Abstract
Vaccination is an effective mean of preventing infectious diseases, including those caused by Helicobacter pylori. Th17 cell responses are critical for the pathogenesis of Helicobacter pylori infection. In view of Th17 responses to multi-epitope vaccine CTB-UE, the IL-17 production in antiserum was examined. CTB-UE immunization decreased IL-17 production, implying that Th17 responses may be inhibited. Furthermore, IL-17 aggravated GES-1 cell injury induced by H. pylori SS1; In contrast, CTB-UE antiserum could alleviate this cell injury, which suggesting that CTB-UE can protect GES-1 cell infected with H. pylori SS1 by inhibiting Th17 responses. Treatment of mice with CTB-UE significantly reduced the H. pylori burden and inflammation in the stomach. On the other hand, the production of IL-17 in the stomach in H. pylori-infected mice was increased; but the production of IL-17 in the stomach was decreased after treatment with CTB-UE. Furthermore, the expression of microRNA-155 in gastric tissue was significantly up-regulated. The results suggested that CTB-UE could relieve the H. pylori-induced gastric inflammatory reaction via up-regulating microRNA-155 to inhibit Th17 responses, implying that the microRNA-155/IL-17 pathway was involved. Further study is required to elucidate the relationship between miRNA-155 and IL-17. We found that the production of IL-17 was significantly increased after the expression of miRNA-155 being down-regulated; however, the production of IL-17 was significantly decreased after the expression of miRNA-155 being upregulated.
Keywords: Epitope vaccine, Helicobacter pylori, IL-17, microRNA-155, Th17
Abbreviations
- H. pylori
Helicobacter pylori
- miRNA
microRNA
- miR-155
microRNA-155
- Th
Helper T lymphocyte
- Th1
Type 1 of helper T lymphocyte
- Th2
Type 2 of helper T lymphocytel
- Th17
Type 17 of helper T lymphocyte
- NC
Normal control group
- MC
Model control group
- HD
High-dose group
- MD
Middle-dose group
- LD
Low-dose group
- IL-17
Interleukin-17
- PCR
Polymerase chain reaction
- RT-PCR
Reverse transcription polymerase chain reaction
- cDNA
Complementary DNA
- ATCC
American Type Culture Collection
- CTB
Cholera toxin B subunit
- dNTP
Deoxyribonucleoside triphosphate
- E. coli
Escherichia coli
- ELISA
Enzyme-linkedimmunosorbent assay
- OD
Optical density
- SDS
Sodium dodecyl sulfate
- PAGE
Polyacrylamide gel electrophoresis
- UreA
Urease A subunit
- UreB
Urease B subunit
- ddH2O
Double distilled water
- Lipo-2000
Lipofectamine 2000
Introduction
Helicobacter pylori (H. pylori), a gram-negative, microaerophilic bacillus, is a pathogenic bacterium that causes chronic infection with a high worldwide infection rate. More than 50% of the world's population is infected with this bacterium. H. pylori is closely correlated with certain diseases of the upper digestive tract. It is an important pathogenic factor in chronic gastritis, gastric and duodenal ulcers, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma. H. pylori infection causes severe local inflammation in the gastric mucosa. It has been listed as a primary pathogenic factor by the World Health Organization.1-3
Vaccine should be an effective method of preventing H. pylori infectious diseases, and the development of such a vaccine has been investigated by a number of groups. The urease B subunit (UreB) of H. pylori is considered an ideal vaccine antigen.4,5 However, previous studies in our laboratory have shown that H. pylori urease A subunit (UreA) is also an effective vaccine antigen.6 In previous studies,14 we constructed a novel multi-epitope vaccine, CTB-UE, composed of the cholera toxin B subunit and tandem copies of the B and Th cell epitopes from the H. pylori urease A and B subunits. We obtained a recombinant protein vaccine using gene cloning, protein expression, and purification technologies. Our previous studies have shown that CTB-UE has a good therapeutic effect on H. pylori infection in BALB/c mice model. But it is still unclear that the multi-epitope vaccine relieves gastric inflammation reaction induced by Helicobacter pylori infection.
However, a novel subset of effector T cells (CD4+), identified by secretion of IL-17, has been defined as Th17 cells. Emerging evidence suggests that IL-17 may play an important role in the inflammatory response to the H. pylori infection and ultimately influence the outcome of the H. pylori-associated disease.7,8 On the other hand, microRNA-155 specifically regulates the immune response of T17 cell, playing a very important role in the immune system.9-12
In this study, we characterized Th17 cell responses regulated by microRNA-155 to H. pylori infection in C57/BL6 mice and GES-1 cell model, and attempted to elucidate that multi-epitope vaccine CTB-UE alleviates the gastric inflammation reaction induced by Helicobacter pylori infection via up-regulating microRNA-155 to inhibit Th17 responses. Aluminum hydroxide, the most commonly used adjuvant, has been used in a wide range of vaccines over the course of many decades. As an adsorbent, Aluminum hydroxide can strongly adsorb the protein antigen and protect protein from gastric acid. In addition, this adjuvant is generally well tolerated and can improve the body's immune response. For these reasons, aluminum hydroxide adjuvant was selected in our study.
Results
Determination of IL-17 production in Antiserum from C57/BL6 mice
IL-17 plays an important role in vaccine-induced immunity against Helicobacter infections.16-18 To evaluate the effect of CTB-UE on Th17 response, we examined the production of interleukin-17 in antiserum from C57/BL6 mice after vaccination with CTB-UE.
As shown in Figure 1, the production of IL-17 in the MC group was significantly higher than in the NC group (P < 0.01). The production of IL-17 in the HD and MD groups was substantially lower than in the NC group (P < 0.05). In contrast, the production of IL-17 in the LD group did not differ significantly from the NC group (P > 0.05).
Figure 1.
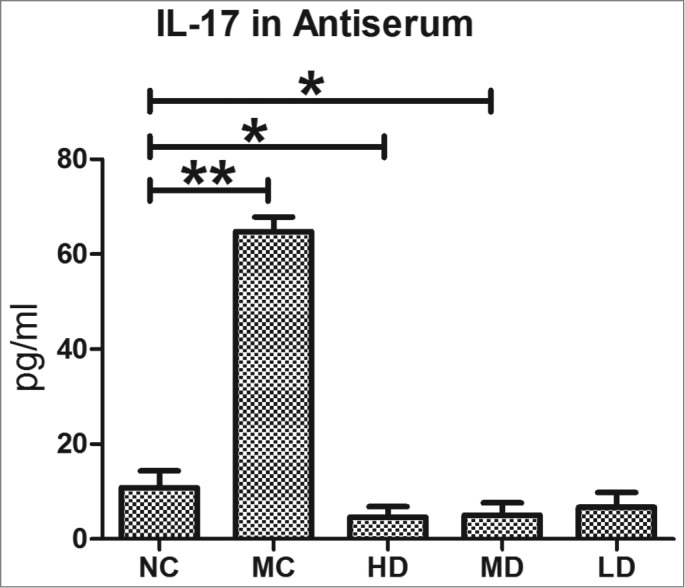
The IL-17 production in Antiserum. IL-17 production in Antiserum from C57/BL6 mice after vaccination with CTB-UE. All bars indicate the mean±SD. Each group contained 8 samples (n = 8/group). *, P < 0.05 and **, P < 0.01 comparing 2 groups. NC: normal control group; MC: model control group; HD: high-dose group (200 μg); MD: middle-dose group (100 μg); LD: low-dose group (50 μg). Each group contains 8 animals.
Determination of LDH activity in H. pylori-infected GES-1 xìbāo huóxìng
GES-1 cells
In the procedure of cellular necrosis and apoptosis, cell membrane structure is damaged and cytoplasm enzymes are released into the culture fluid, including the stable enzyme lactate dehydrogenase (LDH). The degree of injury and survival on cells can be reflected via determination of LDH activity. In this study, we infected GES-1 cells with H. pylori SS1, which leading to cell damage and determined LDH to evaluate GES-1 cells activity.
As shown in Figure 2, the LDH activity in the MC group was significantly higher than in the NC group (P < 0.01). The LDH activity in the AS group was significantly lower than in the MC group (P < 0.01). In contrast, the LDH activity in the I20 and I40 groups was significantly higher compared with the MC group (P < 0.01), and there were significant differences among I10, I20 and I40 groups (P < 0.01), showing an evident dose-effect relationship. The results suggested that IL-17 aggravated GES-1 cell injury induced by H. pylori SS1; In contrast, CTB-UE antiserum could alleviate this cell injury, implying that CTB-UE can protect GES-1 cell infected with H. pylori SS1 by inhibiting Th17 responses.
Figure 2.
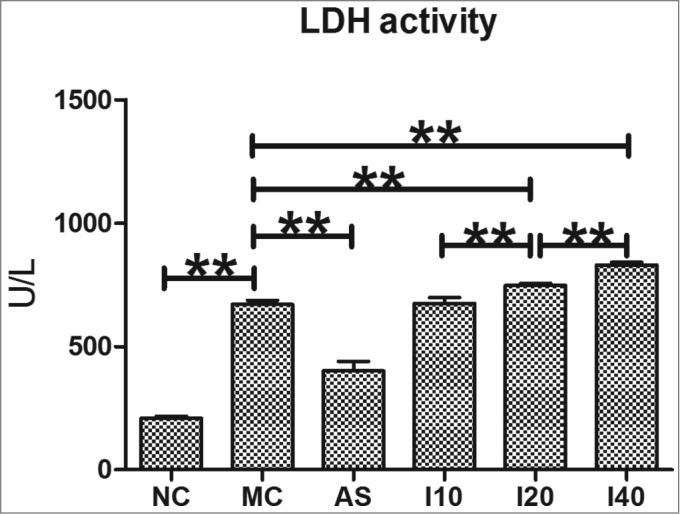
LDH activity in H. pylori-infected GES-1 cells. LDH activity in H. pylori-infected GES-1 cells after treatment with CTB-UE Antiserum. The activity was determined by using LDH micro-ELISA Kits. The bars indicate the mean±SD. Each group contained 3 samples (n = 3/group). *, P < 0.05 and **, P < 0.01 comparing 2 groups. NC: normal control group; MC: model control group; AS: CTB-UE antiserum group; I10: IL-17 treatment at a concentration of 10 ng/mL; I20: IL-17 treatment at a concentration of 20 ng/mL; I40: IL-17 treatment at a concentration of 40 ng/mL. The cell experiments were repeated 3 times.
Urease activity in the stomachs in the C57/BL6 mice model after treatment with CTB-UE
Urease is an important colonisation factor and pathogenic factor in H. pylori infection.19-22 H. pylori can produce a large amount of urease, which decomposes urea into ammonia and carbon dioxide. The ammonia can neutralise stomach acid and protect H. pylori against gastric damage.23 However, urease can directly or indirectly damage the gastric mucosal barrier.24-26 In this study, we examined the changes in urease activity in C57/BL6 mice gastric tissue to evaluate H. pylori burden.
As shown in Figure 3, the urease activity in the MC group was significantly higher than in the NC group (P < 0.01). In contrast, the urease activity in the HD, MD and LD groups was significantly lower compared with the MC group (P < 0.01 or P < 0.05), and there were significant differences among HD, MD, and LD groups (P < 0.01 or P < 0.05), showing an evident dose-effect relationship.
Figure 3.
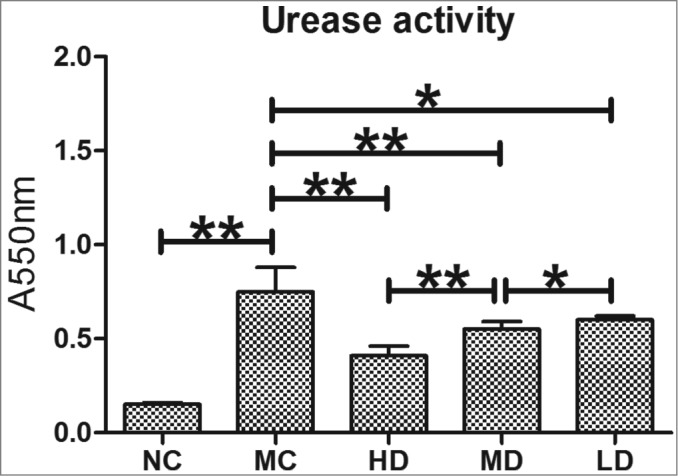
Urease activity in the stomachs in the C57/BL6 mice model after treatment with CTB-UE. Urease activity of H. pylori in the stomachs after treatment with CTB-UE. The activity was determined by measuring the absorbance at 550 nm (A550). The bars indicate the mean±SD . Each group contained 8 samples (n = 8/group). *, P < 0.05 and **, P < 0.01 comparing 2 groups. NC: normal control group; MC: model control group; HD: high-dose group (200 μg); MD: middle-dose group (100 μg); LD: low-dose group (50 μg). Each group contains 8 animals.
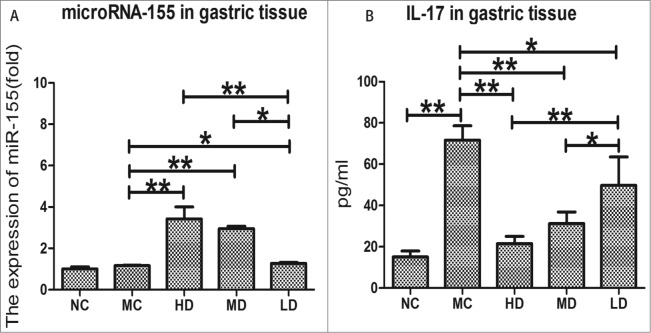
The expression of microRNA-155 in gastric tissue of the C57/BL6 mice model after immunization with CTB-UE
MicroRNA-155 plays an important role in the immune system; it can specifically regulate T17 cell differentiation.9 On the other hand, IL-17 increased both H. pylori load and inflammation, and contributed to pathology in mice.18 In this study, we examined the expression of microRNA-155 and interleukin-17 production in gastric tissue for finding their relationship.
As shown in Figure 4A, compared with the NC group, the expression of microRNA-155 in the MC group was slightly up-regulated but not significantly (P > 0.05). However, the expression of microRNA-155 in the HD, MD, and LD groups was highly upregulated compared with the MC group (P < 0.01 or P < 0.05), and there were significant differences among the HD, MD, and LD groups (P < 0.01 or < 0.05), indicating an evident dose-effect relationship.
Figure 4.
The expression of microRNA-155 and the IL-17 production in gastric tissue. (A). The expression of microRNA-155 in gastric tissue of the C57/BL6 mice model after treatment with CTB-UE. The fold differences in expression are shown. (B). The IL-17 production in gastric tissue of the C57/BL6 mice model after treatment with CTB-UE. The bars indicate the mean±SD . Each group contained 8 samples (n = 8/group). *, P < 0.05 and **, P < 0.01 comparing 2 groups. NC: normal control group; MC: model control group; HD: high-dose group (200 μg); MD: middle-dose group (100 μg); LD: low-dose group (50 μg). Each group contains 8 animals.
The interleukin-17 production in gastric tissue of the C57/BL6 mice model after immunization with CTB-UE
As shown in Figure 4B, the production of IL-17 in the MC group was significantly higher than in the NC group (P < 0.01). In contrast, the production of IL-17 in the HD, MD and LD groups was substantially lower than in the MC group (P < 0.01 or P < 0.05), and there were significant differences among the HD, MD, and LD groups (P < 0.01 or P < 0.05), indicating an evident dose-effect relationship.
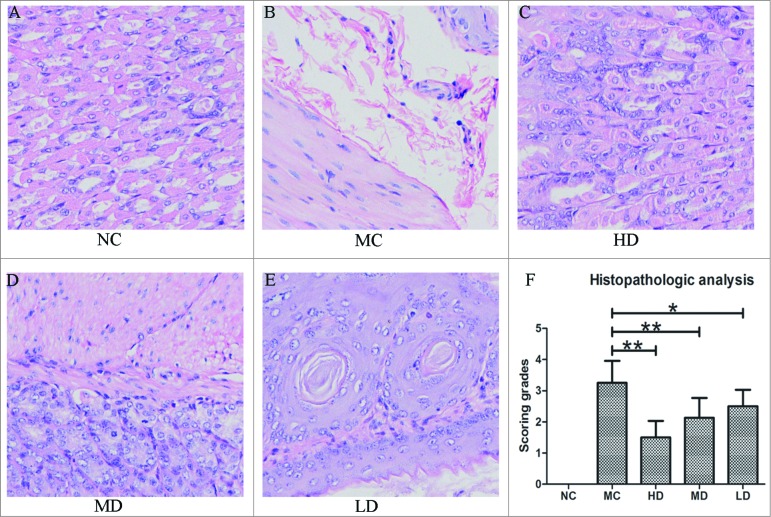
Histopathological analysis of gastric mucosa
No gastric mucosal inflammation was present in the NC group. Neutrophil infiltration was significantly increased in the MC group compared with the NC group. However, increased levels of leukocytes and neutrophils were found in the stomachs of the LD and MD groups. Moreover, the neutrophil infiltration in the HD, MD, and LD groups was significantly lower than in the MC group (Fig. 5A–E).
Figure 5.
Assessment of changes in H. pylori–induced gastric mucosal inflammation in the C57/BL6 mice model after treatment with CTB-UE. (A, B, C, D, E). Representative haematoxylin-eosin–stained stomach sections from the NC, MC, HD, MD, and LD groups (original magnification, × 200). (F). The histopathological changes in the gastric mucosa were determined for individual C57/BL6 mice in each group using ascoring scale. The bars indicate the mean±SD. Each group contained 8 samples (n = 8/group). *, P < 0.05 and **, P < 0.01 comparing 2 groups. NC: normal control group; MC: model control group; HD: high-dose group (200 μg); MD: middle-dose group (100 μg); LD: low-dose group (50 μg). Each group contains 8 animals.
As shown in Figure 5F, the scoring grades in the MC group were significantly higher than in the NC group. In contrast, the scoring grades in the HD, MD, and LD groups were significantly lower than in the MC group (P < 0.01 or P < 0.05).
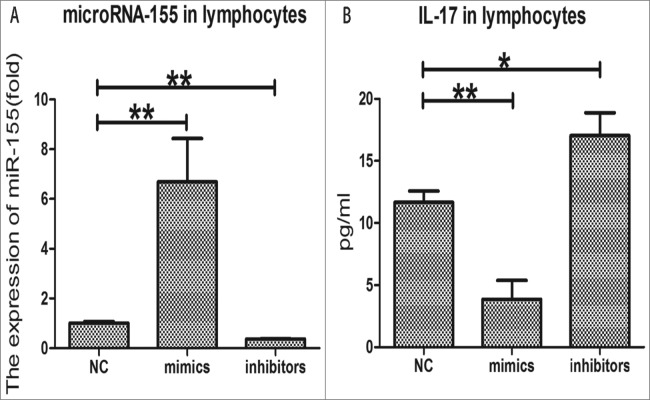
Determination of interleukin-17 production in spleen lymphocytes after the expression of microRNA-155 being up-regulated and downregulated
For further analyzing the relationship between miRNA-155 and IL-17, we transfected lymphocytes with mimics and inhibitors of miRNA-155; then, we determined the IL-17 production in spleen lymphocytes.
As shown in Figure 6A, compared with the NC group, the expression of microRNA-155 in the mimics group was highly upregulated (P < 0.01). However, the expression of microRNA-155 in the inhibitors group was significantly down-regulated compared with the NC group (P < 0.01).
Figure 6.
The IL-17 production in spleen lymphocytes after the expression of microRNA-155 being upregulated and downregulated (A). The expression of microRNA-155 in spleen lymphocytes after transfection with mimics and inhibitors. The fold differences in expression are shown. (B). The IL-17 production in spleen lymphocytes after the expression of microRNA-155 being up-regulated and down-regulated. The bars indicate the mean±SD. Each group contained 8 samples (n = 8/group). *, P < 0.05 and **, P < 0.01 comparing 2 groups. NC: normal control group; MC: model control group; HD: high-dose group (200 μg); MD: middle-dose group (100 μg); LD: low-dose group (50 μg). Each group contains 8 animals.
As shown in Figure 6B, the production of IL-17 in the mimics group was substantially lower than in the NC group (P < 0.01). In contrast, the production of IL-17 in the inhibitors group was significantly higher than in the NC group (P < 0.05).
Discussion
Studies concerning CD4+ T cell responses against H. pylori have predominantly focused on the Th1/Th2 paradigm. Extensive studies have demonstrated that H. pylori infection resulted in a Th1-dominant response and gastric inflammation largely depends on Th1 cell responses.27,28 However, many studies suggest that IL-17 may play an important role in the inflammatory response to the H. pylori infection and ultimately influence the outcome of the H. pylori-associated disease.7,8,18 And many studies have suggested the importance of IL-17 in immune responses to a variety of infectious agents, especially those caused by extracellular pathogens.27,29 In view of Th17 responses to multi-epitope vaccine CTB-UE, we examined the IL-17 production in antiserum. The results suggested that CTB-UE decreased IL-17 production, implying that Th17 responses may be inhibited. Furthermore, we found that IL-17 aggravated GES-1 cell injury induced by H. pylori SS1; In contrast, CTB-UE antiserum could evidently alleviate this cell injury, suggesting that CTB-UE can protect H. pylori-infected GES-1 cell by inhibiting Th17 responses.
Th17 cells have been reported to contribute to the pathogenesis of H. pylori-associated gastric cancer.30 In this study, we created a stable H. pylori infection model in C57/BL6 mice by inoculating the animals with H. pylori SS1. Treatment with CTB-UE resulted in a good therapeutic effect on mucosal inflammation in H. pylori infection. We found that urease activity in the stomachs was significantly decreased and gastric mucosal inflammation was evidently alleviated, implying that CTB-UE reduced the H. pylori burden. In addition, IL-17 production in gastric tissue was substantially lower than in the H. pylori-infected mice. The result was consistent with previous report that the production of IL-17 in the stomach in H. pylori-infected mice was increased.31-34 Furthermore, we found that the expression of microRNA-155 in gastric tissue was significantly up-regulated after treatment with CTB-UE. Our results suggested that the microRNA-155/IL-17 pathway was involved in H. pylori-induced gastric inflammatory reaction. Further study is required to elucidate the relationship between miRNA-155 and IL-17. We found that the production of IL-17 was significantly increased after the expression of miRNA-155 being downregulated; and the production of IL-17 was significantly decreased after the expression of miRNA-155 being upregulated. The results suggested that CTB-UE could relieve the H. pylori-induced gastric inflammatory reaction via up-regulating microRNA-155 to inhibit Th17 responses.
The current studies suggested that Helicobacter pylori could induce Th17 responses to stimulate bacterial growth, and resulted in significant pathology in mice.18,34 They evaluated the role of the Th17 subset in H. pylori infection. H. pylori infection induced significant expression of IL-17 and IFN-ɣ in mouse gastric tissue. But it is still unclear that IL-17 was regulated in this procedure. In our study, IL-17 could aggravate GES-1 cell injury induced by H. pylori; and this cell injury could be evidently alleviated by CTB-UE antiserum. Furthermore, we found that microRNA-155 could regulate IL-17 responses, implying that microRNA-155/IL-17 pathway was involved in H. pylori-induced gastric inflammatory reaction and this vaccine could relieve gastric inflammatory reaction via regulating this pathway.
The Th17/IL-17 pathway has been suggested as a target for therapeutic intervention in treatment of autoimmune and chronic inflammatory disorders associated with Th17.35 Because H. pylori infection leads to chronic gastritis and persistent infection, IL-17 may be potential targets for immunotherapy of H. pylori-related diseases. IL-17 antagonistic strategies of up-regulating microRNA-155 may be an alternative for controlling the H. pylori load, because a multi-epitope vaccine CTB-UE reduces the load and exerts protective effects.
In conclusion, high level of IL-17 contributes to gastric inflammation and H. pylori colonization. Immunization with the multi-epitope vaccine CTB-UE can alleviate gastric mucosal inflammation via up-regulating microRNA-155 to inhibit Th17 responses. Further study is required to elucidate the targets by which microRNA-155 regulates Th17 cells responses. A better understanding of the nature, regulation, and function of microRNA-155/IL-17 pathway against H. pylori infection may help to explore novel and effective vaccine-immunotherapies for gastric diseases induced by this organism.
However, there are still some limitations in our study. We certified that high level of IL-17 contributed to gastric inflammation, but it was still unclear that IL-17 aggravated gastric cells injury in H. pylori-infected mice by some way. We found that the multi-epitope vaccine CTB-UE could alleviate gastric mucosal inflammation via up-regulating microRNA-155 to inhibit Th17 responses, but we still didn't know which signaling pathway and target protein were involved. Further study is required to elucidate this procedure. In addition, we plan to study the pharmacokinetics and therapeutic effect of the CTB-UE vaccine in rats in the future. The data from the C57/BL6 mice will provide necessary information for the further development of therapeutic vaccines against H. pylori.
Materials and Methods
Reagents
RPMI-1640 culture medium and foetal calf serum (FCS) were purchased from Gibco Company (New York, America). The IL-17 ELISA Kit was purchased from Beijing Xinbosheng Biological Technology Co., Ltd (Beijing, China). The recombinant IL-17 was purchased from PEPROTECH Company (New Jersey, America). LDH micro-ELISA Kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The RNApure Tissue Kit was purchased from Beijing Kangwei Biological Technology Co., Ltd (Beijing, China). The miRNA qPCR Detection Primer Set Kit and SYBR qPCR Kit were purchased from Baiaomaike Biological Technology Co., Ltd (Nantong, China).
H. pylori. strain
The mouse-adapted H. pylori strain SS1 (H. Pylori SS1), which was first developed by Lee et al.,13 was obtained from the National Centers for Disease Control and Prevention and maintained in our laboratory (collection no. CPU-BS-09). H. pylori SS1 were cultured on brain–heart infusion (BHI) plates containing 7% FCS, 5 μg/ml trimethoprim, 5 μg/ml polymixin B, and 10 μg/ml vancomycin under microaerophilic conditions at 37°C for 4 d The bacteria were harvested and resuspended in normal sodium, and the final concentration was adjusted to a density of 5 × 108 colony forming units (CFUs) per milliliter before inoculation. Through prior experiments, we found that the H. pylori strain could colonise) the stomach of C57/BL6 mice, resulting in a stable Helicobacter pylori infection model.
Animal model
All experiments were performed using male C57/BL6 adult mice (8-week-old; 18–22g weight). Animals were purchased from the Experimental Animal Center of Zhejiang Province and bred in an axenic environment. All animal experiments were approved by the Animal Ethical and Experimental Committee of China Pharmaceutical University. The H. pylori infection model was initiated through the intragastric administration of H. pylori to C57/BL6 mice. Three days before H. pylori administration, C57/BL6 mice were given a mixture of antibiotics containing 10 mg of AMP, 4 mg of GM, and 3.2 mg of azithromycin by oral gavage (once per day for 3 days). On the fourth day, the C57/BL6 mice were inoculated with 0.3 ml of the H. pylori SS1 dilution (5 × 108 CFUs) by oral gavage 5 times during a 10-day period, with 2 d between each gavage. C57/BL6 mice were bred for 7 weeks before the evaluation of H. pylori infection. In the eighth week, the H. pylori infection model was determined to be successfully established using the following 2 criteria: a positive result for urease activity and histopathological indicators of inflammation in the stomach tissue. In prior experiments, we found that H. pylori SS1 could successfully colonize in the stomach of C57/BL6 mice. Through using antibiotics mixture 3 d before H. pylori administration, the mixed bacterium in the stomach could be cleared, which was beneficial to the colonization of H. pylori. Therefore, in formal experiment, we used antibiotics mixture before H. pylori administration and developed a stable infection model.
Cell culture and H. pylori infection
The GES-1 immortalized gastric epithelial cell line was a gift from the Shanghai Institute of Digestive Disease. The cells were routinely cultured in RPMI-1640 supplemented with 100 U/ml penicillin, 100 U/ml streptomycin, and 10% fetal bovine serum (FBS), in a humidified atmosphere at 37°C in 5% CO2. The GES-1 cells in logarithmic phase were harvested and resuspended in RPMI-1640, and the final concentration was adjusted to a density of 3 × 106 per milliliter before inoculation. One mL cell suspension was inoculated in each well of 6-well culture plate and cultured for 4 hours, then treated with 100 μL H. pylori SS1 (H. pylori SS1 : GES-1 = 100 : 1) for 24 hours.
Multi-epitope vaccine CTB-UE preparation
The high purity (95.7%), multi-epitope vaccine CTB-UE was prepared as previously described.14 Briefly, the predominant B and Th cell epitopes from H. pylori urease A and B subunits were selected according to the design theory of the multi-epitope vaccine and bioinformatics software. The theoretically optimal combination of the intramolecular adjuvant CTB, UreA74–79 (Th cell epitopes from UreA), UreB229–244 and UreB237–251(Th cell epitopes from UreB), UreA183–203 (B cell epitopes from UreA), UreB327–334 (B cell epitopes from UreB), and the linker (DPRVPSS) for the multi-epitope vaccine CTB-UE was established on the basis of modeling and prediction using RANKPEP and DNA star software. The nucleotide sequences of CTB-UE are included in a patent that has been authorised by China State Intellectual Property Bureau (grant no. ZL201110064596.8). The fusion protein CTB-UE expression plasmid pETCUE was constructed by enzyme digestion and PCR. The CTB gene was sub-cloned, and the UE genes containing UreA74–79, UreB229–244,UreB237–251, UreA183–203, and UreB327–334 were inserted into the pET28a expression vector (Novagen, USA).Then, E. coli DH5α (ATCC 53868) were used as a host for the propagation of the plasmids. The E. coli BL21 (DE3) strain, which contains the T7 RNA polymerase gene under the control of the lac promoter in a lysogenic form, was used to express the fusion proteins. The recombinant CTB-UE protein was purified by Ni2+-charged column chromatography (Bio Basic Inc., Markham, Canada) and anion exchange chromatography using DEAE Sepharose FF (Amersham Pharmacia Biotech AB, Sweden). After purification, the purity of the fusion CTB-UE peptide was analyzed by 12% SDS-PAGE and a computer scan. The samples were dialysed in 30 mM Tris–HCl buffer (pH 8.0) in one step and finally concentrated with PEG 20000 and stored at −70°C.
Determination of interleukin-17 production in Antiserum
After overnight fasting, C57/BL6 mice (8 animals per group) were vaccinated intragastrically with 200 μg, 100 μg, 50 μg of vaccine antigen (CTB-UE) in 300 μl aluminum hydroxide adjuvant (Thermo, UK) 4 times at 1-week intervals. The normal control group and model control group were given intragastrically with 300 μl PBS using the same method as control. Two weeks after the final immunization, all mice were exanguinated via angular vein. The serum was isolated to assay for interleukin-17 (IL-17) using ELISA kits according to the manufacturer's instructions. In addition, the Antiserum from 100 μg dose group mice was collected to treat infected GES-1 cells.
Determination of GES-1 xìbāo huóxìng
GES-1 cells activity
The GES-1 cells in logarithmic phase were harvested and resuspended in RPMI-1640, and the final concentration was adjusted to a density of 3 × 106 per milliliter before inoculation. One mL cell suspension was inoculated in each well of 6-well culture plate and cultured for 4 hours, then divided into the following 6 groups: the normal control group (NC); the H. pylori infection group (MC); the CTB-UE antiserum group (AS); IL-17 treatment at a concentration of 10 ng/mL (I10), IL-17 treatment at a concentration of 20 ng/mL (I20) and IL-17 treatment at a concentration of 40 ng/mL (I40) groups. Then, each group cells was treated as follows: In the AS group, 100 μL 200:1 dilution CTB-UE Antiserum was added to the cells; In I10, I20 I40 group, 100 μL IL-17 was added to the cells, followed by a final concentration of 10, 20, or 40 ng/mL; In the NC and MC group, 100 μL RPMI-1640 was added to the cells. After being cultured for 24 hours, except for the NC group, 100 μL H. pylori SS1 dilution was added to each group of cells at a multiplicity of infection (MOI) of 100 : 1 and co-cultured with cell lines for 24 hours. Then, the cellular supernatant was isolated to assay for GES-1 cells activity using LDH micro-ELISA Kits according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute).
Animal experiments
C57/BL6 mice were randomly divided into the following 5 groups (8 animals per group): the normal control group (NC); the model control group (MC); the high-dose group (HD); the middle-dose group (MD); and the low-dose group (LD). Except for the NC group, each group of C57/BL6 mice was inoculated with 0.3 ml of H. pylori SS1 to establish the H. pylori infection model according to the method described above. On the 8th week after infection, each group received various drugs as follows. After overnight fasting, the HD, MD, and LD groups were vaccinated intragastrically with 200, 100, and 50 μg of the vaccine antigen (CTB-UE), respectively, in 300 μl of aluminum hydroxide adjuvant (Thermo, UK); the vaccine was administered 4 times at 1-week intervals. The NC and MC groups were intragastrically administered 300 μl of aluminum hydroxide adjuvant using the same method as the control. Before vaccination, bicarbonate was administered to neutralize stomach acid. Two weeks after the final immunisation, the C57/BL6 mice were sacrificed by excess ether anesthesia, and the stomach was rapidly excised. Half of the stomach was placed into 10% formaldehyde for histopathological analysis. The antral portion of the stomach was immediately placed in urease diagnostic reagent to determine urease activity. The other portion of the stomach was homogenized for real-time quantitative PCR and for the determination interleukin-17 production.
Urease activity determination
The degree of H. pylori colonisation in the C57/BL6 mice stomach was measured by the presence of active urease in the stomach tissue, as previously described.15 Each group of C57/BL6 mice was sacrificed for the determination of urease activity in the stomach. Briefly, the antral portion of the stomach was immediately placed in a microfuge tube containing 300 μl of the urease diagnostic reagent (Fujian Sanqiang Biochemical Co., Ltd.). The stomach sample was incubated for 4 h at room temperature, and the absorbance was measured at 550 nm (A550).
Real-time quantitative PCR for miroRNA-155 expression in gastric tissue
Total RNA was extracted from 30 mg of gastric tissue from C57/BL6 mice in each group using the RNApure Tissue Kit according to the manufacturer's instructions. Extraction was verified by electrophoresis on a 1% agarose gel and an absorbance (A260/280) value of 1.8 to 2.0. cDNA (cDNA) was generated using the miRNA qPCR Detection Primer Set Kit. PCR was performed with 5 μl of RNA template, 5 μl of RT Primer Mix, 10 μl of 5 × RT Buffer, 2.5 μl of dNTPs (10 mM), 2 μl of reverse transcriptase, 25.5 μl of DEPC-H2O in a 50 μl reaction volume and was incubated for one cycle of 95°C for 10 min, 42°C for 60 min, and 30°C for 10 min, followed by 30 cycles. Real-time quantitative PCR was performed with the SYBR qPCR Kit. RT-qPCR was performed with 2 μl of miRNA CDNA, 10 μl of 2 × SYBR Green Mix, 1 μl of forward primer (10μM), 1 μl of reverse primer (10 μM), 6 μl of DEPC-H2O in a 20 μl reaction volume under the conditions of one cycle of initial denaturation at 95°C for 10 min, 95°C for 20 s, 62°C for 30 s and 72°C for 30 s, followed by 40 cycles.
Determination of interleukin-17 production in gastric tissue
A total of 50 mg of gastric tissue from C57/BL6 mice in each group was homogenized in 500 μL of N.S. with a tissue homogenizer. After centrifugation, the homogenate supernatants were harvested to analyze the interleukin-17 (IL-17) levels using ELISA kits according to the manufacturer's instructions.
Histopathological examinations
Half of the tissue cut from each stomach was fixed with 10% formalin and embedded in paraffin. The tissues were stained with haematoxylin and eosin (HE) and assessed for H. pylori-associated inflammation. For evaluation of gastritis, HE-stained sections were scored based on the degree of infiltrating lymphocytes, plasma cells, and neutrophils. The scoring grades were defined as follows: 0, none; 1, a few leukocytes scattered in the deep mucosa; 2, moderate numbers of leukocytes in the deep to mid mucosa and occasional neutrophils in the gastric glands, with microabscesses; 3, dense infiltrates in the deep to mid mucosa, with a few microabscesses; and 4, dense, diffuse infiltrates throughout the lamina propria and into the submucosa, with frequent microabscesses.
Determination of interleukin-17 production in spleen lymphocytes after the expression of miRNA-155 being up-regulated and down-regulated
The spleens from wild C57/BL6 mice were cut and levigated. Lymphocytes were isolated from spleens with lymphocyte separation medium (Dakewe, Shenzhen, China) and seeded in a 6-well plate at 5 × 106 cells /well. Mimics, inhibitors, and scrambled negative control (NC) of miR-155 were purchased from Biomics Biotechnology. Transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Total RNA was extracted using Trizol at the designated time points after transfection. The miroRNA-155 expression level in lymphocyte was determined by SYBR Green qRT-PCR method using miR-155 Detection Kit and EzOmics SYBR qPCR Kit (Biomics, Jiangsu, China) according to the method described above. The cellular supernatant was isolated to assay for interleukin-17 (IL-17) using ELISA kits according to the manufacturer's instructions.
Acknowledgments
We thank all of the staffs for their helpful suggestions and advice during this project.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Major Special Program of New Drug Research and Development (grant no.2012ZX09103301–008) and Natural ScienceFoundation of Jiangsu Province (grant no. BK20130647).
References
- 1. Sette A, Keogh E, Ishioka G, Sidney J, Tangri S, Livingston B, et al. Epitope identification and vaccine design for cancer immunotherapy. J. Curr Opin Investig Drugs 2002; 3:132-139; PMID:12054064 [PubMed] [Google Scholar]
- 2. Kusters JG, AH van Vliet E, Kuipers J. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 2006; 19:449-490; PMID:16847081; http://dx.doi.org/ 10.1128/CMR.00054-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007; 133:659-672; PMID:17681184; http://dx.doi.org/ 10.1053/j.gastro.2007.06.026 [DOI] [PubMed] [Google Scholar]
- 4. Zhao W, Wu W, Xu X. Oral vaccination with liposome-encapsulated recombinant fusion peptide of urease B epitope and cholera toxin B subunit affords prophylactic and therapeutic effects against H. pylori infection in BALB/c mice. Vaccine 2007; 25(44):7664-7673; PMID:17913305; http://dx.doi.org/ 10.1016/j.vaccine.2007.08.034 [DOI] [PubMed] [Google Scholar]
- 5. Zhou WY, Shi Y, Wu C, Zhang WJ, Mao XH, Guo G, et al. Therapeutic efficacy of a multi-epitope vaccine against Helicobacter pylori infection in BALB/c mice model. Vaccine 2009; 27(36):5013-5019; PMID:19446591; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 6. Le Guo, Kunmei Liu, Guangxian Xu, Xiaokang Li, Jiajie Tu, Feng Tang, Xing Y, Xi T. Prophylactic and therapeutic efficacy of the epitope vaccine CTB-UA against Helicobacter pylori infection in a BALB/c mice model. Appl Microbiol Biotechnol 2012; 95:1437-1444; PMID:22569640; http://dx.doi.org/ 10.1007/s00253-012-4122-0 [DOI] [PubMed] [Google Scholar]
- 7. Satoshi Shiomi, Akihiro Toriie, Shigeyoshi Imamura, Hideyuki Konishi, Shoji Mitsufuji, Yoichiro Iwakura, Yamaoka Y, Ota H, Yamamoto T, Imanishi J, et al. IL-17 is Involved in Helicobacter pylori-Induced Gastric Inflammatory Responses in a Mouse Model. Helicobacter 2008; 13(6):518-524; PMID:19166417; http://dx.doi.org/ 10.1111/j.1523-5378.2008.00629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, Eaton KA, Zou W, Berndt BE, Cole TS, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology 2010; 138(3):1046-1054; PMID:19931266; http://dx.doi.org/ 10.1053/j.gastro.2009.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rui-Xue Leng, Hai-Feng Pan, Wei-Zi Qin, Gui-Mei Chen, Dong-Qing Ye. Role of microRNA-155 in autoimmunity. Cytokine & Growth Factor Rev 2011; 22:141-147; PMID:21703910; http://dx.doi.org/ 10.1016/j.cytogfr.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 10. Antony Rodriguez, Elena Vigorito, Simon Clare, Madhuri V Warren, Philippe Couttet, Dalya RSoond, van Dongen S, Grocock RJ, Das PP, Miska EA, et al. Requirement of bic/microRNA-155 for normal immune function. Science 2007; 316:608-611; PMID:17463290; http://dx.doi.org/ 10.1126/science.1139253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 2010; 10:111-122; http://dx.doi.org/ 10.1038/nri2708 [DOI] [PubMed] [Google Scholar]
- 12. Lei Tian, Gert De Hertogh, Maya Fedeli, Kim A. Staats, Susann Schonefeldt, Stephanie Humblet-Baron, Van Den Bosch L, Dellabona P, Dooley J, Liston A. Loss of T cell microRNA provides systemic protection against autoimmune pathology in mice. J Autoimmun 2012; 38: 39-48; PMID:22225602; http://dx.doi.org/ 10.1016/j.jaut.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 13. Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 1997; 112(4):1386-1397; PMID:9098027; http://dx.doi.org/ 10.1016/S0016-5085(97)70155-0 [DOI] [PubMed] [Google Scholar]
- 14. Le Guo, Runting Yin, Kunmei Liu, Xiaobo Lv, Yonghong Li, Xiangguo Duan, Chu Y, Xi T, Xing Y. Immunological features and efficacy of a multi-epitope vaccine CTB-UE against H. pylori in BALB/c mice model. Appl Microbiol Biotechnol 2014; 98(8):3495-507; PMID:24370888; http://dx.doi.org/ 10.1007/s00253-013-5408-6 [DOI] [PubMed] [Google Scholar]
- 15. Oscar G. Gomez-Duarte, Bernadette Lucas, Zheng-Xin Yan, Klaus Panthel, Rainer Haas, Thomas FMeyer. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine 1998; 16(5):460-471; PMID:9491500; http://dx.doi.org/ 10.1016/S0264-410X(97)00247-8 [DOI] [PubMed] [Google Scholar]
- 16. CDC (2006) Helicobacter pylori and peptic ulcer disease: the key to a cure [Google Scholar]
- 17. Yinyao Lin, Samantha R. Slight, Shabaana A. Khader. Th17 cytokines and vaccine-induced immunity. Semin Immunopathol 2010; 32:79-90; PMID:20112107; http://dx.doi.org/ 10.1007/s00281-009-0191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yun Shi, Xiao-Fei Liu, Yuan Zhuang, Jin-Yu Zhang, Tao Liu, Zhinan Yin, Wu C, Mao XH, Jia KR, Wang FJ, et al. Helicobacter pylori-Induced Th17 Responses Modulate Th1 Cell Responses, Benefit Bacterial Growth, and Contribute to Pathology in Mice. J Immunol 2010; 184:5121-5129; PMID:20351183; http://dx.doi.org/ 10.4049/jimmunol.0901115 [DOI] [PubMed] [Google Scholar]
- 19. Eaton KA, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun 1994; 62(9): 3604-3607; PMID:8063376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsuda M, Karita M, Mizote T, Morshed MG, Okita K, Nakazawa T. Essential role of Helicobacter pylori urease in gastric colonization: definite proof using a urease-negative mutant constructed by gene replacement. Eur J Gastroenterol Hepatol 1994; 6(l1): 49-52 [PubMed] [Google Scholar]
- 21. Andrutis KA, Fox JG, Schauer DB, Marini RP, Murphy JC, Yan L, Solnick JV. Inability of an isogenic urease-negative mutant stain of Helicobacter mustelae to colonize the ferret stomach. Infect Immun 1995; 63(9):3722-3725; PMID:7642314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graham David Y, Yoshio Yamaoka. Disease-specific Helicobacter pylori virulence factors: the unfulfilled promise. Helicobacter 2000; 5(Suppl l):S3-S9; PMID:10828748; http://dx.doi.org/ 10.1046/j.1523-5378.2000.0050S1003.x [DOI] [PubMed] [Google Scholar]
- 23. Meyer-Rosberg K, Scott DR, Rex D, Melchers K, Sachs G. The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology 1996; 111(4):886-900; PMID:8831583; http://dx.doi.org/ 10.1016/S0016-5085(96)70056-2 [DOI] [PubMed] [Google Scholar]
- 24. Hu LT, Mobley HL. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun 1990; 58(4):992-998; PMID:2318539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eaton KA, Brooks CL, Morgan DR, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun 1991; 59(7):2470-2475; PMID:2050411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Athanasios Makristathis, Elmar Rokita, Agnes Labigne, Birgit Willinger, Manfred L, Rotter Alexander. Highly significant role of Helicobacter pylori urease in phagocytosis and production of oxygen metabolites by human granulocytes. J Infect Dis 1998; 177(3):803-806; PMID:9498470; http://dx.doi.org/ 10.1086/517814 [DOI] [PubMed] [Google Scholar]
- 27. Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol 2001; 166:7456-7461; PMID:11390498; http://dx.doi.org/ 10.4049/jimmunol.166.12.7456 [DOI] [PubMed] [Google Scholar]
- 28. Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol 2000; 165:1022-1029; PMID:10878379; http://dx.doi.org/ 10.4049/jimmunol.165.2.1022 [DOI] [PubMed] [Google Scholar]
- 29. Wenjun Ouyang, Jay K, Kolls, Yan Zheng. The Biological Functions of T Helper 17 Cell Effector Cytokines in Inflammation. Immunity 2008; 28(4):454-467; PMID:18400188; http://dx.doi.org/ 10.1016/j.immuni.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bin Zhang, Guanghua Rong, Huafeng Wei, Meng Zhang, Jianwei Bi, Liye Ma, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem. Biophys. Res. Commun. 2008; 374:533-537; PMID:18655770; http://dx.doi.org/ 10.1016/j.bbrc.2008.07.060 [DOI] [PubMed] [Google Scholar]
- 31. Caruso R, Fina D, Paoluzi OA, Del Vecchio Blanco G, Stolfi C, Rizzo A, Caprioli F, Sarra M, Andrei F, et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur J Immunol 2008; 38: 470-478; PMID:18200634; http://dx.doi.org/ 10.1002/eji.200737635 [DOI] [PubMed] [Google Scholar]
- 32. Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol 2000; 165:5332-5337; PMID:11046068; http://dx.doi.org/ 10.4049/jimmunol.165.9.5332 [DOI] [PubMed] [Google Scholar]
- 33. Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Jr, Cover TL. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol. Med. Microbiol 2007; 51:577-586; PMID:17919297; http://dx.doi.org/ 10.1111/j.1574-695X.2007.00338.x [DOI] [PubMed] [Google Scholar]
- 34. Yun Shi, Xiao-Fei Liu, Yuan Zhuang, Tao Liu, Zhinan Yin, Chao Wu, Mao XH, Jia KR, Wang FJ, Guo H, et al. Helicobacter pylori-Induced Th17 Responses Modulate Th1 Cell Responses, Benefit Bacterial Growth, and Contribute to Pathology in Mice. The Journal of Immunology 2010; 184(9):5121-5129; PMID:20351183; http://dx.doi.org/ 10.4049/jimmunol.0901115 [DOI] [PubMed] [Google Scholar]
- 35. Dong C. IL-23/IL-17 biology and therapeutic considerations. J Immunotoxicol 2008; 5:43-46; PMID:18382857; http://dx.doi.org/ 10.1080/15476910801897953 [DOI] [PubMed] [Google Scholar]