Abstract
Multi-potent adult progenitor cells (MAPCs) differentiate into endothelial cells (ECs) in the presence of vascular endothelial growth factor (VEGF). The mechanism(s) of VEGF-induced differentiation of MAPCs to ECs are not yet known. We, therefore, examined the role of mitogen-activated protein kinase/extracellular signal-regulated kinase (p42/44-MAPK/ERK1/2) signalling in endothelial differentiation from bone marrow stem cells. We observed that VEGF stimulation of MAPCs for 14 days results in a significant expression of endothelial-specific gene and/or proteins including von Willebrand factor (vWF), vascular endothelial-cadherin (VE-cadherin), VEGF receptor-2 (VEGFR2), and CD31. Up-regulation of EC-specific markers was accompanied by a cobblestone morphology, expression of endothelial nitric oxide synthase (eNOS), and Dil-Ac-LDL uptake, typical for EC morphology and function. VEGF induced a sustained activation of p42 MAPK/ERK, but not that of p44 MAPK/ERK during the course of MAPCs differentiation in a time-dependent manner up to 14 days. VEGF-induced activation of p42 MAPK/ERK also led to the nuclear translocation of MAPK/ERK1/2. Incubation of MAPCs with MAPK/ERK1/2 phosphorylation inhibitor PD98059 blocked the sustained VEGF-induced MAPK/ERK1/2 phosphorylation as well as its nuclear translocation in the differentiating MAPCs. Inhibition of MAPK/ERK1/2 phosphorylation by PD98059 also blocked the expression of EC-specific genes in these cells and their differentiation to ECs. These data suggest that VEGF induces MAPC differentiation into EC via a. MAPK/ERK1/2 signalling pathway-mediated mechanism in vitro.
Keywords: MAPCs, endothelial cell, differentiation, MAPK, ERK, VEGF
Introduction
Endothelial cell injury or dysfunction plays a critical role in the pathogenesis of atherosclerosis and thrombosis [1]. Rapid re-endothelialization of the injured arterial wall is likely to be a key step to prevent the development of atherothrombosis and vascular re-modelling after injury. Accumulating evidence indicates that bone marrow-derived circulating endothelial cells (ECs) and/or endothelial progenitor cells play an important role in the process of endothelialization of vascular grafts and angiogenesis [2].
The multi-potent adult progenitor cell (MAPC) is a novel postnatal stem cell isolated and cultured from human and rodent tissues, including bone marrow, muscle and brain [3]. These cells could be expanded under defined low serum conditions for more than 100 (human) or 400 passages without telomere shortening or karyotypic abnormalities [4, 5]. MAPCs have been shown to have the ability to differentiate into multiple cell lineages including mesodermal, neuroectodermal and endodermal origin in the presence of specific cytokines and growth factors [3, 6, 7]. It has been reported that mouse MAPCs could be induced to differentiate into ECs in the presence of vascular endothelial growth factor (VEGF) [3, 7]. VEGF is a multi-tasking cytokine that promotes endothelial survival, migration, proliferation and angiogenesis [8, 9]. We, and others, have shown that VEGF stimulates endothelial proliferation and survival Wamitogen-activated protein kinase/extracellular signal-regulated kinase (p42/44-MAPK/ERK1/2) signalling [10–13].
Besides survival and proliferation promoting signalling, ERK1/2 pathway also leads to neuronal differentiation of embryonic stem (ES) cell like P19 cells [14], differentiation and morphogenesis events of gastrulation [15]. Inhibition of MAPK/ERK in ES cells blocked their differentiation into neural or mesoder-mal cells [16]. In addition, MAPK/ERK pathway has been shown to be one of the key signalling pathways in the differentiation of various cell types both in vivo and in vitro, including neuronal cells, ECs, adipocyte and cells of visual cortex [4, 17–23].
In the present study, we examined the possibility that VEGF induces the differentiation of mouse MAPCs into ECs via MAPK/ERK1/2 signalling pathway. We show that VEGF induces endothelial differentiation by initiating a sustained activation of MAPK/ERK1/2 in the differentiating mouse MAPCs. Inhibition of ERK1/2 activity prevents endothelial differentiation, suggesting that ERK1/2 signalling pathway plays a key role in the process of mouse MAPC differentiation into EC in vitro.
Materials and methods
Culture of mouse MAPCs
MAPCs were isolated from femurs of 3- to 4-week-old mice using previously established method [6]. The cells were cultured in expansion medium containing 60% low-glucose Dulbecco's minimal essential medium (DMEM-LG) (Gibco-BRL, Grand Island, NY, USA), and 40% MCDB-201 (Sigma Chemical Co, St Louis, MO, USA), supplemented with 1 × SITE Liquid Media Supplement (Sigma Chemical Co, St Louis, MO, USA), 0.2mg/ml linoleic-acid bovine serum albumin (LA-BSA), 0.8mg/ml powdered bovine serum albumin (BSA, Sigma Chemical Co, St Louis, MO, USA), 1 × chemically defined lipid concentrate (Gibco), 10−4 M ascorbic acid 3-phosphate (Sigma Chemical Co, St Louis, MO, USA), 100 units of penicillin and 1000 units of streptomycin (Gibco), 2% foetal bovine serum (FBS, Hyclone Laboratories, Logan, IT) with 10 ng/ml of mouse epithelial growth factor (EGF) (Sigma), 10 ng/ml platelet-derived growth factor-BB (PDGF-BB) (R&D Systems, Minneapolis, MN, USA), 1000 units/ml mouse leukaemia inhibitory factor (LIF, Chemicon, Temecula, CA, USA), and 1 μl/ml β-mercaptoehanol. The cells were maintained in culture flasks coated with 100 ng/ml of fibronectin (FN, Sigma) at 37°C with 5% O2 and 5% CO2 and were strictly kept at a density of 100–200/cm2 to avoid cell-cell contact. The cells were trypsinized and re-plated every 48–72 hrs as needed. The cultured MAPCs will be tested with karyotyping every two weeks for quality control as previously described [24].
Endothelial differentiation from MAPCs
MAPCs were plated with cell density of 67,000/cm2 on a 100 ng/ml FN coated surface in MAPC media as described above. Endothelial differentiation was induced by switching the cells to differentiation media (MAPC media without FBS, PDGF, EGF and LIF) in the presence of 20 ng/ml human VEGF 164 (hVEGF164; R&D systems) and 1 μg/ml-mercaptoethanol. The media was changed every 48 hrs. The cells were maintained at 37°C with 5% O2 and 5% CO2. The process of differentiation was closely monitored with cell morphology and expression of EC-specific markers as described below.
Analysis of endothelial cell-specific markers by immunofluorescence
The differentiating MAPCs at different time intervals were fixed with 4% paraformaldehyde and permeabilized (where required) with 0.1% Triton-X 100 in 0.1% sodium-citrate. Non-specific Ab binding was blocked by incubating the cell preparations with 0.1% BSA and 3% human serum. The preparations were then exposed to the primary antibodies: anti-von Willebrand factor Ab (Santa Cruz, CA, USA) at 1: 300 dilution, α-VE-cad-herin Ab (Santa Cruz) at 1:50 dilution, α-Flk-1 Ab (Santa Cruz) at 1: 50 dilution, α-N0S3 or eNOS (Santa Cruz) at 1: 50 dilution, or rabbit γ globulin (Jackson ImmunoResearch, West Grove, PA, USA) for 30 min. at 37°C. Species-specific secondary Abs conjugated with TRITC/FITC at dilution 1: 50 (Jackson ImmunoResearch, West Grove, PA, USA) were used in addition to the nuclear stain DAPI (Molecular Probes, Eugene, Oregon, USA) at 1: 10,000 dilution. Immunofluorescent images were acquired using a fluorescence microscope (Zeiss Axiovert; Carl Zeiss, Inc., Thornwood, NY, USA).
Quantitative RT-PCR for endothelial cell marker expression
Total RNA was extracted from MAPCs or MAPCs-derived endothelial progeny by using the RNeasy RNA isolation kit (Qiagen Inc, Valencia, CA, USA). Contaminating DNA was eliminated by two sequential steps of DNase (Invitrogen, Carlsbad, CA, USA) treatment. The mRNA was reverse-transcribed, and cDNA underwent 40 rounds of amplification (ABI PRISM 7700, Perkin-Elmer/Applied Biosystems, Foster City, CA, USA) with the following reaction conditions: 40 cycles of a two-step Polymerase chain reaction (95°C for 15 sec. and 60°C for 60 sec.) after initial denaturation (95°C for 10 min.) with 1 μl of DNA solution, 2×SYBR® green PCR master mix reaction buffer (Applied Biosystems). The reactions without addition of cDNA template were used as negative controls. The authenticity and size of PCR products were confirmed by melting curve analysis (using software provided by Perkin-Elmer). The mRNA levels were normalized by using GAPDH as housekeeping gene and compared with the mRNA levels in mouse universal RNA (Ambion, Austin, TX, USA), mouse Embryo or Fisher mouse spleen for endothelial-like differentiation (Ambion, Austin, TX, USA) and mouse ES cell for stem cell markers. The primers used for amplication were listed in Table 1.
Table 1.
Primers’ sequences for RT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| vWF | CCCACCGGATGGCTAGGTATT | GAGGCGGATCTGTTTGAGGTT |
| CD31(PECAM) | GTCATGGCCATGGTCGAGTA | CTCCTCGGCGATCTTGCTGAA |
| VE-cadherin | ATTGAGACAGACCCCAAACG | TTCTGGTTTTCTGGCAGCTT |
| eNOS | GACCCTCACCGCTACAACAT | CTGGCCTTCTGCTCATTTTC |
| Oct-4 | GAGGAGTCCCAGGACATGAA | AGATGGTGGTCTGGCTGAAC |
| KDR(Flk-1) | TCTGTGGTTCTGCGTGGAGA | GTATCATTTCCAACCACCCT |
| GAPDH | TGCACCACCAACTGCTTAG | GATGCAGGGATGATGTTC |
Dil-Ac-LDL uptake assay
Dil-Ac-LDL uptake was performed using a kit (Stoughton, Massachusetts, USA) as per manufactureris recommendations [11]. Briefly, MAPCs and differentiating MAPCs were incubated with endothelial differentiation media containing 10μg/ml Dil-Ac-LDL for 4 hrs at 37°C. The cells were thoroughly washed, and LDL-uptake by the cells was visualized via fluorescence microscopy (Zeiss Axiovert; Carl Zeiss, Inc., Thornwood, NY, USA).
Western blotting analysis
Cell lysates (50 μg protein/lane) were loaded on a 3–15% gradient SDS-PAGE gel and transferred to a polyvinylidene difluoride (PVDF) membrane after electrophoresis. For immunoblotting, the preparations were exposed to α-phospho-p44/42 MAPK/ERK Ab at 1:1000 dilution (Thr202/Tyr204; Cell signalling Technology Beverly MA) and anti-p44/42 MAPK/ERK Ab at 1: 1000 dilution (Cell Signalling Technology Beverly MA), followed by incubation with anti-rabbit IgG alkaline phosphatase-linked 2° antibody at 1: 10,000 dilution (Amersham Life Sciences, Buckinghamshire, United Kindom) [25]. The immunoreactive proteins were visualized with ECF Western blotting system (Amersham); and chemiluminescent signals were acquired using Storm 860 Phosphorlmager (Molecular Dynamics, Sunnyvale, CA, USA).
BrdU incorporation ELISA for cell proliferation
MAPCs and the newly differentiated endothelial-like cells were incubated overnight in 96-well plates coated with 100 ng/ml FN at 5000 cells/well in MAPC media or subculture media for newly differentiated cells (endothelial differentiation media with 10% FBS and 10 ng/ml PDGF). Twenty four hours later the cells were switched to basal media without growth factors and serum for overnight incubation. Then, the cells were incubated with VEGF (20ng/ml) and PD98059 (50 μm) for up to 72 hrs at 37°C with 5% O2 and 5% CO2. Cell proliferation was evaluated using Brdu incorporation ELISA (Roche Science, Indianapolis, IN, USA) as described previously [26]. OD was measured at 370 nm (reference wavelength 492 nm) using VERSAmax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Data are expressed as mean ± standard deviation for all experiments, and a p < 0.05 was considered statistically significant. Statistical analysis was performed using one-way anova (PRISM Version 4.0; GraphPad Software, Inc., San Diego, CA, USA).
Results
VEGF-induced differentiation of MAPCs to ECs
We first examined the time course of differentiation of MAPCs to ECs. MAPCs seeded at 67,000/cm2 in serum-free medium with 20 ng/ml VEGF 165 started showing morphological changes by day 7. By day 14, cells appeared to undergo reorganization and showed cobblestone morphology, a typical appearance for ECs, suggesting that MAPCs differentiated into endothelial-like cells (Fig.1A). These data are consistent with the differentiation of haematopoietic and endothelial progenitor cells to ECs [27–29].
Figure 1.

Differentiation of multi-potent adult progenitor cells (MAPCs) into ECs by day 14 in the presence of vascular endothelial growth factor (VEGF). (A) Phase contrast micrographs of MAPCs and MAPCs derived endothelial cells. Cells at day 0 show typical morphology of MAPCs. On day 7 cells re-organize and start changing in morphology. On Day 14 cells show a cobblestone morphology that is normally observed in ECs. (B) Time course of transcript levels of EC and MAPCs-specific genes detected by Q-RT-PCR. EC-specific transcript levels increased by 3.86 ± 0.40 (vWF), 4.86 ± 0.22 (CD31), 121.1 ± 24.61 (VE-cadherin), 31.26 ± 12.10 (eNOS) and 7.87 ± 0.68 (Flk-1) folders, respectively by day 14 in VEGF-treated cells compared with undifferentiated MAPCs. Stem cell-specific Oct-4 transcripts decreased to 10 ± 0.849% of undifferentiated MAPCs at day14. Results shown are mean ± SD from three independent experiments. *: P < 0.05 compared with undifferentiated MAPCs (dO); **: P < 0.01 compared with undifferentiated MAPCs (dO). (C) Expression of EC-specific markers on differentiated MAPCs. MAPCs at day 0 (left panel) show the presence of blue nuclei stained with DAPI, but do not express vWF (top left panel) or VE-cadherin (bottom left panel). vWF and VE-cadherin in green fluorescence are expressed on differentiated MAPCs on day 14 (top and bottom right panels as indicated by arrows, respectively). (D) Dil-Ac-LDL uptake in MAPCs under differentiation. Undifferentiated MAPC at day 0 did not show the uptake of Dil-Ac-LDL (left panel). MAPCs under differentiation on day 14 show a remarkable uptake of Dil-Ac-LDL shown in red (right panel). Fig. A, C and D, each figure is a representative of results obtained from 3 independent cultures on day 0 and day 14; Fig. B, results are obtained from three independent experiments.
To substantiate the endothelial phenotype, we next examined the expression of EC-specific genes and proteins in the differentiating MAPCs starting from the day of initiation up to 21 days of differentiation. Quantitative RT-PCR analysis showed a consistent increase with time in the transcription levels of EC- specific markers including vWF, VE-cadherin, CD31, eNOS and Flk-1 (Fig 1B). The transcriptional expressions of all five EC-specific markers as presented with their mRNA levels increased significantly (P < 0.05 for CD31, P < 0.01 for VE-cadherin, eNOS and Flk-1 versus day 0, respectively) by day 10. On day 14, we observed a 3.86 ± 0.40, 4.86 ± 0.22,121.1 ± 24.61, 31.26 ± 12.10 and 7.87 ± 0.68 folds increase in the mRNA levels for vWF, CD31, VE-cadherin, eNOS and Flk-1 respectively, in VEGF-treated cells as compared to undifferenti-ated MAPCs. This increase in EC-specific gene expression was accompanied by a decrease in the stem cell-specific marker expression of Oct-4 transcripts. As shown in Fig.1B, Oct-4 expression in the differentiating MAPCs was reduced to 50% after 1 day of induction with VEGF, and further decreased to 9.45 ± 0.25% by day 5, and continued to remain as low as 10 ± 8.5% up to day 21.
In parallel, we observed an up-regulation in the expression of EC specific proteins including vWF and VE-cadherin by immunofluorescent microscopy in the differentiated cells on day 14 (Fig.1C). These observations support the EC-specific gene expression noted above, and suggest that MAPCs indeed differentiate into ECs in the presence of VEGF for 14 days. Uptake of Dil-Ac-LDL is considered to be one of the typical functions for mature ECs (although shared by other cells like monocytes and macrophage) and has been used to test the functional property of stem cell-derived ECs [5, 28, 30, 31]. In out study, an increased uptake of Dil-Ac-LDL in MAPCs-derived ECs was observed at day14 compared to undifferentiated MAPCs. (Fig.1D). Thus, our observations recapitulate the previous observations that MAPCs differentiate into ECs, both phenotypically and functionally, in the presence of VEGF for 14 days [3, 27].
Phospho-MAPK/ERK1/2 are dynamically activated during the differentiation of MAPCs to ECs
We have shown earlier that eNOS is dynamically expressed during the differentiation of MAPCs to ECs [32]. Activation of eNOS plays a critical role in VEGF-induced MAPK/ERK phosphorylation in EC [33]. Therefore, we examined if VEGF-induced activation of eNOS in MAPCs also led to the downstream activation of MAPK/ERK signalling. VEGF induced the phosphorylation of MAPK/ERK in a time-dependent manner in the differentiating MAPCs (Fig.2A). Typically, VEGF stimulates a strong phosphorylation of both p42 and p44 MAPK/ERK1 and 2 within 5–10 min of incubation in EC, and subsides to baseline between 30 and 60 min of incubation [10]. The right-hand lane representing a positive control in Fig.2A showed strong phosphorylation of both MAPK/ERK1 and 2 after 10 min of incubation of human umbilical vein endothelial cell (HUVEC) with VEGF165. However, VEGF induced a comparatively week and delayed activation starting after 15 min of incubation that was sustained throughout the observation period of 4 hrs in MAPCs. The most striking feature of VEGF-induced activation was that it only activated p42 MAPK/ERK2, not p44 MAPK/ERK1, in the differentiating MAPCs from 15 min to 4 hrs. The expression of p42-phospho-MAPK/ERK2 was sustained throughout 14 days in the differentiating MAPCs (Fig.2B), suggesting that it may be critical in the process of differentiation of MAPCs to ECs. Indeed, previous studies have shown that ERK2 may be a major functional intracellular signal in the differentiation process [34]. The total p42/44-MAPK/ERK1 and 2 remained unaltered throughout the time course of differentiation from 0 min. to 14 days of incubation with VEGF, arguing that phosphorylation of MAPK/ERK1/2 is required for the differentiation of MAPCs to ECs.
Figure 2.
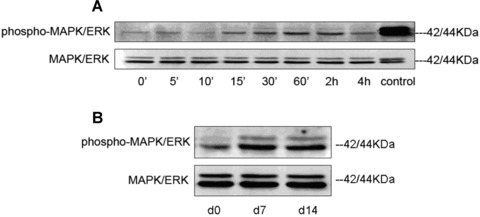
Time course of phospho-MAPK/ERK and MAPK/ERK in undifferentiated and differentiating MAPCs. (A) MAPK/ERK was activated after 15 min. of incubation with VEGF which sustained throughout the observation period of 4 hrs in MAPCs. Control: HUVEC with stimulation by VEGF165 for 10 min. (B) The expression of p42-phospho-MAPK/ERK2 was sustained throughout 14 days in differentiating MAPCs. Membrane was stripped and re-probed with MAPK/ERK that showed the equal loading of the samples. Figures are representative of results obtained from three independent experiments.
Inhibition of the activation of MAPK/ERK1/2 suppresses the endothelial differentiation from MAPCs
In order to elucidate whether MAPK/ERK1/2 is essential for the differentiation process, we examined the effect of PD98059, a selective inhibitor of MEK1 and 2, the upstream regulator of MAPK/ERK 1 and 2 phosphorylation, on the differentiation of MAPCs to ECs. We first determined the effect of PD98059 on the proliferation of MAPCs in the presence and absence of VEGF for 48 hrs. We observed that PD98059 at 50 μm (not at 5 μm), weakly but significantly, inhibited the proliferation of MAPCs in basal media by 8.73 ± 10.56% (n= 3; P > 0.05 at 5 μM) and 18.9 5 ± 2.8% (n= 3; P < 0.01 at 50 μM) respectively, and VEGF-induced proliferation (basal media with 20 ng/ml VEGF) by 6.88 ± 8.96% (n= 3; P > 0.05 at 5 μm) and 15.62 ± 9.16% (n= 3; P < 0.05 at 50 μm) respectively (Fig.3). Cell viability assay with Trypan blue showed that >99% cells were viable in the presence of PD98059 when incubated with VEGF or without VEGF for 48 hrs. A decrease in cell population in the presence of PD98059 may be due to an inhibition of cell proliferation and does not affect cell survival. From these data we inferred that PD98059 could be used for longer period of time with differentiating MAPCs without causing cell death. Therefore, we used 50 μM PD98059 to examine the role of MAPK/ERK phosphorylation in the differentiation of MAPCs to ECs.
Figure 3.
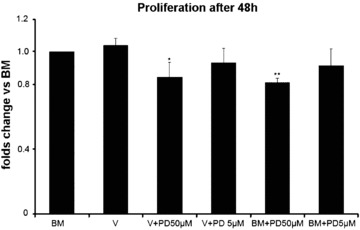
Proliferation assay of MAPCs in the presence and absence of VEGF for 48 hrs. PD98059 at 50 μM inhibited the proliferation of MAPCs and VEGF-induced proliferation by 18.95 ± 2.8% (P < 0.01), 15.62 ± 9.16% (P < 0.05), respectively. BM, differentiation basal media; V 20 ng, VEGF 165 with a final concentration of 20 ng/ml; PD 50 μM/5μM, PD98059 with a final concentration of 50 μM or 5 μM. *: P < 0.05 compared with BM; **: P < 0.01 compared with BM. Results are obtained from three independent experiments.
Time-dependent phosphorylation of MAPK/ERK 1 and 2 from 15 min. to 4 hrs was inhibited in the presence of PD98059 (Fig 4A). MAPK/ERK phosphorylation remained consistently inhibited up to day 14 in the presence of PD98059 (Fig.4B). Inhibition of MAPK/ERK phosphorylation by PD98059 was accompanied by morphological changes in the differentiating MAPCs (data not shown). On day 14, cells exposed to VEGF in the presence of PD98059 showed a lack of morphological change into cobblestone like ECs. The lack of endothelial morphology is further supported by a significantly decreased expression of EC-specific transcripts of VE-cadherin, CD31 and vWF in the cells cultured with VEGF in the presence of PD98059 as compared to those cultured with VEGF alone for 14 days (Fig.5A). Furthermore, Dil-Ac-LDL uptake was dramatically reduced in the cells cultured with VEGF in the presence of PD98059 as compared to VEGF alone (Fig.5B). Thus, inhibition of MAPK/ERK 1 and 2 phosphorylation by PD98059 results in the inhibition of VEGF-induced differentiation of MAPCs to ECs, both morphologically and functionally.
Figure 4.

Inhibition of the activation of MAPK/ERK with PD98059. (A) Time-dependent phosphorylation of MAPK/ERK from 15 min. to 4 hrs was inhibited in the presence of PD98059. (B) MAPK/ERK phosphorylation was consistently inhibited up to day 14 in the presence of PD98059. Membrane was stripped and re-probed with MAPK/ERK which showed the equal loading of the samples. V, VEGF165 20 ng/ml; PD, PD98059 50 μM. Figures are representative of results obtained from three independent experiments.
Figure 5.

Endothelial differentiation from MAPCs was suppressed by PD98059. (A) Transcript levels of endothelial cells (EC) and MAPC-specific genes detected by Q-RT-PCR. EC-specific transcripts of VE-cadherin, CD31 and von Willebrand factor (vWF) on the cells cultured with VEGF in the presence of PD98059 were decreased significantly versus cells cultured with VEGF alone for 14 days. Results shown are mean ± SD from three independent experiments. *: P < 0.05 compared with undifferentiated MAPCs (dO); **: P < 0.01 compared with undifferentiated MAPCs (dO); #: P < 0.05 compared with MAPCs cultured with VEGF alone for 14 days; ##: P < 0.01 compared with MAPCs cultured with VEGF alone for 14 days. PD, PD98059 50 μM. (β) Dil-Ac-LDL uptake in MAPCs cultured with VEGF in the absence (left panel) and presence (right panel) of PD98059 for 14 days. Dil-Ac-LDL uptake (Red) was inhibited in the cells cultured with VEGF in the presence of PD98059 as compared to VEGF alone. PD, PD98059 50 μM. Figures are representative of results obtained from 3 independent experiments.
Nuclear translocation of phospho-MAPK/ERK is required for endothelial differentiation from MAPCs
Phosphorylation of MAPK/ERK1/2 followed by nuclear translocation is required to induce transcriptional changes leading to proliferation, survival and differentiation [35, 36]. MAPCs incubated with VEGF for 14 days showed the presence of phosphor MAPK/ERK (red) in the nuclei (blue) of MAPCs (Fig.6B). In the presence of PD98059, however, VEGF-induced MAPK/ERK 1 and 2 phosphorylation as well as nuclear translocation were significantly inhibited on day 14 (Fig.6C). Thus, VEGF-induced phosphorylation as well as translocation of MAPK/ERK 1 and 2 is required for the differentiation of MAPCs to ECs.
Figure 6.

Nucleus translocation of phospho-MAPK/ERK in MAPCs treated with VEGF for 14 days in the absence of presence or PD98059. (A) Undifferentiated MAPCs at dayO. (B) MAPCs incubated with VEGF only showed the presence of phospho MAPK/ERK (red) in the nuclei (blue) of MAPCs. Arrow showed the translocated phosphor-MAPK/ERK. (C) VEGF-induced phosphorylation as well as nuclear translocation was inhibited on day 14 in the presence of PD98059. Figures are representative of results obtained from three independent experiments.
In brief, our data show that VEGF stimulates sustained activation and nuclear translocation of MAPK/ERK in the differentiating MAPCs. This sustained activation and nuclear translocation of MAPK/ERK leads to the differentiation of MAPCs to ECs.
Discussion
Our observations provide a molecular explanation for endothelial specification of progenitor cells. We show for the first time that phosphorylation of MAPK/ERK mediates VEGF-induced differentiation of MAPCs to ECs. Inhibition of MAPK/ERK phosphorylation abolishes the ability of VEGF to induce MAPCs to EC specification. Therefore, MAPK/ERK phosphorylation is critical to the process of endothelial differentiation and development.
The ability of VEGF to stimulate EC-specific gene expression and to induce the differentiation of mouse MAPCs to ECs are consistent with earlier observations from our laboratory and others utilizing mouse and human MAPCs [3, 6, 32]. We show that the time-dependent differentiation of MAPCs into ECs is accompanied by a consistent but slow up-regulation of EC-specific genes up to day 14. In contrast, a sharp decline was observed in the expression of Oct-4, an ES-cell-specific marker that has been found to be highly expressed in MAPCs [7], within 24 hrs of VEGF stimulation. It is noticeable that MAPCs lose their identity with down-regulation of Oct-4 expression very early upon stimulation with VEGF that is associated with sustained activation of MAPK/ERK during the course of differentiation into ECs. Loss of identity’ may be a key step in the commitment of MAPCs to follow different cell lineages, including ECs. The finding in this study is consistent with previous reports using ES [37, 38]. In our study, addition of VEGF acts by inhibiting the signals that are responsible for the MAPCs, because Oct-4 expression reduces to 50% within a day after the addition of VEGF. Therefore, VEGF on one hand stimulates the expression of EC-specific genes and on the other hand down-regulates the expression of MAPC-specific genes. It appears that the ‘loss of identity’ of MAPCs is a pre-requisite to the induction of EC characteristics. This is further supported by the observations that EC-specific genes are expressed to a much lesser extent up to day 7 and gradually increased up to day 14. Depending upon lineage-specific cues from the microenvironment, these cells can then commit to a specific cell lineage. Furthermore, inhibition of MAPK/ERK phosphorylation with PD98059 prevents VEGF-induced down-regulation of Oct-4 in MAPCs. Thus, phosphorylation of MAPK/ERK negatively regulates Oct-4 expression. These observations though preliminary, provide a rationale for the use of VEGF in addition to other cytokines for the commitment of bone marrow stem cells to different cell lineages.
Indeed VEGF is a multi-tasking cytokine which stimulate cell differentiation, survival, proliferation and migration [9]. Many studies show that VEGF is required for the differentiation of stem cells to haematopoietic cells and cardiac myocytes [39–41]. VEGF (20 ng/ml) promotes the differentiation of ES cells to cardiac myocytes via MAPK/ERK phosphorylation, and PD98059 inhibits VEGF-induced cardiac myocyte differentiation [42]. Mouse ES cells and chick embryo require sustained activation of MAPK/ERK1/2 for a specific period for neural specification [43]. Inhibition of MAPK/ERK1/2 stalls the differentiation of ES cells into neural cells even upon FGF5 up-regulation. Pluripotent stem cells commit to a lineage commitment instead of self renewal upon activation of MAPK/ERK1/2 by FGF4 [16]. Anterior neural organ generation from ectoderm requires phosphorylation of MAPK/ERK, and U0126 (a specific antagonist of MAPK/ERK), inhibits neural differentiation of cultured animal cap ectoderm [44]. These observations support our suggestion that ‘loss of identity’ inferred by VEGF-induced MAPK/ERK phosphorylation precedes the lineage commitment of progenitor cells.
The multi-functionality of VEGF at the cellular level results from its ability to initiate a diverse, complex and integrated network of signalling pathways via Flk1/VEGFR2 that converge at the MAPK/ERK signalling pathway [9]. MAPCs show a low-level expression of Flk1 transcripts that increases with time of differentiation in parallel to an increase in other EC-specific transcripts. It is known that Flk1 is required for the commitment of precursors to differentiating into functional ECs [45]. We noted that PD98059 partially but significantly inhibited the expression of Flk-1 during the course of differentiation of MAPCs into ECs. Therefore, it appears that MAPK/ERK phosphorylation is essential for EC specification via Flk1. Moreover, PD98059 (at 50 μM) inhibited the BrdU incorporation/proliferation only modestly under both VEGF replete and deplete conditions (Fig.3), but completely inhibited the differentiation of MAPC to EC (Fig.5). Therefore, inhibition of MAPK/ERK by PD98059 leads to the inhibition of MAPC differentiation to EC and is not due to the inhibition of cell proliferation.
Previous studies have characterized a critical role of Flk1 in the activation of MAPK/ERK pathway in cell proliferation and survival [46, 47]. Additionally, dimerization of phospho-MAPK/ERK and its nuclear translocation are also required for its activity in EC proliferation and survival. Sustained activation of MAPK/ERK could be detected during an in vitro differentiation process of ES cells. And the inhibition of MAPK/ERK activation by the MEK inhibitor abolished expression of the mesodermal markers [48]. Also it was found that MAPK/ERK activity is essential for the neuronal differentiation of ES cells [14]. Nerve growth factor-induced neuronal differentiation is exclusively dependent on activation of the MEK/ERK pathway in PC12 cells [19, 20]. Our observations demonstrate that MAPK/ERK1/2 phosphorylation is required for EC differentiation, and activated MAPK/ERK1/2 was present in the nucleus of cells incubated with VEGF. PD98059 inhibited the nuclear translocation of phospho-MAPK/ERK1/2 in addition to inhibiting the activation of MAPK/ERK1/2. Thus, nuclear translocation of activated MAPK/ERK has been found to be crucial for the differentiation of various cells [4, 17–22].
However, it has been demonstrated that MAPK/ERK1 and MAPK/ERK2 have distinct biological functions. Previous studies showed that MAPK/ERKr_/_ mice are viable and fertile [49], while disruption of MAPK/ERK2 is embryonic lethal due to defective placenta formation, mesoderm and trophectoderm differentiation [48, 50]. It was found that MAPK/ERK2 knockdown myoblasts are differentiation defective with the presence of IGF-I while MAPK/ERK1 silent myoblasts that synthesize abundant amounts of MAPK/ERK2 are more responsive to the actions of IGF-I, representing a dominant role of MAPK/ERK2 in differentiation process [51]. There is also a suggestion that intracellular signalling that leads to an abundance of MAPK/ERK2 phosphorylation is associated with a positive effect on myogenesis while signals that produce elevated amounts of both phosphorylated MAPK/ERK1 and MAPK/ERK2 are correlated with inhibition of myogenesis [34]. This is consistent with our results where MAPK/ERK2 was strongly activated during the entire endothelial differentiation process while the activation of MAPK/ERK1 was much weaker.
It is believed that sustained MAPK/ERK activation, as opposed to transient activation, is an inherent aspect of differentiation (neurite outgrowth) in PC12 cells [52] and in the differentiation of ES cells. Our data suggest that VEGF stimulates a sustained activation of p42-MAPK/ERK2 in MAPCs that remains up-regulated until the differentiation of MAPCs to ECs up to day 14 and may therefore be critical to the process of differentiation. Our results are consistent with the observations that long-term activation of MAPK/ERK is crucial to the differentiation of PC12 cells and ES cells [52]. More recent studies show that activation of MAPK/ERK is an early marker of arterial progenitors and their subsequent arterial specification [53]. In embryos, cells expressing high levels of phosphorylated MAPK/ERK contribute to artery formation. Conversely, cells contributing to the formation of veins do not show phospho-MAPK/ERK expression. In this study, activation of AKT promotes venous specification. Thus, our observations on the activation of MAPK/ERK signalling in differentiating MAPCs may be of significant contribution in arterial repair and endothelialization of arterial artificial grafts and stents. Therefore, we speculate that strategies leading to the activation of MAPK/ERK may promote angiogenesis from circulating or cardiac progenitor cells and may be beneficial in treating vascular injury and ischaemic cardiovascular diseases.
Acknowledgments
The authors wish to thank Mr. Dennis Mathias for his expertise technical assistance on the preparation of the figures. This work was supported by NIH R01 CA109582–02 (K.G.) and NIH K08 HL075410 (Z.L.).
References
- 1.Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–53. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 4.Di Cristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, Ratto GM, Maffei L. Requirement of ERK activation for visual cortical plasticity. Science. 2001;292:2337–40. doi: 10.1126/science.1059075. [DOI] [PubMed] [Google Scholar]
- 5.Zeng L, Rahrmann E, Hu Q, Lund T, Sandquist L, Felten M, O’Brien TD, Zhang J, Verfaillie C. Multipotent adult progenitor cells from swine bone marrow. Stem Cells. 2006;24:2355–66. doi: 10.1634/stemcells.2005-0551. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 7.Ulloa-Montoya F, Kidder BL, Pauwelyn KA, Chase LG, Luttun A, Crabbe A, Geraerts M, Sharov AA, Piao Y, Ko MS, Hu WS, Verfaillie CM. Comparative transcriptome analysis of embryonic and adult stem cells with extended and limited differentiation capacity. Genome Biol. 2007;8:R163. doi: 10.1186/gb-2007-8-8-r163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta K, Ramakrishnan S, Browne PV, Solovey A, Hebbel RP. A novel technique for culture of human dermal microvascular endothelial cells under either serum-free or serum-supplemented conditions: isolation by panning and stimulation with vascular endothelial growth factor. Exp Cell Res. 1997;230:244–51. doi: 10.1006/excr.1996.3421. [DOI] [PubMed] [Google Scholar]
- 9.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–7. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 10.Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law PY, Hebbel RP. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247:495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Farooqui M, Gupta K. Morphine stimulates vascular endothelial growth factor-like signaling in mouse retinal endothelial cells. Curr Neurovasc Res. 2006;3:171–80. doi: 10.2174/156720206778018767. [DOI] [PubMed] [Google Scholar]
- 12.Meadows KN, Bryant P, Pumiglia K. Vascular endothelial growth factor induction of the angiogenic phenotype requires Ras activation. J Biol Chem. 2001;276:49289–98. doi: 10.1074/jbc.M108069200. [DOI] [PubMed] [Google Scholar]
- 13.Nakatsu MN, Sainson RC, Perez-del-Pulgar S, Aoto JN, Aitkenhead M, Taylor KL, Carpenter PM, Hughes CC. VEGF(121) and VEGF(165) regulate blood vessel diameter through vascular endothelial growth factor receptor 2 in an in vitro angiogenesis model. Lab Invest. 2003;83:1873–85. doi: 10.1097/01.lab.0000107160.81875.33. [DOI] [PubMed] [Google Scholar]
- 14.Reffas S, Schlegel W. Compartment-specific regulation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinases (MAPKs) by ERK-dependent and non-ERK-dependent inductions of MAPK phosphatase (MKP)-3 and MKP-1 in differentiating P19 cells. Biochein J. 2000;352(Pt 3):701–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Kristensen DM, Kalisz M, Nielsen JH. Cytokine signalling in embryonic stem cells. Apmis. 2005;113:756–72. doi: 10.1111/j.1600-0463.2005.apm_391.x. [DOI] [PubMed] [Google Scholar]
- 16.Kunath T, Saba-EI-Lell MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 17.Miranda MB, McGuire TF, Johnson DE. Importance of MEK-1/-2 signaling in monocytic and granulocytic differentiation of myeloid cell lines. Leukemia. 2002;16:683–92. doi: 10.1038/sj.leu.2402400. [DOI] [PubMed] [Google Scholar]
- 18.McCubrey JA, Steelman LS, Hoyle PE, Blalock WL, Weinstein-Oppenheimer C, Franklin RA, Cherwinski H, Bosch E, McMahon M. Differential abilities of activated Raf oncoproteins to abrogate cytokine dependency prevent apoptosis and induce autocrine growth factor synthesis in human hematopoietic cells. Leukemia. 1998;12:1903–29. doi: 10.1038/sj.leu.2401215. [DOI] [PubMed] [Google Scholar]
- 19.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–5. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–52. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 21.Racke FK, Lewandowska K, Goueli S, Goldfarb AN. Sustained activation of the extracellular signal-regulated kinase/ mitogen-activated protein kinase pathway is required for megakaryocytic differentiation of K562 cells. J Biol Chem. 1997;272:23366–70. doi: 10.1074/jbc.272.37.23366. [DOI] [PubMed] [Google Scholar]
- 22.Crompton T, Gilmour KC, Owen MJ. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell. 1996;86:243–51. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 23.Hsu CL, Kikuchi K, Kondo M. Activation of mitogen-activated protein kinase kinase (MEK)/extracellular signal regulated kinase (ERK) signaling pathway is involved in myeloid lineage commitment. Blood. 2007;110:1420–8. doi: 10.1182/blood-2007-02-071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breyer A, Estharabadi N, Oki M, Ulloa F, Nelson-Holte M, Lien L, Jiang Y. Multipotent adult progenitor cell isolation and culture procedures. Exp Hematol. 2006;34:1596–601. doi: 10.1016/j.exphem.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. [PubMed] [Google Scholar]
- 26.Farooqui M, Geng ZH, Stephenson EJ, Zaveri N, Yee D, Gupta K. Naloxone acts as an antagonist of estrogen receptor activity in MCF-7 cells. Mol Cancer Ther. 2006;5:611–20. doi: 10.1158/1535-7163.MCT-05-0016. [DOI] [PubMed] [Google Scholar]
- 27.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–46. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aranguren XL, Luttun A, Clavel C, Moreno C, Abizanda G, Barajas MA, Pelacho B, Uriz M, Arana M, Echavarri A, Soriano M, Andreu EJ, Merino J, Garcia-Verdugo JM, Verfaillie CM, Prosper F. In vitro and in vivo arterial differentiation of human multipotent adult progenitor cells. Blood. 2007;109:2634–42. doi: 10.1182/blood-2006-06-030411. [DOI] [PubMed] [Google Scholar]
- 29.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- 30.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–40. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, Wu JC. Differentiation, survival· and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:146–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Jiang Y, Hao H, Gupta K, Xu J, Chu L, McFalls EO, Zweier JL, Verfaillie CM, Bache RJ. Endothelial Nitric Oxide Synthase Is Dynamically Expressed during Bone Marrow Stem Cell Differentiation into Endothelial Cells. Am J Physiol Heart Circ Physiol. 2007;293:1760–65. doi: 10.1152/ajpheart.01408.2006. [DOI] [PubMed] [Google Scholar]
- 33.Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–6. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Thomson SR, Starkey JD, Page JL, Ealy AD, Johnson SE. Transforming growth factor betal is up-regulated by activated Raf in skeletal myoblasts but does not contribute to the differentiation-defective phenotype. J Biol Chem. 2004;279:2528–34. doi: 10.1074/jbc.M306489200. [DOI] [PubMed] [Google Scholar]
- 35.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebner HL, Blatzer M, Nawaz M, Krumschnabel G. Activation and nuclear translocation of ERK in response to ligand-dependent and -independent stimuli in liver and gill cells from rainbow trout. J Exp Biol. 2007;210:1036–45. doi: 10.1242/jeb.02719. [DOI] [PubMed] [Google Scholar]
- 37.Mitalipov SM, Kuo HC, Hennebold JD, Wolf DP. Oct-4 expression in pluripotent cells of the rhesus monkey. Biol Reprod. 2003;69:1785–92. doi: 10.1095/biolreprod.103.019455. [DOI] [PubMed] [Google Scholar]
- 38.Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–8. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 39.Song YH, Gehmert S, Sadat S, Pinkernell K, Bai X, Matthias N, Alt E. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2007;354:999–1003. doi: 10.1016/j.bbrc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 40.Muguruma Y, Reyes M, Nakamura Y, Sato T, Matsuzawa H, Miyatake H, Akatsuka A, Itoh J, Yahata T, Ando K, Kato S, Hotta T. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells. Exp Hematol. 2003;31:1323–30. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of Human Embryonic Stem Cells in Serum Free Medium Reveals Distinct Roles for BMP4: VEGF, SCF and FGF2 in Hematopoiesis. Stem Cells. 2007;25:2206–14. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Amende I, Hampton TG, Yang Y, Ke Q, Min JY, Xiao YF, Morgan JP. Vascular endothelial growth factor promotes cardiomyocyte differentiation of embryonic stem cells. Am J Physiol Heart Circ Physiol. 2006;291:H1653–8. doi: 10.1152/ajpheart.00363.2005. [DOI] [PubMed] [Google Scholar]
- 43.Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–94. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- 44.Hurtado C, De Robertis EM. Neural induction in the absence of organizer in salamanders is mediated by MAPK. Dev Biol. 2007;307:282–9. doi: 10.1016/j.ydbio.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Edholm D, Lanner F, Breier G, Farnebo F, Dimberg A, Claesson-Welsh L. Lentiviral Rescue of VEGFR-2 Expression in flkl-/- ES Cells Shows Early Priming of Endothelial Precursors. Stem Cells. 2007;12:2987–95. doi: 10.1634/stemcells.2007-0397. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T, Ueno H, Shibuya M. VEGFactivates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–30. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto T, Zhang XM, Chen BY, Yang XJ. VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development. 2006;133:2201–10. doi: 10.1242/dev.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao Y, Li W, Wu J, Germann UA, Su MS, Kuida K, Boucher DM. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci USA. 2003;100:12759–64. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pages G, Guerin S, Grail D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–7. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 50.Saba-EI-Leil MK, Vella FD, Vernay B, Voisin L, Chen L, Labrecque N, Ang SL, Meloche S. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4:964–8. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Johnson SE. ERK2 is required for efficient terminal differentiation of skeletal myoblasts. Blochem Blophys Res Commun. 2006;345:1425–33. doi: 10.1016/j.bbrc.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 52.Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–9. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- 53.Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. CurrBiol. 2006;16:1366–72. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]


