Abstract
Retinal neovascularization is the most common cause of blindness; Retinopathy of pre-maturity (ROP) for children and diabetic retinopa-thy for young age group. ROP still remains as the most serious cause of vision loss in children. We provided that deguelin significantly reduces retinal neovascularization in a mouse model of ROP. Deguelin never affected the transcriptional activity of hypoxia inducible factor (HIF)-1α, however, reduced HIF-1α expression, which led to the decrease of vascular endothelial growth factor expression. Deguelin effectively suppressed endothelial cell proliferation without cytotoxic effect under therapeutic concentration range. In addition, deguelin demonstrated no reduction or retardation in normal retinal development and no retinal toxicity. These data suggest deguelin is a potent inhibitor of retinal neovascularization and may be applied in the treatment of other vasoproliferative retinopathies.
Keywords: deguelin, HIF-1α, oxygen-induced retinopathy, retinal neovascularization
Introduction
Angiogenesis is a process to form new blood vessels, which is tightly regulated by a balance between positive and negative factors [1]. Physiologically, angiogenesis does not occur except during developmental and repair processes. Pathological angio-genesis in the eye is the most common cause of blindness in all age groups. For example, retinopathy of prematurity (ROP) is for children, diabetic retinopathy for young adults and age-related macular degeneration for elderly [2]. ROP is a major cause of blindness which affects premature infants [3]. Despite of outstanding advances in surgical treatment, ROP remains as the most serious problem of vision loss in children even in developed countries. In ROP, retinal neovascularization is followed by the vessel loss leading to retinal hypoxia. New vessels, fragile and leaky, are formed at the junction between the vascularized and the avascular zone of the retina. Over time, these proliferative vessels induce a fibrous scar extending from the retina to the vitreous gel, which result in a retinal detachment and likely blindness [4]. Therefore, the amelioration of pathological angiogenesis in ROP is targeted for either blocking the vessel loss in order to control hypoxic condition or directly inhibiting vessel proliferation [5]. Oxygen-induced ischaemic retinopathy (OIR) in the mouse is a highly reproducible animal model of ROP [6].
Rotenoids of flavonoid family have chemo-preventive activity to inhibit NADH:ubiquinone oxidoreductase activity and to suppress mRNA expression and enzymatic activity of 12-O-tetrade-canoylphorbol 13-acetateinduced ornithine decarboxylase (ODC), which catalyses the decarboxylation of ornithine to yield putrescine, which is converted to the higher polyamines. This activity of ODC and the polyamines are crucial for cellular proliferation [7, 8]. The rotenoid, deguelin, is isolated from several plant species, including Mundulea sericea (Leguminosae) [9]. Deguelin has also shown potential as a chemopreventive agent against breast, colon, skin and non-small cell lung cancers [7–15]. We have demonstrated that deguelin, a natural product isolated from plants in the Mundulea sericea family, inhibits cyclooxygenase-2 expression and phosphatidylinositol 3-kinase (PI3K)/Akt-mediated signalling pathways which contribute to its anti-proliferative effects [10–13]. However, we also found out that deguelin reduces the expression of Hsp90-binding proteins, including hypoxia inducible factor (HIF)-1α protein and induced the degradation of HIF-1α protein independently on reactive oxygen species and PI3K-Akt pathways [14]. Recently, we proposed deguelin as a novel anti-angiogenic anticancer agent to decrease the expression of HIF-1α protein and its target angiogenic cytokine in vascular endothelial cells under hypoxic conditions [15]. Moreover, deguelin inhibited de novo synthesis of HIF-1α protein and reduced the half-life of the synthesized protein [15].
In the present study, we demonstrated deguelin significantly reduces retinal neovascularization in OIR. The anti-angiogenic activity of deguelin was related to the reduction of HIF-1α and vascular endothelial growth factor (VEGF) expression without change in transcriptional activity of HIF-1α. Up to 1 μm deguelin, 10 times of effective therapeutic concentration of it, deguelin never affected the viability of human retinal endothelial cells (HRECs). Moreover, it also showed no significant toxicity in the physiological retinal vascular formation and in the developing retina. In addition to the anti-proliferative activity of deguelin to cancer cells [10–14], we herein suggest that deguelin, a new angiogenesis inhibitor, may have a therapeutic potential in the treatment of retinal neovascularization of ROP as well as in other vasoproliferative retiniopathies such as diabetic retinopathy.
Materials and methods
Animals
C57BL/6 mice were purchased from Samtako (Korea). Care, use, and treatment of all animals in this study were in strict agreement with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.
Cell culture
HRECs were purchased from Applied Cell Biology Research Institute (ACBRI, USA) and cultured in M199 (Gibco BRL, Invitrogen, Carlsbad, CA, USA) supplemented with 20% FBS (Gibco BRL, Invitrogen, Carlsbad, CA, USA), 3 ng/ml basic fibroblast growth factor (bFGF) (Upstate Biotechnology, Charlottesville, VA, USA), 10 U/ml heparin, and antibiotics. HRECs were cultured in plates coated with 0.3% gelatin. Human embryonic kidney, HEK 293, cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10%. For hypoxic condition, cells were incubated at 5% CO2 level with 1% O2 balanced with N2 in hypoxic chamber.
Oxygen-induced retinopathy
OIR was induced as described by Smith et al. [6] with some modifications [16]. Briefly, newborn mice were randomly assigned to experimental and control groups. At postnatal day (P)7, Pups (8–10 pups) in the experimental group were exposed to hyperoxia (75±0.5% O2) for 5 days (P7–P12) and then returned to normoxia (room air) for 6 days. Neovascularization occurs upon return to normoxia and peaks at P17. To assess the anti-angiogenic activity of deguelin, the pups were injected intravitreously with 0.1 μM deguelin in 1 μl PBS into one eye and only 1 μl PBS into the other eye on P14, when retinal neovascularization began.
Qualitative assessment of retinal neovascularization by fluorescein angiography
At P17, deeply anaesthetized mice were perfused through the tail vein with high molecular weight (MW = 500,000) fluorescein conjugated dextran (Sigma-Aldrich Ltd., St. Louis, MO, USA) dissolved in PBS. After 1 hr perfusion, the eyes were enucleated and fixed in 4% paraformaldehyde for 4 hrs. The retinas were dissected, flat-mounted in Dako mounting medium (DakoCytomation, Glostrup, Denmark), and viewed by fluorescein microscopy (BX50, OLYMPUS, Japan) at a magnification of 4x. There were at least six animals in each group.
Quantitative assessment of retinal neovascularization by counting vascular lumens
At P17, the eyes were removed, fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 24 hr, and embedded in paraffin. Sagittal sections of 5 μm, each 30 μm apart, were cut through the cornea parallel to the optic nerve. The sections were stained with haematoxylin and eosin to assess retinal vasculature via light microscopy (Carl Zeiss, Chester, VA, USA). Any vascular lumens on the vitreal side of the inner limiting membrane were counted in at least 10 sections from each eye by two independent observers blind to treatment (Kim JH and Shin JY). The neovascular lumens were defined as the mean number per section found in at least 10 sections (at least five on each side of the optic nerve) per eye. The average intravitreal vessels/section was calculated for each group. There were at least six animals in each group.
Luciferase assay
Luciferase assay using pSV40pro-EpoHRE-Luc vector was performed as described in our previous experiments [17]. The luciferase reporter plas-mid used in these experiments consists in the original VEGF promoter coupled to the luciferase gene reporter. HEK 293 cells in 12-well plates (2 × 105 cells/well) were transiently transfected by the calcium phosphate method. HEK cells were cotransfected with 1 μg of pSV40promoter-EpoHRE-Luc reporter plasmid and 1 μg of the pSV-β-galactosidase plasmid and then allowed to recover for 24 hrs after transfection. Cells were harvested and extracts were prepared with reporter lysis buffer (Promega, Madison, WI, USA). Cell extracts were assayed for luciferase activity with the luciferase assay kit (Promega, Madison, WI, USA) and a luminometer (Turner Designs, Sunnyvale, CA, USA). Extracts were also assayed for galactosidase activity with the β-galactosidase enzyme assay system (Promega) and assayed for protein concentration with the protein assay kit. Each extract was assayed three times, and the mean relative light unit (RLU) was corrected by values obtained from an extract prepared from non-transfected cells. The relative luciferase activity was calculated as RLU/β-galactosidase.
Western blot analysis
Western blotting was performed using standard western blotting methods and visualized by the development with LAS-3000 (Fujifilm, Tokyo, Japan). The protein concentration was measured using a BCA protein assay kit (Pierce, Rockford, IL, USA). Immunoblotting was performed with primary antibodies against HIF-1α (BD Bioscience, Franklin lakes, NJ, USA) and VEGF (Santa-Cruz Biotechnology, Santa-Cruz, CA, USA). To ensure the equal loading of protein in each lane, the blots were stripped and re-probed with an antibody against β-tubulin.
Cell viability assay
Cell viability was evaluated with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. HRECs (1 × 105 cells) were plated in 96-well plates and cultured overnight. Cells were treated with deguelin (0∼10 μM) for 48 hrs. The medium was then replaced with fresh medium containing 0.5 mg/ml MTT for 4 hrs. After incubation, the medium was carefully removed from the plate and Dimethyl Sulfoxide (DMSO) was added to solubilize formazan produced from MTT by the viable cells. Absorbance was measured at 540 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Immunohistochemistry
To evaluate the effect of deguelin on physiologic retinal angiogenesis in development, the immunohistochemistry for von-Willebrand factor (vWF), a endothelial cell marker, was performed in the retina on P8 and P16 with or without intravitreal injection of deguelin. The enucleated mouse eyes used for imunohistochemistry were immersion fixed in 4% formalin and subsequently embedded in paraffin. 4 μm-thick serial sections were prepared from paraffin blocks. Sections were deparaffinized and hydrated by sequential immersion in xylene and graded alcohol solutions, and then treated with proteinase K for 5 min. at 37°C and then treated with normal serum obtained from the same species in which the secondary antibody was developed for 10 min. to block non-specific staining. Slides were incubated overnight at 4°C with rabbit polyclonal antibody against vWF (1:100, Chemicon, Billerica, MA, USA). FITC-conjugated IgG (1:400) was used as a secondary antibody. The slides were mounted and observed under fluorescence microscope (BX50, OLYMPUS, Japan).
TUNEL assay
Deguelin or PBS was intravitreously injected to P5 C57BL/6J mice. The mice were sacrificed at P8 and P16 after 0.1 μM deguelin injection, and enucleated. Enucleated globes were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 24 hrs, and embedded in paraffin. TUNEL staining was performed with a kit (ApopTag; Intergen, Purchase, NY, USA), according to the manufacturer's instructions. TUNEL-positive cells were evaluated in randomly selected fields at a ×400 magnification via light microscope (Carl Zeiss, Chester, VA, USA). There were at least six animals in each group.
Statistical analysis
Statistical differences between groups were evaluated with the Student unpaired t-test (two-tailed). Mean ± SD is shown. P≤ 0.05 was considered significant.
Results
Effect of deguelin in retinal neovascularization in OIR
We determined whether deguelin could reduce retinal neovascularization in oxygen-induced retinopathy. In the mouse OIR model, P7 newborn mice are kept in hyperoxia (75% oxygen) for 5 days. Excessive oxygen results in promoting the obliteration of existing vessels and blocking the development of retinal vasculature. After 5 days of hyperoxia, with back to room air, the undervascularized retina promotes the production of pro-angiogenic factors which drive the growth of abnormal new blood vessels [6, 18, 19]. Based on our previous data [14, 15], we injected deguelin (0.1 μm/1μl) intravitreously on P14 when active neovascularization occurs [18, 19].
To visualize intravitreous neovascularization in OIR, fluorescein angiography using fluorescein-conjugated dextran was performed. P17 deguelin-treated and control mice subjected to OIR showed that peripapillary, central retinal capillaries dropped out, whereas the large, radial retinal vessels extending to the periphery persisted. Retinas from P17 control mice contained many neovascular tufts of intravitreous neovascularization at the junction between the vascualrized and non-vascularized retina. (Fig.1A) In contrast, retinas from P17 deguelin-treated mice showed significantly reduced neovascularization. (Fig.1B)
Figure 1.
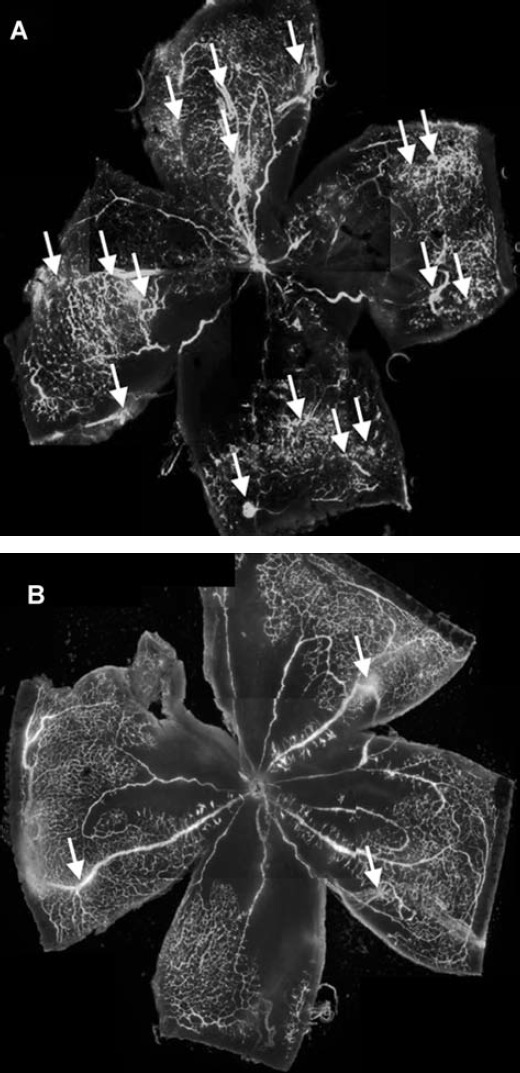
Qualitative assessment of retinal neovascularization by fluorescein angiography. Retinal vasculature in control and deguelin-treated mice during OIR was evaluated by fluorescein angiography using high molecular weight (MW = 500,000) fluorescein-conjugated dextran. Wholemount retinal preparation from P17 control (A) and 0.1 μM deguelin-treated (B) mice subjected to oxygen-induced ischaemic retinopathy (OIR) was performed after 1 hr perfusion of fluorescein-conjugated dextran, respectively. Arrows indicate neovascular tufts of intravitreous neovascularization. These experiments were repeated over three times with similar results.
To quantify the intravitrous neovascularization, vascular lumens between posterior surface of lens and anterior surface of the inner limiting membrane were counted in a masked fashion. Retinas from P17 control mice demonstrated multiple neovascular lumens, (Fig.2A) whereas retinas from P17 deguelin-treated mice demonstrated significantly fewer neovascular lumens. (Fig.2B) We found that deguelin-injected groups (19 ± 3.4) had significant decrease of intravitreous neovascularization compared to controls (4 ± 2.1) (P < 0.05). (Fig.2C)
Figure 2.
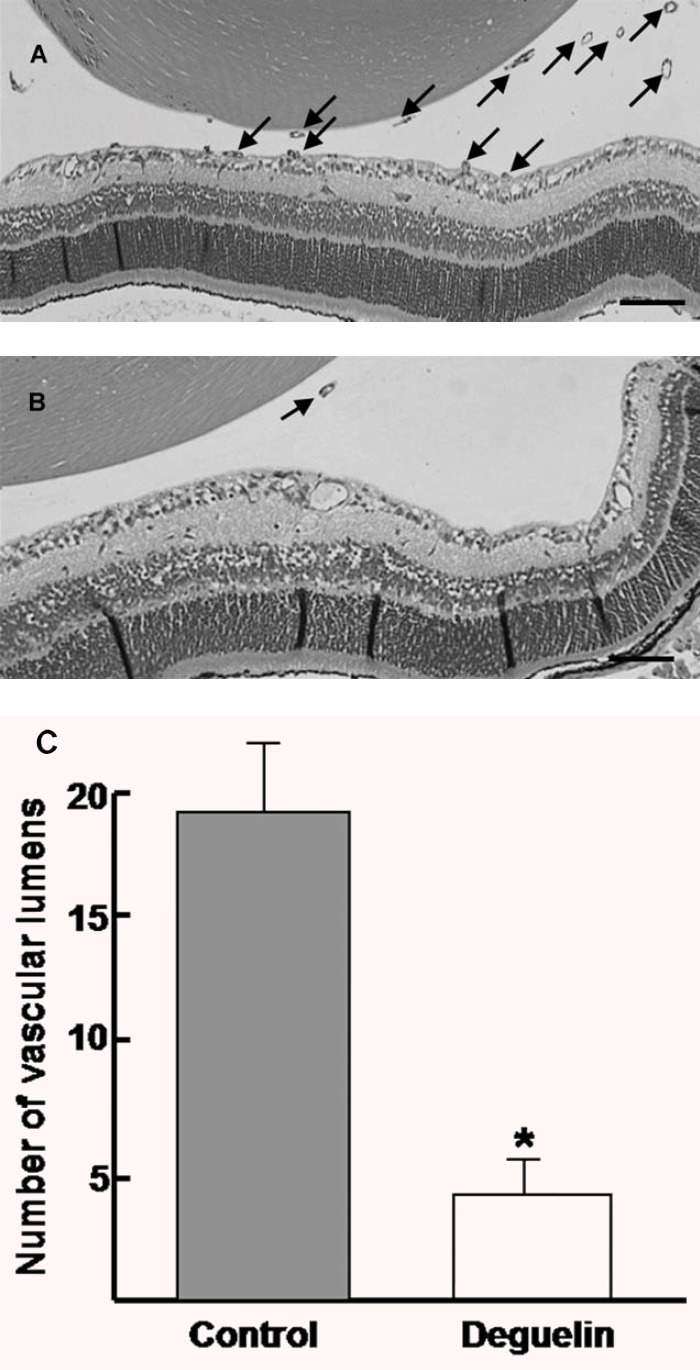
Quantitative assessment of retinal neovascularization by counting vascular lumens. Haematoxylin-stained crosssections prepared from P17 control (A) and 0.1 μM deguelin-treated (B) mice subjected to OIR, respectively. Arrows show the vascular lumens of new vessels growing into the vitreous. (C) Data in each column are the mean ± SD values from at least six eyes. Note that there is a statistically significant difference of retinal neovascularization between control and deguelin-treated mice (*P < 0.05). These experiments were repeated over three times with similar results. Scale bars: 200 μm (A, B).
Effects of deguelin on HIF-1α transcriptional activity and the expression of HIF-1α and VEGF
HIF-1α plays a crucial role in the regulation of angiogenic factors, including VEGF. To investigate whether deguelin is involved in HIF-1α transcriptional activity, we used a luciferase reporter system, pSV40promoter-EpoHRE-Luc reporter. The reporter activity was in 7.5-fold increase under hypoxic conditions compared to normoxia. Interestingly, deguelin never affected the transcriptional activity of HIF-1α up to 1 μm, though HIF-1α transcriptional activity in hypoxia was reduced with treatment of 10 μm deguelin (P < 0.05). (Fig.3A) As our previous report [15], 0.1 μm deguelin reduced HIF-1α expression under hypoxic condition, which led to the decrease of VEGF expression (P < 0.05). (Fig.3B)
Figure 3.
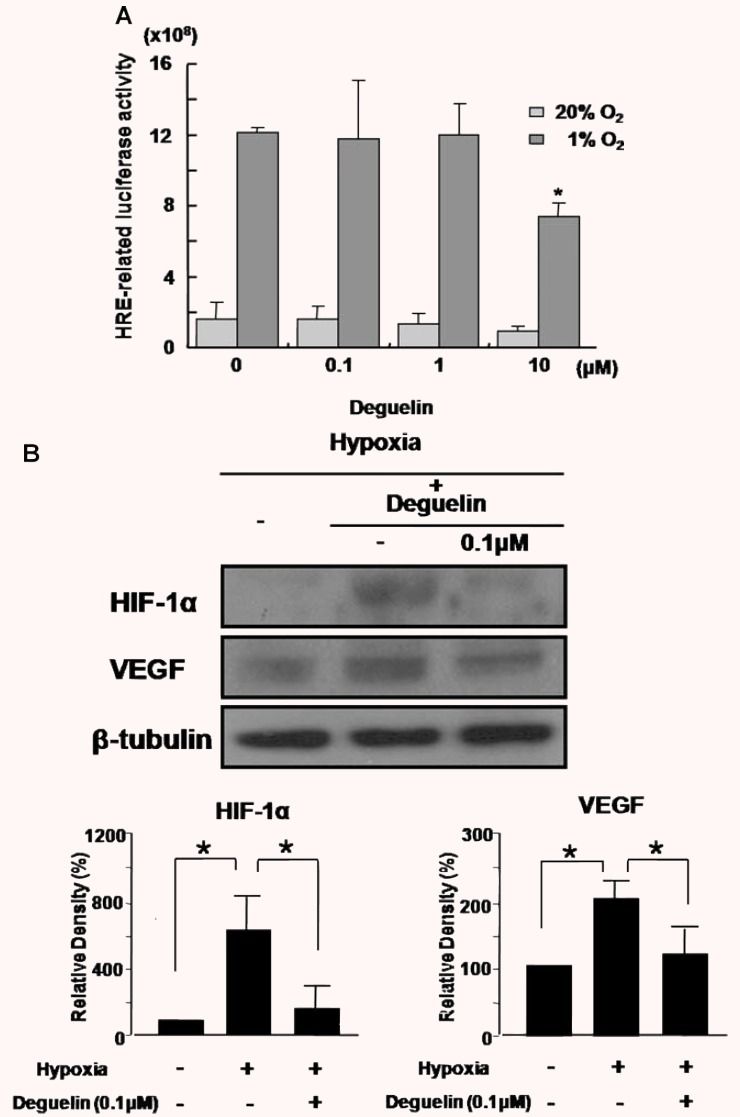
Effects of deguelin on HIF-1α transcriptional activity and the expression of HIF-1α and VEGF. (A) HEK 293 cells were cotransfected with 1 μg of pSV40promoter-EpoHRE-Luc reporter plasmid and 1 μg of the pSV-β-galac-tosidase plasmid and then allowed to recover for 24 hrs after transfection. With treatment of deguelin (0∼10 μM), transfected cells were incubated for 16 hrs before exposing them to hypoxia or maintaining them in normoxia and assayed for luciferase and β-galactosidase activities. The relative luciferase activity refers to the ratio of RLU/β-galactosidase measured in hypoxia-treated cells compared to normoxic cells. (B) HEK 293 cells were incubated for 16 hrs before exposing them to hypoxia or maintaining them in normoxia and assayed for the expression of HIF-1α and vascular endothelial growth factor (VEGF). Quantitative analysis was performed by measuring protein expression relative to the control. Each value represents the mean (±SD) of three independent experiments, each performed in triplicate (*P < 0.05).
Effect of deguelin on the viability of HRECs
To investigate cytotoxic effect of deguelin on HRECs, MTT assay was carried out in various concentrations of deguelin (0∼10 μm). The viability of HRECs treated with deguelin was not affected up to 1 μm. (Fig.4) 0.1 μm deguelin, effective therapeutic concentration to inhibit VEGF expression in our previous report [14, 15], did not affect the viability of HRECs.
Figure 4.
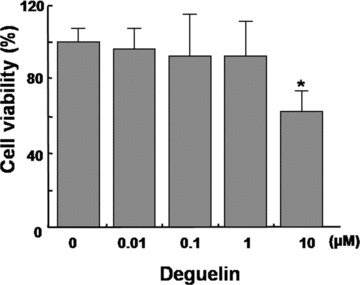
Effect of deguelin on the viability of Human retinal endothelial cells (HRECs). Various concentrations of deguelin (0∼10 μM) were treated on HRECs and cells were incubated for 2∼3 days. Cell viability was measured by MTT assay. Each value represents means ± SE from three independent experiments (*P < 0.05).
Effect of deguelin on physiological retinal angiogenesis in development
After intravitreal injection of 1 μM deguelin, 10 times of effective therapeutic concentration of deguelin, physiologic retinal angiogenesis was evaluated through immunohistochemistry for vWF. In the mouse retinal vasculature development, the primary vascular network spreads approximately halfway across the inner surface of the retina by P4 and reaches the periphery approximately 1 week (P7) after birth. After the vascular network has spread across the entire retina, arteries and veins strictly alternate and, at approximately P7, start to sprout downward, into the inner plexiform layer, where they establish a second vascular network parallel to the first by P14 [20].
As demonstrated in Figure 5A, physiologic retinal angiogenesis on P8 and P16 was not affected by deguelin treatment. In addition, retina with intravitreal injection of deguelin was of normal thickness and all retinal layers were clear without any inflammatory cells in the vitreous, retina or choroid. In control groups, few TUNEL-positive cells were detected in the outer nuclear layer. Compared to control, TUNEL positive cells on P8 and P16 were not increased with deguelin injection. (Figure 5B)
Figure 5.
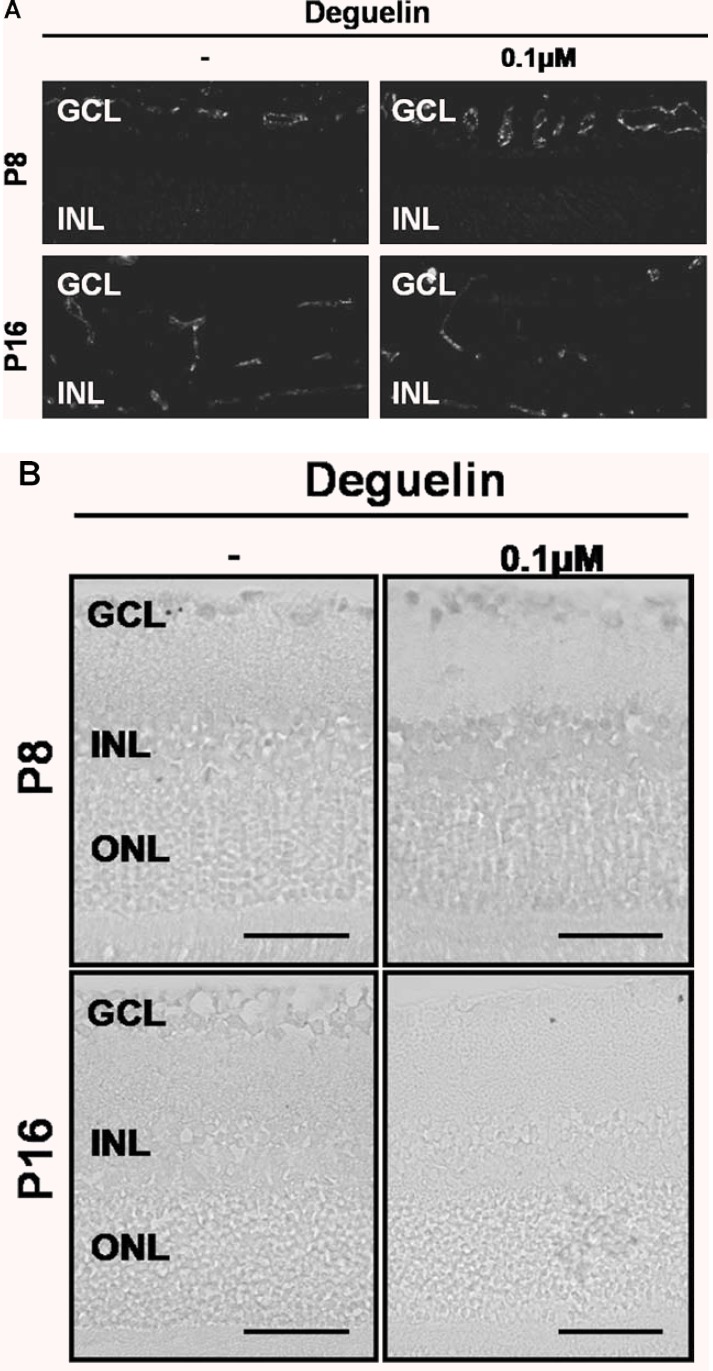
Effect of deguelin on physiological retinal angiogenesis in development. After intravitreal injection of 1 μM deguelin, 10 times of effective therapeutic concentration of deguelin on P4, (A) physiological retinal angiogenesis on P8 and P16 was not affected by deguelin treatment, and (B) TUNEL positive cells on P8 and P16 were not increased with deguelin injection and rarely detected in the field. Figures were selected as representative data from three independent experiments. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars: 50 μm (B).
Discussion
Deguelin, is isolated from several plant species, including Mundulea sericea (Leguminosae). [9] We have demonstrated that deguelin has anti-proliferative activity to cancer cells via regulation of cyclooxygenase-2 expression and phosphatidylinositol 3-kinase (PI3K)/Akt-mediated signalling pathways [10–13]. Recently, we also found out that deguelin reduces VEGF expression through the destabilization of HIF-1α protein. Interestingly, this anti-angiogenic activity of deguelin was independent on reactive oxygen species and PI3K-Akt pathways [14, 15].
In the present study, deguelin did not affect the transcriptional activity of HIF-1α, but reduced the expression of HIF-1α and VEGF in hypoxic condition. However, deguelin induced no reduction or retardation in normal retinal development. VEGF is essential in the physiological and pathological retinal angiogenesis. During physiological retinal angiogenesis, the increased oxygen demand of the developing neural retina generates the physiological hypoxia that induces vessel growth [21]. In response to the hypoxia, VEGF stimulates blood vessel growth. Pathological angiogenesis in ROP basically occurs under the hypoxic condition of the vessel loss. In these physiological and pathological angiogenesis, HIF-1α plays as a master regulator in the conformational change of vessel in response to oxygen concentration [17]. HIF-1α occupies a crucial role in hypoxia-signalling pathway. However, HIF-1α-induced VEGF alone might not be sufficient for retinal angiogenesis.
Insulin-like growth factor 1 (IGF-1) partly regulates retinal angiogenesis through the control of p42/44MAPK-induced VEGF activation [22]. At least, retinal vessel growth in normal development requires both IGF-1 and VEGF [22]. In terms of the difference between effects of deguelin in retinal vascular development and OIR, it may be one reason. Because deguelin is derived from rotenone [8], it is possible that deguelin induces side effect in cardiovascular, respiratory, and nervous system. In addition, we have never observed major toxicity in the mice at the therapeutically effective dose [13, 14], which results from the different mechanism of deguelin from that of other rotenone to inhibit tubulin polymerization. In this study, deguelin showed no affection to cell viability of HUVECs and no retinal toxicity up to 1 μM which is equivalent to 10 times of effective dose (0.1 μM) to OIR. It means that deguelin may reduce retinal neovascularization by inhibition of endothelial cell proliferation via reduction of HIF-1α and VEGF expression, not by reduction of viability of endothelial cells.
In the present study, we presented the inhibitory effect of deguelin on retinal neovascularization of OIR. Deguelin reduced the incidence of clinically significant vascular leakage in OIR. It was consistent with histologic findings of significantly less number of retinal neovascularization in deguelin-treated group. Based on these results, it is possible that deguelin may attenuate retinal neovascularization in OIR through a direct anti-angiogenic effect without cytotoxic effect in a therapeutic range.
In summary, deguelin effectively suppressed endothelial cell proliferation without cytotoxic effect under therapeutic concentration range of 0.01–1 μM. Anti-angiogenic effect of deguelin is directly related to down-regulation of HIF-1α expression. Interestingly, deguelin significantly reduced retinal neovascularization in a mouse model of ROP with no reduction or retardation in normal retinal development and no retinal toxicity. Herein, we provided deguelin as a new anti-angiogenic agent to retinal neovascularization. Furthermore, deguelin may also apply to other vasoproliferative retiniopathies including diabetic retinopathy.
Acknowledgments
This study was supported by R01-2004-000-10212-0 from the Basic Research Program of the Korea Science & Engineering Foundation and by the Bio-signal Analysis Technology Innovation Program (M1064501001-06n4501-00110) of the Ministry of Science and Technology (MOST) and Korea Science AND Engineering Foundation (KOSEF).
References
- 1.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 2.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 3.Silverman WA. Retrolental Fibrofibropla-sia: A Modern Parable. Grune & Stratton; 1980. pp. 37–42. , New York: [Google Scholar]
- 4.Roth AM. Retinal vascular development in premature infants. Am J Ophthalmol. 1977;84:636–40. doi: 10.1016/0002-9394(77)90377-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Smith LE. Retinopathy of Prematurity. Angiogenesis. 2007;10:133–40. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 6.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–11. [PubMed] [Google Scholar]
- 7.Gerhäuser C, Mar W, Lee SK, Suh N, Luo Y, Kosmeder J, Luyengi L, Fong HH, Kinghorn AD, Moriarty RM, Mehta RG, Constantinou A, Monn RC, Pezzuto JM. Rotenoids mediatepotent cancer chemopreventive activity through transcriptional regulation of ornithine decarboxylase. Nat Med. 1995;1:260–6. doi: 10.1038/nm0395-260. [DOI] [PubMed] [Google Scholar]
- 8.Fang N, Casida JE. Anticancer action of cubé insecticide: correlation for rotenoid constituents between inhibition of NADH:ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc Natl Acad Sci USA. 1998;95:3380–4. doi: 10.1073/pnas.95.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhauser C, Lee SK, Kosmeder JW, Moriarty RM, Hamel E, Mehta RG, Moon RC, Pezzuto JM. Regulation of ornithine decarboxylase induction by deguelin, a natural product cancer chemopreventive agent. Cancer Res. 1997;57:3429–35. [PubMed] [Google Scholar]
- 10.Chun KH, Kosmeder JW, 2nd, Sun S, Pezzuto JM, Lotan R, Hong WK, Lee HY. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in pre-malignant human bronchial epithelial cells. J Natl Cancer Inst. 2003;95:291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- 11.Lee HY. Molecular mechanisms of deguelin-induced apoptosis in transformed human bronchial epithelial cells. Biochem Pharmacol. 2004;68:1119–24. doi: 10.1016/j.bcp.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Lee HY, Suh YA, Kosmeder JW, Pezzuto JM, Hong WK, Kurie JM. Deguelin-induced inhibition of cyclooxygenase-2 expression in human bronchial epithelial cells. Clin Cancer Res. 2004;10:1074–9. doi: 10.1158/1078-0432.ccr-0833-3. [DOI] [PubMed] [Google Scholar]
- 13.Lee HY, Oh SH, Woo JK, Kim WY, Van Pelt CS, Price RE, Cody D, Tran H, Pezzuto JM, Moriarty RM, Hong WK. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J Natl Cancer Inst. 2005;97:1695–9. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 14.Oh SH, Woo JK, Yazici YD, Myers JN, Kim WY, Jin Q, Hong SS, Park HJ, Suh YG, Kim KW, Hong WK, Lee HY. Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J Natl Cancer Inst. 2007;99:949–61. doi: 10.1093/jnci/djm007. [DOI] [PubMed] [Google Scholar]
- 15.Oh SH, Woo JK, Jin Q, Kang HJ, Jeong JW, Kim KW, Hong WK, Lee HY. Identification of novel antiangiogenic anticancer activities of deguelin targeting hypoxia-inducible factor-1 alpha. Int J Cancer. 2008;122:5–14. doi: 10.1002/ijc.23075. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Kaufman PL, Gao G, Saunders RA, Ma JX. Intravitreal injection of plasminogen kringle 5, an endogenous angiogenic inhibitor, arrests retinal neovascularization in rats. Diabetologia. 2001;44:757–65. doi: 10.1007/s001250051685. [DOI] [PubMed] [Google Scholar]
- 17.Bae MK, Ahn MY, Jeong JW, Bae MH, Lee YM, Bae SK, Park JW, Kim KR, Kim KW. Jab1 interacts directly with HIF-1alpha and regulates its stability. J Biol Chem. 2002;277:9–12. doi: 10.1074/jbc.C100442200. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228:630–42. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Kim JH, Yu YS, Min BH, Kim KW. The role of clusterin in retinal development and in free radical damage. Br J Ophthalmol. 2007;91:1541–6. doi: 10.1136/bjo.2007.115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly SE, Hores TA, Smith LE, D'Amore PA. Characterization of vascular development in the mouse retina. Microvasc Res. 1988;36:275–90. doi: 10.1016/0026-2862(88)90028-3. [DOI] [PubMed] [Google Scholar]
- 21.Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–14. [PubMed] [Google Scholar]
- 22.Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–5. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]


