Abstract
Despite extensive efforts to control myocyte growth by genetic targeting of the cell cycle machinery and small molecules for cardiac repair, adult myocytes themselves appeared to divide a limited number of times in response to a variety of cardiac muscle stresses. Rare tissue-resident stem cells are thought to exist in many adult organs that are capable of self-renewal and differentiation and possess a range of actions that are potentially therapeutic. Recent studies suggest that a population of cardiac stem cells (CSCs) is maintained after cardiac development in the adult heart in mammals including human beings; however, homeostatic cardiomyocyte replacement might be stem cell-dependent, and functional myocardial regeneration after cardiac muscle damage is not yet considered as sufficient to fully maintain or reconstitute the cardiovascular system and function. Although it is clear that adult CSCs have limitations in their capabilities to proliferate extensively and differentiate in response to injury in vivo for replenishing mature car-diomyocytes and potentially function as resident stem cells. Transplantation of CSCs expanded ex vivo seems to require an integrated strategy of cell growth-enhancing factor(s) and tissue engineering technologies to support the donor cell survival and subsequent proliferation and differentiation in the host microenvironment. There has been substantial interest regarding the evidence that mammalian fibroblasts can be genetically reprogrammed to induced pluripotent stem (iPS) cells, which closely resemble embryonic stem (ES) cell properties capable of differentiating into functional cardiomyocytes, and these cells may provide an alternative cell source for generating patient-specific CSCs for therapeutic applications.
Keywords: cardiac stem cells, heart failure, reprogramming, development
Introduction
Cycling adult myocytes
Intrinsic CSCs
Epicardial CSCs
Developmental origin of CSCs
Signals control CSC proliferation
New advance in cardiac regeneration
Conclusions
Introduction
Despite recent advances in the treatment of heart failure, cardiovascular diseases are the leading cause of morbidity and mortality throughout the world. Recent experiments with cell transplantation therapies have emerged as a potential strategy to treat ventricular dysfunction secondary to ischaemic cardiac injury [1]. The concept that adult cardiac stem cells (CSCs) may play a significant role in cardiac tissue homeostasis or responses to acute injury has been challenged by a series of experiments by fractionation of resident heart-derived cells, suggesting that adult CSCs could contribute at low levels to regenerate multiple cardiovascular lineages following direct transplantation into injured myocardium [2,3]. These observations have created a new avenue for studies aimed at repopulating damaged adult hearts by transferring resident CSCs amplified ex vivo; however, the degree of their regenerative potential was not as high as anticipated. Instead, the particular mechanisms by which such CSCs contribute to myocardial regeneration are still under investigation and whether endogenous CSCs are controlled by similar molecular pathways during cardiac development has yet to be determined. In addition, there is no evidence to provide strong support for dedifferentiation from mature cardiomyocytes or direct differentiation from relatively undifferentiated stem or progenitor cells as a major regenerative process for resident CSC plasticity during cell turnover under normal circumstances or in response to acute muscle damage [4].
Accumulated evidence suggests that transplantation of bone marrow-derived stem cells (BMCs) or skeletal myoblasts may contribute to the preservation of contractile function in some models of cardiac muscle injury [5], although skeletal myoblasts lack direct cardiomyogenic activity when cultured alone in vitro or myocyte formation in vivo[6]. Most importantly, experimental analyses showed that skeletal myoblasts do not couple electronically with the host myocardium without cellular fusion events [7]. Furthermore, recent studies of adult BMCs in adopting cardiac cell fate has been highly controversial [8–11]. The pathophysiological observations from several studies have indicated that BMCs integrated into the myocardium at very low frequency, if any, attributing to the functional improvement after myocardial infarction through paracrine effectors secretion rather than via direct myocyte differentiation and/or fusion in the transplanted hearts [11–13]. Despite these controversies regarding the efficacy of BMCs and myoblasts for cardiac regeneration, a number of randomized, multicenter clinical trials have conducted using autologous cells via intracoronary infusion or intramyocardial injection (Table 1). The results have shown either no benefit or small improvements in cardiac function, which were conferred unlikely from direct differentiation of the injected cells into cardiomyocytes [14, 15].
Table 1.
Randomized, control, clinical trials of stem/progenitor cell therapy for myocardial infarction
| Cell type | Study group | No of cells | Outcome (versus control) |
|---|---|---|---|
| BMMNCs | Wollert et al.[157] | 2.5 × 109 | 6% greater in LVEF at 6 mo |
| BMMNCs | Meyer et al.[158] | 2.5 × 109 | Not significant in LVEF at 18 mo |
| BMMNCs | Janssens et al.[159] | 3× 108 | Not significant in LVEF at 4 mo |
| BMMNCs | Lunde et al.[160] | 8.7 × 107 | Not significant in LVEF at 6 mo |
| BMMNCs | Schachinger et al.[161] | 2.4 × 108 | 2.5% greater in LVEF at 4 mo |
| BMMNCs | Assmus et al.[162] | 2× 108 | 2.9% greater in LVEF at 3 mo |
| SMBs | Menasche et al.[163] | 4 – 8 × 108 | Not significant in LVEF at 6 mo |
BMMNCs: bone marrow mononuclear cells; SMBs: skeletal myoblasts; LVEF: left ventricular ejection fraction; mo: month. BMMNCs were directly infused into coronary arteries. SMBs were injected intramyocardially during open chest surgery.
In the setting of identifying adult CSCs, several cell surface markers have been associated with cardiac progenitors [2, 3, 16]. yet no single marker defines the conclusive pool of CSC population. It is clear that CSCs have more cardiogenic potential than BMCs or skeletal myoblasts, although the contribution of the CSCs to the maintenance or repair of cardiac muscle under physiological and pathological conditions is not sufficient without further modifications in order to establish CSC transplantation as a new therapeutic strategy. In this regard, efficient engraftment of transplanted cells during cell therapy has emerged as a critical determinant to augment cardiac performance.
Based on these previous observations, investigators have demonstrated that specific cell types of stem/progenitors might be suitable for use in transplantation, but the limited success of cell engraftment requires further understanding of the molecular mechanisms of stem cell migration and proliferation, which are related to the improvement of donor cell survival and differentiation in situ. Autologous human CSC transplantation may hold the possibility to improve contractile function via direct cardiac muscle cell regeneration, of which clinical trials are underway, and may have great therapeutic potential to treat human heart failure.
Cycling adult myocytes
Recent studies revealed that adult myocytes possess self-replicative capacity, documented by mitosis and cytokinesis under normal and pathophysiological conditions [17, 18], and a small number of new myocytes could be formed after acute cardiac injury [19]. Early hypotheses proposed that new myocytes might be generated via re-entry to the cell cycle from pre-existing, mature myocytes through altered expression of D-type cyclins [20–22], cyclin-dependent kinase inhibitors [23] and pocket proteins [24]; however the number of cycling cells was relatively low. Further studies demonstrated that forced expression of anti-apoptotic protein bcl-2 [25], proto-oncogene c-myc [26] and telomerase reverse transcriptase [27] may enhance the hyperplastic growth by overriding the determined cellular growth arrest and senescence. Studies using growth factors have suggested that insulin-like growth factor (IGF)-1 [28] and nerve growth factor (NGF) [29, 30] produced myocyte proliferation and might recruit ectopic cells to control cardiac muscle force and cell size during post-natal heart development. The signalling molecules playing important roles in these processes have been postulated through extracellular signal-regulated kinase-1/2- and phosphatidylinositol (PI)-3-kinase-dependent signalling pathways [31–33]. Such studies formed the basis for further investigations of the potential effect of leptin, a product of obesity, on myocyte growth and metabolism in diabetic cardiomyopathy [34, 35].
Innovative strategies targeting single molecules to control myocyte growth are an attractive approach to treat heart failure. The blockade of specific protein kinase p38 has been reported to promote myocyte mitosis and cytokinesis in mice [36]; however significant improvement in global cardiac function after acute injury required additional growth factor treatment with fibrolast growth factor (FGF)-1 possibly allowing myocyte survival and new blood vessel formation in rat heart [37]. A recent report demonstrated that periostin, an adhesion molecule secreted by cardiac fibroblasts, may modulate the structural integrity of ischaemic myocardium by alterations in the amount of collagen with changes in wall stiffness to support infarct tissue healing [38]. Overexpression of periostin led to cardiac dysfunction, whereas inhibition of periostin has been shown to augment cardiac function by altering myocyte—fibroblast interactions during the process of cardiac remodelling [39]. Surprisingly, injection of periostin induced the re-entry of mature myocytes into the mitotic cell cycle leading to reduced ventricular remodelling and improved cardiac performance in vivo[40]. These findings suggested that altered expression of periostin, which may be dose-dependent, could benefit cardiac remodelling through the activation of PI-3 kinase/Akt and integrins as well as regulating myocyte size and collagen content for prevention of cardiac fibrosis. Furthermore, the promotion of myocardial survival and migration by thymosin-β4, which is involved in actin cytoskeletal organization, acting through integrin-linked kinase and Akt signalling, implies crucial roles for these signalling pathways in myocyte biology [41].
Intrinsic CSCs
The recognition of primitive cells in the heart has become an alternative aspect to interpret the mechanisms of the formation of new myocytes, even in the presence or absence of regenerative cues. The identification of cardiogenic progenitor cells has raised the possibility that alternative mechanisms may support cardiac muscle regeneration in the post-natal heart. Within the past few years, many investigators have provided evidence that a subpopulation of progenitor cells exists that possess stem cell-like properties (Table 2) [42]. Recent studies have revealed that during mammalian cardiogenesis, a cell population that expresses the LIM-homeodimain transcription factors Isl1, coexpressed with known early cardiac transcription factors Nkx2-5 and GATA4, arises from the cardiac crescent and marks the secondary heart field [43]. These cells are maintained as a proliferating population throughout early development and then contribute to the formation of the right ventricle and outflow tract [43, 44]. Although cells that expressed Isl were recently identified in the post-natal heart of rodents and human beings and gave rise to cardiac myocytes [45], the failure in identification of Isl1+ cells in the adult human heart has limited the clinical use of these progenitors as therapeutic application [46]. Using a tamoxifen-inducible Cre-lox strategy, Isl1+ progeny could be specifically recognized and purified at a defined time-point [45]. These cells could be expanded in culture and differentiated into β-adrenergic receptor agonist responsive functional myocytes in vitro. A lineage tracing study was used to document Isl + cells in embryonic stem (ES) cells and these cells could be clonally amplified showing unequivocally that a single Isl1+ cell was capable of generating progeny of cardiac, smooth muscle, and endothelial cell lineages [47]. Combinatorial transcriptional signatures of Isl with Nkx2-5 [48] and flkl [49–51], both of which exhibit a differentiation potential for mesodermal lineages such as cardiac muscle during development, revealed that both Isl1+/Nkx2-5+/flk1 and Isl1/Nkx2-5+/flk1 populations could serve as more restricted cardiac muscle progenitors. As described above, Isl1+ cells are not the only cardiac progenitor cell population in the heart during development. Unlike the ES cells-derived Nkx2-5+/c-kit+ population that are capable of differentiating into mature cardiomyocytes [48], the Isl1+ population express neither c-kit nor stem cell antigen (Sca)-1, but they do coexpress Nkx2-5 regardless of the cardiogenic progenitors enriched in the sub-fractionation of flk l [45]. Using Nkx2-5 promoter-driven transgenic mouse lines expressing eGFP specifically in the developing heart, modest expression of c-kit and Sca-1 determined the bipotential cardiovascular progenitors during embryonic cardiogenesis [48], suggesting that the developmental regulation of Nkx2-5, Isl1 and flk l might mutually control the pool of cardiac progenitors during embryogenesis [48, 50]. In addition, recent identification of flk1low/c-kitneg population in human ES cells that contains cardiovascular progenitors provides an opportunity to investigate the roles of flk l prior to the expression of Nkx2-5 and Isl1 in mesodermal specification during human cardiac development [51].
Table 2.
Features of resident cardiac stem/progenitors
| Cell type | Reference | Markers | Source |
|---|---|---|---|
| lsl-1+ progenitors | [45, 47] | Nkx2-5+, GATA4+, Sca-1−, c-kit−, CD31− | Human, rat, mouse, ES cells |
| c-kit+ progenitors | [2, 48, 54, 55, 57, 164] | Nkx2-5+, GATA4+, Sca-1+, MEF2+, CD34−, CD45− | Human, rat, mouse, dog, ES cells |
| Sca-1+ progenitors | [3, 16, 48, 79, 134] | Nkx2-5+, GATA4+, c-kit−, MEF2−, CD34−, CD45−, flk1− | Mouse |
| SP cells | [74, 75, 78] | Sca-1+, c-kitlow, CD34low, CD45low, Nkx2-5−, GATA4− | Mouse |
| Cardiospheres | [63–65] | Sca-1+, c-kit+, CD105+, CD90+, CD29+, CD34low | Human, mouse |
The evidence of these distinct cell populations of cardiac progenitors has raised the possibility that multiple primitive cells may coexist in the heart to support myocardial regeneration. Resident CSCs could also be isolated by the expression of protooncogene c-kit, which encodes a receptor tyrosine kinase, maps to the W locus and is activated by stem cell factor/kit ligand. Ligand binding leads to receptor dimerization and activation of multiple downstream signalling pathways involved in target mobilization, anti-apoptosis and cell proliferation [52]. c-kit was used to isolated cardiac mast cells more than a decade ago [53], but they were negatively sorted for blood lineage markers (Lin) including CD45 and CD34 [2]. These c-kit+/Lin− primitive cells were isolated cells from the adult rat heart with a small subpopulation coexpressing early cardiac transcription factors Nkx2-5, GATA4 and MEF2. As stem cells are defined by their unique capacity to both self-renew in the long-term and to undergo multiple-lineage differentiation, cardiac c-kit+/Lin− cells were prospectively clonogenic at single-cell level, expandable in cell culture and multipotent to generate the entire cardiovascular system in vitro and in vivo[2, 54]. A less frequency (1%), but detectable, of telomerase active human CSCs have been shown to form chimeric organs upon the introduction of human cells into rodent hearts after infarction with a significant improvement of myocardial function [55]. In mouse heart, most of the CSCs were mitotically quiescent and restored in a cardiac niche, created by supporting cells including myocytes and fibroblasts, coupled with the expression of integrins and connexins [56]. Using BrdU pulse-chasing experiments, nearly 10% of CSCs showed slow-cycling properties, indicating that these primitive cells could become activated in response to stress by injury [19] or in the context of myocyte turnover along with homeostasis [18, 56]. Extrinsic mechanical stretch applied to myocytes also triggers numerous intracellular signals to activate preserved CSCs in vivo[57].
Although c-kit may represent a developmental remnant from a multipotent mesodermal cell population including bone marrow, whether these c-kit+/Lin− CSCs in adult hearts represent the progeny of cardiac progenitors from the embryos or a distinct population remains unknown. Studies of W locus mutant mice that strongly implicated a loss-of-function in c-kit [58] showed no developmental abnormalities in the heart, suggesting that c-kit might be dispensable for cardiovascular cell proliferation and differentiation during embryogenesis as well as raising the possibility of its alternative function to identify resident mast cells [59]. Although c-kit+/Lin− CSC populations are associated with high telomerase activity, the absence of cardiac defects in c-kit mutant mice is in sharp contrast to the severe cardiac phenotype observed, ventricular dilation concomitant with cardiac dysfunction, in later generation of telomerase knockout mice [60]. Additional evidence for the crucial role of c-kit+ cells emerged from experiments on bone marrow reconstitution studies showing that bone marrow-derived c-kit+/Lin− cells completely rescued the defective cardiac repair in c-kit mutant mice after infarction [61, 62] indicating somatic c-kit+ cells including cardiac CSCs may be circulating mesodermal progeny of cells residing in the bone marrow and could be mobilized in situ in response to acute injury for myocyte repair. The observation that cardiospheres in human beings and mice (Fig.1), which contain a population of CSCs, exhibited lower levels or the absence of c-kit expression [63–65] has questioned whether single cell surface markers may be adequate to define all CSCs in the developing heart. Lineage-tracing experiments by targeting c-kit could unveil the functions of these cells in the heart throughout embryonic and post-natal development.
Figure 1.
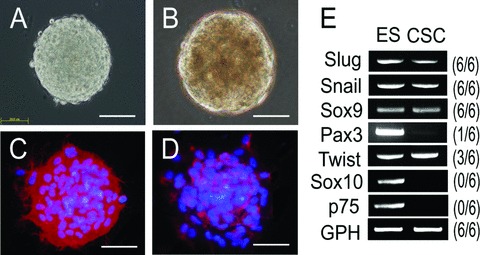
Isolation and characterization of clonal CSCs. Phase contrast and fluorescence micrographs of CSCs isolated from mouse (A) and human hearts (B). Human CSCs were stained with CD105 (C) and CD90 (D). Bars, 50 μm. (E) Single colony RT-PCR for genes characteristic of neural crest stem cells in mouse CSCs. The numbers on the right indicate the number of individual colonies that expressed the corresponding genes out of the colonies examined. ES, embryonic stem cells.
Sca-1, also known as lymphocyte activation protein-6A (Ly-6A). is a glycosyl phosphatidylinositol-anchored cell surface protein and is used widely as an alternative cell surface marker to enrich haematopoietic stem cells (HSCs) in conjunction with Lin−/c-kit+ selection in the presence of interleukin-3 (IL-3) [66]. Studies using reporter gene tracing experiments revealed that foetal Sca-1 expression was first observed at E9 in the ventral dorsal aorta and E11 in the foetal liver [67]; progenitor cells expressing Sca-1 in adult bone marrow-enriched HSCs were capable of generating at least both T and B lymphocytes but lacked myeloid differentiation potential [68, 69]. In adults, Sca-1 is expressed in many somatic tissues including mammary gland [70], prostate [71], lung [72], skeletal muscle [73] and cardiac muscles [3] as a marker of self-amplifying cells within the tissues they reside in. Cardiac Sca-1+ cells exhibited high levels of telomerase activity similar to that in neonatal myocardium and were negative for the expression of CD45 and CD34, markers of HSCs and endothelial progenitor cells (EPCs). These cells have been shown to be highly coexpressed with side population (SP) cells [3, 74, 75], which also enrich HSCs [76]. The expression of Sca-1 in adult hearts identified a cell population capable of differentiating into cardiac muscle, endothelium, smooth muscle, and contributed to adipogenesis and osteopoiesis in vitro[3, 16]. As with Isl1+ and c-kit+ cells in adult myocardium, cardiac Sca-1+ cells are also potent cardiac progenitors and are able to maintain their own functional population by self-renewal. Studies on cell transplantation after acute myocardial infarction showed that the progeny of resident Sca-1+ cells adopted divergent fates with the majority of the population differentiating into cardiac muscle and smooth muscle cells along the ischaemic border zone, whereas a minority remained undifferentiated and retained expression of Sca-1. Intravenously transplanted CSCs homed-in and migrated into the injured cardiac foci and substantial graft-derived cardiogenic activity persisted in the host myocardium for at least 4 weeks [3, 75].
As described above, no single marker conclusively defines lineage-specific stem/progenitor cells, as seen in the enrichment of bone marrow stem cells and skeletal satellite cells [77]. The heterogeneity of marker expression may also reflect functional differences among CSCs in terms of cardiogenic specification. The expression of candidate surface markers or transcription factors may just serve as an indicator during the developmental program in different organ systems but might be needed in conjunction with other components to define a lineage-committed cell population of interest. Negative selection of CD31 from the Sca-1+ cardiac SP cell pool was shown to significantly increase the activity during the first week after myocardial infarction [78]. These Sca-1 +/CD31− cardiac SP cells differentiated into functional cardiomyocytes more efficiently in vitro as compared with CSCs expressing CD31, indicating that a significant heterogeneity exists even among the relatively small percentage of cardiac Sca-1+ SP cells.
To assess whether clonal CSCs are homogenously multipotent, and if CSCs could be derived from a functionally identical primitive cell population that resided in myocardium, we used single-cell deposition analysis without pre-selection by specific cell surface markers or transcription factors to prospectively isolate and characterize CSCs from adult murine heart [79]. Clonally amplified cell clones arising from intrinsic myocardial cells could be expanded in culture and possessed high telomerase activity as observed in Sca-1+ and c-kit+ CSCs [3, 80]. Nearly 70% of clonal CSCs could be recognized by Sca-1 but were rarely detectable by c-kit, indicating that these markers are provisional rather than definitive indicators. Under undifferentiated conditions, all clones exhibited the characteristics of mesenchymal-like cells, as shown by the marker profile CD105+/CD90+/ CD29+/CD44+/CD73+/ CD106+, but these cells lacked both CD45 and CD34 expression and expressed the transcription factors Slug and Snail (Fig.1), which have been suggested to represent mediators of epithelial-mesenchymal transitions (EMT) during neural crest development [81]. These cells could give rise to structurally mature cardiovascular cells regardless of Brachyury expression at baseline, which is a marker for the primitive streak [82]. More detailed study by a retrospective isolation to further delineate the precise identity of CSCs using GFP reporter mice harbouring telomerase reverse transcriptase (TERT) promoter showed that more than 80%, a higher frequency than that of clonal CSCs, of the freshly isolated TERT+ cells in the adult heart expressed Sca-1 [79]. The cardiac niche of telomerase-competent CSCs was marked by TERT expression as previously reported [60, 80] (Fig.2) and revealed clusters of TERT+ but CD45− cells in both atria and ventricles (left atria: 1.60 ± 0.03 cells/mm2, left ventricle: 0.04 ± 0.01 cells/mm2, and right ventricle: 0.26 ± 0.02 cells/mm2, respectively), most abundantly along the right atria (4.80 ± 0.05 cells/mm2) and outflow tract (4.40 ± 0.03 cells/mm2). The TERT+ CSC distribution was in contrast to the results obtained from c-kit+ CSC growth using a BrdU pulse-chasing study within the cardiac niche, showing atria and apex were preferred residential sites [56]. It remains to be determined how these distinct cell populations are related anatomically and physiologically to control the fate of adult myocyte turnover.
Figure 2.
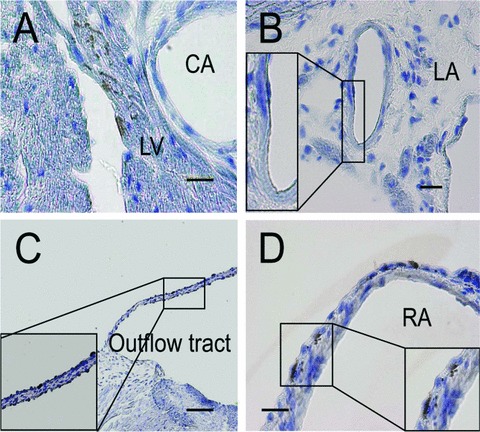
Cardiac stem cell niche in the adult mouse myocardium. (A—D) Immunohistochemistry of TERT promoter-driven GFP transgenic hearts using GFP antibody that was counter-stained with CD45 and eosin. TERT-positive cells in the left ventricle (A), left atria along vessel (B), outflow tract (C), right atria (D). Bars, 50 μm. CA: coronary artery; LV: left ventricle; LA: left atrium; RA: right atrium.
Epicardial CSCs
The embryonic heart tube is formed by fusion of the primary heart fields and comprises an outer myocardial layer, including the epicardium, and an inner endocardial layer [83]. Myocardial precursor cells added to the anterior and posterior poles of the linear heart tube are derived from two lineages that segregate early from a common precursor [84, 85]. The epicardium consists of a sub-population of mesothelial cells adjacent to the inflow tract that proliferate and extend into the pericardial cavity. This subpopulation of epicardium-derived cells undergoes an EMT and migrates to give rise to cardiac fibroblasts, vascular endothelium [86], smooth muscle of the coronary system and the epicardium [87].
A recent study has further shown that epicardium-derived c-kit+/CD34+ cells in mouse and human might be a cell population of myocardial and vascular precursors [88]. These precursor cells were specified in the subepicardial space and proliferated and differentiated following myocardial infarction. The unique function of these cells was verified by a pericardium open/close system in infarction, demonstrating that cardiac function in infarct mice in which the pericardial tissue was kept closed revealed a greater preservation of left ventricular function in contrast to the cardiac function measured in mice where the pericardium was left open during ischaemic injury, in terms of the continuous supply of migrating epicardial progenitor cells towards the ischaemic regions. In addition, c-kit−/CD34+ epicardial cells represent a population with vasculogenic and angiogenic properties in which the expression of CD31 was absent. Although these CD34+ epicardial precursors localized within the subepicardial region, where adipose tissue occupied most of the region, these cells were distinct from circulating blood-derived progenitor cells in terms of their negative expression of CD45 and lower extent of Sca-1 and multi-drug resistance-1 (MDR-1) expression but were positive for CD105 and CD90, suggesting that they could be mesenchymal in origin and had arisen from EMT. This mesodermal mesenchymal supply is critical to provide the heart with vascular and connective tissue progenitor cells [89].
Developmental origin of CSCs
The heart is constructed from cells of a variety of origins including extracardiac sources. The major extracardiac contributions to the heart include cells of epicardial organ and the neural crest [90]. In contrast, the conduction system is not a neural crest derivative but is derived from cardiac myogenic precursors in chicks [91]; a recent study has shown a close relationship between neural crest cells and the developing conduction system in mice using viral and genetic fate-mapping strategies [92]. An additional report has also demonstrated that cardiac neural crest cells originating both from neural tube regions and myogenic precursors invade the myocardium in all segments of the heart including the outflow tract, atrium, atrioventricular junction and ventricle in zebrafish [93]. In the past few years, promoter—reporter constructs or double-transgenic analysis using LacZ or GFP reporter mice and neural crest-specific Cre recombinase has facilitated genetic marking of neural crest cells in mice. Several different transgenic lines carry neural crest-specific Cre recombinase activity including Wnt1 [94] and P0 [95]. The undifferentiated, GFP-labelled neural crest stem cells contain a subset of the SP cell population that reside in the proximal but not distal conduction system, which could form cardiospheres as we have shown previously (Fig.1), and maintain neurogenic, gliagenic and cardiovasculogenic potentials [92, 95].
Selected aspects of growth factor action on cardiac neural crest stem cells have been demonstrated recently. Pinch1, which is composed of five LIM domains, plays an important role in processes as diverse as stem cell migration, proliferation and differentiation. Wnt1-dependent Pinch1-deficient mice displayed severe cardiovascular defects, including an unusual aneurysmal common arterial trunk, ventricular septal defects and defective cushion/valve maturation because neural crest stem cells in the outflow tract cushion underwent markedly increased apoptosis at E11.5 to E13.5 caused by decreased transforming growth factor (TGF)-β signalling [96]. In zebrafish, both Semaphorin (Sema) 3D and its receptor, Neuropilin 1A, morphants showed defects in the neural crest and have disrupted cardiomorphogenesis including hypertrophic cardiomyocytes, decreased ventricular size, and defects in atrioventricular valve development [97]. Interestingly Sema 3A is known to promote the aggregation of neurons into sympathetic ganglia during early embryogenesis. While Sema 3A null mice exhibited sinus bradycardia, abrupt sinus slowing and stellate ganglia defects, cardiac-specific overexpression of Sema3A in mice was associated with reduced sympathetic innervation as well as high susceptibility to ventricular arrhythmias although the contractile function and myocardial structure were preserved at baseline levels [98]. These findings suggested that Sema family members may control the migratory pathways for neural crest stem cells to regulate formation of the primary heart field and subsequent sympathetic nerve system development. Gain-of-function and loss-of-function studies have shown that Sonic hedgehog (Shh) [99] and TGF-β[100] regulate neural crest stem cell differentiation into smooth muscle and neural cells. Moreover, both TrkC and myocardin-related transcription factor-B are essential for normal cardiac neural crest stem cell-oriented outflow tract septation [101] and smooth muscle differentiation [102]. It will be of interest to determine if these factors are involved in the cellular development of other types of CSCs that was not originated from neural crest cells.
Signals control CSC proliferation
Cell-intrinsic properties are not the only determinants of stem cell fate. CSCs are tightly regulated by their microenvironment and tissue-specific signalling (Table 3). Gain-of-function studies revealed that activation of canonical Wnt signalling promoted Isl1+ cardiac progenitor cell expansion with a concomitant increase in FGF signalling [103, 104].
Table 3.
Development and regulatory network of cardiac stem/progenitor cells
| Marker | Origin and function | Mutant phenotype | Regulatory signals |
|---|---|---|---|
| Isl-1 | Embryonic marker of the second heart field [43] | Single atria and ventricle with no RV and OFT formation [43] | Wnt-β-catenin, FGF [103, 104] |
| c-kit | Steel factor receptor expressed on mast cells and stem cells [2, 53] | Not detected in the heart [61, 62] | IGF-1, HGF, HMBG1, Akt [60, 119, 120, 132] |
| Sca-1 | Cell surface protein expressed on activated lymphocytes and stem cells [3, 79, 165] | Not detected in the heart [79, 125] | Akt [79] |
| Abcg2 | ATP-binding cassette (ABC) transporters able to confer SP cells [166] | Not detected in the heart [167] | HIF-2α[168] |
RV: right ventricle; OFT: outflow tract; Abcg2: ABC subfamily G member 2, also known as the breast cancer resistant protein, Bcrp1, expressed in cardiac SP cells; HIF-2α: Hypoxia-inducible factor-2α.
Studies of loss-of-function and gain-of-function by tissue-specific gene targeting in mouse models have implicated Wnt-β-catenin signalling as one of the key regulators of precardiac mosederm-specification, Isl1+ CSC self-renewal and subsequent differentiation. An early study that reported conditional deletion of β-catenin targeting visceral endoderm led to the formation of multiple hearts all along the anterior—posterior axis of the embryo, which is consistent with the regions of ectopic bone morphogenetic protein 2 (BMP2) expression [105]. However deletion of β-catenin under the control of MEF2C, which is activated after initiation of the secondary heart field conducted by Isl1 expression, led to a severe disruption of outflow tract patterning and growth of the right ventricular and interventricular myocardium with significant reductions in cyclin D2 and TGF-β2 expression [106]. This finding is precisely consistent with the cardiac phenotype reported from Isl1 mutant mice [43]. These observations are also analogous to the report from mice in which Wnt-β-catenin signalling is reduced by the suppression of the desmoplakin gene that is responsible for human arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVC) [107].
Conditional deletion of Isl1 resulted in a significant reduction of the number of cardiac progenitors, suggesting that growth factor signalling may be perturbed. Ablation of β-catenin in the Isl1 locus exhibited embryonic lethality due to the absence of outflow tract morphogenesis and right ventricular identities with a drastic reduction in expression of a number of genes including Tbx2/3, Wnt11, Shh and Pitx2 [108, 109]. Furthermore, the fact that a significant decrease in the expression of BMP4/7 and FGF8/10 in Isl1 null mice [43] and the requirement for autocrine FGF8 signalling for normal formation of the primary and secondary heart field-derived structures [110] provided a pivotal insight into how FGFs and BMPs are mutually involved in secondary heart field proliferation and development as upstream or downstream targets of Isl1 [111,112].
c-kit+ CSCs are regulated by a complex network of cytokines including stem cell factor (SCF), which have specific effects known to synergize with other cytokines, e.g. G-CSF and Flt-3 lig-and (FL), to produce rapid expansion and mobilization of progenitor cells and resultant differentiation for cardiac repair [113,114], whereas later cytokines have broader cytoprotective effects than direct control of stem cell self-renewal through cell cycle regulation than has been previously appreciated [115]. Studies have shown a significant cardiac homing of c-kit+ stem cells to the injured myocardium from bone marrow by intramyocardial delivery of SCF or bioengineered stromal cell-derived factor-1 (SDF-1), a chemokine for attracting stem cells [116, 117]. The loss of anti-apoptotic and angiogenic paracrine activities in c-kit deficient mice might directly contribute to perturbed cardiac function after infarction, which could be rescued by c-kit+/Lin− bone marrow cells reconstitution in the absence of cardiac c-kit+ CSC replenishment [61, 62]. Although the exact origin of the majority of the stem cells that spontaneously mobilize to cardiac tissue and differentiate into functional cardiomyocytes after cardiac injury remains poorly understood [4], these studies do not exclude the possible role of intrinsic c-kit+ CSCs participating in myocardial self-repair during injury. Local injection of ACK2, an antibody that blocks c-kit function, may further indicate the balance and dependence on intrinsic and/or extrinsic c-kit+ stem cells as part of the mechanisms of cardiac regeneration in vivo[118].
The functional involvement of insulin-like growth factor-1 (IGF-1)/IGF-1 receptor and hepatocyte growth factor (HGF)-c/Met systems in c-kit+ CSC senescence and telomere attrition is supported by their effects on CSCs to facilitate migration, proliferation and differentiation in situ to reconstitute the damaged myocardium [60, 119]. Recent work has revealed that local injection of high mobility group box 1 (HMGB1), a nuclear protein that is released by cells undergoing necrosis but not by apoptotic cells, specifically promoted resident c-kit+ CSC migration and proliferation acting through HMGB1 receptor RAGE into the ischaemic border zone. However, HMGB1 injection did not recruit cells from the bone marrow into the systemic circulation [120]. Extracellular HMGB1 has been reported to engage multiple receptors including RAGE to induce nuclear factor (NF)-κB activation dependent on extracellular signal-regulated kinase (ERK) [121]. Most importantly, the endogenous levels of HMGB1 increased during tissue injury, suggesting that the response signals could also be early mediators of inflammation to direct c-kit+ mast cells [122]. The intricate interplay of detrimental effects of HMGB1 has been shown by evidence that treatment with a neutralizing antibody to HMGB1 is associated with reduced phosphorylation of c-Jun NH(2)-terminal kinase and increased NF-kappaB DNA binding to reduce damage during liver injury [123].
As with the diminished function and remodelling after myocardial infarction in c-kit mutant mice, Sca-1 deficiency reduced the replicative capacity of clonal CSCs in vitro[79]. It is conceivable that tissue-resident stem cells use tissue-specific signalling, such as Sca-1, to regulate the stem cell balance between self-renewal and growth arrest mediated through different downstream kinases. Eventually, Sca-1 null mice have shown to exhibit age-related bone remodelling and resultant osteoporosis [124], abnormal responses to antigen stimulation in T cells [125], reduction in engraftment of bone marrow transplants [126] and inhibition of myoblast fusion to promote differentiation [127] related to perturbed c-src and Fyn signalling [128,129]. Evidence from a model of Sca-1 knockdown mice revealed that myocardial infarction leads to ventricular remodelling and poor survival; a phenotype that was recapitulated by studies using c-kit mutant mice (Fig.3) [61, 62]. In the Sca-1 deficient system, activation of Akt in the host myocardium appears to be a crucial regulator for survival of cardiomyocytes. Normal Sca-1 function in CSCs is associated with border zone-specific increase in vascular endothelial growth factor (VEGF) and HGF that could promote myocardial tissue and donor cell survival through autocrine/paracrine mechanisms [79]. Using direct transplantation experiments in Sca-1 deficient mice, we also found that clonal CSCs isolated from wild-type but not from Sca-1-deficient hearts were able to significantly improve survival after infarction, suggesting that perturbed cardiac function after infarction in Sca-1 deficient mice could be partially due to the physiological dysfunction of intrinsic CSCs (K. Tateishi and H. Oh, unpublished observations). It is important that Sca-1 may also define a subset of bone marrow stem cells and functional abnormalities such as mobilization and proliferation in this cell population that may account for the cardiac phenotype observed in this study. Similar results have been reported from the transplantation of Akt-modified mesenchymal stem cells into infarct rats, leading to reduced infarct size and prevention of cardiac remodelling through the release of secreted frizzled related protein 2 (Sfrp2) [130,131]. Furthermore, transgenic experiments with cardiac-specific Akt have been shown to promote myocyte proliferation and resident c-kit+ CSC expansion in vivo via up-regulation of HGF in favour of the expression profile in neonatal myocardium [132]. In addition, fibroblast growth factor 2 (FGF2)-deficient mice have been shown to suffer from an impaired hypertrophic response and dilated cardiomyopathy due to a defective capacity of fibroblasts for releasing growth factors to induce cell growth response in cardiomyocytes [133]. Although our results revealed that clonal Sca-1+ CSCs require FGF2 to proliferate in culture [79], study of FGF2 deficient mice has shown that the Sca-1+ CSC pool was comparable with wild-type mice [134]. Interestingly, the full process of cardiac differentiation in both in vivo engraftment and ex vivo culture is dependent on FGF2. It seems that certain redundant pathways might involve other FGF family members in vivo to control stem cell growth, or cell adhesion-mediated extracellular matrix desensitization could occur requiring FGF2 activation for CSCs to self-replicate in vitro[135].
Figure 3.
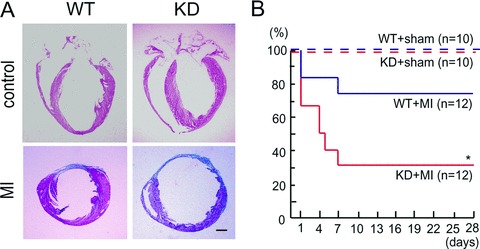
Sca-1 knockdown (KD) mice after acute myocardial infarction (MI) showed cardiac remodelling and reduced survival. (A) H&E staining of the hearts at baseline from wild-type (WT) and Sca-1 KD mice (top). Images of Masson's trichrome staining from hearts 4 weeks after Ml are shown (bottom). Scale bar, 1 mm. (B) Survival analysis of Sca-1 KD mice after MI. Each group started with the numbers indicated. Differences in survival rates between the Sca-1 KD and WT littermate mice after MI were significant by the Peto-Peto-Wilcoxon test. *, P < 0.01.
Although whether the cardiovasculogenic abilities of epicardial progenitor cells strictly depend on a myocardium-derived signal or they are also sensitive to triggers from epicardial tissue itself in mammalian heart remain to be investigated, several signalling molecules have been implicated in the EMT process including ligand FGF17 and its receptor FGFR2 and FGFR4 in zebrafish heart regeneration [136]. Differentiation into the myocardial or epicardial lineage is mediated by the cooperative action of BMP and FGF signalling in chicken embryos [137]. Of the vascular growth factors that could potentially induce EMT, bFGF, epidermal growth factor (EGF), platelet-derived growth factor (PDGF)-BB and VEGF have been shown to stimulate epicardial cells in coronary morphogenesis [87, 89]. Furthermore, thymosin-pβ, a G-actin monomer binding protein involved in reorganization of the actin cytoskeleton, has been shown to promote mobilization and neovascularization not only through cardiac development but also from adult epicardium via direct stimulation of rapid differentiation and migration of Flk-1+ epicardial progenitor cells in terms of an increase in the pro-angiogenic cleavage peptide, N-acetyl-seryl-aspartyl-lysyl-proline [138]. To directly explore whether the embryonic epicardium is a key signalling tissue responsible for transmission of morphogenetic signals during cardiac development, proepicardium-targeted β-catenin-deficient mice have been characterized. Transcription factor GATA5-dependent ablation of β-catenin in the epicardium showed that impaired development of epicardial progenitor growth and concomitant myocardial hypoplasia are secondary to the defective formation of coronary arteries [139]. Although expression of β-catenin in the epicardium did not seem essential for EMT stimulation in this model, it is conceivable that canonical Wnts directly enhance epicardial smooth muscle differentiation in coronary remodelling. It is important to note that α4β1 integrin-mediated cell adhesion functions in the migration of progenitor cells to form the epicardium [140], which is consistent with a previous finding that β-catenin is a bifunctional protein involved in cell—cell adhesion [141].
New advance in cardiac regeneration
Mammalian ES cells, including mouse [142] and human [143], are alternatives for use in cell therapy and were first isolated as undifferentiated cells from the inner cell mass or epiblast of blastocyst-stage embryos. Recent advances in epigenetic reprogramming of somatic cells into ES cells have attracted much attention because of their therapeutic potential allowing the generation of patient-specific pluripotent cell lines to circumvent concerns regarding ethical issues and rejection by the host immune system. Initial studies have attempted to use human ES cells as an alternative to oocytes for reprogramming human somatic nuclei and showed that the hybrid cells generated maintain a stable tetraploid DNA content and the transcriptional state of somatic nuclei can be reprogrammed by cell fusion [144]. This evidence was recapitulated in primate ES cells by nuclear transfer technology and suggested the possibility that differentiated cells can be reprogrammed to an undifferentiated state by trans-acting factors present in the partner ES cells by mechanical reprogramming as well as prompting research to identify specific factors that could mediate this process via molecular reprogramming [145].
Accumulated evidence has provided the stunning result that a simple recipe of just four transcription factors comprising Oct3/4, Sox2, c-Myc and Klf4, can induce both mouse and human fibroblasts into induced pluripotent stem (iPS) cells, which are indistinguishable from ES cells [146–148]. A previous study has shown that another quartet of reprogramming factors, Oct4, Sox2, Nanog and Lin28, could successfully reproduce the pluripotent characteristics from human fibroblasts [149]. The latest report has shown that a modified protocol without c-Myc, which might increase the incidence of tumour development, could yield functionally similar iPS cells in mice [150]. Regardless of the combinations of transcription factors employed, human iPS cells and their derivatives may hold great promise for stem cell-based cardiac repair. Although the tumouri-geneity of the retrovirus vectors themselves and the delivered onco-genes has yet to be investigated in long-term observations, elegant outcome from specific cell lineage differentiation such as blood cells as well as successful creation of iPS cells from liver and stomach cells in animal experiments is quite encouraging [151, 152].
Lastly, modulation of the microenvironment for myocardial tissue engineering subsequent to acute injury by any type of cell transplantation requires enhanced survival and resultant differentiation in vivo. Transplantation of differentiated cardiomyocytes [153], human ES cells [154] or undifferentiated mesenchymai stem cells [155]via direct injection with a scaffold or pre-seeded on cell sheet has demonstrated a significantly greater engraftment and improved cardiac function after infarction. Human ES cell research is crucial as a rational basis for investigating how human iPS cells can give rise to functional cardiomyocytes. The combinations of appropriate tissue engineering strategies with the best characterized and the most functional cardiogenic-stem/progenitors may potentially enhance cardiac muscle regeneration as clinically applicable and practical approaches to treat patients with severe heart failure.
Conclusions
Within the past 4 years, researchers have succeeded in the isolation of somatic CSCs, genetic creation of iPS cells, the most recently, engineering of a cell-based bioartificial heart as a solid organ for transplantation [156] in experimental or preclinical studies. This review highlights the evidence for the existence of endogenous cardiac progenitor cell populations in adult mammalian heart. A subset of the cell populations are neural crest stem cells in genetic origin, undergo to EMT and acquire mesodermal mesenchyme properties during embryonic development and represents an obvious target for therapeutic intervention for cardiovascular regeneration following heart failure. Recent investigations have not yet unveiled the key elements controlling cardiovascular-lineage diversification as well as the orchestrated network conducting cell fate decisions, including self-renewal, migration and differentiation of CSCs. Continued research works may lead to a discovery of the composition of defined factors able to activate CSCs to expand, survive in situ and fully differentiate into the lineages of interest. The rapid progress of tissue engineering technologies may also optimize the efficacy of cell therapy by augmentation of these critical steps during transplantation in patients with cardiac defects or functional failure.
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, and by Grants-in-Aid from the Ministry of Health, Labor and Welfare.
References
- 1.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–83. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 3.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murry CE, Field LJ, Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112:3174–83. doi: 10.1161/CIRCULATIONAHA.105.546218. [DOI] [PubMed] [Google Scholar]
- 6.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci USA. 2003;100:7808–11. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubart M, Soonpaa MH, Nakajima H, Field LJ. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. J Clin Invest. 2004;114:775–83. doi: 10.1172/JCI21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 9.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 10.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 11.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 13.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–50. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Rosenzweig A. Cardiac cell therapy–mixed results from mixed cells. N Engl J Med. 2006;355:1274–7. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 15.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 16.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomy-ocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 17.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA. 1998;95:8801–5. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 20.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, Kim KK, Field LJ. Cyclin D1 overexpression promotes car-diomyocyte DNA synthesis and multinu-cleation in transgenic mice. J Clin Invest. 1997;99:2644–54. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in car-diomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005;96:110–8. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 22.Liao HS, Kang PM, Nagashima H, Yamasaki N, Usheva A, Ding B, Lorell BH, Izumo S. Cardiac-specific overexpres- sion of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ Res. 2001;88:443–50. doi: 10.1161/01.res.88.4.443. [DOI] [PubMed] [Google Scholar]
- 23.Poolman RA, Li JM, Durand B, Brooks G. Altered expression of cell cycle proteins and prolonged duration of cardiac myocyte hyperplasia in p27KIP1 knockout mice. Circ Res. 1999;85:117–27. doi: 10.1161/01.res.85.2.117. [DOI] [PubMed] [Google Scholar]
- 24.MacLellan WR, Garcia A, Oh H, Frenkel P, Jordan MC, Roos KP, Schneider MD. Overlapping roles of pocket proteins in the myocardium are unmasked by germ line deletion of p130 plus heart-specific deletion of Rb. Mol Cell Biol. 2005;25:2486–97. doi: 10.1128/MCB.25.6.2486-2497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limana F, Urbanek K, Chimenti S, Quaini F, Leri A, Kajstura J, Nadal-Ginard B, Izumo S, Anversa P. bcl-2 overexpression promotes myocyte proliferation. Proc Natl Acad Sci USA. 2002;99:6257–62. doi: 10.1073/pnas.092672899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson T, Allard MF, Sreenan CM, Doss LK, Bishop SP, Swain JL. The c-myc proto-oncogene regulates cardiac development in transgenic mice. Mol Cell Biol. 1990;10:3709–16. doi: 10.1128/mcb.10.7.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci USA. 2001;98:10308–13. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA. 1996;93:8630–5. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrade-Rozental AF, Rozental R, Hassankhani A, Spray DC, Federoff HJ. Characterization of two populations of ectopic cells isolated from the hearts of NGF transgenic mice. Dev Biol. 1995;169:533–46. doi: 10.1006/dbio.1995.1167. [DOI] [PubMed] [Google Scholar]
- 30.Hassankhani A, Steinhelper ME, Soonpaa MH, Katz EB, Taylor DA, Andrade-Rozental A, Factor SM, Steinberg JJ, Field LJ, Federoff HJ. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Dev Biol. 1995;169:309–21. doi: 10.1006/dbio.1995.1146. [DOI] [PubMed] [Google Scholar]
- 31.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinosi-tide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–93. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 32.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–49. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 33.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–48. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tajmir P, Ceddia RB, Li RK, Coe IR, Sweeney G. Leptin increases cardiomy-ocyte hyperplasia via extracellular signal-regulated kinase- and phosphatidylinositol 3-kinase-dependent signaling pathways. Endocrinology. 2004;145:1550–5. doi: 10.1210/en.2003-1128. [DOI] [PubMed] [Google Scholar]
- 35.Laustsen PG, Russell SJ, Cui L, Entingh-Pearsall A, Holzenberger M, Liao R, Kahn CR. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol Cell Biol. 2007;27:1649–64. doi: 10.1128/MCB.01110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–87. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:15546–51. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–21. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T, Sugimura K. Periostin as a novel factor responsible for ventricular dilation. Circulation. 2004;110:1806–13. doi: 10.1161/01.CIR.0000142607.33398.54. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–9. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 41.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–72. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 42.Parmacek MS, Epstein JA. Pursuing cardiac progenitors: regeneration redux. Cell. 2005;120:295–8. doi: 10.1016/j.cell.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 43.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–40. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 45.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal Isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN, Menasche P. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–8. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 48.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipo-tential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–7. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 50.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 52.Jiang X, Gurel O, Mendiaz EA, Stearns GW, Clogston CL, Lu HS, Osslund TD, Syed RS, Langley KE, Hendrickson WA. Structure of the active core of human stem cell factor and analysis of binding to its receptor kit. EMBO J. 2000;19:3192–203. doi: 10.1093/emboj/19.13.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sperr WR, Bankl HC, Mundigler G, Klappacher G, Grossschmidt K, Agis H, Simon P, Laufer P, Imhof M, Radaszkiewicz T, Glogar D, Lechner K, Valent P. The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood. 1994;84:3876–84. [PubMed] [Google Scholar]
- 54.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:1668–73. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100:10440–5. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexander WS, Lyman SD, Wagner EF. Expression of functional c-kit receptors rescues the genetic defect of W mutant mast cells. EMBO J. 1991;10:3683–91. doi: 10.1002/j.1460-2075.1991.tb04936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hara M, Ono K, Hwang MW, Iwasaki A, Okada M, Nakatani K, Sasayama S, Matsumori A. Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med. 2002;195:375–81. doi: 10.1084/jem.20002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 61.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H, Zhang J, Cukerman E, Stanford WL, Medin JA, Liu PP. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:2304–9. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angio-genic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 64.Tateishi K, Ashihara E, Honsho S, Takehara N, Nomura T, Takahashi T, Ueyama T, Yamagishi M, Yaku H, Matsubara H, Oh H. Human cardiac stem cells exhibit mesenchymal features and are maintained through Akt/GSK-3beta signaling. Biochem Biophys Res Commun. 2007;352:635–41. doi: 10.1016/j.bbrc.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 65.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomy-ocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 66.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–50. [PubMed] [Google Scholar]
- 67.de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hema-topoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–83. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 68.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lym-phoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 69.Ma X, Robin C, Ottersbach K, Dzierzak E. The Ly-6A (Sca-1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem Cells. 2002;20:514–21. doi: 10.1634/stemcells.20-6-514. [DOI] [PubMed] [Google Scholar]
- 70.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 71.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA. 2005;102:6942–7. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 73.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–52. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 74.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–75. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 75.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomy-ocytes in vitro and in vivo. J Cell Biol. 2007;176:329–41. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 78.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 79.Tateishi K, Ashihara E, Takehara N, Nomura T, Honsho S, Nakagami T, Morikawa S, Takahashi T, Ueyama T, Matsubara H, Oh H. Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. J Cell Sci. 2007;120:1791–800. doi: 10.1242/jcs.006122. [DOI] [PubMed] [Google Scholar]
- 80.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–13. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 81.Le Douarin NM, Dupin E, Ziller C. Genetic and epigenetic control in neural crest development. Curr Opin Genet Dev. 1994;4:685–95. doi: 10.1016/0959-437x(94)90135-p. [DOI] [PubMed] [Google Scholar]
- 82.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806–11. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–35. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 84.Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95:261–8. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- 85.Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6:685–98. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- 86.Wada AM, Smith TK, Osler ME, Reese DE, Bader DM. Epicardial/Mesothelial cell line retains vasculogenic potential of embryonic epicardium. Circ Res. 2003;92:525–31. doi: 10.1161/01.RES.0000060484.11032.0B. [DOI] [PubMed] [Google Scholar]
- 87.Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocar-dial fibroblasts in the avian heart. Dev Biol. 1998;193:169–81. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 88.Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M, Loiaconi V, Pompilio G, Germani A, Capogrossi MC. Identification of myocar-dial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–65. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 89.Guadix JA, Carmona R, Munoz-Chapuli R, Perez-Pomares JM. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev Dyn. 2006;235:1014–26. doi: 10.1002/dvdy.20685. [DOI] [PubMed] [Google Scholar]
- 90.Sieber-Blum M. Cardiac neural crest stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:34–42. doi: 10.1002/ar.a.10132. [DOI] [PubMed] [Google Scholar]
- 91.Cheng G, Litchenberg WH, Cole GJ, Mikawa T, Thompson RP, Gourdie RG. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development. 1999;126:5041–9. doi: 10.1242/dev.126.22.5041. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–54. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- 93.Sato M, Yost HJ. Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Dev Biol. 2003;257:127–39. doi: 10.1016/s0012-1606(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 94.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–6. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 95.Tomita Y, Matsumura K, Wakamatsu Y, Matsuzaki Y, Shibuya I, Kawaguchi H, Ieda M, Kanakubo S, Shimazaki T, Ogawa S, Osumi N, Okano H, Fukuda K. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 2005;170:1135–46. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang X, Sun Y, Schneider J, Ding JH, Cheng H, Ye M, Bhattacharya S, Rearden A, Evans S, Chen J. Pinch1 is required for normal development of cranial and cardiac neural crest-derived structures. Circ Res. 2007;100:527–35. doi: 10.1161/01.RES.0000259041.37059.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sato M, Tsai HJ, Yost HJ. Semaphorin3D regulates invasion of cardiac neural crest cells into the primary heart field. Dev Biol. 2006;298:12–21. doi: 10.1016/j.ydbio.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 98.Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, Lee JK, Matsumura K, Tomita Y, Miyoshi S, Shimoda K, Makino S, Sano M, Kodama I, Ogawa S, Fukuda K. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med. 2007;13:604–12. doi: 10.1038/nm1570. [DOI] [PubMed] [Google Scholar]
- 99.Calloni GW, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Sonic Hedgehog promotes the development of multipotent neural crest progenitors endowed with both mesenchymal and neural potentials. Proc Natl Acad Sci USA. 2007;104:19879–84. doi: 10.1073/pnas.0708806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen S, Lechleider RJ. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res. 2004;94:1195–202. doi: 10.1161/01.RES.0000126897.41658.81. [DOI] [PubMed] [Google Scholar]
- 101.Youn YH, Feng J, Tessarollo L, Ito K, Sieber-Blum M. Neural crest stem cell and cardiac endothelium defects in the TrkC null mouse. Mol Cell Neurosci. 2003;24:160–70. doi: 10.1016/s1044-7431(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 102.Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci USA. 2005;102:8916–21. doi: 10.1073/pnas.0503741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci USA. 2007;104:10894–9. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell. 2002;3:171–81. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- 106.Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci USA. 2007;104:9319–24. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–21. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin L, Bu L, Cai CL, Zhang X, Evans S. Isl1 is upstream of sonic hedgehog in a pathway required for cardiac morphogene-sis. Dev Biol. 2006;295:756–63. doi: 10.1016/j.ydbio.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 109.Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci USA. 2007;104:9313–8. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–33. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–85. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;104:18531–6. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dawn B, Guo Y, Rezazadeh A, Huang Y, Stein AB, Hunt G, Tiwari S, Varma J, Gu Y, Prabhu SD, Kajstura J, Anversa P, Ildstad ST, Bolli R. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocar-dial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–11. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 116.Lutz M, Rosenberg M, Kiessling F, Eckstein V, Heger T, Krebs J, Ho AD, Katus HA, Frey N. Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit + bone marrow-derived stem cells. Cardiovasc Res. 2008;77:143–50. doi: 10.1093/cvr/cvm027. [DOI] [PubMed] [Google Scholar]
- 117.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–92. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 118.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoi-etic stem cell niches. Science. 2007;318:1296–9. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–73. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 120.Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Muller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 121.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proin-flammatory cytokines. Nat Med. 2007;13:719–24. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 123.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci USA. 2003;100:5840–5. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stanford WL, Haque S, Alexander R, Liu X, Latour AM, Snodgrass HR, Koller BH, Flood PM. Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. J Exp Med. 1997;186:705–17. doi: 10.1084/jem.186.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bradfute SB, Graubert TA, Goodell MA. Roles of Sca-1 in hematopoietic stem/progenitor cell function. Exp Hematol. 2005;33:836–43. doi: 10.1016/j.exphem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Epting CL, Lopez JE, Shen X, Liu L, Bristow J, Bernstein HS. Stem cell antigen-1 is necessary for cell-cycle withdrawal and myoblast differentiation in C2C12 cells. J Cell Sci. 2004;117:6185–95. doi: 10.1242/jcs.01548. [DOI] [PubMed] [Google Scholar]
- 128.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 129.Lee SK, Su B, Maher SE, Bothwell AL. Ly-6A is required for T cell receptor expression and protein tyrosine kinase fyn activity. EMBO J. 1994;13:2167–76. doi: 10.1002/j.1460-2075.1994.tb06493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 131.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gude N, Muraski J, Rubio M, Kajstura J, Schaefer E, Anversa P, Sussman MA. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99:381–8. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 133.Pellieux C, Foletti A, Peduto G, Aubert JF, Nussberger J, Beermann F, Brunner HR, Pedrazzini T. Dilated cardiomyopathy and impaired cardiac hypertrophic response to angiotensin II in mice lacking FGF-2. J Clin Invest. 2001;108:1843–51. doi: 10.1172/JCI13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest. 2005;115:1724–33. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Galownia NC, Kushiro K, Gong Y, Asthagiri AR. Selective desensitization of growth factor signaling by cell adhesion to fibronectin. J Biol Chem. 2007;282:21758–66. doi: 10.1074/jbc.M703577200. [DOI] [PubMed] [Google Scholar]
- 136.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–19. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 137.Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Perez Pomares JM, Weesie F, Wessels A, Moorman AF, Van Den Hoff MJ. BMP and FGF regulate the differentiation of multipotential pericardial meso-derm into the myocardial or epicardial lineage. Dev Biol. 2006;295:507–22. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 138.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascular-ization. Nature. 2007;445:177–82. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 139.Zamora M, Manner J, Ruiz-Lozano P. Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proc Natl Acad Sci USA. 2007;104:18109–14. doi: 10.1073/pnas.0702415104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sengbusch JK, He W, Pinco KA, Yang JT. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol. 2002;157:873–82. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toyofuku T, Hong Z, Kuzuya T, Tada M, Hori M. Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin-beta-catenin complex. J Cell Biol. 2000;150:225–41. doi: 10.1083/jcb.150.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 143.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 144.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–73. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 145.Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 146.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 147.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 148.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 149.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripo-tent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 150.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 151.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 152.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 153.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 155.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Mono-layered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 156.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 157.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 158.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–94. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 159.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, ran-domised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 160.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 161.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holscher-mann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 162.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 163.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 164.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–71. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–47. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 166.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 167.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci USA. 2002;99:12339–44. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, Garcia JA, Szweda LI, Garry MG, Garry DJ. Hypoxia-inducible factor-2alpha trans-activates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res. 2008;102:1075–81. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]


