Abstract
We previously reported higher anti-HPV-16 and -18 immune responses induced by HPV-16/18 vaccine compared with HPV-6/11/16/18 vaccine at Month 7 (one month after completion of full vaccination series) in women aged 18–45 y in an observer-blind study NCT00423046; the differences of immune response magnitudes were maintained up to Month 24. Here we report follow-up data through Month 48. At Month 48, in according-to-protocol cohort for immunogenicity (seronegative and DNA-negative for HPV type analyzed at baseline), geometric mean titers of serum neutralizing antibodies were 2.0- to 5.2-fold higher (HPV-16) and 8.6- to 12.8-fold higher (HPV-18) in HPV-16/18 vaccine group than in HPV-6/11/16/18 vaccine group. The majority of women in both vaccine groups remained seropositive for HPV-16. The same trend was observed for HPV-18 in HPV-16/18 vaccine group; however, seropositivity rates in HPV-6/11/16/18 vaccine group decreased considerably, particularly in the older age groups. In the total vaccinated cohort (regardless of baseline serological and HPV-DNA status), anti-HPV-16 and -18 neutralizing antibody levels induced by HPV-16/18 vaccine were higher than those induced by HPV-6/11/16/18 vaccine. CD4+ T-cell response for HPV-16 and HPV-18 was higher in HPV-16/18 vaccine group than in HPV-6/11/16/18 vaccine group. Memory B-cell responses appeared similar between vaccine groups. Both vaccines were generally well tolerated. Overall, the higher immune response observed with the HPV-16/18 vaccine was maintained up to Month 48. A head-to-head study incorporating clinical endpoints would be required to confirm whether the observed differences in immune response between the vaccines influence the duration of protection they provided.
Keywords: Cervarix®, Gardasil®, human papillomavirus, immunogenicity, safety
Abbreviations
- ANOVA
analysis of variance
- AS04
Adjuvant System containing 3-O-desacyl-4’-monophosphoryl lipid A (MPL; 50 μg) adsorbed on aluminum salt (500 μg Al(OH)3)
- ATP
according-to-protocol
- CI
confidence interval
- CMI
cell-mediated immune
- CVS
cervicovaginal secretion
- ED50
effective dose producing 50% response
- ELISA
enzyme-linked immunosorbent assay
- GM
geometric mean
- GMR
geometric mean (titer) ratio
- GMT
geometric mean titer
- HPA
Health Protection Agency
- HPV
human papillomavirus
- IgG
immunoglobulin G
- MSC
medically significant condition
- nAb(s)
neutralizing antibody(ies)
- NOAD
new onset autoimmune disease
- NOCD
new onset chronic disease
- PBMC
peripheral blood mononuclear cells
- PBNA
pseudovirion-based neutralization assay
- SAE
serious adverse event
- TVC
total vaccinated cohort
- VLP
virus-like particle
Introduction
Persistent infection with an oncogenic human papillomavirus (HPV) is a necessary cause of invasive cervical cancer.1 HPV infections are frequently acquired soon after initiation of sexual activity,2,3 women remain at risk while they are sexually active.4 Therefore, long-term protection against HPV infection is required to reduce the prevalence and burden of what is the second most common cancer in women worldwide.5
HPV-16 and HPV-18 are responsible for around 70% of cervical cancer cases globally.6 Prophylactic HPV vaccines designed to induce protection against both HPV-16 and HPV-18 are currently licensed in over 130 countries. Cervarix® (GlaxoSmithKline Vaccines) is a HPV-16/18 AS04-adjuvanted vaccine containing HPV-16 and -18 virus-like particles (VLPs) assembled from the L1 major capsid proteins of HPV-16 and HPV-18. It is formulated with a proprietary immunostimulatory Adjuvant System (AS04) containing 3-O-desacyl-4’-monophosphoryl lipid A (50 μg MPL) adsorbed on aluminum salt (500 μg Al(OH)3).7 The HPV-16/18 vaccine has shown efficacy against incident and persistent HPV-16/18 infection and cervical intraepithelial neoplasia grade 2+ associated with HPV-16/18 for up to 6.4 y.8-10 Gardasil® (Merck and Co., Inc..) is a HPV-6/11/16/18 vaccine containing L1 VLPs for HPV types 6, 11, 16 and 18 and is formulated with amorphous aluminum hydroxyphosphate sulfate adjuvant. To date, efficacy of the HPV-6/11/16/18 vaccine has been demonstrated through 5 y of follow-up.11
Immunogenicity and safety of the HPV-16/18 and HPV-6/11/16/18 vaccines have been compared in a randomized, observer-blind study in healthy women aged 18–45 y (study HPV-010; NCT00423046). The primary objective was analyzed at Month 7, where we showed that geometric mean titers (GMTs) of serum anti-HPV-16 and anti-HPV-18 neutralizing antibodies (nAbs), determined by a pseudovirion-based neutralization assay (PBNA), elicited by the HPV-16/18 vaccine were significantly higher than those elicited by the HPV-6/11/16/18 vaccine.12 These differences were maintained up to Month 24,13 and CD4+ T-cell responses for HPV-16 and HPV-18 were consistently higher with the HPV-16/18 vaccine. We now report extended follow-up of this study cohort up to Month 48.
Results
Study population
Between January and April 2007, 1,106 women were enrolled, randomized into 2 vaccine groups (n = 553 each) and received one or more vaccine dose (total vaccinated cohort [TVC]). Among them, 524 women consented to participate in the extended follow-up and attended the Month 48 visit (259 in the HPV-16/18 vaccine group and 265 in the HPV-6/11/16/18 vaccine group). The according-to-protocol (ATP) cohort for immunogenicity (all subjects who met all eligibility criteria, received 3 vaccine doses and complied with study procedures) comprised 421 women (205 women in the HPV-16/18 vaccine group and 216 in the HPV-6/11/16/18 vaccine group). In the Month 48 immunogenicity analysis, the number of subjects excluded and the reasons for exclusion were similar between the vaccine groups (Fig. 1). In the TVC, the characteristics of subjects who attended the Month 48 visit were comparable between vaccine groups (mean age 31 y in both vaccine groups at study entry, 86% vs. 82% Caucasian in the HPV-16/18 and HPV-6/11/16/18 vaccine groups, respectively).
Figure 1.
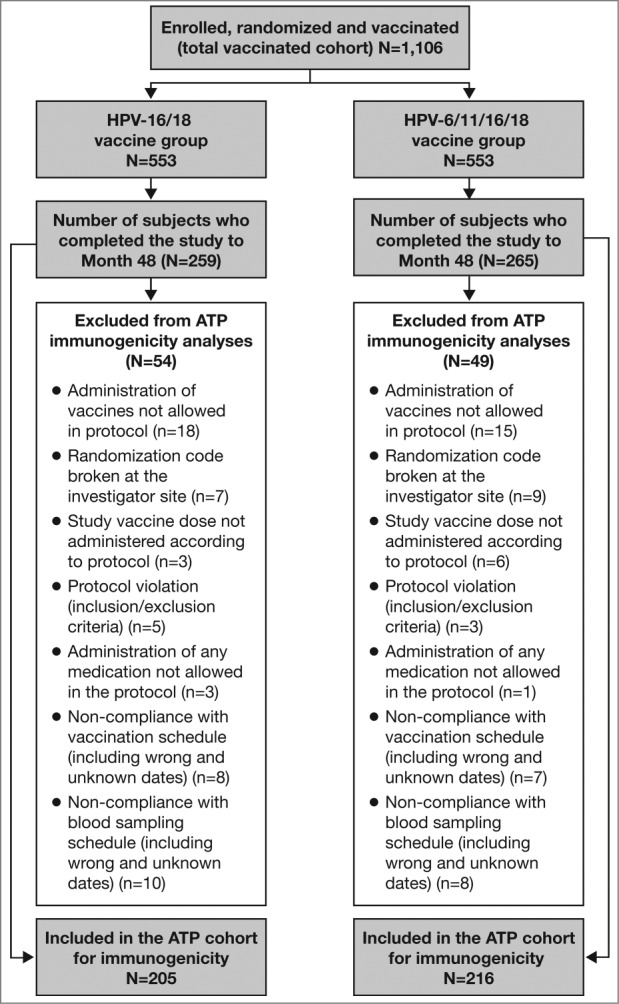
Subject disposition ATP, according-to-protocol. The ATP cohort for immunogenicity included all evaluable subjects who received 3 vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available. This included subjects for whom assay results were available for antibodies against at least one study vaccine antigen (HPV-16 or HPV-18) at the time point under analysis.
Antibody responses in serum
Table 1 shows the GMTs and seropositivity rates of anti-HPV-16 and -18 nAbs in women in the ATP cohort for immunogenicity at each time point who were seronegative and DNA-negative for the HPV type analyzed before vaccination, measured by PBNA. At Month 48, anti-HPV-16 nAb GMTs were 5.2-fold higher in the HPV-16/18 vaccine group than in the HPV-6/11/16/18 vaccine group in women aged 18–26 y In the same age group, anti-HPV-18 nAb GMTs were 12.3-fold higher in the HPV-16/18 vaccine group than in the HPV-6/11/16/18 vaccine group. At the same time point, compared with the HPV-6/11/16/18 vaccine, anti-HPV-16 and -18 GMTs induced by the HPV-16/18 vaccine were 3.0- and 12.8-fold higher, respectively, in women aged 27–35 y and were 2.0- and 8.6-fold higher, respectively, in women aged 36–45 y (Table 1). At Month 48, in the ATP cohort for immunogenicity, the majority of subjects in both vaccine groups were still seropositive for HPV-16. The seropositivity rates for HPV-18 remained high with the HPV-16/18 vaccine, while they decreased considerably with the HPV-6/11/16/18 vaccine (18–26 years: 81.4 % [95% confidence interval (CI): 70.3, 89.7]; 27–35 years: 57.6% [95% CI: 44.1, 70.4]; 36–45 years: 72.1% [95% CI: 59.2, 82.9]). The GMTs and seropositivity rates of anti-HPV-16 and -18 nAbs, as assessed by PBNA, in women in the ATP cohort for immunogenicity (irrespective of serostatus and DNA status prior to vaccination) are shown in Supplementary Table 1. Exploratory analyses performed in the TVC (irrespective of serostatus and DNA status prior to vaccination) showed that anti-HPV-16 and -18 nAb levels induced by the HPV-16/18 vaccine appeared to be higher than those induced by the HPV-6/11/16/18 vaccine across all age groups and at all time points up to Month 48 (p < 0.0085).
Table 1.
Seropositivity rates, GMTs and GMT ratios for serum anti-HPV-16 and anti-HPV-18 type-specific neutralizing antibodies measured by PBNA at Months 7, 12, 18, 24, 36 and 48 (ATP cohort for immunogenicity, seronegative and DNA-negative for the HPV type analyzed prior to vaccination)
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | |||||||
|---|---|---|---|---|---|---|---|---|
| Antigen | Month | N | % SP [95% CI] | GMT [95% CI] | N | % SP [95% CI] | GMT [95% CI] | GMT Ratio* |
| A | 18–26 years | |||||||
| HPV-16 | 7 | 104 | 100 [96.5, 100] | 36792 [29266, 46254] | 103 | 100 [96.5, 100] | 10053 [8136, 12422] | 3.7 [2.6, 5.2] |
| 12 | 101 | 100 [96.4, 100] | 14525 [11070, 19058] | 99 | 100 [96.3, 100] | 3265 [2545, 4190] | 4.5 [3.1, 6.4] | |
| 18 | 100 | 100 [96.4, 100] | 6000 [4681, 7691] | 91 | 100 [96.0, 100] | 1183 [883, 1585] | 5.1 [3.5, 7.4] | |
| 24 | 97 | 100 [96.3, 100] | 5184 [4015, 6694] | 89 | 97.8 [92.1, 99.7] | 894 [672, 1188] | 5.8 [4.0, 8.5] | |
| 36 | 60 | 100 [94.0, 100] | 3845 [2804, 5272] | 62 | 98.4 [91.3, 100] | 653 [460, 927] | 5.9 [3.7, 9.4] | |
| 48 | 54 | 100 [93.4, 100] | 3901 [2745, 5543] | 57 | 98.2 [90.6, 100] | 750 [505, 1115] | 5.2 [3.1, 8.8] | |
| HPV-18 | 7 | 118 | 100 [96.9, 100] | 16487 [13383, 20310] | 131 | 100 [97.2, 100] | 2258 [1809, 2818] | 7.3 [5.1, 10.4] |
| 12 | 112 | 100 [96.8, 100] | 4472 [3528, 5669] | 127 | 96.1 [91.1, 98.7] | 596 [469, 757] | 7.5 [5.4, 10.5] | |
| 18 | 109 | 100 [96.7, 100] | 2256 [1762, 2890] | 114 | 93.0 [86.6, 96.9] | 249 [195, 318] | 9.1 [6.4, 12.8] | |
| 24 | 106 | 100 [96.6, 100] | 1652 [1296, 2105] | 109 | 84.4 [76.2, 90.6] | 175 [133, 231] | 9.4 [6.5, 13.6] | |
| 36 | 64 | 100 [94.4, 100] | 1594 [1177, 2158] | 76 | 78.9 [68.1, 87.5] | 128 [92.6, 177] | 12.5 [8.0, 19.5] | |
| 48 | 59 | 100 [93.9, 100] | 1711 [1180, 2482] | 70 | 81.4 [70.3, 89.7] | 139 [98.7, 196] | 12.3 [7.5, 20.3] | |
| B | 27–35 years | |||||||
| HPV-16 | 7 | 90 | 100 [96.0, 100] | 23908 [18913, 30222] | 85 | 100 [95.8, 100] | 4958 [3896, 6311] | 4.8 [3.3, 7.1] |
| 12 | 91 | 100 [96.0, 100] | 7419 [5592, 9843] | 85 | 98.8 [93.6, 100] | 1756 [1290, 2390] | 4.2 [2.8, 6.4] | |
| 18 | 87 | 100 [95.8, 100] | 2908 [2229, 3793] | 83 | 98.8 [93.5, 100] | 690 [506, 941] | 4.2 [2.8, 6.3] | |
| 24 | 84 | 100 [95.7, 100] | 2269 [1766, 2916] | 79 | 97.5 [91.2, 99.7] | 619 [447, 856] | 3.7 [2.5, 5.5] | |
| 36 | 63 | 100 [94.3, 100] | 1898 [1419, 2538] | 49 | 100 [92.7, 100] | 502 [347, 726] | 3.8 [2.4, 6.0] | |
| 48 | 54 | 100 [93.4, 100] | 2046 [1469, 2850] | 51 | 96.1 [86.5, 99.5] | 678 [433, 1060] | 3.0 [1.8, 5.2] | |
| HPV-18 | 7 | 102 | 100 [96.4, 100] | 9502 [7519, 12008] | 101 | 98.0 [93.0, 99.8] | 1043 [790, 1378] | 9.1 [6.0, 13.8] |
| 12 | 105 | 99.0 [94.8, 100] | 2266 [1765, 2910] | 102 | 90.2 [82.7, 95.2] | 280 [209, 376] | 8.1 [5.5, 11.8] | |
| 18 | 101 | 100 [96.4, 100] | 1302 [1011, 1677] | 99 | 74.7 [65.0, 82.9] | 133 [101, 176] | 9.8 [6.7, 14.2] | |
| 24 | 98 | 100 [96.3, 100] | 1028 [801, 1320] | 94 | 72.3 [62.2, 81.1] | 116 [87.4, 155] | 8.9 [6.1, 12.9] | |
| 36 | 75 | 100 [95.2, 100] | 943 [713, 1247] | 61 | 70.5 [57.4, 81.5] | 102 [69.6, 149] | 9.3 [5.9, 14.6] | |
| 48 | 66 | 100 [94.6, 100] | 982 [741, 1302] | 59 | 57.6 [44.1, 70.4] | 76.9 [52.7, 112] | 12.8 [8.1, 20.2] | |
| C | 36–45 years | |||||||
| HPV-16 | 7 | 96 | 100 [96.2, 100] | 17302 [13605, 22002] | 83 | 100 [95.7, 100] | 7634 [5916, 9853] | 2.3 [1.5, 3.4] |
| 12 | 89 | 100 [95.9, 100] | 7110 [5386, 9386] | 83 | 100 [95.7, 100] | 2678 [1987, 3610] | 2.7 [1.8, 4.0] | |
| 18 | 90 | 100 [96.0, 100] | 2344 [1808, 3039] | 82 | 100 [95.6, 100] | 995 [733, 1350] | 2.4 [1.6, 3.5] | |
| 24 | 87 | 100 [95.8, 100] | 2059 [1592, 2661] | 80 | 100 [95.5, 100] | 875 [637, 1201] | 2.4 [1.6, 3.5] | |
| 36 | 61 | 100 [94.1, 100] | 1794 [1278, 2519] | 57 | 100 [93.7, 100] | 824 [567, 1196] | 2.2 [1.3, 3.6] | |
| 48 | 51 | 98.0 [89.6, 100] | 2081 [1378, 3144] | 54 | 98.1 [90.1, 100] | 1019 [645, 1608] | 2.0 [1.1, 3.8] | |
| HPV-18 | 7 | 110 | 100 [96.7, 100] | 9846 [7835, 12372] | 91 | 100 [96.0, 100] | 1439 [1105, 1873] | 6.8 [4.6, 10.2] |
| 12 | 104 | 100 [96.5, 100] | 3032 [2321, 3962] | 91 | 98.9 [94.0, 100] | 434 [325, 579] | 7.0 [4.7, 10.3] | |
| 18 | 103 | 99.0 [94.7, 100] | 1427 [1084, 1878] | 91 | 86.8 [78.1, 93.0] | 182 [137, 241] | 7.9 [5.3, 11.6] | |
| 24 | 100 | 99.0 [94.6, 100] | 1040 [786, 1377] | 88 | 77.3 [67.1, 85.5] | 136 [99.0, 186] | 7.7 [5.0, 11.6] | |
| 36 | 71 | 97.2 [90.2, 99.7] | 904 [625, 1306] | 61 | 73.8 [60.9, 84.2] | 103 [74.6, 143] | 8.8 [5.3, 14.4] | |
| 48 | 61 | 96.7 [88.7, 99.6] | 785 [529, 1164] | 61 | 72.1 [59.2, 82.9] | 91.5 [67.0, 125] | 8.6 [5.2, 14.1] | |
CI, confidence interval; GMT, geometric mean titer; N, number of subjects with available results; PBNA, pseudovirion-based neutralization assay; SP, seropositivity (defined as neutralizing antibody titer ≥40 ED50 [effective dose producing 50% response]).
Month 7–Month 48 data are presented for the ATP cohort for immunogenicity corresponding to the time point under analysis.
*GMT ratio, GMT in the HPV-16/18 group divided by GMT in the HPV-6/11/16/18 group; Month 7 GMT ratios are provided with 97.6% CI while Month 12–Month 48 GMT ratios are provided with 95% CI.
The ATP cohort for immunogenicity included all evaluable subjects who received 3 vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available. This included subjects for whom assay results were available for antibodies against at least one study vaccine antigen (HPV-16 or HPV-18) at the time point under analysis.
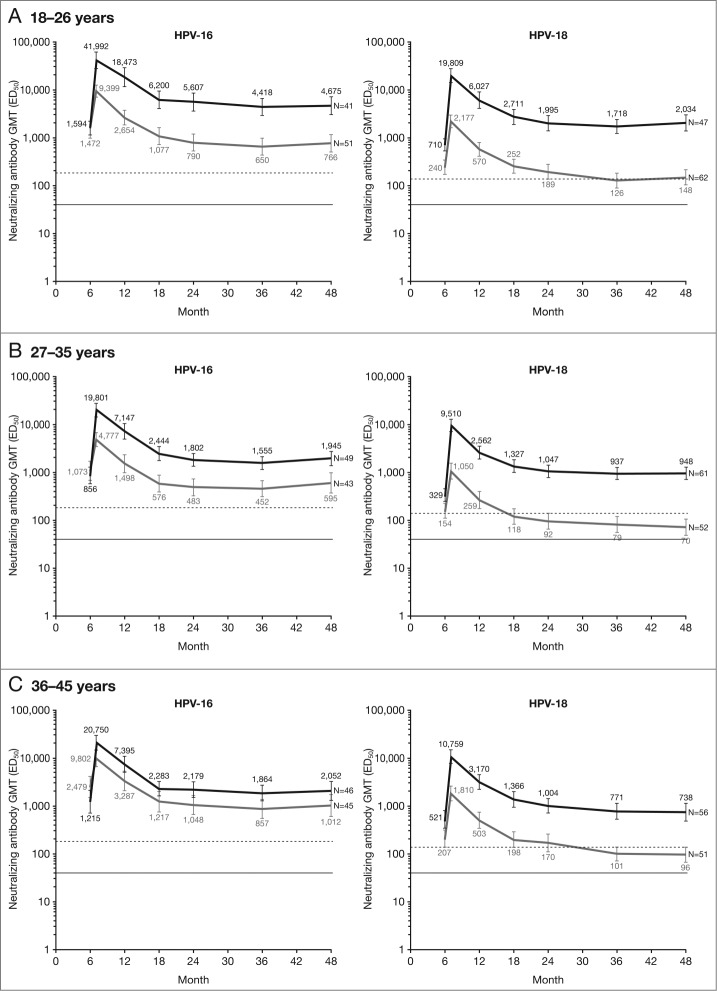
In ATP subjects seronegative and DNA-negative for the HPV type analyzed at baseline and with valid results available at each time point (ATP kinetic cohort), the kinetics of the antibody responses induced by both vaccines showed a similar pattern. Anti-HPV-16 and -18 nAb titers peaked at Month 7, slowly declined and then plateau from Month 18 onwards (Fig. 2). In all age groups, the HPV-16/18 vaccine induced higher plateauing levels of anti-HPV-16 and -18 nAbs than did the HPV-6/11/16/18 vaccine. This was particularly evident for anti-HPV-18 neutralizing antibodies, where plateau levels induced by the HPV-16/18 vaccine remained markedly higher than the level associated with natural infection through to Month 48, while plateau levels of anti-HPV-18 nAbs induced by the HPV-6/11/16/18 vaccine were close to or below the level associated with natural infection (Fig. 2).
Figure 2.
GMTs for serum anti-HPV-16 and anti-HPV-18 type-specific neutralizing antibodies at Months 6, 7, 12, 18, 24, 36 and 48 (PBNA, ATP kinetic cohort; seronegative and DNA-negative for the HPV type analyzed prior to vaccination) Black lines, Human Papillomavirus Types 16 and 18 Vaccine (Recombinant, AS04-adjuvanted, adsorbed) (Cervarix®); gray lines, Human Papillomavirus Types 6, 11, 16 and 18 Vaccine, Recombinant (Gardasil®). Error bars denote 95% confidence intervals of geometric mean titers (GMTs). Dashed line, neutralizing antibody GMTs measured by pseudovirion-based neutralization assay (PBNA) in women in the total vaccinated cohort of the HPV-010 study who had cleared natural infection prior to vaccination (i.e., those who were seropositive and DNA-negative at Month 0): 180.1 ED50 for HPV-16 and 137.3 ED50 for HPV-18.12 Solid line, PBNA limit of detection (40 ED50). ED50, effective dose producing 50% response. The according-to-protocol (ATP) kinetic cohort is a sub-cohort of the ATP cohort for immunogenicity (seronegative and DNA-negative at baseline) that included all subjects without any elimination codes and with valid results available for the HPV type(s) and the assay considered in the analysis for each time point.
Vaccine-induced HPV type-specific serum immunoglobulin G (IgG) antibody responses measured by enzyme-linked immunosorbent assay (ELISA) corroborated the PBNA results. The kinetics of antibody response assessed by ELISA followed a similar trend to those assessed by PBNA; the plateauing levels of anti-HPV-16 and -18 antibodies induced by the HPV-16/18 vaccine appeared higher than those induced by the HPV-6/11/16/18 vaccine in all age groups (Supplementary Fig. 1).
Antibody response in cervicovaginal secretions
HPV-specific antibody responses in cervicovaginal secretion (CVS) samples were evaluated in a subset of women. Due to the limited CVS samples available, the results are presented for all 3 age groups combined in the TVC. At Month 48, anti-HPV-16 positivity rates in CVS were 72.1% (95% CI: 56.3, 84.7) in the HPV-16/18 vaccine group and 54.3% (95% CI: 39.0, 69.1) in the HPV-6/11/16/18 vaccine group. Anti-HPV-18 positivity rates in CVS at Month 48 were 55.8% (95% CI: 39.9, 70.9) in the HPV-16/18 vaccine group and 39.1% (95% CI: 25.1, 54.6) in the HPV-6/11/16/18 vaccine group. GMTs of anti-HPV-16 and -18 IgG antibodies measured in CVS, calculated in the positive subjects, are given in Table 2.
Table 2.
Positivity rates and GMTs of anti-HPV-16 and anti-HPV-18 type-specific IgG antibodies measured in cervicovaginal secretions by ELISA at Months 0, 7, 12, 18, 24, 36 and 48 (TVC, irrespective of serostatus and DNA status for the HPV type analyzed prior to vaccination)
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antigen | Month | N | n | % P [95% CI] | GMT* [95% CI] | N | n | % P [95% CI] | GMT* [95% CI] |
| HPV-16 | 0 | 57 | 2 | 3.5 [0.4, 12.1] | 15.6 [0.8, 321.3] | 64 | 0 | 0 [0.0,0.0] | – [–, –] |
| 7 | 65 | 62 | 95.4 [87.1, 99.0] | 200.4 [141.6, 283.6] | 82 | 74 | 90.2 [81.7, 95.7] | 92.9 [68.1, 126.5] | |
| 12 | 67 | 57 | 85.1 [74.3, 92.6] | 121.1 [88.6, 165.5] | 66 | 52 | 78.8 [67.0, 87.9] | 59.0 [43.5, 79.9] | |
| 18 | 51 | 42 | 82.4 [69.1, 91.6] | 102.1 [70.6, 147.8] | 65 | 41 | 63.1 [50.2, 74.7] | 68.8 [47.7, 99.1] | |
| 24 | 54 | 42 | 77.8 [64.4, 88.0] | 86.9 [60.7, 124.6] | 61 | 34 | 55.7 [42.4, 68.5] | 43.4 [27.8, 67.5] | |
| 36 | 60 | 43 | 71.7 [58.6, 82.5] | 57.7 [41.5, 80.4] | 62 | 42 | 67.7 [54.7, 79.1] | 51.5 [36.2, 73.2] | |
| 48 | 43 | 31 | 72.1 [56.3, 84.7] | 41.6 [29.3, 59.1] | 46 | 25 | 54.3 [39.0, 69.1] | 72.8 [38.5, 137.8] | |
| HPV-18 | 0 | 57 | 3 | 5.3 [1.1, 14.6] | 18.9 [8.8, 40.6] | 64 | 4 | 6.3 [1.7, 15.2] | 11.4 [1.4, 96.4] |
| 7 | 65 | 60 | 92.3 [83.0, 97.5] | 96.7 [73.1, 128.0] | 82 | 57 | 69.5 [58.4, 79.2] | 38.1 [28.0, 51.8] | |
| 12 | 67 | 54 | 80.6 [69.1, 89.2] | 57.6 [43.2, 76.7] | 67 | 35 | 52.2 [39.7, 64.6] | 36.4 [24.9, 53.1] | |
| 18 | 51 | 37 | 72.5 [58.3, 84.1] | 40.2 [25.7, 62.9] | 65 | 22 | 33.8 [22.6, 46.6] | 28.7 [16.5, 49.8] | |
| 24 | 54 | 38 | 70.4 [56.4, 82.0] | 49.0 [33.3, 72.0] | 61 | 22 | 36.1 [24.2, 49.4] | 20.8 [11.7, 36.8] | |
| 36 | 60 | 41 | 68.3 [55.0, 79.7] | 27.5 [20.3, 37.2] | 62 | 23 | 37.1 [25.2, 50.3] | 30.6 [17.8, 52.7] | |
| 48 | 43 | 24 | 55.8 [39.9, 70.9] | 24.1 [16.0, 36.2] | 46 | 18 | 39.1 [25.1, 54.6] | 26.1 [13.4, 50.9] | |
CI, confidence interval; GMT, geometric mean titer; N, number of subjects with available results; n, number of subjects with an antibody titer ≥ the limit of quantification; P, positivity (defined as antibody titer ≥0.58 ELISA units/mL for HPV-16 and ≥0.35 ELISA units/mL for HPV-18); TVC, total vaccinated cohort.
Month 7–Month 48 data are presented for the TVC corresponding to the time point under analysis.
*GMTs were calculated on positive subjects (n values) because data for all subjects in the subset did not follow a normal distribution. Dashes (–) indicate where there were insufficient values (i.e., n≤ 1) to calculate GMTs.
At Month 48, the Pearson correlation coefficients (r) between HPV-specific antibody titers in CVS and serum were: for HPV-16, 0.88 in the HPV-16/18 vaccine group and 0.82 in the HPV-6/11/16/18 vaccine group; for HPV-18, 0.47 in the HPV-16/18 vaccine group and 0.66 in the HPV-6/11/16/18 vaccine group.
CD4+ T-cell responses
At Month 48, in all 3 age groups combined, the proportion of responders (women with ≥500 HPV type-specific memory CD4+ T-cells/million cells) appeared to be higher in the HPV-16/18 vaccine group than in the HPV-6/11/16/18 vaccine group for both HPV-16 (92% vs. 36%) and HPV-18 (79% vs. 33%) (Table 3). The geometric mean (GM) frequency of circulating antigen-specific CD4+ T-cells in all subjects (responders and non-responders) was 1.5-fold higher (95% CI: 0.5, 4.8) for HPV-16 and 2.9-fold higher (95% CI: 1.3, 6.4) for HPV-18, in the HPV-16/18 vaccine group compared with the HPV-6/11/16/18 vaccine group (Table 3).
Table 3.
Proportion of responders and geometric means for (a) HPV-16- and (b) HPV-18 type-specific CD4+ T-cell responses at Months 7, 12, 18, 24, 36 and 48 (ATP cohort for immunogenicity; seronegative, DNA-negative and HPV type-specific CD4+ T-cell negative prior to vaccination)
| Positivity rates | GM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | |||||||||||
| Antigen | Month | N | n | % P | N | n | % P | p-value* | N | GM (95% CI) | N | GM (95% CI) | GMR (95% CI) | p-value† |
| HPV-16 | 7 | 41 | 36 | 87.8 | 33 | 21 | 63.6 | 0.0245 | 41 | 1080 (853, 1369) | 33 | 665 (511, 865) | 1.63 (1.14, 2.32) | 0.0029 |
| 12 | 34 | 27 | 79.4 | 27 | 13 | 48.2 | 0.0151 | 34 | 794 (630, 1001) | 27 | 448 (345, 580) | 1.77 (1.25, 2.51) | 0.0008 | |
| 18 | 40 | 37 | 92.5 | 25 | 10 | 40.0 | < 0.0001 | 40 | 1149 (898, 1471) | 25 | 397 (291, 543) | 2.89 (1.94, 4.31) | < 0.0001 | |
| 24 | 33 | 30 | 90.9 | 20 | 12 | 60.0 | 0.0128 | 33 | 1070 (837, 1367) | 20 | 441 (322, 605) | 2.42 (1.63, 3.61) | < 0.0001 | |
| 36 | 20 | 16 | 80.0 | 15 | 6 | 40.0 | 0.0322 | 20 | 627 (292, 1346) | 15 | 285 (118, 688) | 2.20 (0.69, 7.07) | 0.0020 | |
| 48 | 13 | 12 | 92.3 | 14 | 5 | 35.7 | 0.0044 | 13 | 732 (314, 1707) | 14 | 498 (220, 1127) | 1.47 (0.45, 4.76) | 0.0057 | |
| HPV-18 | 7 | 43 | 34 | 79.1 | 40 | 23 | 57.5 | 0.0571 | 43 | 907 (668, 1232) | 40 | 507 (370, 697) | 1.79 (1.15, 2.78) | 0.0102 |
| 12 | 38 | 26 | 68.4 | 32 | 11 | 34.4 | 0.0078 | 38 | 461 (285, 747) | 32 | 315 (186, 533) | 1.46 (0.72, 2.99) | 0.0131 | |
| 18 | 42 | 33 | 78.6 | 33 | 14 | 42.4 | 0.0018 | 42 | 842 (572, 1240) | 33 | 314 (203, 486) | 2.68 (1.50, 4.81) | 0.0006 | |
| 24 | 35 | 26 | 74.3 | 25 | 10 | 40.0 | 0.0152 | 35 | 694 (488, 987) | 25 | 294 (194, 446) | 2.36 (1.37, 4.08) | 0.0003 | |
| 36 | 22 | 12 | 54.6 | 17 | 5 | 29.4 | 0.1930 | 22 | 352 (189, 653) | 17 | 243 (120, 492) | 1.45 (0.57, 3.70) | 0.0210 | |
| 48 | 14 | 11 | 78.6 | 15 | 5 | 33.3 | 0.0253 | 14 | 740 (415, 1319) | 15 | 257 (147, 449) | 2.88 (1.29, 6.44) | 0.0088 | |
ATP, according-to-protocol; CI, confidence interval; GM, geometric mean (calculated from responder [defined as subjects with detectable HPV type-specific memory CD4+ T-cells, i.e., > 500 HPV-specific CD4+ T-cells/million CD4+ T-cells] and non-responder data); GMR, geometric mean ratio; N, number of subjects with available results; n, number of positive subjects; P, positivity (defined as > 500 HPV-specific CD4+ T-cells/million CD4+ T-cells).
Month 7–Month 48 data are presented for the ATP cohort for immunogenicity corresponding to the time point under analysis.
*For the comparison of proportions of responders, p-values were calculated using Fisher's exact test. †For statistical assessment of CD4+ T-cell GM ratios, p-values were computed using a Kruskal-Wallis model.
Memory B-cell responses
At Month 48, in all 3 age groups combined, the proportion of memory B-cell responders (subjects with detectable HPV type-specific memory B-cells, i.e., >0 antigen-specific memory B-cell/million memory B-cells) in the HPV-16/18 and HPV-6/11/16/18 vaccine groups appeared similar for HPV-16 (71% vs. 70%) and HPV-18 (65% vs. 60%), respectively (Table 4). The mean frequency of circulating antigen-specific memory B-cells was not substantially different between vaccine groups for HPV-16 (GM ratio, 1.2 [95% CI: 0.4, 4.2]) and HPV-18 (GM ratio, 2.2 [95% CI: 0.8, 6.5]), as assessed in responders only (Table 4).
Table 4.
Proportion of responders and geometric means for (a) HPV-16- and (b) HPV-18 type-specific memory B-cell responses at Months 7, 12, 18, 24, 36 and 48 (ATP cohort for immunogenicity; seronegative, DNA-negative and with no detectable HPV type-specific B-cells prior to vaccination)
| Positivity rates | GM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | |||||||||||
| Antigen | Month | N | n | % P | N | n | % P | p-value* | N | GM (95% CI) | N | GM (95% CI) | GMR (95% CI) | p-value† |
| HPV-16 | 7 | 51 | 46 | 90.2 | 39 | 36 | 92.3 | 1.0000 | 46 | 996 (713, 1391) | 36 | 326 (224, 476) | 3.05 (1.84, 5.05) | < 0.0001 |
| 12 | 44 | 40 | 90.9 | 33 | 25 | 75.8 | 0.1109 | 40 | 305 (217, 428) | 25 | 217 (142, 333) | 1.40 (0.81, 2.42) | 0.2183 | |
| 18 | 45 | 39 | 86.7 | 29 | 17 | 58.6 | 0.0112 | 39 | 255 (178, 367) | 17 | 231 (133, 401) | 1.10 (0.57, 2.14) | 0.7649 | |
| 24 | 36 | 30 | 83.3 | 24 | 16 | 66.7 | 0.2122 | 30 | 312 (205, 475) | 16 | 233 (131, 415) | 1.34 (0.65, 2.73) | 0.4174 | |
| 36 | 20 | 11 | 55.0 | 16 | 11 | 68.8 | 0.5007 | 11 | 366 (209, 639) | 11 | 195 (111, 340) | 1.88 (0.85, 4.14) | 0.1106 | |
| 48 | 14 | 10 | 71.4 | 10 | 7 | 70.0 | 1.0000 | 10 | 310 (139, 688) | 7 | 257 (99.1, 668) | 1.20 (0.35, 4.18) | 0.7546 | |
| HPV-18 | 7 | 53 | 48 | 90.6 | 52 | 34 | 65.4 | 0.0021 | 48 | 513 (373, 704) | 34 | 163 (112, 238) | 3.14 (1.92, 5.14) | < 0.0001 |
| 12 | 47 | 38 | 80.9 | 44 | 17 | 38.6 | < 0.0001 | 38 | 228 (158, 331) | 17 | 117 (67.3, 205) | 1.95 (1.00, 3.80) | 0.0507 | |
| 18 | 47 | 35 | 74.5 | 42 | 19 | 45.2 | 0.0087 | 35 | 236 (160, 347) | 19 | 79.5 (47.0, 135) | 2.97 (1.54, 5.70) | 0.0015 | |
| 24 | 38 | 29 | 76.3 | 34 | 18 | 52.9 | 0.0489 | 29 | 258 (171, 389) | 18 | 102 (60.3, 171) | 2.54 (1.31, 4.93) | 0.0071 | |
| 36 | 22 | 12 | 54.6 | 21 | 8 | 38.1 | 0.3640 | 12 | 267 (123, 583) | 8 | 131 (50.4, 340) | 2.04 (0.60, 7.00) | 0.2387 | |
| 48 | 17 | 11 | 64.7 | 15 | 9 | 60.0 | 1.0000 | 11 | 259 (127, 531) | 9 | 117 (52.7, 258) | 2.23 (0.76, 6.48) | 0.1336 | |
Month 7–Month 48 data are presented for the ATP cohort for immunogenicity corresponding to the time point under analysis.
ATP, according-to-protocol; CI, confidence interval; GM, geometric mean (calculated on responders only [defined as subjects with detectable HPV-type specific memory B-cells, i.e., >0 cell/million cells] as data were log-transformed for statistical analyses); GMR, geometric mean ratio; N, number of subjects with available results; n, number of positive subjects; P, positivity (defined as >0 cell/million cells).
*For the comparison of proportions of responders, p-values were calculated using Fisher's exact test. †For statistical assessment of memory B-cell GM ratios, p-values were calculated using an ANOVA model.
Safety
From Month 0 to Month 48, the proportions of subjects reporting serious adverse events (SAEs), new onset chronic diseases (NOCDs), new onset autoimmune diseases (NOADs) and medically significant conditions (MSCs, conditions prompting physician visits) in the TVC appeared to be similar between vaccine groups (Table 5). Up to Month 48, 102 SAEs were reported by 71 subjects; 2 SAEs (one per vaccine group) considered to be possibly related to vaccination and one fatal SAE (metastatic renal cell carcinoma) were reported previously.12,13 Overall, 66 subjects experienced NOCDs, 19 of these subjects reported NOCDs that were identified as NOADs. The most common NOCD/NOAD was hypothyroidism. Up to Month 48, 139 pregnancies were reported (HPV-16/18 vaccine group, n = 77; HPV-6/11/16/18 vaccine group, n = 62). In the HPV-16/18 and HPV-6/11/16/18 vaccine groups, respectively, 57 (74%) and 42 (68%) pregnancies resulted in a live infant with no apparent congenital anomalies. No infants were born with congenital anomalies in the HPV-16/18 vaccine group compared with 2 (3%) in the HPV-6/11/16/18 vaccine group. The number of spontaneous abortions (with no apparent congenital anomaly) appeared similar between vaccine groups (HPV-16/18 vaccine, 10 [13%]; HPV-6/11/16/18, 9 [15%]).
Table 5.
Safety outcomes up to Month 48; proportion of subjects reporting at least one event (TVC; irrespective of serostatus and DNA status prior to vaccination)
| Proportion of subjects with ≥ 1 event,% (95% CI) | ||
|---|---|---|
| HPV-16/18 vaccine (N = 553) | HPV-6/11/16/18 vaccine (N = 553) | |
| Medically significant conditions | 45.4 (41.2, 49.6) | 39.1 (35.0, 43.3) 216] |
| New onset of chronic diseases* | 6.0 (4.1, 8.3) | 6.0 (4.1, 8.3) |
| New onset of autoimmune diseases‡ | 1.3 (0.5, 2.6) | 2.2 (1.1, 3.8) |
| Serious adverse events | 6.7 (4.8, 9.1) | 6.1 (4.3, 8.5) |
CI, confidence interval; TVC, total vaccinated cohort.
*All adverse events reported were compared with a pre-defined list of potential chronic diseases derived from the Medical Dictionary for Regulatory Activities; determination of whether a chronic disease was of new onset was based on blinded review of the reported symptoms and the subject's pre-vaccination medical history by a GlaxoSmithKline physician.
‡New onset autoimmune diseases were identified from events categorized as new onset chronic diseases using a list detailing potential autoimmune events, which excluded allergy-related events or isolated signs and symptoms, plus events not considered to be autoimmune in origin.
Discussion
The higher anti-HPV-16 and -18 serum nAb levels induced by the HPV-16/18 vaccine versus the HPV-6/11/16/18 vaccine reported at Months 7 and 2412,13 remained up to Month 48 after first dose. In the ATP cohort for immunogenicity, GMTs of anti-HPV-16 and -18 nAbs measured by PBNA remained several fold higher with the HPV-16/18 vaccine than the HPV-6/11/16/18 vaccine across all age groups and at all time points up to Month 48. The magnitudes of difference in GMTs at Month 48 between vaccines were comparable with those at all other time points.13 As reported at Month 24,13 the levels of vaccine-induced anti-HPV-16 and -18 antibodies peaked at Month 7, declined and then plateau from Month 18. In the ATP kinetic cohort, higher anti-HPV-16 nAb titers than those observed in women who had previously cleared their HPV infection (measured by PBNA in the TVC) were induced by both vaccines, through to Month 48.12 However, vaccine-induced anti-HPV-18 nAb titers remained higher than those observed after natural infection in the HPV-16/18 vaccine group, while they declined to levels similar to or below those associated with previously cleared HPV infection in the HPV-6/11/16/18 vaccine group. In the ATP kinetic cohort at Month 48, higher ELISA antibody titers were detected with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine for HPV-16 and HPV-18, across all age groups. Using exploratory analyses in the TVC, the cohort that is most representative of the general population, the HPV-16/18 vaccine demonstrated higher anti-HPV-16 and -18 serum nAbs at all measured time points through to Month 48 when compared with the serum nAb levels elicited by the HPV-6/11/16/18 vaccine. As an immune correlate of protection has not yet been defined, a head-to-head study incorporating clinical endpoints would be required to confirm whether the observed differences in immune response between the vaccines influence the duration of protection they provide.
The distinct immune responses between the HPV vaccines have been recently confirmed in an independent phase IV head-to-head study conducted by the Health Protection Agency (HPA) in England.14 In this trial involving 12–15 y old girls, serum nAb responses to vaccine (HPV-16 and -18) and non-vaccine (HPV-31 and -45) types were found to be higher in the HPV-16/18 vaccine arm compared with the HPV-6/11/16/18 vaccine arm, up to 12 months after the first vaccination. Although the follow-up period drastically differs between the HPV-010 study (48 months) and the HPA trial, it appears that differences in the level of antibody responses remained consistent over time in each study. However, although nAbs are believed to play a significant role in mediating protection against HPV infection and disease, any attempt to extrapolate the observed differences in immunogenicity to clinical efficacy data should be treated with caution. Nonetheless, these independent data strengthen the current body of evidence that the HPV-16/18 vaccine has a higher immunogenicity profile compared with the HPV-6/11/16/18 vaccine.
The proportion of HPV-16-specific CD4+ T-cell responders was higher after the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine at all time points through to Month 48. Similarly, GMs of circulating HPV-16-specific CD4+ T-cells were higher after the HPV-16/18 vaccine at all time points. The proportion of subjects demonstrating an HPV-18-specific CD4+ T-cell response was higher after vaccination with the HPV-16/18 vaccine than after the HPV-6/11/16/18 vaccine at most time points (except Months 7 and 36). GMs of circulating HPV-18-specific CD4+ T-cells were higher after the HPV-16/18 vaccine at all time points. The observed enhanced CD4+ T cell response in the HPV-16/18 vaccine group is likely to be related to the ability of the MPL component of the AS04 adjuvant to enhance antigen presentation to CD4+ T cells, in turn resulting in increased differentiation of B cells into antibody-producing plasma cells and memory B cells.7 The AS04-adjuvant was previously shown to induce an enhanced memory B-cell response compared with aluminum salt only formulations;15 however, this was not a direct comparison to the HPV-6/11/16/18 vaccine formulation, and a direct comparison of the immune response induced by AS04 and amorphous aluminum hydroxyphosphate sulfate (AAHS) adjuvant has not been performed.
At all time points up to Month 24, the proportion of responders with detectable HPV-18-specific circulating memory B-cells was higher after vaccination with the HPV-16/18 vaccine than after the HPV-6/11/16/18 vaccine; however, from Month 36 through to Month 48, the proportion of responders with detectable HPV-16- and HPV-18-specific circulating memory B-cells was similar after each vaccine. At Month 48, GMs of HPV-16- and -18-specific circulating memory B-cells in responders were similar to the plateau levels reported from Month 12 and were comparable between vaccines.13 We note that the B-cell ELISPOT assay is performed using peripheral blood samples and thus may be less relevant at the later time points, by which time memory B-cells might have migrated from the blood and into the different lymphatic compartments (e.g., spleen). It is still unclear precisely where memory B-cells usually reside preferentially and what the significance of their frequency is when measured in blood.16 At Month 48, memory B-cell responses remain detectable and may account for the immune memory demonstrated in trials with the HPV-16/18 and HPV-6/11/16/18 vaccines.17,18 Giving a fourth dose of the HPV-16/18 vaccine 6.8 y after first vaccination has been shown to cause a rapid and considerable increase in antibody titer and in the GMs of T- and B-cells.16 Similarly, a fourth dose of the HPV-6/11/16/18 vaccine given 5 y after first dose resulted in a rapid increase in anti-HPV antibody levels.18
High serum antibody titers have been associated with enhanced antibody concentrations in CVS, thus likely providing a first defense against HPV infection and subsequent disease.19 At Months 7 and 24, a greater proportion of women who received HPV-16/18 vaccine had detectable HPV type-specific nAbs in CVS compared with those who received HPV-6/11/16/18 vaccine. This finding is consistent with the higher serological immune response observed with the HPV-16/18 vaccine (in the ATP cohort for immunogenicity).12,13 At Month 48, serological immune response was higher with the HPV-16/18 vaccine than with the HPV-6/11/16/18 vaccine. At this time point, limited CVS samples were available so mucosal antibody levels were assessed in the TVC; positivity rates of anti-HPV-16 and -18 IgG antibodies in CVS appeared higher in the HPV-16/18 vaccine group compared with the HPV-6/11/16/18 vaccine group, while CVS antibody levels were of similar magnitude. Taken together, our results at Month 48 are in line with those observed at previous time points.
Antibody levels in serum and CVS are poorly correlated, especially for HPV-18. Any extrapolation on transudation rates from serum to CVS should be treated with caution as the relevance of CVS findings at Month 48 is limited by the sensitivity of the analyses, scores being close to the limit of detection, and the small number of samples analyzed at the later time points. Whether the CVS antibody titers observed in this study would be sufficient to offer protection at the site of infection is unknown, and an immune correlate of protection remains to be determined.
To induce optimal protection against HPV, adolescents are vaccinated prior to sexual debut; therefore, a limitation of this study is the exclusion of adolescents. However, as noted above, an independent head-to-head study has been performed in 12–15 y old girls and reported a broader and higher magnitude of serum nAb response with the HPV-16/18 vaccine when compared with the HPV-6/11/16/18 vaccine.14 Further, a head-to-head study comparing the immunogenicity of the 2 vaccines when administered to 9–14 y old girls according to a 2-dose schedule is ongoing (NCT01462357). A separate study has already shown that, for the HPV-16/18 vaccine, a 2-dose schedule in 9–14 y old girls appears comparable to the standard 3-dose schedule in women aged 15–25 years, up to 4 y after the first dose.20 Also a limitation of the present analysis was the lack of long term follow-up data on cross-reactivity against non-vaccine HPV types, such as HPV-31 and HPV-45—these data were available only up to Month 24, as previously reported.21 We also note that our study was reliant on the accurate reporting of medical and sexual history.
Over the 48-month period of the trial, sample size decreased due to subject attrition and this led to a smaller subgroup for analysis of positivity rates and GMTs in CVS and for analysis of T-cell and B-cell responses. The relatively high drop-out rate is likely due to the subjects not wishing to participate in the extended follow-up; however, the drop-out rate is unlikely to affect the validity of results as demographic data at Month 7 and Month 48 are similar.
Tolerability was generally good for both vaccines, with clinically acceptable safety profiles for the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine up to Month 48. This is consistent with ongoing post-licensure vaccine monitoring data.22 Between Months 24–48, no fatal SAEs or SAEs related to vaccination were reported.
Overall, the higher immune response previously observed with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine was maintained up to Month 48. The higher immune response observed with the HPV-16/18 vaccine may be partially explained by the AS04 adjuvant. These findings may be of benefit to healthcare providers and public health officials in informing policy choices with regards to primary prevention of cervical cancer. Longer-term assessment and registries are required to ascertain if any clinical efficacy differences exist between the vaccines. Follow-up data to Month 60 are complete and are reported along with data modeling long-term persistence of antibody responses.23
Patients and Methods
Study design, immunogenicity and safety assessments
This long-term follow-up through Month 48 was conducted in 36 centers in the United States; ClinicalTrials.gov NCT00423046.
Study participants, ethics, study design and vaccine composition have previously been reported.12,13 Briefly, women stratified by age (18–26, 27–35 and 36–45 years) were randomized (1:1 ratio in each age group) to receive 0.5 mL of either HPV-16/18 vaccine or HPV-6/11/16/18 vaccine according to their recommended 3-dose schedules (Months 0, 1, 6 or Months 0, 2 and 6, respectively). The treatment allocation at the investigator site was performed using a web-based central randomization system. The study was conducted in an observer-blind manner; to maintain the blind, women received one dose of placebo (aluminum hydroxide) at either Month 1 or 2, as appropriate. Follow-up data to Month 60 are complete and disclosed.23
Blood samples for assessment of serological humoral responses (measured by ELISA24,25 and PBNA25) were collected at yearly visits during follow-up. At the preselected sites, peripheral blood mononuclear cell (PBMC) samples were obtained from a subset of women in all age groups and both vaccine groups for assessment of cell-mediated immune (CMI) responses. In addition, CVS samples were collected for assessment of mucosal HPV antibody response by ELISA. Anti-HPV nAbs were measured by PBNA using pseudovirions. Total HPV antibodies were measured by ELISA using the purified HPV type-specific recombinant VLPs. CD4+ T-cell responses were evaluated by in vitro stimulation with a pool of HPV peptides followed by quantification by cytokine flow cytometry.13,26 Memory B-cells were evaluated by B-cell ELISPOT assay using L1 VLP antigens of the HPV-16/18 AS04-adjuvanted vaccine.15 Methodology for PBNA and PBMC isolation, antibody extraction from CVS samples, and immunological assays has been described previously.13,26 In the absence of a serological correlate of protection, GMTs of anti-HPV-16 and -18 nAbs (measured by PBNA) induced by natural infection were used to evaluate vaccine-induced antibody responses. These antibody responses were defined as GMTs in women in the TVC who were DNA-negative but seropositive at Month 0 for the antigen under analysis, indicating clearance of natural infection.12 For each antigen, positivity in the PBNA was defined as a serum dilution greater than or equal to the assay threshold of 40 ED50 (effective dose producing 50% response).
SAEs, NOCDs, NOADs, pregnancies, and other MSCs were recorded in the TVC throughout the study, as previously described.12
Statistical analysis
The objective of this follow-up analysis through Month 48 was to compare the serological nAb responses and CMI responses to HPV-16 and -18 induced by the 2 vaccines by means of descriptive and exploratory analyses. For nAb responses, GMT ratios with 2-sided 95% CI (GMT in HPV-16/18 vaccine group divided by GMT in the HPV-6/11/16/18 vaccine group) were calculated in the ATP cohort for immunogenicity (all subjects who received 3 vaccine doses and for whom data concerning immunogenicity endpoint measurements were available at Month 48; seronegative and DNA-negative at baseline for the HPV type analyzed). Exploratory analyses were performed on the total vaccinated cohort (TVC, all subjects who received ≥ 1 dose of vaccine; regardless of serostatus and DNA status at baseline) and the p-value associated with an analysis of variance (ANOVA) test was calculated to compare the 2 vaccine groups. In the exploratory analysis of CD4+ T-cell and memory B-cell responses, the proportion of responders in each vaccine group was compared using a Fisher's exact test. The GM ratio between vaccine groups was obtained using an ANOVA model on the log10-transformed frequencies. The ANOVA model included the vaccine group as fixed effect. The GM ratio and its 95% CI were derived as exponential transformation. For the statistical assessment of CD4+ T-cell GM ratios, p-values were computed using a Kruskal-Wallis model. For the statistical assessment of memory B-cell GM ratios, p-values were calculated using an ANOVA model. Additional objectives at Months 36 and 48 were to evaluate the response to HPV-16 and -18 induced by the 2 vaccines in serum and in CVS by ELISA.
Notes
Cervarix is a registered trade mark of the GlaxoSmithKline group of companies.
Gardasil is a registered trade mark of Merck & Co., Inc..
Supplementary Material
Acknowledgments
The first author (M.E.) and the sponsor clinical team wrote the first draft of the manuscript with the support of medical writers (Meridian HealthComms Ltd., Plumley, UK) and publication managers (Dirk Saerens and Jérôme Leemans, Keyrus Biopharma, Belgium, Bruno Baudoux, Business & Decision Life Sciences, Belgium) working on behalf of GlaxoSmithKline Vaccines. All authors contributed to the development of the subsequent drafts, with the writing and editorial assistance of the sponsor. All authors had full access to the data and gave final approval before submission. The authors received no financial support or other form of compensation for the development of the manuscript. GlaxoSmithKline Biologicals SA took in charge all the costs associated with the development and publishing of the present publication.
We thank all study participants and their families. We are indebted to investigators and co-investigators who are not named as authors but who substantially contributed to the HPV-010 study at Months 36 and 48†. We are also grateful to all teams of GlaxoSmithKline Vaccines for their contribution to this study, in particular the US team, i.e., Latt Htun-Myint and Joan Adler (Medical Monitors); at GlaxoSmithKline Vaccines (Belgium) Global Vaccine Clinical laboratories, the Human Cellular Immunity team for the characterization of the cell-mediated immune responses, the Viral functional assays Development Unit team for the pseudovirion-based neutralization assays, the Clinical Immunology and Applied Microbiology team for the enzyme-linked immunosorbent assay analysis of sera and the Immunochemistry Development Unit team for the testing of HPV-16 and HPV-18 antibodies in cervicovaginal secretions. We also acknowledge Marie-Pierre David and Marie Lebacq (GlaxoSmithKline Vaccines, Belgium) for input on statistical analyses; Stéphanie Genevrois (GlaxoSmithKline Vaccines, Belgium) and Sanchaita Ukil (GlaxoSmithKline Vaccines, India) for scientific writing of the clinical study reports; Stéphanie Grzesiak for global study management (GlaxoSmithKline Vaccines, Belgium); Sanjoy Datta and Dominique Descamps (GlaxoSmithKline Vaccines, Belgium) for their contribution to the study design and conception.
†HPV-010 Study Group: Mark Blatter (Primary Physicians Research, Inc., Pittsburgh, PA), Christopher V. Chambers (Thomas Jefferson University, Philadelphia, PA), Marina Fernandez (Center for Clinical Trials of San Gabriel, West Covina, CA), Bradley Fox (Liberty Family Practice, Erie, PA), David L. Fried (Omega Medical Research, Warwick, RI), Sidney A. Funk (Radiant Research, Atlanta, GA), Cheryl A. Hansen (Ridgeview Research, Chaska, MN), James A. Hedrick (Kentucky Pediatric and Adult Research, Bardstown, KY), Bethany Hoffman (Aspen Medical Group, St. Paul, MN), Terry D. Klein (Heartland Research Assoc., Wichita, KS), Jacob Lalezari (Quest Clinical Research, San Francisco, CA), Michael J. Noss (Radiant Research, Cincinnati, OH), James H. Silverblatt (Lake Medical Research, LLC, Willoughby Hills, OH), Rhoda S. Sperling (Mount Sinai School of Medicine, New York, NY), Karen G. Swenson (Professional Quality Research, Austin, TX), Troy Thompson (Southwestern Medical Clinic PC, Stevensville, MI), Mark Turner (Advanced Clinical Research, Boise, ID), Michael W. Warren (Research Across America, Lancaster, PA), Robert Yoachim (Heartland Research Associates, Arkansas City, KS).
Disclosure of Potential Conflicts of Interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf. Institutions of A.C., M.L. and P.T. received grants from the GlaxoSmithKline group of companies to conduct this study. A.C.'s previous institution received funding for other clinical trials sponsored by the GlaxoSmithKline group of companies. M.E. has not received payments from any companies. Montefiore Medical Center received payment for M.E. time spent for the development of educational presentations (GlaxoSmithKline group of companies) and consulting activities (Merck, Bristol-Myers Squibb, Advaxis, Inovio, Photocure, Roche, Aura, and the GlaxoSmithKline group of companies), and for M.E. travel to meetings for the study (GlaxoSmithKline group of companies). A.C. received consulting fees for advisory board membership from the GlaxoSmithKline group of companies and payment for lectures including services on speaker bureaus from Merck and the GlaxoSmithKline group of companies. L.L. is a consultant outsourced from XPE Pharma & Science to the GlaxoSmithKline group of companies. M.L. received consultancy fees from the GlaxoSmithKline group of companies and Merck. M.L. chairs an adjudication committee for another GlaxoSmithKline vaccine and received grants from Merck. M.L. holds the patent and receives royalties from a Merck product. N.C. received payment from the GlaxoSmithKline group of companies for lectures including services on speaker bureaus. F.D., F.S., G.C., G.D. and P.M. are employees of the GlaxoSmithKline group of companies. F.D., F.S. and P.M. own stock in the GlaxoSmithKline group of companies. G.D. receives restricted shares from the GlaxoSmithKline group of companies, and previously received royalties from Wyeth Vaccines.
Funding
The study reported here (HPV-010; NCT00423046) was funded by GlaxoSmithKline Biologicals SA, which was involved in all stages of the study, from design to final report. A.C. was coordinating investigator and, together with M.E., M.L., N.C. and P.T., participated in the recruitment and/or follow-up of subjects. M.E. was involved in the original study design in collaboration with G.D. (GlaxoSmithKline Vaccines, USA). G.C. (GlaxoSmithKline Vaccines, Belgium) contributed toward data analyses and interpretation, and prepared the statistical analysis report. F.D. and P.M. led the PBNA and CMI analyses, respectively, at GlaxoSmithKline Vaccines (Belgium). F.S. and L.L. supervised the conduct of the study at GlaxoSmithKline Vaccines (Belgium), and together with A.C. critically reviewed the study report.
References
- 1. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12-9; PMID:10451482; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 2. Winer RL, Lee S-K, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003; 157:218-26; PMID:12543621; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 3. Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, Xi L-F, Stern ME, Lee S-K, O'Reilly SF, Hawes SE, et al. . Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis 2007; 196:1125-36; PMID:17955429; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 4. Castellsague X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol 2009; 115:S15-23; PMID:19819540; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 5. WHO/ICO Information Centre on HPV and Cervical Cancer. HPV and cervical cancer in the 2007 report. Vaccine 2007; 25 Suppl 3:C1-26; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 6. Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128:927-35; PMID:20473886; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 7. Garcon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, Van Mechelen M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs 2011; 25:217-26; PMID:21815697; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 8. Paavonen J, Jenkins D, Bosch F, Naud P, Salmeron J, Wheeler C, Chow S, Apter D, Kitchener H, Castellsague X, et al. . Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161-70; PMID:17602732; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 9. Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. . Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301-14; PMID:19586656; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 10. The GlaxoSmithKline Vaccine HPV-007 Study Group . Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975-85; PMID:19962185; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 11. Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Hoye J, Steinwall M, Riis-Johannessen G, et al. . High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459-66; PMID:17117182; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. . Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5:705-19; PMID:19684472; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 13. Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Meric D, Dessy FJ, et al. . Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin 2011; 7:1343-58; PMID:22048173; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, Andrews N, Miller E, Beddows S. A randomized, observer-blinded immunogenicity trial of Cervarix® and Gardasil® Human Papillomavirus vaccines in 12-15 year old girls. PLoS One 2013; 8:e61825; PMID:23650505; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, et al. . Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006; 24:5937-49; PMID:16828940; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 16. Cao Y, Gordic M, Kobold S, Lajmi N, Meyer S, Bartels K, Hildebrandt Y, Luetkens T, Ihloff AS, Kroger N, et al. . An optimized assay for the enumeration of antigen-specific memory B cells in different compartments of the human body. J Immunol Methods 2010; 358:56-65; PMID:20302874; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 17. Moscicki AB, Wheeler CM, Romanowski B, Hedrick J, Gall S, Ferris D, Poncelet S, Zahaf T, Moris P, Geeraerts B, et al. . Immune responses elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in previously vaccinated adult women. Vaccine 2012; 31:234-41; PMID:23063422; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 18. Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Hoye J, Steinwall M, Riis-Johannessen G, et al. . Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007; 25:4931-9; PMID:17499406; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 19. Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, Poncelet S, Zahaf T, Hardt K, Descamps D, et al; HPV Study Group for Adult Women. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine 2009; 27:581-7; PMID:19022320; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 20. Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Behre U, et al. . Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: Results from a randomized study. Hum Vaccin Immunother 2014; 10:1155-65; PMID:24576907; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Lebacq M, van der Most R, et al. . Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin 2011; 7:1359-73; PMID:22048172; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centres for Disease Control and Prevention. Vaccine Safety: Human Papillomavirus (HPV) Vaccine [Internet]. Updated December 20, 2013; cited January 29, 2014. Available from: http://www.cdc.gov/vaccinesafety/vaccines/HPV/Index.html. [Google Scholar]
- 23. Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, Lalezari J, David M-P, Lin L, Struyf F, et al. . Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: end-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother (in submission). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. . Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757-65; PMID:15541448; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 25. Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425-34; PMID:18948732; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 26. Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clinical Immunol 2011; 31:443-54; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.