Abstract
IL-24, a member of the IL-10 family of cytokines, is produced by monocytes and Th2 cells. Interestingly, immune cells do not appear to express specific IL-24 receptor chains (IL-20R1/IL-20R2 and IL-22R/IL-20R2), it is therefore unlikely that IL-24 has classical immune-modulating properties. Skin, on the other hand, seems to represent a major target tissue for IL-24 and related cytokines such as IL-19, -20, and -22. However, the initial interest in IL-24 did not arise from its physiological signalling properties through its cognate receptors but rather because of its tentative ability to selectively kill different cancer cells. In an attempt to further investigate the signalling events underlying the IL-24-induced cancer cell death, we found that melanoma cell lines did not react in the expected and previously described way. Using several different forms and delivery modes of IL-24, we were unable to detect any apoptosis-inducing properties of this cytokine in melanoma cells. In the present ‘Point of view’ we will briefly summarizse these findings and put them in context of published reports stating that IL-24 might be a long sought after treatment for several types of cancer.
Keywords: IL-24, cytokine, melanoma, cancer treatment
Background
Soon after its discovery in 1995, IL-24 (then called mda-7) has been described as a potential therapeutic option for cancer (reviewed in [1–4]). First thought to be a tumour suppressor, Zhang et al. [5] clarified already in 2000 that mda-7/IL-24 is in fact a secreted protein, further substantiated by the identification of the specific receptor complexes [6, 7], which led to the re-naming of mda-7 to IL-24 cytokine. Nevertheless, around 100 publications, the majority of which come from three groups at Columbia University, the M.D. Anderson Cancer Center in Texas and Introgen Therapeutics, have suggested that IL-24 has the ability to selectively and efficiently kill cancer cells in vitro and in vivo. These astonishing properties led to the rapid development of a therapeutic regimen by which a modified adenovirus delivers IL-24 to cancer patients (INGN-241, Introgen Therapeutics, Inc., Houston, TX, USA). Initial results of clinical trials, which commenced in the year 2000, were released in 2004. Although these reports claimed low toxicity in preclinical and phase 1 studies [8] and some efficacy in malignant melanoma therapy in a phase 2 trial [9], further reports on clinical developments around IL-24 are lacking thus far. According to information released on Introgens’ homepage (http://www.introgen.com), INGN-241 is still being tested in a phase 3 clinical study in head and neck cancer patients as well as in phase 2 studies in melanoma and other solid tumours. After the completion of these trials, it will have to be evaluated whether IL-24, delivered by a modified and replication-incompetent adenovirus (INGN-241) can live up to the high expectations that have been raised in the past [2, 3, 10].
IL-24: a normal cytokine?
IL-24, together with IL-10, -19, -20, -22, -26, -28, and -29, are members of the group of IL-10-like cytokines. IL-19, IL-20, IL-22, and IL-24 have been proposed to form a unique IL-19 subfamily characterized by their structural features and aggregation state as monomers [11–13]. Although sequence homologies within this group do not exceed 25%, there is some conservation of structural features, namely the presence of six to seven α-helices in all family members [11, 13, 14]. Another common denominator of this group is the sharing of receptor chains, best exemplified by IL-24 and IL-20, which can both bind to the same two receptor complexes:
IL-20R1/IL-20R2 and/or IL-22R/IL-20R2 [6]. The biological functions and structural characteristics of individual members of the IL-10-like cytokine group have been reviewed in more detail elsewhere [11, 13–15]. In brief, physiological properties that involve engagement of the respective receptor complexes and subsequent signalling through the Jak/STAT pathway, range from triggering anti-inflammatory responses (IL-10) and possibly antiviral responses (IL-28, and -29) to pro-inflammatory reactions (IL-22). Functions of IL-19, -20, and -24 are less well defined but several reports indicate pro-inflammatory [16] roles in diseased skin, which is the major target tissue for these cytokines [14, 17–20]. In this context, it has recently been shown by microarray analysis on human keratinocytes that IL -24 similar to the other IL-10-like cytokines up-regulates a set of inflammatory and immune-modulating genes that is characteristic of expression patterns seen in psioriatic skin lesions [21]. Together with elevated expression levels of IL-19, -20, -22, and IL-24 in psoriasis [22], this indicates that these cytokines may play an important role in the proliferation and differentiation of keratinocytes suggestive of an involvement in the immunopathology of psoriasis and other inflammatory skin diseases. Taken together, accumulating evidence is pointing towards a physiological role of IL-24 (and related cytokines) in the regulation of immune and inflammatory responses of skin tissue.
IL-24: a potential cure for cancer?
IL-24 has been discovered by subtraction hybridization, a method that involves the reversal of de-differentiated cancer-like growth characteristics by treatment of cells with IFN-β and a protein kinase C activator [23]. Compared to untreated melanoma cells, genes that were up-regulated during the reversal phase towards a more benign growth behaviour were hoped to play an active role in preventing or stopping cancer cells from outgrowing their healthy counterparts [24–26]. One of the candidates found to be augmented during enforced terminal differentiation of melanoma cells was IL-24, then called mda-7 (melanoma differentiation antigen 7). Shortly after its discovery, two lines of evidence suggested that IL-24 may indeed be a promising cytokine to be studied further as a cancer therapeutic agent. First, IL-24 reduced colony numbers when transiently transfected into melanoma cells while healthy cells remained unaffected by such a treatment [23, 27] and secondly, IL-24 was found to be down-regulated with progression of melanomas in vivo[25]. This study, however, solely relied on immunohistochemistry for scoring IL-24 expression, and to our knowledge, it has remained the only report thus far showing in vivo down-regulation of IL-24 with progression of cancer. The down-regulation of a gene with cancer progression together with properties such as loss of heterozygosity (LOH) and germline mutations, would make IL-24 a tumour suppressor gene. However, Wang and Liang have already clarified in 2003 [28] that there is no convincing evidence to this day for IL-24 to be considered a tumour suppressor.
Despite the already mentioned advanced stages of clinical testing, many fundamental questions regarding the physiological and ‘supra-physiological’ properties of IL-24 remain open or controversial. For instance, conflicting data regarding IL-24 produced from stably transfected Hek cells exist. Some reports claim that Hek-IL-24 efficiently kills cancer cells [29–32] while others were unable to alter the growth characteristics of cancer cells with Hek-IL-24 [33–35]. Similarly, opposing data can also be found concerning the ability of bacterially expressed GST-IL-24 to drive cancer cells into apoptosis. Some studies reported that GST-IL-24 can kill prostate, breast, renal, and pancreatic cancer cell lines [36–38] while we and others have not detected these effects of bacterially expressed IL-24 [33, 39, 40]. Another enigma around IL-24 is its ability to distinguish between healthy and cancer cells. How different forms of recombinant IL-24 can reliably differentiate between a normal and a cancer cell remains completely elusive. Finally, IL-24 secreted from cancer cells infected with an adenovirus encoding IL-24 has been described to bind to receptors on neighbouring cells. On surrounding non-cancer cells, this interaction activates the Jak/STAT pathway without any cytotoxic effects. On receptor-positive cancer cells, however, the binding of IL-24 triggered apoptosis of melanoma and other cancer cells, independent of Jak/STAT signalling, an effect that which has been termed ‘bystander effect’[10, 39]. Taken together, IL-24 has been suggested to distinguish between cancer and normal cells and by doing so it can kill the cancer cells in a receptor-independent or -dependent fashion, either through direct interaction or via secondary bystander effects, respectively. In this context, we recently found that the melanoma cell lines used in the above study (Wm35, Mewo, and A375) [39] hardly expressed IL-24 receptors and consequently did not show phosphorylation of STAT3 following IL-24 treatment [33]. Hence, the receptor-mediated killing of these cells by IL-24 or possible bystander effects are difficult to reconcile with the fact that sufficient amounts of the three possible receptor chains were not present on such cells. In the same study, we further investigated different forms and modes of IL-24 delivery to melanoma cells but could not identify any condition under which this cytokine killed melanoma cells [33]. To substantiate our data, we have used four different ways of treating a panel of melanoma cell lines with IL-24: (1) by GST-IL-24 fusion protein (previously shown to kill cancer cells [36]); (2) by IL-24 secreted from transfected Hek cells, also previously published to drive cancer cells selectively and efficiently into apoptosis [39]. Thirdly, we have used intracellular over-expression, as we reckoned that a higher threshold amount of IL-24 might be necessary to trigger the unfolded protein response (UPR) pathway, which drives target cells into apoptosis [41] and finally, we have applied various amounts of commercial IL-24. None of the above forms and delivery regimens killed cancer cells more than appropriate control treatments. Taken together, we neither saw a selective ability of IL-24 itself to distinguish between healthy and cancer cells, nor did we detect IL-24-induced killing of melanoma cells. Therefore, we assume that only the combination of IL-24 with an adenoviral backbone might have the beneficial and desired selective cancer killing effects. (Fig.1) illustrates and summarizes the effects that the described different forms and delivery modes of IL-24 might have.
Figure 1.
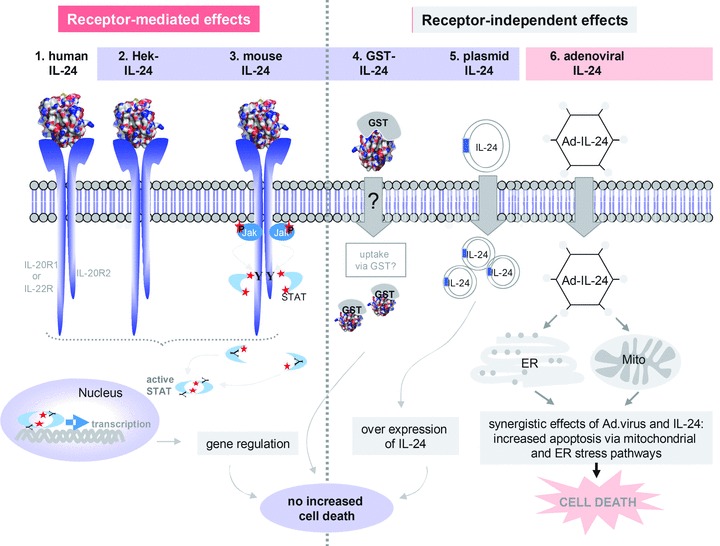
Summary of possible IL-24-induced effects. The figure depicts schematically our view of the effects that different sources and delivery modes of IL-24 can have on receptor-positive or -negative cells. The left side of the scheme shows receptor-mediated activation of the Jak/STAT pathway and subsequent regulation of STAT target genes on receptor-positive (IL-20R1/IL-20R2 and/or IL-22R/IL-20R2) cells by different forms of IL-24: 1. human IL-24: human endogenous IL-24, 2. Hek-IL-24: human IL-24 expressed from Hek cells, 3. mouse IL-24: commercially available human IL-24 expressed from mouse cells. On the right- hand side receptor-independent effects are shown: 4. GST-IL-24: recombinant fusion protein expressed from bacteria, 5. plasmid IL-24: plasmid-encoded IL-24, over-expressed following nucleofection, 6. Ad-IL-24: IL-24, with or without signal peptide, transduced by modified adenoviruses. Only IL-24 in combination with an adenoviral expression backbone may kill target melanoma or other cancer cells while intracellular overexpression of IL-24 or any other kind of IL-24 on its own does not lead to increased cell death. In this context, we have recently shown the lack of apoptosis-inducing effects of different forms of IL-24 (light blue box, no. 2–5) on melanoma cells.
Adenovirus: contributions to IL-24 oncolytic effects?
In order to improve IL-24 delivery to cancer cells, various replication-deficient adenoviruses have been developed that all aim at selectively transducing target cells. Third generation adenoviruses driven by promoters, which only function in cancer cells have boosted the selectivity of IL-24 delivery even further [42, 43]. Over the past decade, many studies have claimed that intracellular over-expression of an adenovirus encoded IL-24 (Ad-IL-24) drives a multitude of different cancer cells and types into apoptosis or regression, respectively. There is evidence that the combination of IL-24 with an adenoviral backbone has the desired oncolytic effects while the empty vector control does not [44–46], however, a useful but still lacking control for the specificity of IL-24 and for synergistic effects of the Ad backbone would be an adenovirus expressing IL-20, as IL-20 is a related cytokine that uses the same receptor complexes.
High doses of Ad vectors lead to a rapid initiation of acute inflammatory responses by activating the innate immune system. Many pro-inflammatory cytokines such as IL-6, -8, -12, TNF-a, IFN-γ and others are dose-dependently secreted following transduction with Ad virus and this might be advantageous in evoking a rapid oncolytic response following IL-24 or any other gene therapy [47]. Hence, it is very possible that the adenoviral infection itself may lead to a local up-regulation of cancer-cytotoxic proteins such as interferons, death receptor ligands, and possibly the IL-24 receptor chains. Increased amounts of cognate receptors may be necessary to set off and augment the effects of the IL-24 secreted by transduced cells on surrounding cells (bystander effect). Therefore, it can be speculated that the adenoviral backbone triggers an initial immune response, which may result in up-regulation of pro-inflammatory cytokines and possibly IL-24 receptors. The largely over-expressed IL-24 might then cause unfolded protein (UPR) and ER stress responses, which in synergy with the adenoviral priming of the immune system, could drive the targeted cancer cells into apoptosis. Interestingly and depending on cancer cell types, Ad-IL-24 appears to activate a multitude of distinct signalling pathways, which can converge in an ER stress response eliciting cell death: (1) p38MAPK signalling, which results in up-regulation of growth arrest and DNA damage inducible genes (GADD) in melanoma and other cancer cells [48], (2) PKR activation in non-small cell lung carcinomas (NSCLC), (3) JNK signalling cascades in prostate, NSCLC, and glioma cells, (4) activation of the Fas/FasL pathway in ovarian cancer cells, and other pathways (reviewed in [10]). Interestingly, a recent study described opposing effects for IL-24, sharply contradicting the above cited effects on p38MAPK signalling: rather than inducing selective cancer cell death, IL-24-mediated activation of p38MAPK led to enhanced survival of malignant chronic lymphocytic leukaemia cells [49]. Even though the transduction of cancer cells with Ad-IL-24 can affect many different signalling pathways, the apoptosis-inducing properties of the recombinant virus are independent of classic receptor-driven Jak/STAT signalling, which seems to exclusively mediate the physiological properties of IL-24.
Whether the above- described different scenarios will indeed lead to an efficient IL-24 gene therapy in future will have to await the outcome of the ongoing clinical trials. However, upon analysis of the presently available and at times conflicting and questionable data, it remains to be shown whether IL-24 will ever qualify to be called the ‘magic bullet against cancer’.
References
- 1.Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–37. [PubMed] [Google Scholar]
- 2.Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, Nemunaitis J, Cunningham C, Yacoub A, Dent P, Fisher PB. mda-7/IL-24: exploiting cancer's achilles Achilles’ heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, Yang HY, Sahin AA, Hunt KK, Fuson KL, Poindexter N, Roth JA, Ramesh R, Grimm EA, Mhashilkar AM. MDA-7/IL-24 is a unique cytokine-tumor suppressor in the IL-10 family. Int Immunopharmacol. 2004;4:649–67. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, Fisher PB. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111:596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R, Tan Z, Liang P. Identification of a novel ligand-receptor pair constitutively activated by ras oncogenes. J Biol Chem. 2000;275:24436–43. doi: 10.1074/jbc.M001958200. [DOI] [PubMed] [Google Scholar]
- 6.Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–9. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Tan Z, Zhang R, Kotenko SV, Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277:7341–7. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–59. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J, Merritt J, Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–72. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–38. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 11.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 12.Zdanov A. Structural features of the interleukin-10 family of cytokines. Curr Pharm Des. 2004;10:3873–84. doi: 10.2174/1381612043382602. [DOI] [PubMed] [Google Scholar]
- 13.Zdanov A. Structural studies of the interleukin-19 subfamily of cytokines. Vitamins and hormones. 2006;74:61–76. doi: 10.1016/S0083-6729(06)74003-1. [DOI] [PubMed] [Google Scholar]
- 14.Renauld JC. Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat Rev Immunol. 2003;3:667–76. doi: 10.1038/nri1153. [DOI] [PubMed] [Google Scholar]
- 15.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 16.Sabat R, Sterry W, Philipp S, Wolk K. Three decades of psoriasis research: where has it led us. Clinics in Dermatology. 2007;25:504–9. doi: 10.1016/j.clindermatol.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, Haugen H, Jelinek L, Kelly JD, Madden K, Maurer MF, Parrish-Novak J, Prunkard D, Sexson S, Sprecher C, Waggie K, West J, Whitmore TE, Yao L, Kuechle MK, Dale BA, Chandrasekher YA. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 18.Otkjaer K, Kragballe K, Funding AT, Clausen JT, Noerby PL, Steiniche T, Iversen L. The dynamics of gene expression of interleukin-19 and interleukin-20 and their receptors in psoriasis. Br J Dermatol. 2005;153:911–8. doi: 10.1111/j.1365-2133.2005.06800.x. [DOI] [PubMed] [Google Scholar]
- 19.Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol. 2006;15:991–1004. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 20.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 21.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–40. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 22.Romer J, Hasselager E, Norby PL, Steiniche T, Thorn Clausen J, Kragballe K. Epidermal overexpression of interleukin-19 and -20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine a or calcipotriol. J Invest Dermatol. 2003;121:1306–11. doi: 10.1111/j.1523-1747.2003.12626.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–86. [PubMed] [Google Scholar]
- 24.Madireddi MT, Su ZZ, Young CS, Goldstein NI, Fisher PB. Mda-7, a novel melanoma differentiation associated gene with promise for cancer gene therapy. Adv Exp Med Biol. 2000;465:239–61. doi: 10.1007/0-306-46817-4_22. [DOI] [PubMed] [Google Scholar]
- 25.Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, Grimm EA. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol. 2002;20:1069–74. doi: 10.1200/JCO.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- 26.Madireddi MT, Dent P, Fisher PB. Regulation of mda-7 gene expression during human melanoma differentiation. Oncogene. 2000;19:1362–8. doi: 10.1038/sj.onc.1203424. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci U S A. 1996;93:9160–5. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Liang P. Interleukin-24 and its receptors. Immunology. 2005;114:166–70. doi: 10.1111/j.1365-2567.2005.02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng M, Bocangel D, Doneske B, Mhashilkar A, Ramesh R, Hunt KK, Ekmekcioglu S, Sutton RB, Poindexter N, Grimm EA, Chada S. Human interleukin 24 (MDA-7/IL-24) protein kills breast cancer cells via the IL-20 receptor and is antagonized by IL-10. Cancer Immunol Immunother. 2006;56:205–15. doi: 10.1007/s00262-006-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekmekcioglu S, Ellerhorst JA, Mumm JB, Zheng M, Broemeling L, Prieto VG, Stewart AL, Mhashilkar AM, Chada S, Grimm EA. Negative association of melanoma differentiation-associated gene (mda-7) and inducible nitric oxide synthase (iNOS) in human melanoma: MDA-7 regulates iNOS expression in melanoma cells. Mol Cancer Ther. 2003;2:9–17. [PubMed] [Google Scholar]
- 31.Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, Dent P, Fisher PB. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–66. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- 32.Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, Zheng M. mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Mol Ther. 2005;11:724–33. doi: 10.1016/j.ymthe.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Kreis S, Philippidou D, Margue C, Rolvering C, Haan C, Dumoutier L, Renauld JC, Behrmann I. Recombinant interleukin-24 lacks apoptosis-inducing properties in melanoma cells. PLoS ONE. 2007;2:e1300. doi: 10.1371/journal.pone.0001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S, Grimm EA. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 35.Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L, Grimm EA, Renauld JC, Kotenko S, Chada S. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–13. [PubMed] [Google Scholar]
- 36.Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, Dent P, Fisher PB. Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analysed via a bacterial fusion protein. Oncogene. 2004;23:7679–90. doi: 10.1038/sj.onc.1207958. [DOI] [PubMed] [Google Scholar]
- 37.Sauane M, Lebedeva IV, Su ZZ, Choo HT, Randolph A, Valerie K, Dent P, Gopalkrishnan RV, Fisher PB. Melanoma differentiation associated gene-7/inter-leukin-24 promotes tumor cell-specific apoptosis through both secretory and nonsecretory pathways. Cancer Res. 2004;64:2988–93. doi: 10.1158/0008-5472.can-04-0200. [DOI] [PubMed] [Google Scholar]
- 38.Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, Linehan WM, Su ZS, Sarkar D, Lebedeva IV, Valerie K, Gopalkrishnan RV, Grant S, Fisher PB, Dent P. MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Mol Cancer Ther. 2003;2:623–32. [PubMed] [Google Scholar]
- 39.Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, Zheng M, Grimm EA, Ekmekcioglu S. Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10:1085–95. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Sieger KA, Mhashilkar AM, Stewart A, Sutton RB, Strube RW, Chen SY, Pataer A, Swisher SG, Grimm EA, Ramesh R, Chada S. The tumor suppressor activity of MDA-7/IL-24 is mediated by intracellular protein expression in NSCLC cells. Mol Ther. 2004;9:355–67. doi: 10.1016/j.ymthe.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biology. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, Dent P, Curiel DT, Fisher PB. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007;67:5434–42. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci USA. 2005;102:14034–9. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708–18. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- 45.Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, Pestka S, Fisher PB. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14:35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 46.Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R, Barral P, Lee SG, Wang D, Vozhilla N, Park ES, Chatman L, Boukerche H, Ramesh R, Inoue S, Chada S, Li R, De Pass AL, Mahasreshti PJ, Dmitriev IP, Curiel DT, Yacoub A, Grant S, Dent P, Senzer N, Nemunaitis JJ, Fisher PB. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review) Int J Oncol. 2007;31:985–1007. [PubMed] [Google Scholar]
- 47.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Current Gene Therapy. 2006;6:215–26. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA. 2002;99:10054–9. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sainz-Perez A, Gary-Gouy H, Portier A, Davi F, Merle-Beral H, Galanaud P, Dalloul A. High Mda-7 expression promotes malignant cell survival and p38 MAP kinase activation in chronic lymphocytic leukemia. Leukemia. 2006;20:498–504. doi: 10.1038/sj.leu.2404073. [DOI] [PubMed] [Google Scholar]


