Abstract
Ultraviolet radiation is known to cause oxidative DNA damage and is thought to be a major factor implicated in the pathogenesis of pterygium. Among all the photo-oxidative DNA products, the 8-hydroxydeoxyguanosine (8-OHdG) is regarded a sensitive and stable biomarker for evaluating the degree of DNA damage. The protein p53 is a major cell stress regulator that acts to integrate signals from a wide range of cellular stresses. UV radiation has a carcinogenic effect resulting in DNA damaged cells with loss of normal growth control. This assumption is supported by the association between UV-B exposure and activation of survivin, a member of the inhibitor of apoptosis protein family (IAP), highly up-regulated in almost all types of human malignancy. In this study we demonstrate, for the first time in pterygium, the immunohistochemical presence of survivin, and investigate the correlation between survivin, p53 and 8-OHdG. Our results demonstrate that oxidative stress could lead to a significant activation of survivin expression, suggesting that this might be an important event in the development of pterygium, inducing and supporting a hyperproliferative condition. Survivin expression in pterygium would counteract UV-B-induced apoptosis and would cooperate with loss of p53. The co-operation between survivin and functional loss of p53 might provide a general mechanism for aberrant inhibition of apoptosis that could be responsible for the development of pterygium and its possible progression to neoplasia.
Keywords: survivin • oxidative stress • pterygium • immunohistochemistry
Introduction
Pterygium is a surface ocular lesion that begins growing from lim-bal epithelium and invades the cornea centripetally followed by con-junctival epithelium,exhibiting degenerative and hyperplastic changes as well as proliferative, inflammatory features and a rich vasculature.
Recent studies have provided evidence that a genetic component might be involved in the pathology of pterygium [1–3], while anti-apoptotic mechanisms [4, 5], cytokines [6], growth factors [7–10], angiogenic factors [11, 12], extracellular matrix remodelling [13], immunologic mechanisms [14, 15] and viral infections [3,16–18] have all been implicated in its pathogenesis. Studies by Clear et al.[19] suggest that pterygia may represent a precancerous condition of the mucosal epithelium, analogous to cutaneous actinic keratosis. Since pterygium can recur aggressively after removal, it has been suggested to be a neoplastic-like growth disorder and not merely a simple degenerative condition of the conjunctiva [9, 20, 21]. To support this hypothesis, in a previous study [22] we reported in pterygium the presence of conjunctival melanocytic pigmented lesions, and, among these, two primary acquired melanosis with atypia.
Epidemiological studies indicate that chronic exposure to the sunlight, and most probably ultraviolet B (UV-B) irradiation, is an important factor in the development of pterygia [23–26]. It is known that UV irradiation has a carcinogenic effect resulting in DNA-damaged cells with loss of normal growth control [27, 28]. Recent studies by Aziz et al.[29] demonstrated that UV-B exposure leads to a significant activation of survivin, a protein only expressed in embryonic or proliferating normal adult tissues, and highly up-regulated in almost all types of human malignancy [30–32]. As its name infers, survivin is cytoprotective, belonging to the inhibitor of apoptosis (IAP) gene family, but it has also a role as an essential cell division protein [33, 34]. Overexpression of survivin seems to inhibit apoptosis initiated via the extrinsic or intrinsic pathways, relative to its ability to inhibit terminal caspase-3 and -7 [35, 36]. In vitro, UV irradiation has been shown to induce typical caspase-dependent cell death in normal conjunctival cells [37].
The primary goal of the present study was to demonstrate, for the first time in pterygium, the immunohistochemical presence of survivin. Because of its strong expression in several preneoplastic lesions [38, 39], including hypertrophic actinic keratosis [40] and in the vast majority of cancers [30, 41], the presence of survivin would emphasize the idea of the origin of pterygium from an anti-apoptotic mechanism, and the trend of this lesion towards a neoplastic-like growth disorder versus a simple degenerative condition of the conjunctiva. Moreover, since in our previous study [42] we demonstrated the concomitant presence of altered p53 in 8-hydroxydeoxyguanosine (8-OHdG)-immunoreactive cells, the other purpose of the study was to verify a possible correlation between survivin, p53 and 8-OHdG, in order to provide a further evidence of an apparent genetic instability which is in contrast to the pterygium's benign clinical course.
Materials and methods
Patients and study design
Primary pterygia were harvested from 31 patients (11 males and 20 females). All patients were of mixed race, between Indium and Hispanic. Ages ranged between 21 and 68 years (mean age, 43.13; standard deviation, 13.5). Nineteen patients lived in the countryside and 12 resided in an urban setting. All the patients were outdoors workers. The patients underwent excision by bare sclera technique at the Department of Pathology Cancer Center of SOLCA, Cuenca, Ecuador. Twenty-four lesions were located on the nasal side and only the head of primary pterygium was used as pterygium sample. Pterygium morphology was clinically graded as atrophic (nine cases), intermediate (15 cases) or fleshy (seven cases), according to an assessment of pterygium translucency. Normal conjunctiva samples as controls were collected from medial bulbar conjunctiva of 10 patients (six males and four females) without pterygium and pinguecula while undergoing cataract surgery. Ages ranged between 25 and 70 years (mean age 52.1; standard deviation 16.02); the younger patients were surgically treated for traumatic cataract. Seven patients lived in the countryside and three resided in an urban setting. Patients did not receive any medication prior to surgery, except for a topical anaesthetic, and no drugs or chemical agents were used during surgical operation. The study protocol was approved by the local Research Ethic Committee and informed consent was obtained from all participants, according to the World Medical Association Declaration of Helsinki; complete information on patients was available in all cases.
Immunohistochemistry
Tissue segments were fixed by immersion in cold 10% formalin in 0.2 M phosphate buffer, pH 7.3 for 4–6 hrs, and processed for paraffin embedding. Microtome sections (6–7 μm) were treated for the immunohistochemical demonstration of survivin and p53 using the streptavidin-biotin alkaline phosphatase method. Briefly, they were re-hydrated in phosphate-buffered saline (PBS) and water-bath heating-based antigen retrieval was performed by immersion in 10 mM citrate buffer solution (pH 6.0) at 95°C for 40 min. After gradual cooling for 20 min., sections were treated for 45 min. with 10% normal goat or normal horse serum in PBS, respectively. Rabbit polyclonal antibody to recombinant human survivin protein (Novus Biologicals, Littleton, CO, 1:1000) and mouse monoclonal antibody to human p53 protein (clone DO-7, Dako Glostrup, Denmark, 1:50) were used as primary antisera and incubated for 60 min. at room temperature, while biotinylated anti-rabbit and antimouse IgG were used as secondary antisera (Vector Laboratories, Burlingame, CA, USA, 1:200) by incubation for 30 min. at room temperature. The sections were further incubated in alkaline phosphatase streptavidin (Vector Laboratories, Burlingame, CA, USA: 1:1000) for 30 min. at room temperature, and reacted with Fast Red Substrate System (Dako Glostrup).
Adjacent microtome sections were treated for the immunohistochemical demonstration of 8-OHdG, using the Dako EnVision™+ System/AR Water-bath heating-based antigen retrieval was performed by immersion in 10 mM citrate buffer solution (pH 6.0) at 95°C for 30 min. The slides were incubated in 10 mM Tris-HCI pH 7.5,1 mM ethylenediaminetetraacetic acid (EDTA), 0.4 mM NaCI, 100 μg/ml RNase for 15 min. at 37°C. To denature DNA, slides were soaked in 4 N hydrochloric acid for 7 min. and then neutralized by incubation in 50 mM Tris-base for 5 min. at room temperature. To block non-specific sites, specimens were incubated in PBS containing 10% foetal bovine serum for 1 hr at 37°C; they were then treated with mouse monoclonal anti-8-OHdG (Trevigen, Gaithersburg, USA, 1:300) in PBS, pH 7.5 containing 10% foetal bovine serum overnight at 4°C. Samples were further incubated with antimouse immunoglobulins (Ig) conjugated to alkaline phosphatase labelled dextran polymer (Dako EnVision™+ System, Carpinteria, CA, USA) for 30 min. at room temperature, and reacted with Fast Red as chromogen.
In the experiment, all section were thoroughly rinsed in PBS between each step, and finally counterstained with Mayer haematoxylin and mounted in glycerol gelatin (Sigma, St. Louis, MO, USA).
Sections of human cutaneous melanoma were used as positive control tissues for survivin and p53 staining, while human skin was used as control for 8-OHdG. Negative controls were obtained by omission of the primary antibody or by replacing the primary antibody with an isotype-matched antibody.
Micrographs were captured by a digital camera Canon PowerShot A620, (Canon Inc. China) on a microscope Zeiss Axiophot, and processed by Adobe Photoshop (7.0 version) software.
Evaluation of immunoreactivity
Results were independently evaluated by three observers in a blinded fashion. Four to six 200x fields covering almost the whole of each of four sections per sample were examined with a 144-intersection point square reticulum (0.78 mm2) inserted in the eyepiece, and scored for the percentage of epithelial immunoreactive cells. The cut-off level for immunohisto-chemical analysis was set at 10%, meaning that those samples with more than 10% of cells stained were considered to be positive. Patients were divided into six groups: p53-positive, p53-negative, 8-OHdG-positive, 8-OHdG-negative, survivin-positive and survivin-negative.
Statistics
The results were assessed with parametric analysis of variance (Fisher's exact test); data were computed by the SPSS statistical software package, version 15.0 (SPSS Inc., Chicago, IL, USA); a P-value of less than 0.05 was considered statistically significant.
Results
Relevant clinical features of the patients are summarized in Table 1.
Table 1.
This table presents clinical variables of the study's participants and the immunohistochemical analysis. The asterisk indicates the median value
| Samples | Variables | No. of patients |
|---|---|---|
| Pterygium | ||
| Gender | ||
| Male | 11 | |
| Female | 20 | |
| Age | ||
| <42* | 17 | |
| 3 ≥ 42* | 14 | |
| Residency | ||
| Urban | 12 | |
| Rural | 19 | |
| 8-OHdG expression | ||
| Positive | 21 | |
| Negative | 10 | |
| p53 expression | ||
| Positive | 11 | |
| Negative | 20 | |
| Survivin expression | ||
| Positive | 14 | |
| Negative | 17 | |
| Conjunctiva | ||
| Gender | ||
| Male | 6 | |
| Female | 4 | |
| Age | ||
| <55* | 5 | |
| ≥55* | 5 | |
| Residency | ||
| Urban | 3 | |
| Rural | 7 | |
The data on 8-OHdG and p53 expression are imported from our previous paper [42].
8–OHdG analysis
Trevigen's anti-8-OHdG antibody allows detection of 8-hydrox-yguanosine in DNA, cells and tissue samples. The levels of 8-OHdG have been shown to correlate with mutagenesis and car-cinogenesis in which oxidative damage is involved as causative mechanisms [43]. In our study, 21 (21/31, 67.74%) primary pterygia samples were positive for 8-OHdG staining, with immunoreactivity localized to the nuclei of the epithelium; no immunostaining was observed in the lamina propria (Fig.1A). In normal conjunctiva group, all specimens were negative (Fig.1B). Positive control section from human skin demonstrated immunoreactivity for 8-OHdG in the epithelial layer (Fig.3A). Reactivity was absent in sections incubated without primary antibody and no reactivity developed when the isotype control antibody was used (Fig.3A1).
Figure 1.
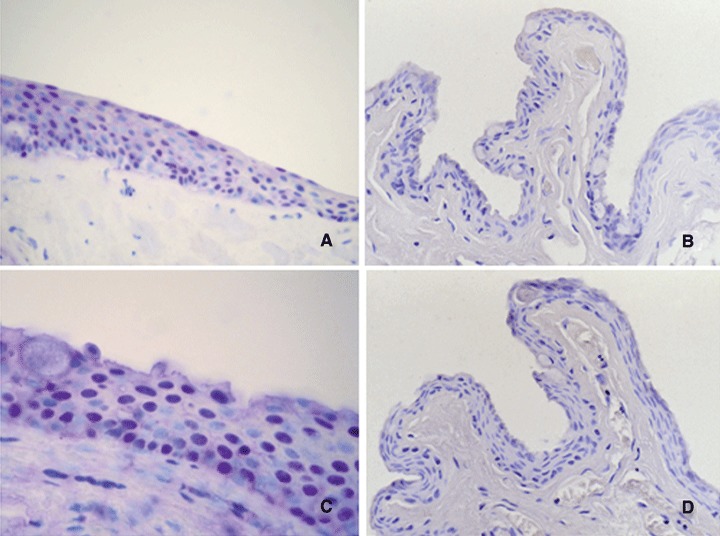
Immunohistochemical staining for 8-OHdG (A, B) and p53 (C, D). In pterygium 8-OHdG (A) and p53 expression (C) was localized to the nuclei of the epithelial layer. No differences in the pattern of staining between 8-OHdG and p53 were observed. No immunostaining for 8-OHdG (B) or p53 (D) in normal conjunctiva samples was observed. Each section was counterstained with haematoxylin. Original magnification: A, B, D, 400X; C, 630x.
Figure 3.
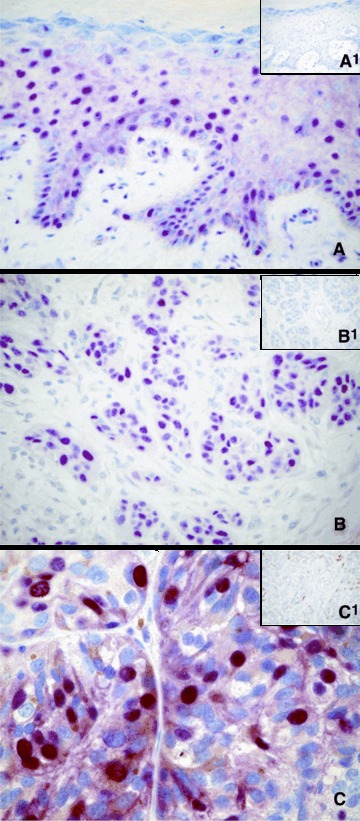
Immunohistochemical staining for 8-OHdG (A), p53 (B), survivin (C). Sections from normal human skin (A) and human cutaneous malignant melanoma (B, C) were included as positive controls. Sections incubated without a primary antibody (inset B1), or with an isotype control antibody (insets A1 and C1) displayed no immunoreactivity. Each section was counterstained with haematoxylin. Original magnification: A, B, and insets A1, B1, C1, 400x; C, 630x.
P53 analysis
Analysis of overexpression of the p53 protein was performed using the monoclonal antibody D07, which is directed against an epitope located between amino acids 19–26, and recognizes both wild-type and mutant forms of the protein. Positive staining was detected in 11 (11/31, 35.48%) pterygia. The expression of protein p53 was limited to the nuclei of the epithelial layer; no substantial staining was visible in the subepithelial fibrovascular layers (Fig.1C). No immunostaining for p53 in normal conjunctiva samples was observed (Fig.1D). Positive control section from human cutaneous melanoma demonstrated immunoreactivity for p53 in nuclei of tumoural cells (Fig.3B). Reactivity was absent in sections incubated without primary antibody (Fig.3B1) and no reactivity developed when the isotype control antibody was used.
Relationship between 8-OHdG and p53
The relationship between 8-OHdG and p53 is shown in Table 2. In the group of pterygia with 8-OHdG immunostaining, there were 11 (11/21, 52.38%) samples with p53 expression. All samples positive for p53 (11/31, 35.48%) were also positive for 8-OHdG immunostaining, and all specimens negative for 8-OHdG (10/31, 32.26%) were also negative for p53. Fisher's exact test demonstrated a significant correlation between 8-OHdG expression and p53 staining (P = 0.0049).
Table 2.
Relationship between 8-OHdG and p53
| p53 positive | p53 negative | Total | |
|---|---|---|---|
| 8-OHdG positive | 11 | 10 | 21 |
| 8-OHdG negative | 0 | 10 | 10 |
| Total | 11 | 20 | 31 |
| P = 0.0049 |
Survivin analysis
Novus Biologicals' anti-survivin antibody recognizes the full-length recombinant human survivin. In our study, 14 (14/31, 45.16%) primary pterygia were positive for survivin staining, with immunoreactivity localized to the nuclei and cytoplasm of the epithelial cells (Fig.2A and B). In each sample, some cells showed positivity only in nucleus, other, only in cytoplasm, while further cells showed immunoreactivity in both nucleus and cytoplasm. Cytoplasmic immunostaining was weaker than the nuclear. The positive cells were scattered throughout the epithelium; no substantial staining was visible in the subepithelial fibrovascular layers. No immunostaining for survivin in normal conjunctiva was present (Fig.2C). Positive control section from human cutaneous melanoma demonstrated immunoreactivity for survivin in nuclei and cytoplasm of tumoural cells (Fig.3C). Reactivity was absent in sections incubated without primary antibody and no reactivity developed when the isotype control antibody was used (Fig.3C1).
Figure 2.
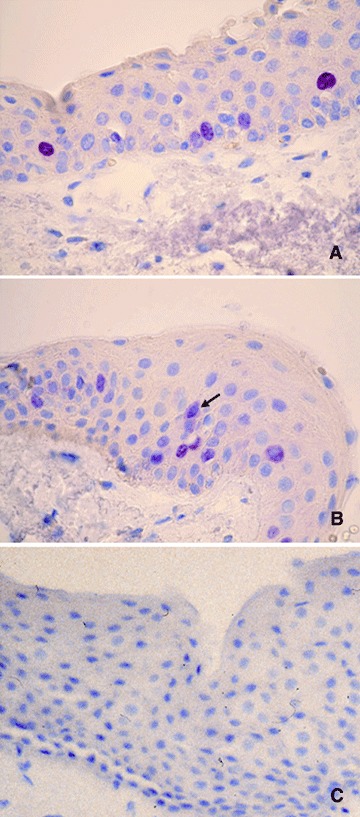
Immunohistochemical staining for survivin. The immunoreactiv-ity was localized to the nuclei and cytoplasm of the epithelial cells. Note in B a cell with immunoreactivity in both nucleus and cytoplasm (arrow). No immunostaining in normal conjunctiva was detected (C). Each section was counterstained with haematoxylin. Original magnification: A-C, 400x.
Relationship between survivin, p53 and 8-OHdG
All samples positive for p53 (11/31) and for survivin (14/31) were also positive for 8-OHdG immunostaining, and all specimens negative for 8-OHdG (10/31, 32.26%) were also negative for p53 and survivin. When analysed by Fisher's exact test, the expression of total survivin showed a strong significant correlation both with p53 (P = 0.0004) and 8-OHdG immunostaining (P = 0.0005). The relationships between total survivin and p53 and 8-OHdG are showed in Tables 3 and 4.
Table 3.
Relationship between survivin and p53
| survivin positive | survivin negative | Total | |
|---|---|---|---|
| p53 positive | 10 | 1 | 11 |
| p53 negative | 4 | 16 | 20 |
| Total | 14 | 17 | 31 |
| P = 0.0004 |
Table 4.
Relationship between survivin and 8-OHdG.
| survivin positive | survivin negative | Total | |
|---|---|---|---|
| 8-OHdG positive | 14 | 7 | 21 |
| 8-OHdG negative | 0 | 10 | 10 |
| Total | 14 | 17 | 31 |
| P= 0.0005 |
Discussion
UV light is one of the major factors implicated in the pathogenesis of pterygium, but the mechanism by which UV radiation induces this disease remains unknown.
The detrimental effects of UV irradiation are either directly due to a UV phototoxic effect or indirectly to formation of radical oxygen species (ROS). ROS have been associated with initiation and progression in a multi-stage model of carcinogenesis [43]. A most typical oxidative lesion product is 8-OHdG, since guanine is the most easily oxidized naturally occurring base. 8-OHdG frequently mispairs with adenine during DNA replication, which results in G:C to T:A transversion mutations; therefore, it has been established as a sensitive marker of oxidative DNA damage [44]. G:C to TAtransversions, induced by the presence of 8-OHdG during DNA replication, have also been observed in the ras oncogene and p53 in human skin cancers of sun-exposed areas and in UV-induced mouse skin cancers [45, 46]. Based upon the premise that pterygium is a UV-related lesion, the demonstration of the presence of 8-OHdG highlights the importance of the role played by oxidative stress in the development of the lesion.
The protein p53 is a major cell stress regulator that monitors the genetic integrity of the cell and is required for inducing the expression of genes that are responsible for inhibiting DNA synthesis or inducing apoptosis in response to DNA damage [47, 48]. Detection of DNA damage or replication errors may lead to cell cycle arrest and apoptosis if the damage cannot be repaired. This mechanism prevents the accumulation of potentially oncogenic DNA mutations. In normal unstressed cells, the p53 protein has a short half-life and is maintained at low, often undetectable, levels. Mutations in the p53 gene are believed to lead to an increased stability of the protein, allowing its more pronounced immunohisto-chemical detection. UV radiation can cause mutation in genes such as p53. In our previous report [42], we observed overexpres-sion of p53 in 8-OHdG immunoreactive pterygia, providing evidence that pterygium is a tumour-like growth disorder, related to faulty apoptosis; noteworthy is the finding that all specimens negative for 8-OHdG were also negative for p53. Moreover, other researchers demonstrated by immunohistochemistry the overex-pression of p53 in pterygium, but only Tsai and co-authors [49] were able to detect mutations in all their samples, being one a G:C to T:A transversion, which is typical of oxidative lesions. In their analysis on apoptosis-related gene expression, Tan and collegues [4] showed the presence of apoptotic cells only in restricted areas of the epithelium, consistent with aberrant expression of p53 and the anti-apoptotic effect of bcl-2 in the remaining part of the epithelium. This datum would indicate pterygium as a result of disruption of the normal process of apoptosis. Also, Schneider et al.[50] showed accumulation of p53 in Caucasian pterygia, but they failed to detect mutations in p53 gene, concluding that the increased amount of p53 protein in pterygial cells does not stop cell proliferation or cause cells to die by apoptosis; they proposed that p53 protein in pterygia appears to be inactive, at least in this function. They explained the high levels of the protein with a UV-induced defect in p53 activation.
As other tissues, homeostasis in conjunctiva is essentially regulated by a delicate balance between cellular proliferation and cellular apoptosis, thus degenerative processes are linked to either modified proliferative capacity or apoptosis. Recent studies [51] on normal apoptotic conjunctiva demonstrated the absence of Apaf-1 (cytosolic ‘apoptotic protease activating factor 1’), which is shown to activate, by intrinsic pathway, caspase-3 in a cytochrome c-dependent manner. This datum would indicate that Apaf-1 does not participate in the apoptotic cascade in conjuncti-val cells. However, studies in vitro by Buron et al.[37] demonstrated in conjunctival cells the involvement of the extrinsic pathway and caspase-8 activation in UV-induced chromatin condensation. Investigations by Aziz et al.[29] on human epidermal ker-atinocytes demonstrated that UV-B exposure leads to a significant activation of survivin, whose overexpression seems to inhibit apoptosis initiated via the extrinsic or intrinsic pathways, relative to its ability to inhibit caspases [35]. In the present study we demonstrated a significative survivin overexpression in 8-OHdG-and p53-positive cells, localized in both nuclei and cytoplasm of epithelial cells. It is known, in fact, that there are different survivin proteins with unique subcellular localizations and functions [52]; among these, nuclear survivin appears to play a key role in cell division, whereas cytoplasmic survivin seems essential for the inhibition of apoptosis [53]. Thus, our immunohistochemical localization in both nuclei and cytoplasm, obtained by an antibody recognizing all survivin proteins, would represent the combined expression of all variants.
In normal cells, wild-type p53 transcriptionally represses survivin, [54–56] and overexpression of survivin is able to counteract p53-dependent apoptosis induced by ultraviolet light [54]. In our opinion, survivin, which transcription in pterygium is no more silenced by UV-inactivated p53, could exert its anti-apoptotic effect by directly inhibiting terminal caspase-3. Since there is strong evidence that p53 also exerts its effects on the inhibition of new blood vessel development [57], an intense angiogenesis [12]. typical of the histopathology of pterygium, could be another sign of the loss of p53 function.
In conclusion, this is the first report on the presence of survivin in pterygium; our results are in agreement with previous data by Aziz et al.[29] on mouse skin about the up-regulation of survivin by UV-B and increase in p53 expression. In this study, we demonstrate that oxidative stress may be associated with a significant activation of survivin expression, suggesting that this might be an important event in the development of a relevant number of ptery-gia, inducing and supporting a hyperproliferative condition. Survivin expression in pterygium would counteract UV-B-induced apoptosis and would cooperate with loss of p53. The co-operation between survivin and functional loss of p53 might provide a general mechanism for aberrant inhibition of apoptosis. Such aberrant inhibition of apoptosis may initiate pterygium growth and stimulate not only local invasiveness, but also a possible progression towards potentially malignant neoplasia, since we demonstrated in our previous paper [22] the presence in pterygium of primary acquired melanosis with atypia, with a high risk for the development of invasive melanoma. The possibility of restoring p53 function by pharmaceutical manipulation or reducing or eliminating the effect of the anti-apoptotic molecule survivin could offer hope for more effective treatment against pterygium.
Acknowledgments
This study was supported by grants from the Ministero degli Affari Esteri (MAE), and Fondazione Banco di Sardegna, Italy. Particular thanks are due to Dr. Alfredo Campoverde, Mrs. Maria Itala Mosso and Mr. Massimo Annis for their expert technical assistance. The authors are grateful to the Sociedad de Oftalmologia de Cuenca for their help in enrolment and follow-up.
References
- 1.Saw SM, Tan D. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999;6:219–28. doi: 10.1076/opep.6.3.219.1504. [DOI] [PubMed] [Google Scholar]
- 2.Coroneo MT, Di Girolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol. 1999;10:282–8. doi: 10.1097/00055735-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Detorakis ET, Drakonaki EE, Spandidos DA. Molecular genetic alterations and viral presence in ophthalmic pterygium. Int J Mol Med. 2000;6:35–41. [PubMed] [Google Scholar]
- 4.Tan DT, Tang WY, Liu YP, Goh HS, Smith DR. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br J Ophthalmol. 2000;84:212–6. doi: 10.1136/bjo.84.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakoonwatanyoo P, Tan DT, Smith DR. Expression of p63 in pterygium and normal conjunctiva. Cornea. 2004;23:67–70. doi: 10.1097/00003226-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3430–7. [PubMed] [Google Scholar]
- 7.Nolan TM, DiGirolamo N, Sachdev NH, Hampartzoumian T, Coroneo MT, Wakefield D. The role of ultraviolet irradiation and heparin-binding epidermal growth factor-like growth factor in the pathogenesis of pterygium. Am J Pathol. 2003;162:567–74. doi: 10.1016/S0002-9440(10)63850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kria L, Ohira A, Amemiya T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 1998;236:702–8. doi: 10.1007/s004170050144. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Xie Y, Zhang M. Overexpression of type I growth factor receptors in pterygium. Chin Med J. 2002;115:418–21. [PubMed] [Google Scholar]
- 10.Van Setten G, Aspiotis M, Blalock TD, Grotendorst G, Schultz G. Connective tissue growth factor in pterygium: simultaneous presence with vascular endothe-lial growth factor – possible contributing factor to conjunctival scarring. Graefes Arch Clin Exp Ophthalmol. 2003;241:135–9. doi: 10.1007/s00417-002-0589-1. [DOI] [PubMed] [Google Scholar]
- 11.Marcovich AL, Morad Y, Sandbank J, Huszar M, Rosner M, Pollack A, Herbert M, Bar-Dayan Y. Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res. 2002;25:17–22. doi: 10.1076/ceyr.25.1.17.9959. [DOI] [PubMed] [Google Scholar]
- 12.Ribatti D, Nico B, Maxia C, Longo V, Murtas D, Mangieri D, Perra MT, De Giorgis M, Piras F, Crivellato E, Sirigu P. Neovascularization and mast cells with tryptase activity increase simultaneously in human pterygium. J. Cell. Mol. Med. 2007;11:585–9. doi: 10.1111/j.1582-4934.2007.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT, Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:671–9. [PubMed] [Google Scholar]
- 14.Pinkerton OD, Hokama Y, Shigemura LA. Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol. 1984;98:225–8. doi: 10.1016/0002-9394(87)90358-8. [DOI] [PubMed] [Google Scholar]
- 15.Perra MT, Maxia C, Zucca I, Piras F, Sirigu P. Immunohistochemical study of human pterygium. Hlstol Hlstopathol. 2002;17:139–49. doi: 10.14670/HH-17.139. [DOI] [PubMed] [Google Scholar]
- 16.Detorakis ET, Sourvinos G, Spandidos DA. Detection of herpes simplex virus and human papilloma virus in ophthalmic pterygium. Cornea. 2001;20:164–7. doi: 10.1097/00003226-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher MJ, Giannoudis A, Herrington CS, Hiscott P. Human papillomavirus in pterygium. Br J Ophthalmol. 2001;85:782–4. doi: 10.1136/bjo.85.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piras F, Moore PS, Ugalde J, Perra MT, Scarpa A, Sirigu P. Detection of human papillomavirus DNA in pterygia from different geographical regions. BrJ Ophthalmol. 2003;87:864–6. doi: 10.1136/bjo.87.7.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clear AS, Chirambo MC, Hutt MS. Solar keratosis, pterygium, and squamous cell carcinoma of the conjunctiva in Malawi. Br J Ophthalmol. 1979;63:102–9. doi: 10.1136/bjo.63.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dushku N, Reid TW. P53 expression in altered limbal basal cells of pingueculae: pterygia, and limbal tumors. Curr Eye Res. 1997;16:1179–92. doi: 10.1076/ceyr.16.12.1179.5036. [DOI] [PubMed] [Google Scholar]
- 21.Tan DT, Lim AS, Goh HS, Smith DR. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997;123:404–5. doi: 10.1016/s0002-9394(14)70141-2. [DOI] [PubMed] [Google Scholar]
- 22.Perra MT, Colombari R, Maxia C, Zucca I, Piras F, Corbu A, Bravo S, Scarpa A, Sirigu P. Finding of conjunctival melanocytic pigmented lesions within pterygium. Hlstopathology. 2006;48:387–93. doi: 10.1111/j.1365-2559.2006.02346.x. [DOI] [PubMed] [Google Scholar]
- 23.Moran DJ, Hollows FC. Pterygium and ultraviolet radiation: a positive correlation. BrJ Ophthalmol. 1984;68:343–6. doi: 10.1136/bjo.68.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackenzie FD, Hirst LW, Battistutta D, Green A. Risk analysis in the development of pterygia. Ophthalmology. 1992;99:1056–61. doi: 10.1016/s0161-6420(92)31850-0. [DOI] [PubMed] [Google Scholar]
- 25.Cullen AP. Photokeratitis and other photo-toxic effects on the cornea and conjunctiva. Int J Toxicol. 2002;21:455–64. doi: 10.1080/10915810290169882. [DOI] [PubMed] [Google Scholar]
- 26.Kau HC, Tsai CC, Lee CF, Kao SC, Hsu WM, Liu JH, Wei YH. Increased oxidative DNA damage, 8-hydroxydeoxy- guanosine, in human pterygium. Eye. 2006;20:826–31. doi: 10.1038/sj.eye.6702064. [DOI] [PubMed] [Google Scholar]
- 27.Hussein MR. Ultraviolet radiation and skin cancer: molecular mechanisms. J Cutan Pathol. 2005;32:191–205. doi: 10.1111/j.0303-6987.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 28.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 29.Aziz MH, Ghotra AS, Shukla Y, Ahmad N. Ultraviolet-B radiation causes an upregula-tion of survivin in human keratinocytes and mouse skin. Photochem Photobiol. 2004;80:602–8. doi: 10.1562/0031-8655(2004)080<0602:URCAUO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med. 2001;7:542–7. doi: 10.1016/s1471-4914(01)02243-2. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apopto-sis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071–4. [PubMed] [Google Scholar]
- 32.Swana HS, Grossman D, Anthony JN, Weiss RM, Altieri DC. Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N. Engl. J. Med. 1999;341:452–3. doi: 10.1056/NEJM199908053410614. [DOI] [PubMed] [Google Scholar]
- 33.Colnaghi R, Connell CM, Barrett RM, Wheatley SP. Separating the anti-apoptot-ic and mitotic roles of survivin. J Biol Chem. 2006;281:33450–6. doi: 10.1074/jbc.C600164200. [DOI] [PubMed] [Google Scholar]
- 34.Noton EA, Colnaghi R, Tate S, Starck C, Carvalho A, Ko Ferrigno P, Wheatley SP. Molecular analysis of survivin isoforms: evidence that alternatively spliced variants do not play a role in mitosis. J Biol Chem. 2006;281:1286–95. doi: 10.1074/jbc.M508773200. [DOI] [PubMed] [Google Scholar]
- 35.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, Pilotti S, Zunino F, Daidone MG. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Lite Sci. 2002;59:1406–12. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannetti L, Console U, Magnoni C, Lo Muzio L. Apoptosis: escaping strategies in human skin cancer. Oncol Rep. 2004;11:401–5. [PubMed] [Google Scholar]
- 37.Buron N, Micheau O, Cathelin S, Lafontaine PO, Creuzot-Garcher C, Solary E. Differential mechanisms of conjunctival cell death induction by ultraviolet irradiation and benzalkonium chloride. Invest Ophthalmol Vis Sci. 2006;47:4221–30. doi: 10.1167/iovs.05-1460. [DOI] [PubMed] [Google Scholar]
- 38.Grossman D, McNiff JM, Li F, Altieri DC. Expression of the apoptosis inhibitor, survivin, in nonmelanoma skin cancer and gene targeting in a keratinocyte cell line. Lab Invest. 1999;79:1121–6. [PubMed] [Google Scholar]
- 39.Chiodino C, Cesinaro AM, Ottani D, Fantini F, Giannetti A, Trentini GP, Pincelli C. Communication: expression of the novel inhibitor of apoptosis survivin in normal and neoplastic skin. J Invest Dermatol. 1999;113:415–8. doi: 10.1046/j.1523-1747.1999.00711.x. [DOI] [PubMed] [Google Scholar]
- 40.Park HR, Min SK, Cho HD, Kim KH, Shin HS, Park YE. Expression profiles of p63: p53, survivin, and hTERT in skin tumors. J Cutan Pathol. 2004;31:544–9. doi: 10.1111/j.0303-6987.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5:1087–98. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 42.Perra MT, Maxia C, Corbu A, Minerba L, Demurtas P, Colombari R, Murtas D, Bravo S, Piras F, Sirigu P. Oxidative stress in pterygium: relationship between p53 and 8-hydroxydeoxyguanosine. Mol Vis. 2006;12:1136–42. [PubMed] [Google Scholar]
- 43.Nishigori C. Cellular aspects of photocar-cinogenesis. Photochem Photobiol Sci. 2006;5:208–14. doi: 10.1039/b507471a. [DOI] [PubMed] [Google Scholar]
- 44.Calderon-Garciduenas L, Wen-Wang L, Zhang YJ, Rodriguez-Alcaraz A, Osnaya N, Villarreal-Calderon A, Santella RM. 8-hydroxy-2’-deoxyguanosine, a major mutagenic oxidative DNA lesion, and DNA strand breaks in nasal respiratory epithelium of children exposed to urban pollution. Environ Health Perspect. 1999;107:469–74. doi: 10.1289/ehp.107-1566580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierceall WE, Goldberg LH, Tainsky MA, Mukhopadhyay T, Ananthaswamy HN. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196–202. doi: 10.1002/mc.2940040306. [DOI] [PubMed] [Google Scholar]
- 46.Hattori Y, Nishigori C, Tanaka T, Uchida K, Nikaido O, Osawa T, Hiai H, Imamura S, Toyokuni S. 8-hydroxy-2’-deoxyguano-sine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure. J Invest Dermatol. 1996;107:733–7. doi: 10.1111/1523-1747.ep12365625. [DOI] [PubMed] [Google Scholar]
- 47.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 48.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 49.Tsai YY, Cheng YW, Lee H, Tsai FJ, Tseng SH, Chang KC. P53 gene mutation spectrum and the relationship between gene mutation and protein levels in pterygium. Mol Vis. 2005;11:50–5. [PubMed] [Google Scholar]
- 50.Schneider BG, John-Aryankalayil M, Rowsey JJ, Dushku N, Reid TW. Accumulation of p53 protein in pterygia is not accompanied by TP53 gene mutation. ExpEyeRes. 2006;82:91–8. doi: 10.1016/j.exer.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Giebel J, Woenckhaus C, Fabian M, Tost F. Age-related differential expression of apoptosis-related genes in conjunctival epithelial cells. Ada Ophthalmol Scand. 2005;83:471–6. doi: 10.1111/j.1600-0420.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 52.Li F, Ling X. Survivin study: an update of “what is the next wave”? J Cell Physio! 2006;208:476–86. doi: 10.1002/jcp.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–27. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chein. 2002;277:3247–57. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 55.Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, Nielsen LL, Pickett CB, Liu S. Human survivin is neg-atively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21:2613–22. doi: 10.1038/sj.onc.1205353. [DOI] [PubMed] [Google Scholar]
- 56.Zhou M, Gu L, Li F, Zhu Y, Woods WG, Findley HW. DNA damage induces a novel p53-survivin signalling pathway regulating cell cycle and apoptosis in acute lym-phoblastic leukemia cells. J Pharmacol Exp Ther. 2002;303:124–31. doi: 10.1124/jpet.102.037192. [DOI] [PubMed] [Google Scholar]
- 57.Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med. 2007;85:1175–86. doi: 10.1007/s00109-007-0221-2. [DOI] [PubMed] [Google Scholar]


