Abstract
Soft tissue sarcomas (STS) are rare, heterogeneous tumors of mesenchymal origin. Despite optimal treatment, a large proportion of patients will develop recurrent and metastatic disease. For these patients, current treatment options are quite limited. Significant progress has been made recently in the use of immunotherapy for the treatment of other solid tumors (e.g. prostate cancer, melanoma). There is a strong rationale for immunotherapy in STS, based on an understanding of disease biology. For example, STS frequently have chromosomal translocations which result in unique fusion proteins and specific subtypes have been shown to express cancer testis antigens. In this review, we discuss the current status of immunotherapy in STS, including data from human studies with cancer vaccines, adoptive cell therapy, and immune checkpoint blockade. Further research into STS immunology is needed to help design logical, subtype-specific immunotherapeutic strategies.
Keywords: adoptive cell therapy, cancer vaccines, immunotherapy, immune checkpoint blockade, soft tissue sarcoma
Introduction
Soft tissue sarcomas (STS) are tumors of mesenchymal as opposed to epithelial origin that account for approximately 1% of all solid tumors in adults.1 STS may occur anywhere in the body, although the most common anatomic sites are the extremities (60–70%) and the abdomen and retroperitoneum (20%). Most patients present with localized disease for which surgical resection is the mainstay of treatment. Radiation therapy and chemotherapy are adjuncts to surgery that are given either pre- or postoperatively, particularly in patients with locally-advanced and high risk disease. Despite optimal treatment, a large proportion of STS patients will still develop either locoregional recurrence or distant metastasis (typically to the lungs) or both.
For patients with recurrence and those who initially present with metastatic disease, cytotoxic chemotherapy is the primary treatment modality but effective options are quite limited. With current cytotoxic chemotherapy, tumor responses are seen in only a minority of patients and estimated median overall survival is approximately 8–12 months from the start of first-line therapy.2-4 In most STS patients, first-line therapy consists of doxorubicin alone or a doxorubicin-containing combination, frequently with ifosfamide. Second-line therapy is most commonly gemcitabine-docetaxel. Recently, pazopanib, a multi-tyrosine kinase receptor inhibitor, received FDA approval for use in STS on the basis of improved progression-free survival by 3 months compared to placebo.5 Despite some of these advances, overall mortality in STS patients with advanced disease is high.
Immunotherapy, which involves active utilization of the patient's immune system, has tremendous potential in the treatment of solid tumors. In fact, for specific tumor types (e.g., prostate cancer, melanoma), significant progress and notable successes have been achieved recently with the use of immunotherapy for the treatment of advanced disease. Durable radiologic tumor responses and improvement in clinical outcome, including prolonged survival, have been observed even in patients with heavy tumor burden and metastatic disease.
This review will provide a brief overview of tumor immunology and immunotherapy and then discuss the current status of immunotherapy in STS. We will emphasize data in soft tissue as opposed to bone sarcomas (osteosarcoma, Ewing's, chondrosarcoma). We will focus on studies done in patients and specifically discuss cancer vaccines, adoptive cell therapy and immune checkpoint blockade.
Overview of tumor immunology and immunotherapy
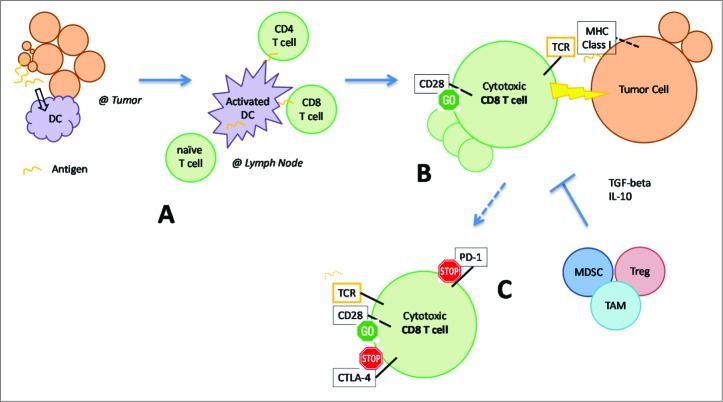
In several solid tumor types, there are data to suggest that an antigen-specific adaptive immune response to cancer naturally exists in patients.6-8 A simplified schematic is provided [Figure 1]. In the tumor microenvironment, antigen presenting cells acquire and process peptides released from cell turnover during tumor growth. The most potent antigen presenting cells are dendritic cells (DCs). DCs then migrate to the draining lymph nodes where they sensitize and activate T cells against these peptide antigens. T cells, in particular antigen-specific, cytotoxic CD8 T cells, are then capable of eliminating antigen-expressing tumor cells found in the peripheral tissues. This process is restricted by the display of the antigen on the tumor cell surface in the context of an appropriate, matched major histocompatibility complex (MHC; e.g. human leukocyte antigen or HLA) molecule.
Figure 1.
Adaptive immune response to cancer and mechanisms which inhibit the anti-tumor immune response. See manuscript text for details. DC, dendritic cell; TCR, T cell receptor; MHC, major histocompatibility complex; MDSC, myeloid derived suppressor cell; Treg, regulatory T cell; TAM, tumor associated macrophage. Immunotherapeutic strategies enhance various components of the immune response: (A) cancer vaccines manipulate DCs to enhance antigen presentation, (B) adoptive cell therapy utilizes expanded populations of cytotoxic T cells, (C) immune checkpoint blockade “removes the brakes” (CTLA4, PD-1) on T cells to maintain their activation status and cytotoxic function.
Multiple inhibitory mechanisms, however, may be responsible for the inability of the immune system to eliminate tumor cells [Figure 1, bottom and right].6-8 Inappropriate antigen presentation or lack of appropriate activation signals from DCs can result in the opposite effect, tolerance of T cells to the target antigen. Immunosuppressive cytokines (TGF-beta, IL-10) and alternate immune cell types (myeloid derived suppressor cells, regulatory T cells, tumor-associated macrophages) present at the tumor microenvironment may directly inhibit the anti-tumor immune response. Tumor cells may lose expression of the target antigen and thus avoid detection. Activated T cells also express inhibitory signals known as immune checkpoint molecules, which arise naturally and dampen the immune response, to the advantage of tumor cells. Among the most extensively studied immune checkpoint molecules are CTLA4 and PD-1.
Nonetheless, the immune system can potentially be exploited to yield a very valuable treatment option for cancer. Along with the ability to eliminate “foreign” cells with target specificity, the adaptive immune system has long term memory and can respond again when re-exposed to cells expressing antigen. In addition, there is evidence to suggest that the immune system can adapt to shifts in antigen expression over time (e.g., immunoediting). Immunotherapy can therefore potentially offer durable simultaneous locoregional and distant control, impacting both macroscopic and importantly, microscopic disease.9 These characteristics make immunotherapy ideal for solid tumors and potentially STS, which are often very challenging to manage (e.g., frequent recurrence) with limited treatment options. Interestingly, one of the earliest examples of cancer immunotherapy from the late 19th century (Coley's toxin) was in fact, motivated by a sarcoma patient with tumor regression after clearance of a bacterial infection.10
STS subtype heterogeneity
Although rare, STS are complex tumors which we are only beginning to understand. STS is not a single type of tumor but rather encompasses a variety of tumors which arise from different tissues (e.g., fat, muscle, vessels) within the body. For several STS tumors, the tissue of origin is unclear or unknown. In the past decade, there has been increased recognition of the tremendous heterogeneity of STS, with now over 50 distinct subtypes recognized by the World Health Organization. Importantly, each subtype has unique disease biology, clinical behavior and response to therapy.
As an example of STS heterogeneity, within liposarcoma (tumors of adipocyte origin), 3 subtypes are recognized: 1) well- and dedifferentiated, 2) myxoid and 3) pleomorphic.11 Well-differentiated liposarcomas are low grade tumors that arguably never metastasize unless there is evidence of dedifferentiation, but have a high rate of locoregional recurrence and are resistant to chemotherapy and radiation therapy. In contrast, myxoid liposarcomas can metastasize to the lung, bones, as well as interestingly, other fat-bearing areas of the body;12,13 however these tumors are generally very sensitive to chemotherapy and radiation therapy. Pleomorphic liposarcomas are almost uniformly high grade with aggressive clinical behavior and virtually no response to all forms of therapy.
In support of STS heterogeneity, characteristic genetic and cytogenetic changes have been identified for several subtypes4,14 and indeed many experts in the field have started to advocate for further “molecular subclassification” in this disease.15 Specific chromosomal translocations frequently exist, which result in characteristic fusion proteins [Table 1]. These translocations can be especially helpful for diagnostic purposes. With our liposarcoma example, well-differentiated and dedifferentiated liposarcomas have high amplification of chromosome region 12q13–15, but no actual translocation. Myxoid liposarcomas have a translocation between chromosome 12 and 16, resulting in the fusion protein, FUS-DD1T3. Without genetic and cytogenetic characterization, there can be significant overlap in the histologic appearance between the liposarcoma subtypes which can frequently lead to misdiagnosis.16
Table 1.
Known chromosomal translocations and fusion proteins in soft tissue sarcoma
| Histologic subtype | Translocation | Fusion Protein |
|---|---|---|
| Alveolar soft parts sarcoma | t(X;17)(p11;q25) | ASPL-TFE3 |
| Angiomatoid fibrous histiocytoma | t(12;16)(q13;p11) | FUS-ATF1 |
| Clear cell sarcoma | t(12;22)(q13;q12) | EWSR1-ATF1 |
| t(2;22)(q33;q12) | EWSR1-CREB1 | |
| Dermatofibrosarcoma protuberans | t(17;22)(q21;q13) | COL1A1-PDGFB |
| Desmoplastic small round cell tumor | t(11;22)(p13;q12) | WT1-EWSR1 |
| Endometrial stromal sarcoma | t(7;17)(p15;q21) | JAZF1-SUZ12 |
| Epithelioid hemangioendothelioma | t(1;3))(p36.3;q25) | WWTR1-CAMTA1 |
| Fibromyxoid sarcoma (low grade) | t(7;16)(q33;p11) | FUS-CREB3L2 |
| t((11;16)(p11;p11) | FUS-CREB3L1 | |
| Inflammatory myofibroblastic tumor | t(2;19)(p23;p13) | TPM4-ALK |
| t(1;2)(q22;p23) | TPM3-ALK | |
| Myxoid liposarcoma | t(12;16)(q13;p11) | FUS-DD1T3 |
| t(12;22)(q13;q12) | EWSR1-DD1T3 | |
| Rhabdomyosarcoma (alveolar) | t(2; 13)(q34;q14) | PAX3-FOXO1 |
| t(1;13)(p36;q14) | PAX7-FOXO1 | |
| t(X;2)(q13;q35) | PAX3-AFX | |
| Synovial sarcoma | t(X;18)(p11;q11) | SYT-SSX |
Rationale for immunotherapy in STS
From an immunotherapy standpoint, the fusion proteins which result from chromosomal translocations (not found on normal cells) represent attractive potential targets in STS. Although there may be tolerance toward epitopes within the separate proteins, the area of fusion represents a foreign antigen that can be targeted either by the natural immune response or actively induced through immunotherapy. Worley et al. explored the immunogenicity of these antigens for several STS subtypes including synovial sarcoma, clear cell sarcoma and desmoplastic round cell tumors.17 Peptides corresponding to the characteristic fusion proteins were designed and assessed for the ability to bind to various HLA class I molecules. After appropriate HLA matching, the authors were able to use normal peripheral blood lymphocytes and generate in vitro, antigen-specific T cells with cytotoxic function against tumor cells that expressed the full length fusion protein.
Several STS subtypes also naturally have high expression of cancer testis antigens, which are ideal candidates for targeting by immunotherapy, since in adults these peptides are found only in germ cells (e.g., in testis tissue) and not in somatic tissue cells. NY-ESO-1 is the most notable of these cancer testis antigens that have been been described in STS. In synovial sarcoma, for both monophasic and biphasic variants, Jungbluth et al. reported that by immunohistochemistry, 20 out of 25 cases (80%) expressed NY-ESO-1.18 Recently, in myxoid liposarcoma, virtually uniform (>90%) expression of NY-ESO-1 was reported independently by Pollack et al. and Hemminger et al.19,20
Mutations in proteins expressed by tumor cells are also potential targets for immunotherapy. Barretina et al. reported results of a collaborative effort in which detailed genomic analyses were performed on 207 STS tumors encompassing 7 major subtypes.21 Mutations were identified by mass spectrometry-based genotyping of tumor tissue in comparison to matched normal DNA. Mutated genes found with the highest frequency were PIK3CA in 18% of myxoid liposarcomas, TP53 in 17% of pleomorphic liposarcomas and NF1 in 11% of myxofibrosarcomas and 8% of pleomorphic liposarcomas. It is important to note that within each of these genes, several types of mutations were observed. Overall, although genetic mutations exist in STS, they are certainly not nearly as frequent as those seen in traditionally “immunogenic” solid tumors such as melanoma.22 This observation is an important consideration that could limit the efficacy of immunotherapy in STS. The frequency of mutations in STS however, also varies tremendously by subtype, including some (e.g., angiosarcoma, leiomyosarcoma, radiation-induced sarcoma) with complex genetic abnormalities.4
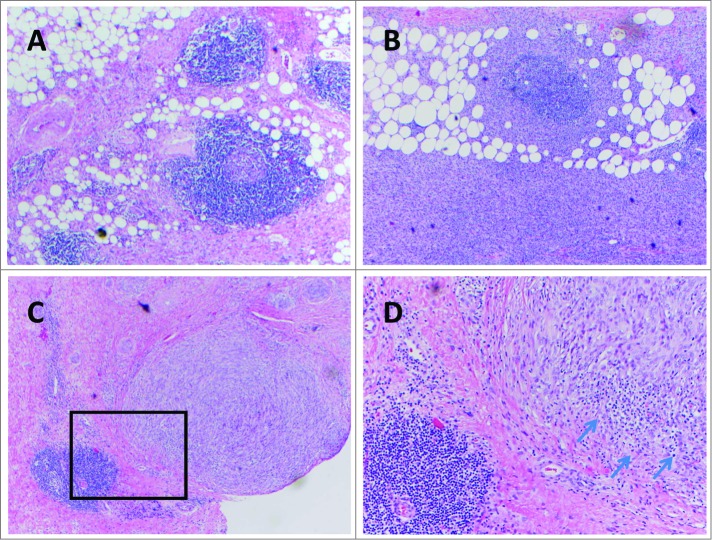
Whether or not a defined target is known (e.g., chromosomal fusion protein, cancer testis antigen, or mutation), multiple studies have suggested that there is indeed a naturally-occurring immune infiltrate in STS.23 Soilleux et al. studied myxofibrosarcomas and found intratumoral myeloid cells with dendritic morphology that had expression of DC-SIGN, a marker for immature DCs.24 A wide range of DC-SIGN positive cells were observed from tumor to tumor (3–61%, median 22%), with no correlation between the percentage of DC-SIGN positive cells and tumor grade. Orui et al. provided detailed characterization of the immune infiltrates in a variety of STS subtypes. Cytotoxic CD8 T cells were detected in all cases and half had DCs.25 The T cells expressed granzyme B, indicative of their cytotoxic function. The DCs were found to be CD1a negative but CD83 positive, indicative of a mature phenotype. Fujii et al. characterized the T cells found in cutaneous angiosarcoma.26 Both CD4 and CD8 T cells were identified in all tumors, including FoxP3 positive cells which may represent regulatory T cells. Higher frequencies of CD8 T cells were associated with improved overall and distant metastasis-free survival. In well-differentiated liposarcoma, Tseng et al. reported evidence to suggest an adaptive immune response with presence of intratumoral lymphoid structures, reminiscent of immature lymph nodes.27 Examples in both well- and dedifferentiated liposarcoma are shown [Figure 2].These lymphoid structures have been characterized in other solid tumors.8 In liposarcoma, CD8 T cells appear to be scattered throughout the tumor, whereas CD4 T cells and CD20 B cells were localized to these lymphoid structures. Recently, DC-LAMP positive, mature DCs have been identified juxtaposed next to CD4 T cells, suggestive of antigen presentation (unpublished data).
Figure 2.
Examples of tertiary lymphoid structures seen in well-differentiated / dedifferentiated liposarcoma (A, B, C) (hematoxylin and eosin staining, all 100 X). (D) magnification (400 X) of inset (C), showing separate immune cell infiltration (arrows) into a tumor nodule.
The presence of a naturally-occurring immune infiltrate in STS is encouraging and suggests that immunotherapy may potentially be feasible in these subtypes. Multiple approaches to immunotherapy have been explored for the treatment of other solid tumors; we will now highlight human data available in STS for cancer vaccines, adoptive cell therapy and immune checkpoint blockade. These data are also summarized [Table 2].
Table 2.
Human studies of immunotherapy in soft tissue sarcoma
| Strategy | STS pts | STS subtypes* | Best Clinical Outcome | |
|---|---|---|---|---|
| Cancer Vaccines | ||||
| Mahvi et al., 2002 | Autologous whole tumor cells expressing GM-CSF | 6 | Leiomyosarcoma | 100% negative DTH |
| Liposarcoma | 100% PD | |||
| Matsuzaki et al., 2002 | Autologous DCs pulsed w/SYT-SSX peptide | 1 | Synovial sarcoma | Brief SD, then PD |
| Dillman et al., 2004 | Autologous whole tumor cells, GM-CSF or IFN-gamma | 16 | Not specified | 50% positive DTH |
| Kawaguchi et al., 2012 | SYT-SSX peptide, +/-IFN-alpha | 21 | Synovial sarcoma | with IFN: 50% SD |
| without IFN: 11% SD | ||||
| Finkelstein et al., 2012 | Radiation therapy, intratumoral DC injection | 17 | MFH/UPS | 71% progression-free+ |
| Spindle cell sarcoma | ||||
| Synovial sarcoma | ||||
| Fibrosarcoma | ||||
| Takahashi et al., 2013 | Peptides x 4, based on pre-existing IgG titers, Freund's | 16 | Leiomyosarcoma | 30% SD |
| MFH/UPS | ||||
| Synovial sarcoma | ||||
| Liposarcoma | ||||
| Adoptive Cell Therapy | ||||
| Robbins et al., 2011 | Transduced TCR for NY-ESO-1 | 6 | Synovial sarcoma | 67% OR |
| Ratnavelu et al., 2013 | Expanded blood NK cells | 1 | Epithelioid sarcoma | Clinical tumor regression, improved quality of life |
| Immune Checkpoint Blockade | ||||
| Maki et al., 2013 | Anti-CTLA4 | 6 | Synovial sarcoma | 100% PD |
* - most frequently represented subtypes in the study; + - localized disease only; STS, soft tissue sarcoma; GM-CSF, granulocyte macrophage-colony stimulating factor; DC, dendritic cell; IFN, interferon; TCR, T cell receptor; MFH/UPS, malignant fibrous histiocytoma / undifferentiated pleomorphic sarcoma; DTH, delayed type hypersensitivity; PD, progressive disease; SD, stable disease; OR, objective response.
Cancer vaccines
Cancer vaccines attempt to elicit an immune response against tumor cells through active manipulation of DCs [Figure 1, A]. The target antigen can be pre-specified or unknown; in the latter case, encompassing a wide variety of nonspecific tumor antigens (e.g., released by tumor lysis). Adjuvants such as GM-CSF are frequently given to enhance DC activation and antigen presentation. A number of vaccination strategies have been evaluated in solid tumors, however only recently in 2010, Sipuleucel-T became the first FDA approved cancer vaccine. Sipuleucel-T is a DC-based vaccine containing prostatic acid phosphatase, an important tumor antigen in prostate cancer. In a large, randomized controlled trial in patients with metastatic, hormone-resistant disease, treatment with Sipuleucel-T was well tolerated and improved median overall survival by 4 months compared to placebo; objective tumor responses (defined as complete and partial responses), however, were rare.28
In STS, without knowledge of an appropriate target antigen, many of the earlier studies of vaccines used whole tumor cells as the antigen source and focused more on the feasibility and utility of adjuvants. In a phase I study to assess toxicity, Mahvi et al. transferred DNA plasmids encoding for GM-CSF into irradiated autologous tumor cells as part of a vaccination strategy in patients with melanoma and sarcoma.29 Of the 16 patients enrolled, 6 had STS including 4 with leiomyosarcoma and 1 each with liposarcoma and an unspecified sarcoma. The vaccine was well tolerated, however only one (melanoma) patient demonstrated a positive delayed type hypersensitivity (DTH) test indicative of an induced systemic immune response. All other patients including those with STS had a negative DTH test and clinical disease progression. In a similar vaccination strategy, Dillman et al. first established short term autologous tumor cell lines, which were then irradiated and given back to sarcoma patients along with GM-CSF or IFN-gamma.30 After vaccination, 8 out of 16 (50%) of the patients experienced positive DTH conversion. These patients had longer median survival compared to those who never converted (16.6 vs. 8.3 months). Unfortunately, no objective tumor responses were observed.
In synovial sarcoma, the SYT-SSX fusion protein is found in >90% of tumors31 and as a result, several groups have attempted to vaccinate patients against SYT-SSX derived peptides. Sato et al. demonstrated the preclinical feasibility of this strategy in synovial sarcoma patients.32 Peripheral blood precursors were pulsed with SYT-SSX fusion derived peptides, which generated antigen-specific T cells with in vitro cytotoxicity against HLA-matched synovial sarcoma cells. Matsuzaki et al. treated a metastatic synovial sarcoma patient with autologous DCs pulsed with SYT-SSX derived peptides.33 This patient tolerated treatment well and even experienced symptomatic improvement despite substantial disease burden in the lungs. Tumor-specific immune responses were observed based on in vitro assays and the patient had a brief (1 month) period of radiologic disease stabilization before disease progression. Recently, the Japanese Musculoskeletal Oncology Group reported their phase I experience using vaccination with a 9-mer SYT-SSX derived peptide with and without systemic interferon-alpha for patients with metastatic synovial sarcoma.34 The vaccines were well tolerated and antigen-specific cytotoxic T cell responses were seen in vitro. Disease stabilization was observed in 6 out of 12 (50%) of patients who had received vaccine with interferon versus 1 out of 9 (11%) patients who had received the vaccine alone. Although transient tumor shrinkage was seen in one patient, no objective responses were reported.
Vaccination against several different known tumor antigens simultaneously may be more effective than targeting a single antigen. Takahashi et al. explored this concept with a personalized vaccination strategy in patients with refractory sarcomas, 80% of which were soft tissue in origin.35 Patients were vaccinated with 4 of 31 possible peptides, chosen based on pre-existing immunity as assessed by peptide-specific IgG titers. Both humoral and cellular immune responses against the vaccinated peptides were observed. No objective responses were seen; however with personalized vaccination, 6 out of 20 total patients (30%) experienced disease stabilization including one synovial sarcoma patient who had progression-free survival of 33 months.
In situ vaccination is an approach in which local treatment (e.g., radiation therapy) can provide a mechanism for release of tumor antigens. Gamma radiation, in particular, has theoretical advantages from an immunotherapy standpoint based on 1) induction of tumor cell apoptosis, through which DCs can efficiently acquire, process and present antigen,36 2) local release of immunologically active cytokines and 3) increase in tumor vascular permeability to allow for immune cell tumor infiltration.37 Finkelstein et al. combined preoperative gamma radiation (50 Gy) with intratumoral DC injection in patients with high risk extremity STS.9,38 Using this in situ vaccination strategy, 9 out of 17 patients (53%) demonstrated an in vitro immune response to autologous tumor lysate. Development of an immune response to vaccination was positively correlated with the presence of CD4 T cells and negatively correlated with myeloid derived suppressor cells in the tumor microenvironment. At one year, 12 of 17 (71%) patients were progression-free; however, it is important to note that all of the study patients had completely resected, localized disease and longer follow-up is needed.
Adoptive cell therapy
In contrast to cancer vaccines, adoptive cell therapy “bypasses” DCs [Figure 1, B] by giving patients autologous T cells that typically have been expanded and enhanced in vitro with cytokines and other growth factors. Adoptive cell therapy for patients with solid tumors was developed and has been widely used in melanoma by Rosenberg et al. at the National Cancer Institute (NCI) in Bethesda, Maryland.39 With this immunotherapeutic approach, tumor-infiltrating lymphocytes or “TILs” are isolated from surgical specimens, selected for in vitro reactivity against tumor cells, and then expanded several thousand-fold in vitro using IL-2. The expanded TILs are then given back to the patient along with systemic IL-2 and after preconditioning with myeloablative cyclophosphamide-based systemic chemotherapy with or without whole body irradiation. This preconditioning treatment is designed to remove the theoretically tumor tolerant native immune system to “create space” for the new “army” of expanded TILs. In patients with metastatic melanoma, up to 50–70% objective response rates have been reported with adoptive cell therapy using TILs.40,41
Adoptive cell therapy is potentially applicable to any solid tumor type. In early studies at the NCI, Topalian et al. reported on isolation and expansion of TILs from both melanoma and non-melanoma tumors.42 TILs were successfully isolated from 2 patients with leiomyosarcoma, 2 with liposarcoma, 3 with malignant fibrous histiocytoma / undifferentiated pleomorphic sarcoma and 2 with spindle cell sarcoma. The TILs from one patient with leiomyosarcoma underwent a 2000-fold in vitro expansion with IL-2 and TILs from another patient with spindle cell sarcoma expanded 30,000-fold. Schiltz et al. also attempted to isolate and expand TILs from a variety of human solids tumors and were successful in 5 out of 7 (71%) cases of sarcoma.43 Similarly, Tseng et al. have recently been able to isolate and expand TILs from well-differentiated and dedifferentiated liposarcoma, with 300- to almost 2000-fold expansion (unpublished data). It is important to note that in comparison to melanoma, the success and consistency of TIL isolation and expansion in STS appears to be highly variable and may in fact, require subtype specific modifications to the technique. To our knowledge, there have not been any reports of STS patients who have been treated with TIL adoptive cell therapy.
Another approach to adoptive cell therapy involves harvesting T cells from peripheral blood as opposed to tumor. T cells that demonstrate recognition of a specific peptide (e.g., NY-ESO-1) can be isolated and expanded, then given back to the patient with the same preconditioning used in adoptive cell therapy with TIL.44 Alternatively, naïve T cells from peripheral blood can be transduced with genetically engineered alpha-beta T cell receptors (TCR) that have antigen-specificity. Using this latter approach, Robbins et al. modified T cells from melanoma and synovial sarcoma patients to have receptor specificity against NY-ESO-1.45 These patients all had metastatic disease refractory to multiple prior chemotherapy treatments. Among the synovial sarcoma patients treated with adoptive cell therapy using TCR-transduced T cells, 4 out of 6 (67%) demonstrated objective responses (all partial, no complete). One patient had a durable partial response lasting 18 months. Recently, Abate-Daga et al. reported on the preclinical development of a modified TCR with specificity for SSX2, a portion of the SYT-SSX fusion protein found in synovial sarcoma.46 T cells transduced with this TCR demonstrated antigen-specific recognition and target lysis in vitro.
NK cell-based adoptive cell therapy also has been reported and may ultimately prove efficacious in STS. One advantage of NK-immunotherapy is the absence of MHC/HLA restriction. Ratnavelu et al. described a patient with locally-advanced, chemotherapy-refractory epithelioid sarcoma who experienced clinical disease regression and marked improvement in quality of life after receiving autologous, in vitro expanded peripheral blood NK cells.47 The patient survived for 25 months by the time of publication. Sangiolo et al. recently reported on the preclinical feasibility of isolation and expansion of putative “cytokine-induced killers” from the peripheral blood of bone and soft tissue sarcoma patients.48 These cells, which express both CD3 and CD56, demonstrate NKG2D-dependent cytotoxicity against autologous tumor cells and may also have the ability to target cancer stem cells.48
Immune checkpoint blockade
Immune checkpoint blockade is a relatively recent immunotherapeutic strategy in solid tumors that “removes the brakes” (CTLA4, PD-1) to maintain the activation status and cytotoxic function of T cells and other immune cells that are already present in the tumor microenvironment [Figure 1, C]. Specific tumor antigens are not targeted per se by immune checkpoint blockade; however, this immunotherapeutic strategy enhances immune function of in situ T cells that are likely already sensitized to a multitude of antigens, potentially including tumor-specific antigens. In melanoma, FDA approval was granted in 2011 to the anti-CTLA4 antibody, ipilimumab, after demonstration of improvement in median overall survival by Hodi et al.49 which was subsequently confirmed by Robert et al.50 This year, 2014, pembrolizumab, an anti-PD-1 antibody was granted FDA approval for the treatment of ipilimumab-resistant melanoma.
Several observations made from trials of immune checkpoint blockade suggest that this immunotherapeutic strategy may prove useful for STS. Immune checkpoint blockade can result in objective response in a significant number of patients even with advanced stages of disease and heavy tumor burden. Wolchok et al. recently reported that combination anti-CTLA4 and anti-PD-1 therapy resulted in an overall objective response rate of 53% including sustained tumor regression by over 80% in a number of melanoma patients with advanced disease.51 Importantly, immune checkpoint blockade antibodies do not act on the tumor cells directly and therefore can be theoretically applied to any solid tumor. This concept has been supported in the phase I trials of anti-PD-1 therapy in which objective responses were observed even in patients with tumors that were previously regarded as “non-immunogenic” tumors (e.g., non-small cell lung cancer).52
To our knowledge, only one study has been reported to date with immune checkpoint blockade in STS patients. Maki et al. treated patients with synovial sarcoma with ipilimumab (anti-CTLA4) in a pilot, phase II study designed to assess objective tumor response.53 Patients received 3 cycles of ipilimumab at the FDA approved dose used for melanoma and then were restaged by cross-sectional imaging. Unfortunately, the trial was closed due to poor patient accrual. Among the 6 patients enrolled, no serologic evidence of an immune response (to NY-ESO-1) was seen and all patients demonstrated disease progression after therapy.
Anti-PD-1 therapy represents an alternative strategy, since the mechanism by which PD-1 inhibits the immune response is distinct from CTLA4. Moreover, in contrast to CTLA4, PD-1 and its natural ligand PD-L1 may be potential biomarkers for response to therapy and can be used to guide patient selection.52 Kim et al. recently characterized expression of PD-1 and PD-L1 across several STS subtypes.54 In total, 105 cases of STS were analyzed by immunohistochemistry. The largest subtypes represented were leiomyosarcoma (n = 20), synovial sarcoma (n = 16), undifferentiated sarcoma (n = 11), and myxoid liposarcoma (n = 10). For all STS combined, intratumoral infiltration of PD-1 positive lymphocytes was seen in 65% of cases and PD-L1 tumor expression in 58%; however, by subtype, there was significant variation in the frequency of marker expression. For PD-1, 100% of undifferentiated sarcomas had positive expression, compared to 10% (one case) in myxoid liposarcoma. These data suggest that certain STS subtypes may be more amenable to immune checkpoint blockade using anti-PD-1 or anti-PD-L1.
Discussion and Conclusions
Current treatment options for patients with STS are limited, in particular for those with locally-advanced, recurrent, or metastatic disease. Several aspects of STS disease biology suggest that there is strong rationale for immunotherapy, including the presence of chromosomal translocations which result in unique fusion proteins, high expression of cancer testis antigens (e.g., NY-ESO-1), and to a lesser extent, the moderate frequency of genetic mutations. In addition, a naturally-occurring immune infiltrate has been reported for several STS subtypes. The currently available human data to support the efficacy of immunotherapy in STS, however, is limited. The numbers of patients assessed thus far is too small to draw any conclusions.
In STS, for which multimodality treatment is frequently used, it is important to note that immunotherapy would be complementary to current standard treatment. The combination of immunotherapy with surgery, radiation therapy and systemic chemotherapy should continue to be explored. Surgery is useful to macroscopically debulk gross disease and obtain tissue to study the local tumor and immune microenvironment. Both radiation therapy and systemic chemotherapy can induce tumor lysis for antigen release and facilitate immune cell infiltration into tumors. We also advocate combination immunotherapy, such as vaccination to induce an immune response followed by immune checkpoint blockade to maintain and enhance the immune response.
Critical challenges of evaluating new therapies in STS, including immunotherapy, are the rarity of the disease and tremendous heterogeneity of subtypes. Multicenter national and even international collaborations are needed to efficiently accrue enough patients to assess efficacy or alternatively, to understand why a particularly therapy does not work. As with other forms of systemic therapy (e.g., chemotherapy), STS subtypes are likely to exhibit varying sensitivity to a particular immunotherapeutic strategy. Therefore, clinical trials should ideally be conducted for a specific STS subtype rather than collectively for all STS. The inclusion of a few isolated cases of STS under the broad category of “advanced solid tumors” should be discouraged.
Similarly, preclinical research should focus on an understanding of the native immune response and inhibitory mechanisms present in the tumor microenvironment that are unique and specific to each STS subtype. With the differences in disease biology, clinical behavior and response to therapy, it is very likely that the immune response is also distinct between STS subtypes. These immunologic differences, whether obvious or subtle, need to be recognized and appropriately incorporated into the design of immunotherapeutic strategies tailored for each STS subtype. Direct study of tumors, including paraffin embedded and fresh tissue (e.g., from surgical resection) is important in this regard; fortunately, STS are often quite large in size with ample tissue for research.
Immunocompetent (not xenograft) animal models can also be helpful for providing insight into the immune response in the tumor microenvironment and evaluating the potential efficacy of immunotherapy. Validation of these models for accurate recapitulation of human disease, even on a genetic or cytogenetic level, is critical. Immunocompetent, genetically-engineered mouse models have been described for leiomyosarcoma,55 rhabdomyosarcoma,56,57 and malignant fibrous histiocytoma / undifferentiated pleomorphic sarcoma58; the latter 2 models have been validated using cross species genomic analysis for similarity to human disease.59,60 To our knowledge, characterization of the immune response or utilization of these mouse models for preclinical immunotherapy studies have not been reported. Dogs, which naturally develop many subtypes of STS, are another potentially useful animal model.61,62 Studies in dogs, in contrast to inbred laboratory mice, preserve the background genetic diversity seen in humans.63
In our opinion, the potential for immunotherapy in the treatment of STS is only beginning to be explored. Further research into STS immunology is needed to help design logical, subtype-specific immunotherapeutic strategies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med 2005; 353:701-11; PMID:16107623; http://dx.doi.org/ 10.1056/NEJMra041866 [DOI] [PubMed] [Google Scholar]
- 2.Karavasilis V, Seddon BM, Ashley S, Al-Muderis O, Fisher C, Judson I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer 2008; 112:1585-91; PMID:18278813; http://dx.doi.org/ 10.1002/cncr.23332 [DOI] [PubMed] [Google Scholar]
- 3.Bramwell VH, Anderson D, Charette ML, Group SDS. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003:CD003293; PMID:12917960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol 2014; 11:187-202; PMID:24642677; http://dx.doi.org/ 10.1038/nrclinonc.2014.26 [DOI] [PubMed] [Google Scholar]
- 5.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP, Beppu Y, et al. . Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012; 379:1879-86; PMID:22595799; http://dx.doi.org/ 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 6.Talmadge JE. Immune cell infiltration of primary and metastatic lesions: mechanisms and clinical impact. Semin Cancer Biol 2011; 21:131-8; PMID:21145968; http://dx.doi.org/ 10.1016/j.semcancer.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 7.Gajewski TF. Cancer immunotherapy. Mol Oncol 2012; 6:242-50; PMID:22248437; http://dx.doi.org/ 10.1016/j.molonc.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridman WH, Remark R, Goc J, Giraldo NA, Becht E, Hammond SA, Damotte D, Dieu-Nosjean MC, Sautès-Fridman C. The immune microenvironment: a major player in human cancers. Int Arch Allergy Immunol 2014; 164:13-26; PMID:24852691 [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein SE, Fishman M, Conley AP, Gabrilovich D, Antonia S, Chiappori A. Cellular immunotherapy for soft tissue sarcomas. Immunotherapy 2012; 4:283-90; PMID:22401634; http://dx.doi.org/ 10.2217/imt.12.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coley WB. II. Contribution to the knowledge of sarcoma. Ann Surg 1891; 14:199-220; PMID:17859590; http://dx.doi.org/ 10.1097/00000658-189112000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng WW, Somaiah N, Lazar AJ, Lev DC, Pollock RE. Novel systemic therapies in advanced liposarcoma: a review of recent clinical trial results. Cancers (Basel) 2013; 5:529-49; PMID:24216990; http://dx.doi.org/ 10.3390/cancers5020529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guadagnolo BA, Zagars GK, Ballo MT, Patel SR, Lewis VO, Benjamin RS, Pollock RE. Excellent local control rates and distinctive patterns of failure in myxoid liposarcoma treated with conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70:760-5; PMID:17892916; http://dx.doi.org/ 10.1016/j.ijrobp.2007.07.2337 [DOI] [PubMed] [Google Scholar]
- 13.Asano N, Susa M, Hosaka S, Nakayama R, Kobayashi E, Takeuchi K, Horiuchi K, Suzuki Y, Anazawa U, Mukai M, et al. . Metastatic patterns of myxoid/round cell liposarcoma: a review of a 25-year experience. Sarcoma 2012; 2012:345161; PMID:22550416; http://dx.doi.org/ 10.1155/2012/345161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennicelli JL, Barr FG. Chromosomal translocations and sarcomas. Curr Opin Oncol 2002; 14:412-9; PMID:12130926; http://dx.doi.org/ 10.1097/00001622-200207000-00008 [DOI] [PubMed] [Google Scholar]
- 15.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer 2003; 3:685-94; PMID:12951587; http://dx.doi.org/ 10.1038/nrc1168 [DOI] [PubMed] [Google Scholar]
- 16.de Vreeze RS, de Jong D, Nederlof PM, Ariaens A, Tielen IH, Frenken L, Haas RL, van Coevorden F. Added Value of Molecular Biological Analysis in Diagnosis and Clinical Management of Liposarcoma: A 30-Year Single-Institution Experience. Ann Surg Oncol 2010; 17:686-93; PMID:20183915; http://dx.doi.org/ 10.1245/s10434-009-0806-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worley BS, van den Broeke LT, Goletz TJ, Pendleton CD, Daschbach EM, Thomas EK, Marincola FM, Helman LJ, Berzofsky JA. Antigenicity of fusion proteins from sarcoma-associated chromosomal translocations. Cancer Res 2001; 61:6868-75; PMID:11559563 [PubMed] [Google Scholar]
- 18.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, Chen YT, Stockert E, Ladanyi M, Old LJ, et al. . Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer 2001; 94:252-6; PMID:11668506; http://dx.doi.org/ 10.1002/ijc.1451 [DOI] [PubMed] [Google Scholar]
- 19.Pollack SM, Jungbluth AA, Hoch BL, Farrar EA, Bleakley M, Schneider DJ, Loggers ET, Rodler E, Eary JF, Conrad EU 3rd, et al. . NY-ESO-1 is a ubiquitous immunotherapeutic target antigen for patients with myxoid/round cell liposarcoma. Cancer 2012; 118:4564-70; PMID:22359263; http://dx.doi.org/ 10.1002/cncr.27446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemminger JA, Iwenofu OH. NY-ESO-1 is a sensitive and specific immunohistochemical marker for myxoid and round cell liposarcomas among related mesenchymal myxoid neoplasms. Mod Pathol 2013; 26:1204-10; PMID:23599152; http://dx.doi.org/ 10.1038/modpathol.2013.65 [DOI] [PubMed] [Google Scholar]
- 21.Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho A, et al. . Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet 2010; 42:715-21; PMID:20601955; http://dx.doi.org/ 10.1038/ng.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al. . Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013; 499:214-8; PMID:23770567; http://dx.doi.org/ 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maki RG. Immunity against soft-tissue sarcomas. Curr Oncol Rep 2003; 5:282-7; PMID:12781069; http://dx.doi.org/ 10.1007/s11912-003-0067-x [DOI] [PubMed] [Google Scholar]
- 24.Soilleux EJ, Rous B, Love K, Vowler S, Morris LS, Fisher C, Coleman N. Myxofibrosarcomas contain large numbers of infiltrating immature dendritic cells. Am J Clin Pathol 2003; 119:540-5; PMID:12710126; http://dx.doi.org/ 10.1309/JEB7DGHH01J11VUM [DOI] [PubMed] [Google Scholar]
- 25.Orui H, Ishikawa A, Okada K, Nishida J, Mitsui H, Kashiwa H, Tsuchiya T, Ogino T, Yamakawa M. Dendritic cell and effector cell infiltration in soft tissue sarcomas with reactive lymphoid hyperplasia. J Orthop Sci 2003; 8:669-77; PMID:14557933; http://dx.doi.org/ 10.1007/s00776-003-0692-0 [DOI] [PubMed] [Google Scholar]
- 26.Fujii H, Arakawa A, Utsumi D, Sumiyoshi S, Yamamoto Y, Kitoh A, Ono M, Matsumura Y, Kato M, Konishi K, et al. . CD8⁺ tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer 2014; 134:2393-402; PMID:24243586; http://dx.doi.org/ 10.1002/ijc.28581 [DOI] [PubMed] [Google Scholar]
- 27.Tseng WW, Demicco EG, Lazar AJ, Lev DC, Pollock RE. Lymphocyte composition and distribution in inflammatory, well-differentiated retroperitoneal liposarcoma: clues to a potential adaptive immune response and therapeutic implications. Am J Surg Pathol 2012; 36:941-4; PMID:22446945; http://dx.doi.org/ 10.1097/PAS.0b013e31824f2594 [DOI] [PubMed] [Google Scholar]
- 28.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. . Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 29.Mahvi DM, Shi FS, Yang NS, Weber S, Hank J, Albertini M, Schiller J, Schalch H, Larson M, Pharo L, et al. . Immunization by particle-mediated transfer of the granulocyte-macrophage colony-stimulating factor gene into autologous tumor cells in melanoma or sarcoma patients: report of a phase I/IB study. Hum Gene Ther 2002; 13:1711-21; PMID:12396624; http://dx.doi.org/ 10.1089/104303402760293556 [DOI] [PubMed] [Google Scholar]
- 30.Dillman R, Barth N, Selvan S, Beutel L, de Leon C, DePriest C, Peterson C, Nayak S. Phase I/II trial of autologous tumor cell line-derived vaccines for recurrent or metastatic sarcomas. Cancer Biother Radiopharm 2004; 19:581-8; PMID:15650450; http://dx.doi.org/ 10.1089/1084978042484812 [DOI] [PubMed] [Google Scholar]
- 31.Eilber FC, Dry SM. Diagnosis and management of synovial sarcoma. J Surg Oncol 2008; 97:314-20; PMID:18286474; http://dx.doi.org/ 10.1002/jso.20974 [DOI] [PubMed] [Google Scholar]
- 32.Sato Y, Nabeta Y, Tsukahara T, Hirohashi Y, Syunsui R, Maeda A, Sahara H, Ikeda H, Torigoe T, Ichimiya S, et al. . Detection and induction of CTLs specific for SYT-SSX-derived peptides in HLA-A24(+) patients with synovial sarcoma. J Immunol 2002; 169:1611-8; PMID:12133991; http://dx.doi.org/ 10.4049/jimmunol.169.3.1611 [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaki A, Suminoe A, Hattori H, Hoshina T, Hara T. Immunotherapy with autologous dendritic cells and tumor-specific synthetic peptides for synovial sarcoma. J Pediatr Hematol Oncol 2002; 24:220-3; PMID:11990310; http://dx.doi.org/ 10.1097/00043426-200203000-00012 [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi S, Tsukahara T, Ida K, Kimura S, Murase M, Kano M, Emori M, Nagoya S, Kaya M, Torigoe T, et al. . SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: a study from the Japanese Musculoskeletal Oncology Group. Cancer Sci 2012; 103:1625-30; PMID:22726592; http://dx.doi.org/ 10.1111/j.1349-7006.2012.02370.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi R, Ishibashi Y, Hiraoka K, Matsueda S, Kawano K, Kawahara A, Kage M, Ohshima K, Yamanaka R, Shichijo S, et al. . Phase II study of personalized peptide vaccination for refractory bone and soft tissue sarcoma patients. Cancer Sci 2013; 104:1285-94; PMID:23829867; http://dx.doi.org/ 10.1111/cas.12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman RM, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol 1999; 60:562-7; PMID:10426272; http://dx.doi.org/ 10.1016/S0198-8859(99)00030-0 [DOI] [PubMed] [Google Scholar]
- 37.Indelicato DJ, Finkelstein SE. Dendritic cell immunotherapy in soft tissue sarcoma. Immunotherapy 2012; 4:1023-9; PMID:23148754; http://dx.doi.org/ 10.2217/imt.12.106 [DOI] [PubMed] [Google Scholar]
- 38.Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, Noyes DR, Cheong D, Gonzalez RJ, Heysek RV, et al. . Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys 2012; 82:924-32; PMID:21398051; http://dx.doi.org/ 10.1016/j.ijrobp.2010.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. . Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298:850-4; PMID:12242449; http://dx.doi.org/ 10.1126/science.1076514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. . Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233-9; PMID:18809613; http://dx.doi.org/ 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. . Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17:4550-7; PMID:21498393; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topalian SL, Muul LM, Solomon D, Rosenberg SA. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods 1987; 102:127-41; PMID:3305708; http://dx.doi.org/ 10.1016/S0022-1759(87)80018-2 [DOI] [PubMed] [Google Scholar]
- 43.Schiltz PM, Beutel LD, Nayak SK, Dillman RO. Characterization of tumor-infiltrating lymphocytes derived from human tumors for use as adoptive immunotherapy of cancer. J Immunother 1997; 20:377-86; PMID:9336745; http://dx.doi.org/ 10.1097/00002371-199709000-00007 [DOI] [PubMed] [Google Scholar]
- 44.Yee C. The use of endogenous T cells for adoptive transfer. Immunol Rev 2014; 257:250-63; PMID:24329802; http://dx.doi.org/ 10.1111/imr.12134 [DOI] [PubMed] [Google Scholar]
- 45.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. . Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29:917-24; PMID:21282551; http://dx.doi.org/ 10.1200/JCO.2010.32.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abate-Daga D, Speiser DE, Chinnasamy N, Zheng Z, Xu H, Feldman SA, Rosenberg SA, Morgan RA. Development of a T cell receptor targeting an HLA-A*0201 restricted epitope from the cancer-testis antigen SSX2 for adoptive immunotherapy of cancer. PLoS One 2014; 9:e93321; PMID:24681846; http://dx.doi.org/ 10.1371/journal.pone.0093321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratnavelu K, Subramani B, Pullai CR, Krishnan K, Sugadan SD, Rao MS, Veerakumarasivam A, Deng X, Hiroshi T. Autologous immune enhancement therapy against an advanced epithelioid sarcoma: a case report. Oncol Lett 2013; 5:1457-60; PMID:23761810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sangiolo D, Mesiano G, Gammaitoni L, Leuci V, Todorovic M, Giraudo L, Cammarata C, Dell'Aglio C, D'Ambrosio L, Pisacane A, et al. . Cytokine-induced killer cells eradicate bone and soft-tissue sarcomas. Cancer Res 2014; 74:119-29; PMID:24356422; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1559 [DOI] [PubMed] [Google Scholar]
- 49.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. . Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/ 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 51.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. . Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/ 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maki RG, Jungbluth AA, Gnjatic S, Schwartz GK, D’Adamo DR, Keohan ML, Wagner MJ, Scheu K, Chiu R, Ritter E, et al. . A Pilot Study of Anti-CTLA4 Antibody Ipilimumab in Patients with Synovial Sarcoma. Sarcoma 2013; 2013:168145; PMID:23554566; http://dx.doi.org/ 10.1155/2013/168145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ, et al. . Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One 2013; 8:e82870; PMID:24349382; http://dx.doi.org/ 10.1371/journal.pone.0082870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernando E, Charytonowicz E, Dudas ME, Menendez S, Matushansky I, Mills J, Socci ND, Behrendt N, Ma L, Maki RG, et al. . The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med 2007; 13:748-53; PMID:17496901; http://dx.doi.org/ 10.1038/nm1560 [DOI] [PubMed] [Google Scholar]
- 56.Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med 1998; 4:619-22; PMID:9585239; http://dx.doi.org/ 10.1038/nm0598-619 [DOI] [PubMed] [Google Scholar]
- 57.Keller C, Arenkiel BR, Coffin CM, El-Bardeesy N, DePinho RA, Capecchi MR. Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: cooperativity of Ink4a/ARF and Trp53 loss of function. Genes Dev 2004; 18:2614-26; PMID:15489287; http://dx.doi.org/ 10.1101/gad.1244004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP, Nielsen GP, Quade BJ, Chaber CJ, Schultz CP, et al. . A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med 2007; 13:992-7; PMID:17676052; http://dx.doi.org/ 10.1038/nm1602 [DOI] [PubMed] [Google Scholar]
- 59.Nishijo K, Chen QR, Zhang L, McCleish AT, Rodriguez A, Cho MJ, Prajapati SI, Gelfond JA, Chisholm GB, Michalek JE, et al. . Credentialing a preclinical mouse model of alveolar rhabdomyosarcoma. Cancer Res 2009; 69:2902-11; PMID:19339268; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mito JK, Riedel RF, Dodd L, Lahat G, Lazar AJ, Dodd RD, Stangenberg L, Eward WC, Hornicek FJ, Yoon SS, et al. . Cross species genomic analysis identifies a mouse model as undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma. PLoS One 2009; 4:e8075; PMID:19956606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ettinger SN. Principles of treatment for soft-tissue sarcomas in the dog. Clin Tech Small Anim Pract 2003; 18:118-22; PMID:12831074; http://dx.doi.org/ 10.1053/svms.2003.36628 [DOI] [PubMed] [Google Scholar]
- 62.Mayer MN, LaRue SM. Soft tissue sarcomas in dogs. Can Vet J 2005; 46:1048, 50, 52; PMID:16363335 [PMC free article] [PubMed] [Google Scholar]
- 63.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer 2008; 8:147-56; PMID:18202698; http://dx.doi.org/ 10.1038/nrc2273 [DOI] [PubMed] [Google Scholar]