Abstract
Mutations in or ablation of the gene encoding caveolin-3, a protein of muscle cell caveolae, result in forms of muscular dystrophy and cardiomyopathy. Another member of the caveolin gene family, caveolin-1, is widely considered not to be expressed in myocytes, yet ablation of the gene encoding this protein in mice also results in cardiomyopathy. By applying the high-resolution electron-microscopical imaging technique of freeze-fracture replica immunolabelling, we report here evidence that caveolin-1 is expressed in human cardiac myocytes, localized to both caveolae and non-caveolar domains in the plasma membrane. Disorders of the myocyte resulting from defects in caveolin-1 may thus arise directly, at the level of the myocyte, rather than via other cell types as previously proposed.
Keywords: freeze-fracture electron microscopy, immunogold cytochemistry, caveolin-1, caveolin-3, cardiomyocytes
Caveolins are the structural proteins of invaginated plasma membrane domains termed caveolae, which function in signal transduction, lipid regulation and mechanosensing [1]. Three members of the caveolin gene family have been identified, caveolins-1 and -2, which are highly expressed in non-muscle cells such as endothelial cells and fibroblasts, and caveolin-3, which is muscle specific. Mutations in the caveolin-3 gene in human beings have been identified in disorders both of skeletal muscle (e.g. limb girdle muscular dystrophy and rippling muscle disease) and of the heart as a form of familial hypertrophic cardiomyopathy [2, 3]. Knockout of the caveolin-3 gene in mice correspondingly results in hypertrophic cardiomyopathy [4]. Intriguingly, however, the phenotype of the caveolin-1 knockout also includes hypertrophic cardiomyopathy and heart failure, despite the widely held view that caveolin-1 is not expressed in cardiomyocytes [5–7]. Thus, current explanations for caveolin-1 ablation-induced cardiomyopathy invoke mechanisms that involve non-myocyte types in the heart, in particular activation of the Ras-p42/44 Mitogen-Activated-Protein (MAP) kinase cascade in fibroblasts and of nitric oxide synthase in endothelial cells [6, 7].
The widely held assumption that caveolin-1 is not expressed in cardiomyocytes comes from biochemical analysis of tissue lysates and imaging of tissue sections by immunofluorescence light microscopy. The former technique provides averaged data for all the cell types within a tissue, which in the heart will include cardiomyocytes, endothelial cells, fibroblasts, pericytes, smooth muscle cells and neurons. Although immunofluorescence microscopy does permit discrimination between cell types, unequivocal conclusions are often hindered by its relatively low resolution, which may make it difficult to ascribe label between closely apposed cells, and by the technical vagaries of applying immunofluorescence labelling techniques to chemically fixed samples.
Here, we report the localization of caveolin-1 in human cardiomyocyte plasma membranes using the high-resolution imaging technique of freeze-fracture electron microscopy combined with immunogold labelling. This technique involves splitting membranes at low temperature to give extensive planar views of the membrane interior, replicating the membrane's structural detail by vacuum deposition of platinum and carbon, and then, after removing all but a molecular layer of the tissue, applying antibody labelling techniques with minute electron-dense gold particles to localize caveolin molecules. Because the heart tissue is stabilized by a purely physical means (i.e. rapid freezing), chemical fixation, with its attendant adverse effect on epitope preservation and ability to label caveolins, is avoided. Full descriptions of the methodology have been reported elsewhere [8].
Figure 1 shows the localization of caveolin-3 by this technique. The curving grey expanse in the top half of the image shows an en face view of the plasma membrane of a cardiomyocyte. This view (termed a ‘P face’) is of the half-membrane leaflet of the plasma membrane that remains attached to the protoplasm of the cell after freeze fracture, the membrane half in which caveolins reside. The abundant small black dots are the gold label marking sites at which caveolin-3 is detected. Beneath the cardiomyocyte, the fracture plane has crossed the extracellular space and through a capillary endothelial cell, revealing views of its plasma membrane on the tissue front and luminal side. The endothelial membranes show no caveolin-3 labelling above background level. The differential patterns of labelling for caveolin-3 between the myocyte and the endothelial cell are thus precisely as predicted from existing studies.
Figure 1.
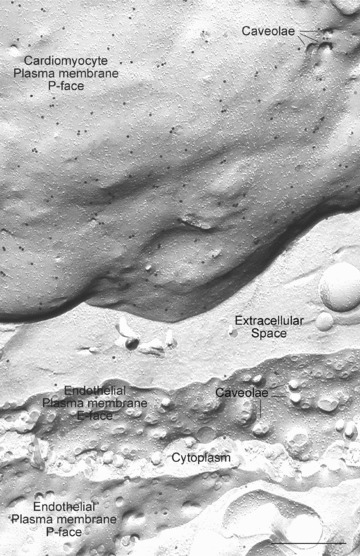
Image of a cardiomyocyte plasma membrane and an adjacent capillary endothelial cell prepared by freeze-fracture electron microscopy with immunogold labelling for caveolin-3. In freeze fracture, membranes are split along the centre of the lipid bilayer to give two half-membrane leaflets which are viewed en face. The half-membrane leaflet left attached to the protoplasm of the cell is termed the P half; that left attached to the extracellular space is termed the E half. The images of these two leaflets viewed in the electron microscope are termed the P face and E face, respectively. The expanse of curving plasma membrane in the top half of the picture is a P-face view of a cardiomyocyte plasma membrane. This essentially means that the observer is looking down on the cell, at its plasma membrane, after the outermost half membrane leaflet (E half) has been fractured away. The protoplasm of the cell lies immediately beneath the membrane leaflet viewed. The numerous black dots are the caveolin-3 label. Beneath the cardiomyocyte, the fracture plane crosses the extracellular space to reveal a capillary endothelial cell in the lower half of the image. After splitting the endothelial plasma membrane on the tissue front (seen in E-face view), the fracture crosses the cytoplasm of the endothelial cell to reveal an expanse of luminal plasma membrane in P-face view. There is negligible label for caveoilin-3 in the endothelial plasma membranes. This example comes from rat left ventricle; similar results are found for human ventricular myocytes. The antibody used here was a mouse monoclonal anti-caveolin-3 (BD Transduction Laboratories; #610420, Heidelberg, Germany), detected with goat antimouse 18-nm gold complexes. Scale Bar: 0.5 μm.
With caveolin-1, however, not only does the endothelial cell plasma membrane show an abundance of label in keeping with the established findings (not shown) but label is also consistently and reproducibly visualized on the human cardiomyocyte plasma membrane, as illustrated in Fig.2. The cardiomyocyte plasma membranes are readily identified in these examples because the fracture plane, after exposing expanses of the P face, cross fractures into the adjacent cytoplasm, revealing characteristic myofilament arrays. The label for caveolin-1 is abundantly distributed in the membrane, and often aggregated at the rims of caveolae (Fig.2 inset).
Figure 2.
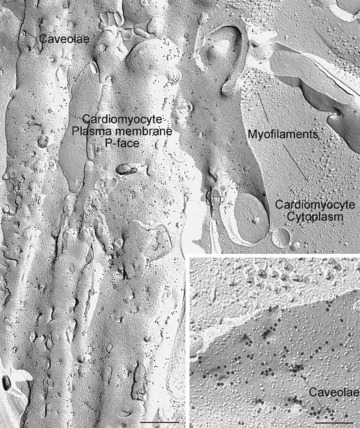
Image of a human cardiomyocyte plasma membrane prepared by freeze-fracture electron microscopy with immunogold labelling for caveolin-1. Extensive caveolin-1 label is seen covering the plasma membrane (P face). To the right, the fracture has penetrated into the cell's cytoplasm revealing bundles of myofilaments, confirming the identity of the cell as a cardiomyocyte. Inset shows at higher magnification that the caveolin-1 label occurs as rings at the necks of caveolae, as well as in intervening areas. The caveolae appear as circular indentations in the membrane because the fracture path skips across the caveolar necks where the membrane curves downwards to form the flask-shaped invagination. From human left ventricular myocardium using mouse monoclonal anti-caveolin-1 (BD Transduction Laboratories; catalogue #610406, clone # 2297) detected with goat antimouse 18-nm gold complexes. The specificity of the primary antibody for caveolin-1 and its lack of cross reactivity with caveolin-3 have previously been documented [15]. Sample from the ventricular apex of a patient with dilated cardiomyopathy given a left ventricular assist device. Scale bar main image: 0.5 μm; inset 0.2 μm.
This high-resolution imaging technique has never been applied previously to the detection and localization of caveolins in non-chemically fixed human cardiac muscle. Our findings stand in marked contrast to explicit statements that caveolin-1 in not expressed in muscle cells [5, 6, 9], though it should be noted that some authors are more circumspect, implying without explicitly stating that muscle cells lack caveolin-1 [1, 10, 11], and a few studies, in keeping with the results presented here, have concluded that caveolin-1 may in fact be co-expressed with caveolin-3, at least in some types of myocyte of some species [12–14]. In all immunolabelling studies, an awareness of the possibility of cross-reactivity of antibodies to closely related proteins is essential, and the conflicting data on caveolin-1 localization would benefit from re-appraisal with this in mind. Nevertheless, the present findings raise the possibility that the mechanisms by which car-diomyopathies may arise from mutations in or ablation of the caveolin-1 gene could reside, at least in some instances, in direct disruption of function in the myocyte itself, rather than indirectly via other cells types as previously supposed [6, 7].
Acknowledgments
We thank Karin Schlattmann and Christina Köppler for competent and indispensable technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 492) and the British Heart Foundation (PG/08/048/25093).
References
- 1.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–94. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 2.McNally EM, de Sa Moreira E, Duggan DJ, Bonneman CG, Lisanti MP, Lidov HG, Vainzof M, Passos-Bueno MR, Hoffman EP, Zatz M, Kunkel LM. Caveolin-3 in muscular dsystrophy. Hum Mol Genet. 1998;7:871–7. doi: 10.1093/hmg/7.5.871. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi T, Arimura T, Ueda K, Shibata H, Hohda S, Takahashi M, Hori H, Koga Y, Oka N, Imaizumi T, Yasunami M, Kimura A. Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2004;313:178–84. doi: 10.1016/j.bbrc.2003.11.101. [DOI] [PubMed] [Google Scholar]
- 4.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 5.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Nat Acad Sci USA. 2002;99:11375–80. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, Pereira DS, Kitsis RN, Russell RG, Weiss LM, Tang B, Jelicks LA, Factor SM, Shtutin V, Tanowitz HB, Lisanti MP. Caveolin-1 null mice develop cardiac hyptertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–74. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 7.Wunderlich C, Schober K, Lange SA, Drab M, Braun-Dullaeus RC, Kasper M, Schwencke C, Schmeisser A, Strasser RH. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340:702–8. doi: 10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto K. SDS-digested freeze-fracture replica labeling electron microscopy to study the two-dimensional distribution of integral membrane proteins and phospholipids in biomembrane: practical procedure, interpretation and application. Histochem Cell Biol. 1997;107:87–96. doi: 10.1007/s004180050092. [DOI] [PubMed] [Google Scholar]
- 9.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–79. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 10.Aravamudan B, Volonte D, Ramani R, Gursoy E, Lisanti MP, London B, Galbiati F. Transgenic overexpression of caveolin-3 in the heart induces a cardiomyopathic phenotype. Hum Mol Genet. 2003;12:2777–88. doi: 10.1093/hmg/ddg313. [DOI] [PubMed] [Google Scholar]
- 11.Ratajczak P, Damy T, Heymes C, Oliviero P, Marotte F, Robidel E, Sercombe R, Boczkowski J, Rappaport L, Samuel JL. Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc Res. 2003;57:358–69. doi: 10.1016/s0008-6363(02)00660-0. [DOI] [PubMed] [Google Scholar]
- 12.Hagiwara Y, Nishini Y, Yorifuji H, Kikuchi T. Immunolocalization of caveolin-1 and caveolin-3 in monkey skeletal, cardiac and uterine smooth muscles. Cell Struct Funct. 2002;27:375–82. doi: 10.1247/csf.27.375. [DOI] [PubMed] [Google Scholar]
- 13.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenyl cyclase signaling components. J Biol Chem. 2006;281:26391–9. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 14.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21:1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 15.Volonte D, McTiernan CF, Drab M, Kasper M, Galbiati F. Caveolin-1 and caveolin-3 form heterooligomeric complexes in atrial cardiac myocytes that are required for doxorubicin-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;294:H392–401. doi: 10.1152/ajpheart.01039.2007. [DOI] [PubMed] [Google Scholar]


