Abstract
Bone-forming cells are known to be coupled by gap junctions, formed primarily by connexin43 (Cx43). The role of Cx43 in osteoclasts has so far only been studied in rodents, where Cx43 is important for fusion of mononuclear precursors to osteoclasts. Given the potential importance for human diseases with pathologically altered osteoclasts, we asked whether a similar influence of Cx43 can also be observed in osteoclasts of human origin. For this purpose, Cx43 mRNA expression was studied in a time course experiment of human osteoclast differentiation by RT-PCR. Localization of Cx43 in these cells was determined by immunohistochemistry and confocal microscopy. For the assessment of the effect of gap junction inhibition on cell fusion, gap junctions were blocked with heptanol during differentiation of the cells and the cells were then evaluated for multinuclearity. Paraffin sections of healthy bone and bone from patients with Paget's disease and giant cell tumour of the bone were used to study Cx43 expression in vivo. We found mRNA and protein expression of Cx43 in fully differentiated osteoclasts as well as in precursor cells. This expression decreased in the course of differentiation. Consistently, we found a lower expression of Cx43 in osteoclasts than in bone marrow precursor cells in the histology of healthy human bone. Blockade of gap junctional communication by heptanol led to a dose-dependent decrease in multinuclearity, suggesting that gap junctional communication precedes cell fusion of human osteoclasts. Indeed, we found a particularly strong expression of Cx43 in the giant osteoclasts of patients with Paget's disease and giant cell tumour of the bone. These results show that gap junctional communication is important for fusion of human mononuclear precursor cells to osteoclasts and that gap junctional Cx43 might play a role in the regulation of size and multinuclearity of human osteoclasts in vivo.
Keywords: human osteoclasts, gap junction, connexin, Cx43, cell fusion
Introduction
Bone-forming cells are known to be coupled by gap junctions formed primarily by connexin43 (Cx43) and to a lesser degree by connexin 45 (Cx45) [1–4]. It has been shown that expression of Cx43 in osteoblasts, osteoclasts and osteocytes is highly responsive to mechanical loading, suggesting a role of gap junctions in mechanical signal transduction of bone [5, 6]. Gap junctional channels allow direct intercellular communication via diffusion of ions, metabolites and small soluble molecules (up to 1 kD in size), including Ca2+, cAMP, adenosine-5'-triphosphate (ATP) and inositol (1,4,5)-triphosphate [7]. The role of Cx43 was also studied by targeted deletion of the gene in mice [8]. Cx43-deficient mice display a skeletal phenotype characterized by retarded intramembranous and enchondral bone formation and craniofacial abnormalities, delayed tooth eruption and osteoblast dysfunction [8]. The role of Cx43 for osteoblastic bone formation has been further studied in detail by osteoblast-specific Cx43 gene deletion, showing that functional Cx43 is required for normal bone mass acquisition and maintenance and is involved in the mechanism of action of parathyroid hormone (PTH)-induced anabolism [9, 10]. Unfortunately, skeletal remodelling could not be studied in this model because all Cx43-deficient mice died shortly after birth due to major heart malformation. Therefore, the role of Cx43 in osteoclasts has so far only been studied in rodent bone marrow cell cultures [11, 12]. Results from these studies suggest that Cx43 acts downstream of RANK/RANKL interaction in osteoclast precursors and is crucial for mediating their fusion and differentiation to active osteoclasts. Given the potential importance of these findings for the pathogenesis of human bone loss diseases accompanied by pathologically enlarged osteoclasts, we asked whether a similar influence of Cx43 can also be observed in osteoclasts of human origin.
Materials and methods
Reagents
Standard laboratory chemicals were from Merck (Darmstadt, Germany) and Sigma (Deisenhofen, Germany). Na-heparin was obtained from Roche (Mannheim, Germany). Ficoll-Paque came from Amersham Pharmacia Biotech (Uppsala, Sweden). α-minimal essential medium (MEM), foetal bovine serum (FBS), penicillin-streptomycin-solution, recombinant human macrophage colony-stimulating factor (M-CSF) and trypsin (10x) were purchased from Sigma (Deisenhofen, Germany). Phosphate buffered saline (PBS) was from GIBCO BRL, Life Technologies Inc. (Rockville, MD, USA). Soluble recombinant human receptor-activator for NfcB Ligand (RANKL) came from Peprotech Ltd. (London, U.K.). Anti-Cx43 antibody came from Sigma (Deisenhofen, Germany). Rhodamine-phalloidine and secondary fluorescent antibodies were obtained from Molecular Probes (Leiden, The Netherlands). Fluorsave came from DAKO Diagnostika (Hamburg Germany). Heptanol came from Fluka (now Sigma-Aldrich, Switzerland).
Dentin
Dentin (ivory) was kindly provided by German customs in accordance with the international laws for the protection of species.
Media, solutions, buffer
Cells were cultivated in α-MEM containing 0.22% sodium bicarbonate, 10% FBS and 1% penicillin/streptomycin, 20 ng/ml M-CSF and 40 ng/ml RANKL. Fixation of cells was done in PBS containing 3.7% formaldehyde. For trypsination α-MEM was used containing 0.22 g sodium carbonate, 10% Trypsin-ethylenediaminetetraacetic acid (EDTA) (10x) and 1% penicillin/streptomycin. PBS was used as immunochemistry solution containing 0.1% bovine serum albumin (BSA) and 0.05% saponin. For the blocking solution, 150 μl of serum of the host of the secondary antibody were added to 2.85 ml immunochemistry solution. TRAP buffer was a solution with 40 mM sodium acetate and 10 mM sodium tartrate. TRAP staining solution was 5 mg naphtol AS-MX phosphate, 500 μl N-N-dimethylformamide and 30 mg fast red violet LB salt in 50 ml TRAP-buffer. Heptanol was dissolved in ethanol as described previously [13] so that the final concentration in the cell culture medium equalled 0.15% ethanol with 0 mM, 0.3 mM, 3 mM and 30 mM heptanol, respectively. As heptanol is volatile and known to cause only a reversible inhibition, it was given to the cell cultures every day from day 1 to day 28. Additionally, we also performed all experiments with a water control to rule out an independent effect of the ethanol. There was no difference between water and ethanol / 0 mM heptanol group in any of the experiments, so for the sake of easier readability of the figures we only included the ethanol control.
RT-PCR analysis
Total RNA was isolated from cultured human osteoclasts following the TRIzol-protocol (Invitrogen, Karlsruhe, Germany). cDNA synthesis from 1 |xg of total RNA was performed with the cDNA-CYCLE-Kit (Invitrogen) according to the manufacturer's instructions. The obtained cDNA was dissolved in a total volume of 100 μl of sterile water. To normalize the cDNA amount in the samples, 3 μl of the resulting cDNA of each probe was used for PCR analysis of the housekeeping gene β-actin. PCR reactions were performed with 27 cycles. Products were detected in ethidium bromide (0.1%)-stained 1.0% agarose gels and cDNA volumes were adjusted for consecutive analyses by densitometry (Quantity One, Bio-Rad, Munich, Germany). Programs and primers for the measurement of steady-state levels of mRNA were as follows. 4 min. at 94°C, 27 cycles (β-actin) / 36 cycles (Connexin 43 [Cx43], carbonic anhydrase type 2[CA-2]) 30 sec. at 94°C, 45 sec. a primer specific temperature (Cx43 55,9°C; CA-2 56.0°C; β-actin 60.0°C) and 45 sec. at 72°C, with an additional 5 min. at 72°C for the final extension in the end.
Primers:
Cx43 sense 5’-TCAAGCCTACTCAACTGCTGGAG-3’ anti-sense 5’-CCCTCGCATTTTCACCTTACC-3’ 406 nt product.
CA-2: sense 5’-GGACAAGGTTCAGAGCATACTGTGG -3’ anti-sense 5’-ACATTCCAGAAGAGGAGGGGTG-3’ 312 nt product.
β-actin: sense 5’-GCACCACACCTTCTACAATGAGC-3’ anti-sense 5’-AATGTCACGCACGATTTCCCGC-3’ 379 nt product
All PCR reactions were performed in an iCycler (Bio-Rad).
Primers and PCR-chemicals
Small DNA low-melt agarose came from Biozym Diagnostik GmbH (Oldendorf, Germany). Recombinant Taq DNA Polymerase and 10× PCR buffer were from Invitrogen. DNeasy Tissue Kit was obtained from Qiagen (Hilden, Germany). 100 bp DNA-ladder came from Biowhittaker Molecular Applications (Vallensbaek Strand, Denmark). 25mM dNTP-solution was bought from Biozym Diagnostik GmbH (Oldendorf, Germany). Primers were bought from Invitrogen.
Isolation of haematopoietic stem cells and cell culture
Human mononuclear cells were isolated and cultured essentially as previously described [14]. Briefly, buffy coats were obtained from healthy volunteers and were generously provided by Dr. P. Kühnl (Department for Transfusion Medicine, Hamburg University School of Medicine, Germany). Density gradient centrifugation with Ficoll-Paque was used to separate the mononuclear osteoclast precursor cells from other formed elements in blood.
To dispose the culture of contaminating lymphocytes, the cells were purified for adherence. The non-adherent cells were washed off at day 1 of culture, so that only the adherent monocytes were used for cultivation. All cells were cultivated in α-MEM containing 0.22% sodium bicarbonate, 10% FBS and 1% penicillin/streptomycin and 20 ng/ml M-CSF for 28 days. For the generation of osteoclasts 40 ng/ml RANKL was added to the culture medium.
Pit assay on dentin
Dentin was cut to a thickness of 1 mm. For the assessment of resorption, cells were cultivated for 28 days on dentin. At the end of the culture period, cells were washed off with PBS. The dentin chips were then shortly rinsed in H2O2 to remove residual cell components, and then washed again in PBS. Pits were stained with toluidine blue taking advantage of the fact that the resorption process uncovers the embedded collagen fibres.
TRAP-staining and cytomorphometry
Cells were fixed for 5 min. in 3.7% buffered formaldehyde and air-dried for 2 min. The cells were stained in TRAP-staining solution for 10 min. For the determination of cell numbers and nuclei per cell, we counted the cells in five randomly assigned independent fields of view, using a Zeiss Axioskop microscope (Carl Zeiss AG, Hamburg, Germany) at a magnification of 200×. Cells with more than three nuclei were counted as multinuclear while cells with only one nucleus were counted as mononuclear cells. For easier readability, the mean value of the control group was set to 100% and all values were then normalized to this number.
Immunofluorescence and confocal laser-microscopy
For actin staining, cells on microscopic cover slips were dehydrated in -20°C acetone for 5 min. and air-dried on parafilm. They were then incubated in rhodamine-phalloidin 568, diluted in PBS 1:40 for 20 min. in the dark. For double labelling, additional steps were also performed in the dark to prevent bleaching. Cover slips were rinsed in PBS and then incubated for 20 min. in blocking solution. The primary antibody (rabbit against human Cx43; Sigma C6219) was diluted 1:500 in PBS and the cells were incubated in it for 120 min. Then the cover slips were washed 3×5 min. in immunochemistry solution. The secondary antibody (Alexa Fluor 488) was diluted 1:200 and was used to incubate the cells for 60 min. After 3×5 min. of washing, the cells were mounted with Fluosave and stored overnight at 4°C.
Immunohistochemistry on human bone sections
A collection of transiliac bone biopsies from non-genotyped patients with various disorders was established at the Institute of Pathology at the Hamburg University over the last 30 years. For immunohistochemical staining, bones of three healthy patients, two patients with Paget's disease and two patients with giant cell tumour of the bone were decalcified in 4% EDTA, embedded in paraffin and cut in 5 μm thick sections using a Microtec rotation microtome (Techno-Med, Munich, Germany). The sections were then deparaffinized with xylol, ethanol and water and stained for Cx43 with the following protocol: 5 min. PBS, 30 min. 0.05% trypsin 2×5 min. PBS, 30 min. peroxidase-blocking reagent (DAKO), 3×5 min. washing in PBS, 30 min. 2.5% BSA in PBS, overnight primary rabbit-anti-human-Cx43 antibody (Sigma C6219) in 1% BSA in PBS (1:3000), 3 × 5 min. washing with PBS, 30 min. secondary antibody (anti-rabbit IgG, biotynilated, 1:200), 3×5 min. washing in PBS, 7 min. developing in DAB (DAKO) 10 min. aqua dest., 1 min. haematoxylin, 10 min. water.
Digital imaging
For confocal microscopic analysis we used an Olympus confocal laser microscope (Olympus I × 70) with a combined argon/krypton laser at 488 nm and 568 nm wavelengths. The pits on dentin and the cells in cell culture were visualized on a Zeiss Axiovert 25 microscope and then digitally photographed with a Canon 10D digital camera. The cells on cover slips were photographed on a Zeiss Axioskop microscope using a Zeiss Axiocam digital camera.
Statistics
All cell culture experiments were done with groups of at least n = 5 and were repeated at least twice. Statistical analysis was done using anova with Bonferroni post-hoc test and with Pearson's correlation. P > 0.05 was accepted as statistically significant. Error bars represent standard deviation (SD).
Results
Cx43 expression in human osteoclasts and precursor cells
First we studied the mRNA expression of Cx43 by semi-quantitative RT-PCR in a time course experiment of osteoclast differentiation. We started with mononuclear precursors from buffy coats (Fig.1A) and stimulated the cells over 28 days with RANKL and M-CSF, when fully differentiated osteoclasts were observed (Fig.1B) which had the ability to resorb dentin (Fig.1C) [14]. As internal control of the differentiation process, we determined the expression of CA-2 at day 1 and after 3, 14 and 28 days. As expected, we found a time-dependent up-regulation of CA-2 in our cultures, suggesting progressive osteoclast differentiation (Fig.1D). When we looked at Cx43 expression at the same time-points, we found a strong expression of Cx43 in the early phase of differentiation to osteoclasts and a subsequent down-regulation in the later stages (Fig.1D). When we performed the same cultures only with M-CSF, without the addition of RANKL, Cx43 expression was completely depressed already at day 3 of culture (Fig.1E), suggesting that one of the effects how RANKL leads to osteoclast differentiation might be by prolonging expression of Cx43. This observation prompted us to study protein expression and localization of Cx43 in the cultures using an anti-human Cx43 antibody. To visualize the cytoskeleton, double staining with rhodamine-phalloidin was performed. Indeed we found strong expression of Cx43 in some, but not all of the freshly isolated mononuclear cells at day 1 of cell culture, even before the addition of RANKL (Fig.2A–C). When we sectioned these cells with the confocal microscope at different planes (Fig.2A bottom of cells and Fig.2C top of cells), we saw that Cx43 was distributed throughout the whole cell. Next, we looked at Cx43 protein expression at day 28 of cell culture in presence of M-CSF and RANKL. The staining in the precursors with fewer than three nuclei was much stronger than in the fully differentiated multinucleated osteoclasts at day 28, confirming our results from the RT-PCR on a protein level (Fig.2D and E). Strong staining with the typical punctuate distribution of gap junction channels was observed at cell-cell contacts between some of the multinucleated cells with mononuclear precursors, which were localized on top of them (arrows Fig.2F and higher magnification Fig.2G).
Figure 1.
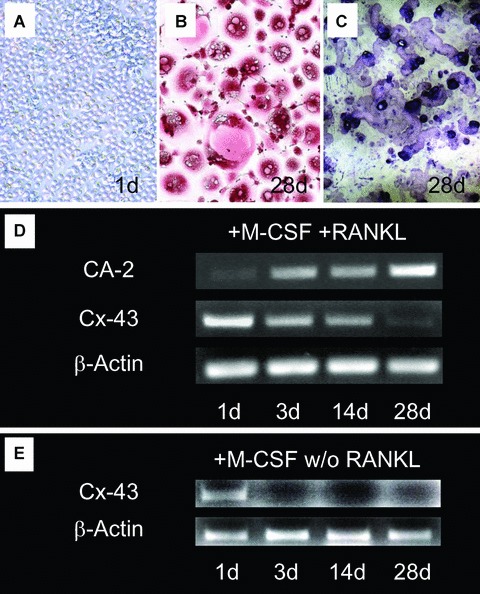
Generation of human osteo-clasts from mononuclear precursor cells (A; day 1; 100×) to multinu-cleated, TRAP+ cells (B; day 28; red: TRAP staining; 100×) with the ability to resorb dentin (C; day 28; toluidine blue staining; 100x). Time course of mRNA expression of carbonic anhydrase type 2 (CA-2) and connexin 43 (Cx43) over 28 days of culture in presence of macrophage-colony stimulating factor (M-CSF) and RANKL (D). A progressive increase of CA-2 indicates differentiation of the cells to active osteoclasts. Expression of Cx43 progressively decreases in the course of differentiation (semi-quantitative RT-PCR, normalized to β-actin as housekeeping gene). (E) Cultivation without the addition of RANKL leads to loss of Cx43 signal already at day 3 (semi-quantitative RT-PCR, normalized to β-actin as housekeeping gene).
Figure 2.
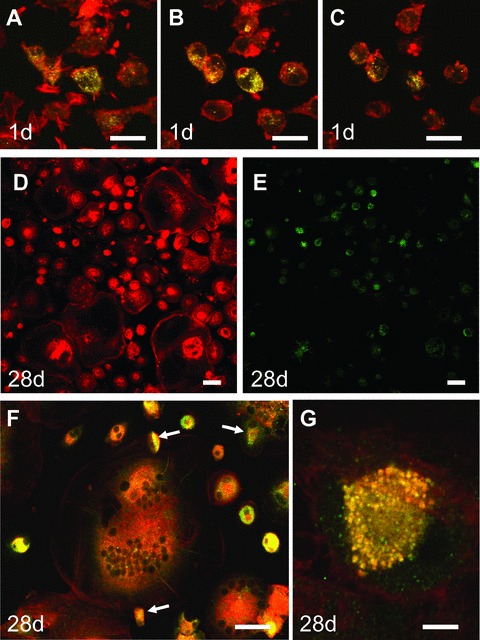
(A-C) Connexin 43 (Cx43) expression in mononuclear precursor cells at day 1 of cell culture before addition of M-CSF and RANKL (A: bottom, C: top). (D and E) Overview over the cell culture treated for 28 days with M-CSF and RANKL showing lower expression of Cx43 in fully differentiated osteoclasts with actin rings. (F) Cx43 staining in multinuclear osteoclasts and osteoclast precursors is localized between osteoclasts and mononuclear precursors on top of the cell (arrows) and within the cytoplasm. (G) High magnification of cell-cell contact between osteoclast and mononuclear precursor on top of the osteoclast shows the typical punctuate distribution of gap junctions (con-focal microscopy, red: actin, green: Cx43, yellow: double-labelling, scale bar = 100 μm [A–E]; 50 μm [F], 10 μm [G]).
Gap junctional influence on cell number and multinuclearity of human osteoclasts
To further study the effect of gap junctional communication on the differentiation of human osteoclasts, we blocked the gap junctions with heptanol (Fig.3). There was no significant effect of heptanol treatment on the number of cells in our cultures (data not shown) or the number of TRAP positive (TRAP+) cells (Fig.3C). We also did not observe obvious differences in dead or disaggregated cells, ruling out a toxic effect of the used concentrations. We found significantly fewer nuclei per TRAP+ cell in cultures treated with 3 mM (P = 0.001 versus control / P = 0.045 versus 0.3 mM) or 30 mM heptanol (P> 0.001 versus control / P = 0.01 versus 0.3 mM) (Fig.3D). There was a small, but significant negative correlation between heptanol concentration and the number of nuclei per cell (r = 0.372; P > 0.001). There was also a significant negative correlation of the concentration of heptanol in the cell culture with the number of TRAP+ multinuclear cells (r =−0.674; P > 0.001); however the number of TRAP+ multinuclear cells only was significantly decreased at a concentration of 30 mM heptanol (P = 0.003 versus control; P = 0.008 versus 0.3 mM) (Fig.3E). There was a significant positive correlation of heptanol concentration with the number of TRAP+ mononuclear cells (r = 0.615; P = 0.001). We found a significant increase of these cells at a concentration of 3 mM when compared to 0.3 mM (P = 0.036) and a significant increase at a concentration of 30 mM when compared to control or 0.3 mM (P = 0.01 and P = 0.005, respectively) (Fig.3F). Additionally, we found a significant negative correlation with between Trap+ multinuclear and Trap+ mononuclear cells (r =−0.568; P = 0.002). Taken together, these data suggest a dose-dependant effect of heptanol treatment on multin-ucleation of the cells.
Figure 3.
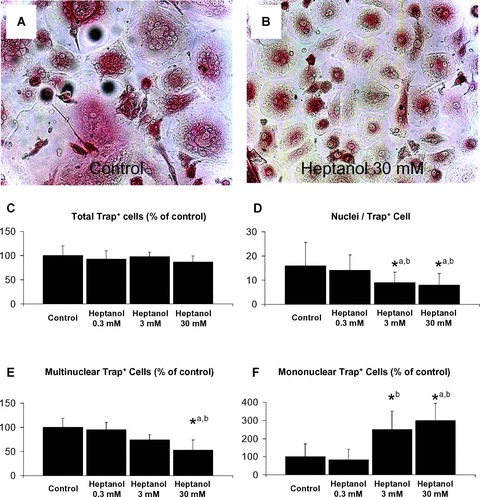
(A/B) Inhibition of gap junctions leads to a significant decrease of multinuclear Trap+ cells and nuclei / Trap+ cells after 28 days of culture (light microscopy, red: Trap-staining; (A) control, (B) 30 mM heptanol, magnification 200×). This difference is not caused by cytotoxic effects of heptanol, as the total number of Trap+ cells is unchanged (C). There is a significant dose-dependant effect on nuclei / Trap+ cells (D), number of multinuclear Trap+ cells (E) and number of mononuclear Trap+ cells (F) (*P < 0.05; a: versus control; b: versus heptanol 0.3 mM).
Expression of Cx43 in human bone
To study, if the observed down-regulation of Cx43 expression in the course of differentiation in vitro can also be found in vivo, we first analysed osteoclasts in sections of human bone from healthy donors. Indeed, we found stronger staining intensity in mononuclear cells of the bone marrow (Fig.4A: BMCs) than in the osteoclasts (Fig.4A: OC, Fig. S1: arrows), confirming our in vitro results. However, in sections of patients with giant cell tumour of bone (Fig.4B) and Paget's disease (Fig.4C); we observed a very strong staining for Cx43, especially in the pathologically enlarged osteoclasts.
Figure 4.
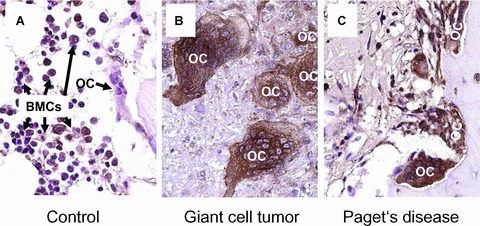
Expression of Cx43 (brown staining) in normal bone (A), giant cell tumour of the bone (B) and Paget's disease (C). While the expression in normal osteoclasts (A; OC, Fig. S1: arrows) is detectably lower than in the surrounding bone marrow precursor cells (A; BMCs: bone marrow cells), there is a strong expression of Cx43 in pathologically enlarged osteoclasts (B, C; OC).
Discussion
We report here the expression of Cx43 in human osteoclasts and osteoclast precursor cells in vitro and in vivo. So far, Cx43 expression has only been studied in fully differentiated osteoclasts of rodents [11–13, 15]. To analyse the expression of Cx43 in the course of differentiation to active osteoclasts, we used a culture system that allowed the generation of fully differentiated osteoclasts with the ability to resorb dentin. To monitor the progressive differentiation of the cells to osteoclasts, we used the expression of CA-2 which has been shown to play an important role in osteoclastic bone resorption, since patients with an inactivating mutation of CA-2 develop osteopetrosis [16]. When we looked at mRNA expression of Cx43 in this model, we found a time-dependent decrease in the course of differentiation. Immunostaining of human osteoclasts with Cx43 antibodies revealed the localization of Cx43 throughout the cells at the start of culture and in cell–cell contacts and in the cytoplasm of osteoclasts. Similar findings were reported for osteoclasts from mice [11, 13]. Consistent with the mRNA expression results, we found the strongest staining for Cx43 in osteoclast precursors. It has been suggested that gap junctions may be necessary for the fusion of mononuclear precursor cells to osteoclasts [13, 17] and that it acts downstream of RANKL. Indeed, when we cultivated our precursor cells without the addition of RANKL, a necessary molecule for the generation of osteoclasts in vitro[18], we found a complete down-regulation of Cx43 mRNA already at day 3 of culture, while there was still Cx43 detectable at day 28 in the cultures with addition of RANKL. These results suggest that the differentiation to osteoclast may be associated with gap junctional communication. To further analyse this association, we blocked gap junctions with different concentrations of heptanol, which is a commonly used inhibitor of gap junctional communication [13, 19–21]. Heptanol leads to a decreased probability of open gap junctions [22]. In contrast to a previous report in a mouse bone marrow culture system [13], we could not detect a toxic effect of 30 mM heptanol in our cultures. We see two possible explanations for this discrepancy. First, human cells may be able to tolerate higher concentrations of heptanol. This might be due to their longer differentiation time (6 days in mice versus 28 days in human cells) and the volatility of heptanol. Indeed, when we treated the cells with heptanol every other day instead of every day we did not even observe an effect with 30 mM heptanol (data not shown). Second, the mouse bone marrow cell culture system also always includes some degree of contamination by osteoblasts and other cells from mesenchymal origin, while our cell culture system is purely based on mononuclear precursor cells from the haematopoietic system. As it is known that there is a strong influence of gap junctions on osteoblasts and osteocytes [1, 3, 8] and the regulation of osteoclastogene-sis by bone marrow stromal cells [11], an indirect effect mediated by these cells might be explanatory. We found a dose-dependent decrease of multinuclear TRAP+ cells and a parallel increase of mononuclear TRAP+ cells upon treatment with heptanol. These findings may be interpreted as inhibited fusion of mononuclear cells in the face of reduced gap junctional communication and are in line with similar data reported for the mouse model [13, 23]. In the light of this interpretation, the observed decrease of Cx43 upon differentiation in our cultures would lead to a declining probability of new fusion events. This might be an explanation for the self-limitation of the size of osteoclasts and the number of nuclei per osteoclast in healthy bone. In this case, a missing down-regulation of Cx43 in the course of differentiation to osteoclasts should lead to giant osteoclasts with an increased number of nuclei. Indeed, we found a very strong expression of Cx43 in the giant osteoclasts from patients with Paget's disease and patients with giant cell tumour of the bone, further supporting our hypothesis. It may be worthwhile to perform osteoclast differentiation experiments with precursor cells from patients suffering from Paget's disease and giant cell tumour of the bone in the future, to better characterize the role of gap junctional communication in these diseases.
Taken together, our results show a function of gap junctional communication for the fusion of human mononuclear cells to osteoclasts and suggest a possible explanation for the regulation of size and multinuclearity of human osteoclasts in vivo.
Supporting Information
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
References
- 1.Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993;91:1888–96. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Hick E, Veenstra RD, Wang HZ, Warlow PM, Westphale EM, Laing JG. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J. 1994;13:744–50. doi: 10.1002/j.1460-2075.1994.tb06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donahue HJ, Mcleod KJ, Rubin CT, Andersen J, Grine EA, Hertzberg EL, Brink PR. Cell-to-cell communication in osteoblastic networks: cell line-dependent hormonal regulation of gap junction function. J Bone Miner Res. 1995;10:881–9. doi: 10.1002/jbmr.5650100609. [DOI] [PubMed] [Google Scholar]
- 4.Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–62. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su M, Borke JL, Donahue HJ, LI Z, Warshawsky NM, Russell CM, Lewis JE. Expression of connexin 43 in rat mandibu-lar bone and periodontal ligament (Pdl) cells during experimental tooth movement. J Dent Res. 1997;76:1357–66. doi: 10.1177/00220345970760070501. [DOI] [PubMed] [Google Scholar]
- 6.Gluhak-Heinrich J, GU S, Pavlin D, Jiang JX. Mechanical loading stimulates expression of connexin 43 in alveolar bone cells in the tooth movement model. Cell Commun Adhes. 2006;13:115–25. doi: 10.1080/15419060600634619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeager M, Unger VM, Falk MM. Synthesis, assembly and structure of gap junction intercellular channels. Curr Opin Struct Biol. 1998;8:517–24. doi: 10.1016/s0959-440x(98)80131-0. [DOI] [PubMed] [Google Scholar]
- 8.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–44. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro CH, Stains JP, Sheikh S, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Development of mice with osteoblast-specific connexin43 gene deletion. Cell Commun Adhes. 2003;10:445–50. doi: 10.1080/cac.10.4-6.445.450. [DOI] [PubMed] [Google Scholar]
- 10.Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–98. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 11.Matemba SF, Lie A, Ransjo M. Regulation of osteoclastogenesis by gap junction communication. J Cell Biochem. 2006;99:528–37. doi: 10.1002/jcb.20866. [DOI] [PubMed] [Google Scholar]
- 12.Ilvesaro J, Tavi P, Tuukkanen J. Connexin-mimetic peptide gap 27 decreases osteo-clastic activity. Bmc Musculoskelet Disord. 2001;2:10. doi: 10.1186/1471-2474-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilvesaro J, Vaananen K, Tuukkanen J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J Bone Miner Res. 2000;15:919–26. doi: 10.1359/jbmr.2000.15.5.919. [DOI] [PubMed] [Google Scholar]
- 14.Schilling AF, Linhart W, Filke S, Gebauer M, Schinke T, Rueger JM, Amling M. Resorbability of bone substitute biomaterials by human osteoclasts. Biomaterials. 2004;25:3963–72. doi: 10.1016/j.biomaterials.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 15.Ransjo M, Sahli J, Lie A. Expression of connexin 43 mrna in microisolated murine osteoclasts and regulation of bone resorption in vitro by gap junction inhibitors. Biochem Biophys Res Commun. 2003;303:1179–85. doi: 10.1016/s0006-291x(03)00502-3. [DOI] [PubMed] [Google Scholar]
- 16.Sly WS, Hewett-Emmett D, Whyte MP, YU YS, Tashian RE. Carbonic anhydrase ii deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci USA. 1983;80:2752–6. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SJ, Boyde A. Questions of quality and quantity–a morphological view of bone biology. Kaibogaku Zasshi. 1994;69:229–43. [PubMed] [Google Scholar]
- 18.Buckley KA, Fraser WD. Receptor activator for nuclear factor kappab ligand and osteoprotegerin: regulators of bone physiology and immune responses/potential therapeutic agents and biochemical markers. Ann Clin Biochem. 2002;39:551–6. doi: 10.1177/000456320203900602. [DOI] [PubMed] [Google Scholar]
- 19.Miura T, Yano T, Naitoh K, Nishihara M, Miki T, Tanno M, Shimamoto K. {Delta}-opioid receptor activation before ischemia reduces gap junction permeability in ischemic myocardium by pkc-{epsilon}-mediated phosphorylation of connexin-43. Am J Physiol Heart Circ Physiol. 2007;293:1425–31. doi: 10.1152/ajpheart.01115.2006. [DOI] [PubMed] [Google Scholar]
- 20.Mao HJ, Chen BP, Ren GY, Jin JS, Fan FY, Gao Q, Bruce I, Xia Q. The effects of heptanol on electrical coupling during ischemia in the perfused isolated rat heart. Conf Proc Ieee Eng Med Biol Soc. 2005;1:122–5. doi: 10.1109/IEMBS.2005.1616357. [DOI] [PubMed] [Google Scholar]
- 21.Palani D, Manchanda R. Effect of heptanol on noradrenaline-induced contractions in rat vas deferens. J Smooth Muscle Res. 2006;42:49–61. doi: 10.1540/jsmr.42.49. [DOI] [PubMed] [Google Scholar]
- 22.Takens-Kwak BR, Jongsma HJ, Rook MB, Van Ginneken AC. Mechanism of heptanol-induced uncoupling of cardiac gap junctions: a perforated patch-clamp study. Am J Physiol. 1992;262:1531–8. doi: 10.1152/ajpcell.1992.262.6.C1531. [DOI] [PubMed] [Google Scholar]
- 23.Ilvesaro J, Tuukkanen J. Gap-junctional regulation of osteoclast function. Crit Rev Eukaryot Gene Expr. 2003;13:133–46. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


