint blockade," which is achieved by targeting inhibitory receptors of lymphocytes.9 The first in class drug is Ipilimumab, an antibody specific for CTLA-4. Targeting this receptor results in strongly enhanced T cell activation. This therapy is now widely used for the treatment of melanoma patients,10 and further anti-CTLA-4 targeting drugs are in development. A second inhibitory receptor is PD-1 which interacts with PD-L1 and PD-L2. Therapeutic targeting of PD-1 or PD-L1 is highly promising, not only for melanoma but also for patients with several types of carcinomas such as Non Small Cell Lung Cancer (NSCLC) and kidney cancer,11,12 as reviewed in the paper by A. Daud. Further approaches are currently in clinical development, for example by targeting Lag-3, Tim-3 or KIRs (Killer-cell Immunoglobulin-like Receptors), and many more strategies are the subject of intense research. The broad applicability of immunotherapy for so many patients has elicited great enthusiasm.
Figure 1.
Clinical benefit depends on CD8 T cells and tumor resistance. The success of immunotherapy and other treatments correlates with increasing activation of cytotoxic T cells (CTLs), and decreasing escape and/or resistance mechanisms of tumor tissues. Immunotherapy can be “active” e.g., vaccination or “checkpoint targeting," or “passive” e.g. ACT (adoptive cell therapy by transfusion of autologous CD8 and CD4 T cells, with or without genetic modifications). Abbreviations: CTL, cytotoxic T lymphocytes.
Vaccine Development
A promising field in cancer immunotherapy is vaccine development. A vaccine usually induces a broad immune response by stimulating simultaneously the innate and adaptive immune system. Successful cancer vaccines are already in use against hepatocellular carcinoma (hepatitis B vaccine), cervical cancer (HPV vaccine) and prostate cancer (Sipuleucel T). The review by J. Rosenblatt and colleagues focuses on clinical data assessing the role of dendric cell vaccines in hematological malignancies. G. Peoples et al. investigate the immunogenicity of novel dentritoma which are fused DC-tumor cell hybrids in patients. Another review discusses preclinical and clinical data generated by CureVac's mRNA vaccine against prostate cancer (J. Bedke et al.). Wu et al. discuss in their review how the DNA vaccine vector platform can be improved in order to overcome self-tolerance which so far limits the use of versatile, safe and cheap plasmid DNA platform in cancer. The article by M. Sioud addresses how RNA interference can positively impact the vaccination with DCs or adoptive T cell transfer. A review by G. Parmiani highlights the recent development in combining peptide-based vaccination with checkpoint blockade inhibitors. The immunogenicity of IMA901 (tumor associated peptides) in renal cell cancer patients is reviewed by C. Reinhardt and colleagues. Two papers evaluate either Listeria or modified vaccinia virus Ankara expressing 5T4 or oncoprotein E7 as cancer antigens (Ch. Chu; J. Rowe). Finally, K. Manoutcharian and S.-C. Cheong describe in their original articles the discovery of new cancer epitopes in a breast cancer model in mice or for oral squamous cell carcinoma in man, respectively, inducing promising T cell responses.
Tumor Biology
A large part of research is focused on aspects of tumor biology. The review by R. Consolini provides insights in defects of dendritic cells (DCs) in colorectal cancer, emphasizing that many cancers hamper the important role of DCs in the activation of T cells. Among the various immune suppressive actors, myeloid cells likely play a prominent role (see the reviews by S.-J. Liu and C. Mesa). Tumor biology studies will continue to be important for the understanding of malignancy mechanisms, and for the detection of novel therapy targets such as antigens (as reviewed by F. Salazar Onfray) and innate immune activation (R. Wang). The negative effects on T cells are most prominent in the TME, but they may also be substantial at the systemic level,13 making the patients more vulnerable to tumor progression, and less likely to respond to therapy. Currently, only little is known about the underlying mechanisms. One possible mediator is osteopontin which is produced by tumor cells and can trigger the recruitment of bone marrow cells that activate stromal fibroblasts.14,15
Clinical Trials and Combination Treatments
Given the rich knowledge and the multiplicity of disease driving mechanisms, it is not surprising that the number of proposed treatment modalities is rapidly increasing. This special issue provides several examples of novel treatment approaches. Furthermore, treatment combinations are attracting much attention. As in infectious diseases, cancers often require multi-targeting in order to reduce the likelihood of escape variant outgrow. The website www.clinicaltrials.com is a worldwide reference and information source. Regulatory bodies require that all trials are registered in this database, assuring comprehensiveness. The website provides all essential information to screen for treatment opportunities. It allows patients, physicians, researchers and many more to keep up-to-date with ongoing activities and trends. For example, the site reveals the high emphasis on combination treatments, taking advantage of specific mechanisms of chemotherapy, radiation, small molecules and immunotherapy. The rational development is based on optimal preclinical research models, and carefully designed multidisciplinary clinical trials with clinical and biological endpoints that may rapidly provide the necessary data to conclude on the potential usefulness of the chosen combinations.
Regulatory and Economic Aspects
As other fields of pharmaceutical development, immunotherapy is facing increasing regulatory hurdles. More complicated and time-consuming procedures are necessary to fulfill the requirements established for safety and quality control. This is particularly challenging for the development of combination therapies because they imply multiple drugs. For vaccines, the number of pharmaceutical components is even higher, as vaccines are composed of multiple components (antigens, adjuvants, delivery vehicles). The components must be tested in various combinations and doses. This is only possible if regulators permit that for phase I/II studies, vaccine formulations can be prepared on site (in accredited institutions) and directly administered to patients, without the requirement of certification of the vaccine formulations as pharmaceutical product. The article by B. Heelan provides guidelines and recommends a 2-way dialog with regulators, on a case-by-case basis, to streamline clinical development plans. Nevertheless, each of the vaccine components must be quality checked and certified, based on GCP principles. Dynamic development in phase I/II studies will provide essential information to best identify the final design of optimal vaccine formulations. Subsequently, they can be tested in larger phase II/III trials.
Finally, the economical impact of novel therapies is often not sufficiently studied, as reported by Y.-C. T. Shih. Appropriate studies will identify those therapies that are most cost effective. The trend that novel treatments are often very expensive does not only apply to the field of immunotherapy, as it can be observed elsewhere in health care. Higher costs seem justified when the treatments are highly effective.
Driving Implementation Sciences in Oncology
In the 1980s, the HIV pandemic elicited widespread R&D efforts, strongly promoting insights and rapid progress in the biology and the clinical aspects of retrovirally induced diseases.16. Today, one of the largest fields of intense R&D is cancer: Enormous efforts are taken by academia, industry and administrations to tackle the numerous problems of malignant diseases. At an impressive pace, the knowledge and understanding on biological and clinical processes is increasing. Despite that many biological mechanisms are still poorly understood, diagnostic and therapeutic procedures are increasingly based on scientific rationales. Targeted approaches for patients will accelerate the identification of efficient therapies that are still missing for many patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fridman W-H, Pagès F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 2.Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, et al. . Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest 2011; 121:2350-60; PMID:21555851; http://dx.doi.org/ 10.1172/JCI46102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert-Tissot C, Nguyen LT, Ohashi PS, Speiser DE. Mobilizing and evaluating anticancer T cells: pitfalls and solutions. Expert Rev Vaccines 2013; 12:1325-40; PMID:24127850; http://dx.doi.org/ 10.1586/14760584.2013.843456 [DOI] [PubMed] [Google Scholar]
- 4.Taylor CF, Field D, Sansone S-A, Aerts J, Apweiler R, Ashburner M, Ball CA, Binz P-A, Bogue M, Booth T, et al. . Promoting coherent minimum reporting guidelines for biological and biomedical investigations: the MIBBI project. Nat Biotechnol 2008; 26:889-96; PMID:18688244; http://dx.doi.org/ 10.1038/nbt.1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JA, Spidlen J, Boyce K, Cai J, Crosbie N, Dalphin M, Furlong J, Gasparetto M, Goldberg M, Goralczyk EM, et al. . MIFlowCyt: The minimum information about a flow cytometry experiment. Cytometry 2008; 73A:926-30; PMID:18752282; http://dx.doi.org/ 10.1002/cyto.a.20623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJM, et al. . T cell assays and MIATA: the essential minimum for maximum impact. Immunity 2012; 37:1-2; PMID:22840835; http://dx.doi.org/ 10.1016/j.immuni.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 7.Butterfield LH, Palucka AK, Britten CM, Dhodapkar MV, Håkansson L, Janetzki S, Kawakami Y, Kleen TO, Lee PP, Maccalli C, et al. . Recommendations from the iSBTc-SITC/FDA/NCI workshop on immunotherapy biomarkers. Clin Cancer Res 2011; 17:3064-76; PMID:21558394; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McShane LM, Altman DG, Sauerbrei W. Identification of clinically useful cancer prognostic factors: what are we missing? JNCI J Natl Cancer Inst 2005; 97:1023-5; PMID:16030294; http://dx.doi.org/ 10.1093/jnci/dji193 [DOI] [PubMed] [Google Scholar]
- 9.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006; 90:297-339; PMID:16730267; http://dx.doi.org/ 10.1016/S0065-2776(06)90008-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley JL. Combination checkpoint blockade–taking melanoma immunotherapy to the next level. N Engl J Med 2013; 369:187-9; PMID:23724866; http://dx.doi.org/ 10.1056/NEJMe1305484 [DOI] [PubMed] [Google Scholar]
- 12.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 2014; 11:24-37; PMID:24247168; http://dx.doi.org/ 10.1038/nrclinonc.2013.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgaertner P, Jandus C, Rivals J-P, Derré L, Lövgren T, Baitsch L, Guillaume P, Luescher IF, Berthod G, Matter M, et al. . Vaccination-induced functional competence of circulating human tumor-specific CD8 T-cells. Int J Cancer 2012; 130:2607-17; PMID:21796616; http://dx.doi.org/ 10.1002/ijc.26297 [DOI] [PubMed] [Google Scholar]
- 14.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, et al. . Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 2008; 133:994-1005; PMID:18555776; http://dx.doi.org/ 10.1016/j.cell.2008.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray M-A, Carpenter AE, Jirström K, Magnusson K, Ebert BL, et al. . Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest 2011; 121:784-99; PMID:21266779; http://dx.doi.org/ 10.1172/JCI43757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Sadr WM, Philip NM, Justman J. Letting HIV transform academia - embracing implementation science. N Engl J Med 2014; 370:1679-81; PMID:24785205; http://dx.doi.org/ 10.1056/NEJMp1314777 [DOI] [PubMed] [Google Scholar]