Abstract
The development of an efficient vaccine against high-risk HPV types can reduce the incidence rates of cervical cancer by generating anti-tumor protective responses. Traditionally, the majority of prophylactic viral vaccines are composed of live, attenuated or inactivated viruses. Among them, the design of an effective and low-cost vaccine is critical. Inactivated vaccines especially heat-killed yeast cells have emerged as a promising approach for generating antigen-specific immunotherapy. Recent studies have indicated that yeast cell wall components possess adjuvant activities. Moreover, a non-pathogenic protozoan, Leishmania tarentolae (L.tar) has attracted a great attention as a live candidate vaccine. In current study, immunological and protective efficacy of whole recombinant killed Pichia pastoris and Leishmania tarentolae expressing HPV16 L1 capsid protein was evaluated in tumor mice model. We found that Pichia-L1, L.tar-L1 and Gardasil groups increase the IgG2a/IgG1 ratio, indicating a relative preference for the induction of Th1 immune responses. Furthermore, subcutaneous injection of killed Pichia-L1 generated the significant L1-specific IFN-γ immune response as well as the best protective effects in vaccinated mice as compared to killed L.tar-L1, killed Pichia pastoris, killed L.tar and PBS groups. Indeed, whole recombinant Leishmania tarentolae could not protect mice against C3 tumor mice model. These data suggest that Pichia-L1 may be a candidate for the control of HPV infections.
Keywords: cervical cancer, human papillomavirus, killed vaccine, Leishmania tarentolae expression system, L1 capsid protein, Pichia pastoris expression system
Abbreviations
- HPV
human papillomaviruses
- GFP
green fluorescent protein
- FACS
fluorescence-activated cell sorting
- KBMA
killed but metabolicallyactive
- AOX1
alcohol oxidase I gene
- Yeast-HBsAg
yeast expressing hepatitis B surface antigen
- L.tar
Leishmania tarentolae
- DAB
3,3′-diaminobenzidine
- rL1
recombinant L1
- 2-ME
mercaptoethanol
- ConA
concanavalin A
- SD
standard deviation
Introduction
Human papillomaviruses (HPVs) are responsible for genital warts in both women and men, especially cervical carcinoma in women.1 Considering the high prevalence of HPV infection in women and the lack of anti-viral agents against HPV, the development of a prophylactic HPV vaccine has been an important strategy to prevent cervical cancer.2 However, the design of an effective and low-cost vaccine is critical. Currently, many successful prophylactic viral vaccines have been developed. These vaccines are often based on live, attenuated or inactivated (/ killed) viruses. The killed vaccines are made as whole cell vaccines (such as Influenza, polio, rabies and hepatitis A) or as subunit vaccines.3 Advantages of killed vaccines include: a) No reversion to virulence; b) Safe in pregnant animals; c) Safe in immuno-compromised animals and d) No shedding of the virus. However, 2 doses are often needed for initial response by killed vaccines. Killed antigens generally require an adjuvant for induction of a strong Th1 immune response in murine models.4 Recent studies have suggested that yeast cell wall components possess adjuvant activities.5 The reports have demonstrated that vaccination with saccharomyces cerevisiae expressing tumor-associated antigens can induce antigen-specific T-cell responses and protect animals against tumor challenge. Furthermore, heat-killed recombinant S. cerevisiae shows no toxicity in clinical studies.6,7 However, there are some restrictions in S. cerevisiae expression systems including a) hyperglycosylation of recombinant proteins, which is allergenic and b) limitation in various carbon sources utilized by this species.8 Recently, the Pichia pastoris system has been suggested for heterologous protein expression, because of the powerful genetic techniques, high expression levels, rapid growth rate and well-established fermentation technology, along with its economy of use.9 Subsequently, a novel protein expression system was also developed based on non-pathogenic Leishmania tarentolae, a protozoan parasite of lizards. This system allows not only easy handling like E. coli and yeast, but also full eukaryotic protein folding and the mammalian-type post-translational modification of target proteins.10-12 To date, several heterologous proteins (intracellular or secreted mode) have been expressed in L. tarentolae such as Amylase, Cu/Zn Superoxide dismutase, Erythropoietin, Green Fluorescent Protein (GFP), HIV-1 gag, Interferon-γ, Laminin-322, Luciferase, Red Fluorescent Protein, Proprotein convertase 4, Retinoic acid receptor γ, T7 RNA polymerase, Tissue plasminogen activator, G-protein coupled receptor 56.11 Currently, killed Leishmania vaccines have been tested against human cutaneous and visceral leishmaniasis.13-15 In addition, some studies indicated that the recombinant Leishmania tarentolae live vector was capable of eliciting effective humoral and cellular immune responses in mice.16,17
In the present study, we evaluated and compared the ability of 2 killed vaccines (Pichia pastoris and Leishmania tarentolae strains expressing high levels of full length HPV16 L1, termed Pichia-L1 and L.tar-L1, respectively) to induce B- and T-cell mediated immune responses and to eradicate C3 tumor cells in vivo. In both systems, the expression cassettes including L1 gene (pLEXSY-L1 and pPICZB-L1) were integrated in Leishmania and Pichia genome through homologous recombination. 9,16-19 Our results indicated that whole recombinant Pichia-L1 and L.tar-L1 enhance L1-specific antibody titers (IgG1 and IgG2a) and the IgG2a/IgG1 ratio in mice. Furthermore, Pichia-L1 stimulated the secretion of L1-specific IFN-γ from mouse splenocytes as compared to other groups. Indeed, Pichia-L1 could significantly elicit the protective effects against C3 tumor mice model in comparison with L.tar-L1 vaccinated group. These data suggest the possibility of developing whole killed Pichia pastoris expressing L1 as an effective vaccine to control HPV infections.
Results
In vitro pEGFP-L1 delivery using PEI
For confirmation of the L1 expression in vitro, PEI 25 kDa was used as a transfection reagent. GFP fluorescence was observed in cells that received 5 μg of pEGFP-L1 plasmid. The transfection efficiency using different concentrations of PEI (NrE = 5, 7, 10) was almost similar. The percentage of L1-GFP expressing cells was ∼71.5% as compared to negative control (∼2.29%) using flow cytometry.
Development of the recombinant L. tarentolae strain expressing HPV16 L1 protein
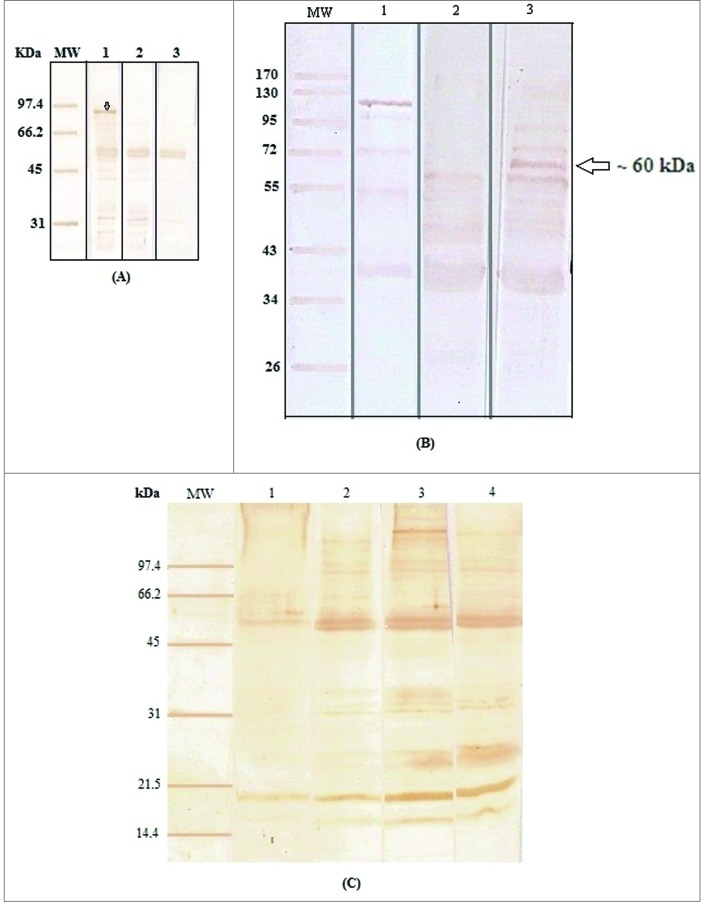
To generate L. tarentolae expressing L1-GFP protein, the 2265 bp fragment encoding the L1-GFP was cloned into pLEXSY-neo2. Then, the linearized recombinant plasmid was electroporated into wild type L. tarentolae. The transformants were selected by plating on solid medium containing G418. Integration of gene in the genomic DNA of the recombinant cells was confirmed by PCR analysis. The expected 1 kb band was only observed from transformed cells indicating the correct integration of expression construct into the ssu locus. The expected PCR products of L1 and L1-GFP were appeared as ∼1515 bp and ∼2265 bp fragment for L1-GFP positive clones, respectively. To verify mRNA synthesis, RT-PCR was performed on total mRNA extracted from wild and transgenic cells. The desired bands for L1 and L1-GFP were obtained in recombinant Leishmania promastigotes (data not shown). In addition, the expression of L1-GFP using GFP was monitored by Epi-fluorescent microscopy and flow cytometry. The expression of genes was readily evident from the intense green fluorescence of the parasites. Fluorescence-activated cell sorting (FACS) analysis indicated a clear quantitative separation between transfected and wild type parasites. L1-GFP expression in transgenic L. tarentolae was detectable in 65% of the cells as compared to GFP expression in L.tar-GFP (∼98.7%). The stability of protein expression was monitored over a period of 4 months post-electroporation (data not shown). Furthermore, L1 expression was also detectable in cell extracts of transgenic parasites compared to wild type by western blotting. As shown in Figure 1A, the dominant band of ∼82 kDa was detected in transgenic parasites expressing L1-GFP. No such corresponding band was revealed in the wild-type promastigotes.
Figure 1.
A) Detection of L1-GFP protein in the recombinant Leishmania by western blotting. Western blot analysis using an anti-L1 antibody showed high levels of expression of the full-length L1-GFP protein (∼82 kDa, line 1) in contrast with L.tar-GFP (line 2) and wild type L.tar (line 3). These data were obtained using anti-GFP antibody, while GFP was detected at 27 kDa for L.tar-GFP as a positive control; (B) Identification of HPV16 L1 expressed in Pichia-L1 by protein gel blotting. line 1: yeast vector sample without L1-specific band; line 2: Before induction of Pichia-L1; line 3: After induction of Pichia-L1 (∼72 h); (C) Killed promastigotes of L. tarentolae in stationary phase (day 7): Line 1: autoclaved; Line 2: heat-killed in 60°C; Line 3: suspension in formalin and Line 4: freeze-thawed parasites. MW: Protein molecular weight marker.
Expression of HPV16 L1 protein in Pichia pastoris under the control of the AOX promoter
To generate Pichia pastoris expressing L1 protein, the 1543 bp fragment encoding L1 was cloned into pPICZ-B. Then, the recombinant pPICZB-L1 was linearized and electroporated into GS115 yeast strain. The transformants were selected by plating on solid medium containing 500 μg/ml zeocin. Integration of genes in the genomic DNA of the recombinant cells was confirmed by PCR analysis using AOX primers. The expected 2100 bp band was only observed in transgenic cells indicating the correct integration of expression construct into the genome in contrast with negative clones (∼600 bp). In addition, a 55 kDa L1 protein (+5 kDa His-tag) was detected in the lysates of Pichia pastoris strain using protein gel blotting. L1 band was not detected in negative control extract (GS115 transformed with empty pPICZB vector) and in the non-induced clone, showing that the expression of the heterologous protein was specifically induced in the presence of methanol as indicated in Figure 1B.
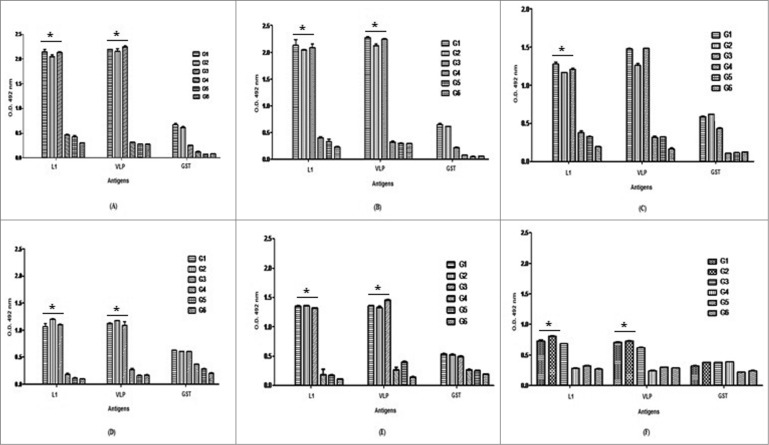
Figure 2.
Analysis of IgG2a (A–C) and IgG1-specific antibody levels (D–F) with respect to rL1, rVLP and rGST per group using ELISA. Serum samples were obtained from immunized mice after second (A & D), third (B & E) immunization and 7 days after C3 challenge (C & F). The significant increases in L1-specific IgG1 and IgG2a isotypes were observed in mice immunized with Pichia-L1, L.tar-L1 and Gardasil as compared to control groups 4 to 6 (٭P < 0.05). Data shows antibody titers of pooled mice sera with independent experiments and error bars indicate SD. N = 8 per group.
Generation of the recombinant L1 protein, killed leishmania and killed yeast
The HPV16 L1 protein was expressed and analyzed in SDS-PAGE by Coomassie Blue staining as previously described.20 The recombinant GST-L1 and GST proteins migrated as 82 kDa and 27 kDa proteins in SDS-PAGE, respectively. These recombinant proteins (rGST and rGST-L1) were purified, concentrated, assessed and kept in −70°C for next use in antibody and cytokine assays. Preparation of the killed Leishmania with different approaches showed that the protein content in 0.1% formalin is more intact than that in other methods especially autoclaving. Figure 1C demonstrates western blot analysis of total proteins detected in various killing methods.
Whole recombinant yeast and Leishmania indicated the mixture of IgG1 and IgG2a isotypes with high intensity toward IgG2a response
C57BL/6 mice were subcutaneously vaccinated 3 times at 2-week intervals with 5 mg of Pichia-L1 and yeast, 100 μg of L.tar-L1 and L.tar as well as one fifth of a dose of Gardasil. The quantity of anti-HPV16 L1 antibodies (IgG1 and IgG2a) in the sera of the vaccinated mice was determined by ELISA at different times (after second and third immunizations as well as 7 days after C3 challenge). Vaccination with Pichia-L1 and L.tar-L1 generated L1-specific IgG2a and IgG1 antibodies as compared to control groups especially before challenge (P < 0.05). The same result was also obtained for the group immunized with Gardasil (P > 0.05). Indeed, anti-L1 IgG2a and IgG1 isotypes increased significantly after the second immunization in test groups. It is interesting that IgG1 levels were reduced in the sera of groups 1 to 3 after C3 challenge (Fig. 2). As known, Th bias of the immune response can be analyzed by determination of IgG isotypes. Herein, the significant increases in L1-specific IgG1 and IgG2a isotypes were observed in mice immunized with yeast-L1, L.tar-L1 and Gardasil. Moreover, groups 1 to 3 greatly enhanced the IgG2a/ IgG1 ratio compared to control groups (P < 0.05) indicating a relative preference for the induction of Th1 immune responses (Table 1).
Table 1.
The mean of IgG2a/IgG1 ratios against rL1 and rVLP for test groups after second and third immunizations (Booster 1 and 2) and post-challenge
| Booster 1 | Booster 2 | Challenge | ||||
|---|---|---|---|---|---|---|
| Group | L1 | VLP | L1 | VLP | L1 | VLP |
| 1 | 2.03 ± 0.05 | 1.95 ± 0.00 | 1.58 ± 0.01 | 1.67 ± 0.01 | 1.76 ± 0.02 | 2.08 ± 0.00 |
| 2 | 1.70 ± 0.01 | 1.84 ± 0.01 | 1.51 ± 0.01 | 1.60 ± 0.02 | 1.45 ± 0.00 | 1.74 ± 0.02 |
| 3 | 1.94 ± 0.02 | 2.07 ± 0.02 | 1.58 ± 0.02 | 1.54 ± 0.01 | 1.76 ± 0.01 | 2.40 ± 0.00 |
Pichia-L1 stimulates the secretion of HPV16 L1-specific IFN-γ
Protection following successful immunization requires the generation and maintenance of antigen-specific CD4+ Th cells. Herein, splenocytes from immunized mice were assayed for the secretion of IFN-γ (Th1-like) and IL-4 (Th2-like) cytokines upon re-stimulation with rL1, rVLP and rGST, before C3 tumor challenge. As shown in Figure 3, splenocytes from mice immunized with Pichia-L1 produced higher levels of L1-specific IFN-γ (∼152 pg/ml) as well as VLP-specific IFN-γ (∼101 pg/ml), confirming the induction of Th1 response. However, neither IFN-γ nor IL-4 was detected in mice immunized with yeast or L.tar vectors. Moreover, mice immunized with killed Pichia-L1 (group 1) exhibited approximately a 3.2-fold higher L1-specific and VLP-specific IFN-γ responses as compared to the IFN-γ response measured following vaccination with killed L.tar-L1 (group 2) (P < 0.05). Overall, killed Pichia-L1 stimulated the most potent L1-specific IFN-γ responses of vaccine candidates. There were no significant differences for IL-4 production in different groups compared to that in control groups at before challenge (P > 0.05). The levels of IFN-γ and IL-4 with respect to ConA, as a positive control were between 400–600 pg/ml and 100–200 pg/ml in all groups, respectively (data not shown).
Figure 3.
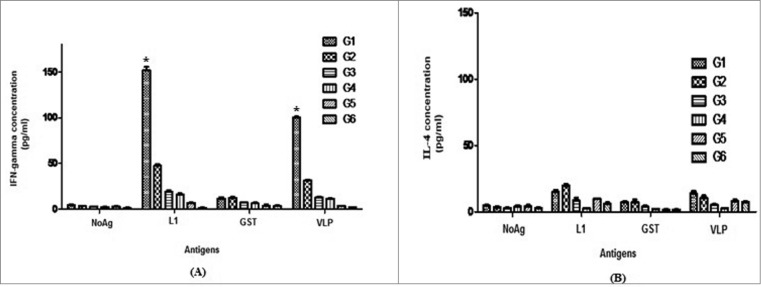
The levels of IFN-γ (A) and IL-4 (B) were measured by a sandwich ELISA in culture supernatants of splenocytes following in vitro re-stimulation with the recombinant proteins of GST-L1, GST and HPV16 VLP L1. Secretion of L1 (/ VLP)-specific IFN-gamma from spleen cells in the Pichia-L1 group is significantly higher than other groups (٭P < 0.05). There were no significant differences for IL-4 production in different groups at before challenge (P > 0.05). Data are presented as the mean ± SD.
Vaccination with Pichia-L1 enhances protection of mice against C3 tumors
To evaluate the anti-tumor effects of killed vaccine candidates, in vivo tumor protection experiments were performed by challenging vaccinated mice with C3 tumor cells. The data showed that ∼60% of mice receiving Pichia-L1 (group 1) remained tumor-free 50 days after the C3 challenge, in contrast with zero percent (0%) for mice receiving L.tar-L1 (group 2). In addition, all of the mice receiving killed L.tar vector and PBS developed a tumor growth at 7 days after tumor challenge (Fig. 4). Control groups (5 and 6) showed progressive tumor growth. Vaccination with Gardasil significantly resulted in prevention of TC-1 tumor growth (∼80% survival rates) in comparison with other groups (P < 0.05, Fig. 4). The growth of tumor delayed about 20 days in mice vaccinated with Gardasil and 7 days in group 1 vaccinated with Pichia-L1 after challenge. Interestingly, group 4 receiving yeast vector showed ∼20% tumor-free mice as compared to mice immunized with L.tar (group 5), PBS (group 6) and L.tar-L1 (group 2), indicating the protective efficacy and adjuvant activity of killed yeast components. Immunization with whole killed Pichia-L1 significantly retarded the tumor growth rate compared to other groups between days 35 and 50 except to group 3 vaccinated with Gardasil (P < 0.05, Fig. 4).
Figure 4.
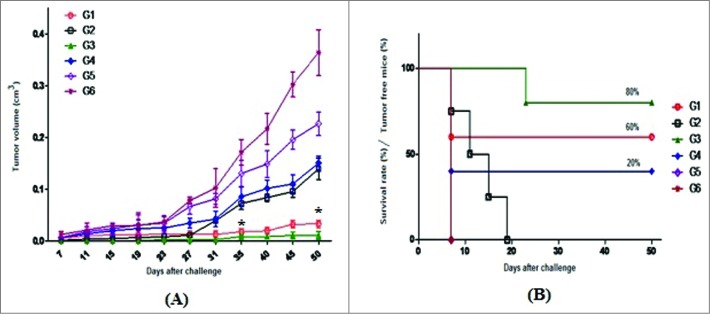
Prophylactic anti-tumor effect of various killed vaccines: At 2 weeks after the last immunization, mice were subcutaneously challenged with 0.5 × 10 6 C3 tumor cells. Tumor growth was then measured twice a week and tumor volumes calculated (A); In addition, the survival rate was determined as a Kaplan–Meier plot (B). Mice in PBS and L.tar-vaccinated groups experienced rapid and exponential tumor growth, which was delayed by only 7 days after C3 challenge. Mice in the group 1 (Pichia-L1) had the significant differences with other groups except to group 3 (Gardasil) between days 35 and 50 after C3 challenge (*P < 0.05). Tumor volume is presented as the mean ± SD.
Discussion
Availability of a safe and immunogenic vaccine would represent the best strategy for control of viral diseases such as human papillomavirus.2,3,21 The studies support the idea that an HPV16L1 VLP-based vaccine will induce the strongly neutralizing antibodies necessary for effective prophylaxis. This was the basis for the development of 2 highly efficient VLP-based HPV vaccines named Gardasil (Merck MSD) and Cervarix (GlaxoSmithKline) 2,3,22,23 The major drawbacks of these vaccines are the high production costs, the need for a cold chain and sterile needles for the intramuscular administration.23 Therefore, the production, assembly and characterization of HPV16 L1 structural protein in P. pastoris, has been studied by a Brazilian team for the development of cheap and efficient prophylactic vaccine in future.9
Currently, inactivated whole pathogen vaccines are the most common form of vaccine.24 However, these vaccines typically require multiple doses and regular boosters. Moreover, their efficacy frequently depends on use of potent adjuvants.24-26 Recently, a new class of vaccines termed killed but metabolically active (KBMA) was produced. KBMA vaccines are whole pathogenic or attenuated organisms killed through photochemical inactivation and cannot cause disease, yet retain sufficient metabolic activity to initiate a potent immune response.27 In current study, the immunogenicity and protective effects of 2 different vaccine modalities such as whole killed Pichia pastoris and Leishmania tarentolae expressing HPV16 L1 were evaluated in tumor mice model. Our goal of this study is not development of a vaccine for tumor therapy. The novelty of the study is limited to use killed vectors expressing L1 protein for protective vaccine design. At first, we constructed a Pichia-L1 vaccine using the pPICZ-B vector and L.tar-L1 vaccine using the pLEXSY vector. Herein, the efficient and tightly regulated promoter from the alcohol oxidase I gene (AOX1) was used to drive the expression of HPV16 L1 gene in Pichia pastoris.
From the yeast expression systems, Saccharomyces cerevisiae has been considered to be safe for consumption in humans without any side effects and a good expression system for heterologous proteins.28 Recent studies in mice showed that the recombinant saccharomyces cerevisiae induced potent antigen-specific proliferative and cytotoxic T cell responses associated with Th1-type cytokine secretion. Furthermore, prophylactic administration of the recombinant saccharomyces cerevisiae led to eradication of tumor cells in mice model.29 The studies suggest that oral vaccination with the yeast expressing apxIIA gene could induce the protection against porcine pleuropneumonia by eliciting both mucosal and systemic immune responses.28 Moreover, the protection against systemic murine coccidioidomycosis was observed by vaccination with heat-killed Saccharomyces cerevisiae.30
In this study, mice immunization was done by different modalities at 3 times. Then, the induction of L1 antibody titres and also T-cell responses were investigated for analyzing protection against tumor formation by C3 tumor cells, a cell line containing the entire HPV16 genome.31 We showed that a tumor-specific Th1-type response was established with whole killed Pichia-L1 vaccinated mice. As known, Th1 response is characterized by the production of IFN-γ and an enhancement of IgG2a and Th2 response is determined by the production of IL-4 and an enhancement of IgG1. Analysis of serum antibody isotypes revealed that Pichia-L1 not only enhances the levels of IgG1 and IgG2a, but also increases the IgG2a/IgG1 ratio, indicating a relative preference for the induction of Th1 immune responses. Furthermore, analysis of L1-specific cytokines secreted by spleen cells showed that Pichia-L1 increases the secretion of IFN-γ from spleen cells with no production of IFN-γ observed in mice receiving yeast vector, indicating that the cytokines are L1-specific. Similar data has been obtained by immunization with heat-killed whole recombinant Hansenula polymorpha yeast expressing hepatitis B surface antigen (yeast-HBsAg) in mice model. Indeed, yeast-HBsAg enhanced both HBsAg-specific Th1 and Th2 immune responses, while alum as an adjuvant only increased Th2 immune responses, suggesting that yeast-HBsAg may be an ideal candidate for an effective vaccine against chronic HBV infection.5
On the other hand, Leishmania tarentolae is a promising host for large-scale recombinant protein production for both pharmaceutical purpose and structural studies. 11,32-34 In the present study, Leishmania tarentolae was transfected with an expression cassette containing HPV16 L1 gene. This expression cassette harboring L1 gene was integrated in Leishmania 18S rRNA genes through homologous recombination.32-34 In this study, vaccination with killed parasites (L.tar-L1) generated L1-specific antibody and cellular responses, but it could not induce any protection in C57BL/6 mice model as compared to whole killed Pichia-L1. The reasons for this difference have not been still known. It may be related to differences in the quality of the early immune responses to killed parasites or yeast. Because, the modulation of the early responses with appropriate adjuvants could enhance efficacy of killed parasite vaccines. For instance, the researchers demonstrated that CpG ODN, when used as a vaccine adjuvant with heat-killed leishmanial antigens, could induce long-term protection against an intracellular infection.15 Our data confirm the potent adjuvant activities of yeast cell wall components in comparison with parasite components as previously described.35 Indeed, yeast can be easily engineered to express multiple antigens and its inherent adjuvant properties avoid the need for additional adjuvants.5 In addition, Formalin-killed promastigotes have been used as antigens in vaccine candidates against leishmaniasis.4 In current study, we also used formalin-killed promastigotes of Leishmania tarentolae expressing HPV16 L1 for the first time. Most studies have focused on the use of autoclaved L. major promastigotes with or without adjuvants against leishmaniasis. Regarding to our data, autoclaving of parasites in stationary phase denatures proteins and this may interfere with the appropriate conformational structure of important antigen epitopes resulting in less efficacious vaccines as described by others.4,14
Our results showed that ∼60% of mice receiving Pichia-L1 remained tumor-free 50 days after the C3 challenge, in contrast with zero percent (0%) for mice receiving L.tar-L1. Furthermore, immunization with whole killed Pichia-L1 significantly retarded the tumor growth rate compared to other groups especially L.tar-L1 vaccinated mice. However, even when an immune response is effectively induced in a vaccinated group, the immunological outcomes do not necessarily correlate with clinical improvement as reported by others.36 In addition, the route of immunogen administration influences how and where an immune response is started.28 More importantly, the induced T-cell responses may play an important role in establishing long-lived B-cell immune memory. Our data indicated that Pichia-L1 could significantly enhance the anti-tumor immunity against the growth of C3 tumors; although, Gardasil represented a higher survival rate in mice. The studies showed that the quadrivalent HPV vaccine (Gardasil) results in protective immunity against type-specific HPV infection using the production of a systemic neutralizing anti-HPV response indicating the importance of this response.22,23 The exact role of different immune mechanisms in the protective efficacy of the HPV L1 VLP vaccine remains to be determined. Therefore, a HPV-pseudovirion neutralizing assay would be necessary to evaluate the vaccine candidates used in this experiment, in future.
Tumor protection studies comparing live versus heat-killed yeast cells were performed with yeast expressing ovalbumin and with yeast expressing the HIV-Gag protein.7 The results demonstrated that protective immunity was equivalently elicited with heat-killed yeast compared to live yeast and was also not dependent on the antigen expressed. However, the use of heat-inactivated yeast eliminates potential safety risks associated with the administration of live cells 7 confirming our results. Determination of exact dose with high stabiliy, manipulation of vector and the use of other strains for higher protein expression would be critical for selecting Pichia-L1 as a vaccine candidate. The durability of the protection by killed whole yeast vaccines is unknown and should be determined in future. Furthermore, the comparison of live pichia-L1 and killed pichia-L1 will be suitable for determination of the best strategy.
In summary, our data demonstrated that vaccination with Pichia-L1 not only induces Th1 immune responses, but also has protective effects as compared to L.tar-L1 vaccinated mice. Indeed, whole recombinant Leishmania tarentolae could not protect mice against C3 tumor mice model. The potential advantages of this approach are low cost and the absence of additional adjuvant. Therefore, Pichia-L1 may be a novel approach for prevention and control of HPV infections.
Materials and Methods
In vitro expression of HPV16 L1 in COS-7 cells
For the generation of L1-expressing plasmid [pEGFP-L1], the L1 DNA was amplified from pUF-L1 (kindly provided by Prof. Martin Muller, German Cancer Research Center) by PCR using the primer sets as following:
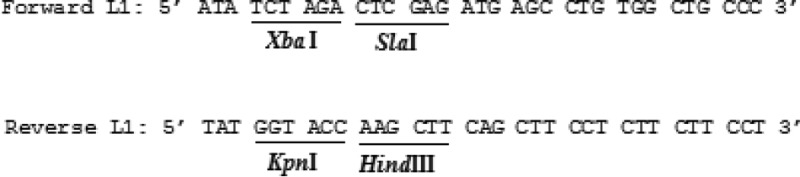
The amplified L1 DNA was then cloned into the unique XhoI and HindIII cloning sites of the pEGFP-N1 expression vector (Clontech, CA). DNA constructs containing L1-GFP was purified in large-scale using Midi-kit (Qiagen). The presence of the inserted L1 fragment was confirmed by PCR and restriction enzyme digestion as detected on a 0.8% agarose gel electrophoresis. The accuracy of this construct was confirmed by DNA sequencing. DNA concentration was determined by the absorbance measured at 260 nm.
After DNA preparation, COS-7 cells were incubated at 37°C in the presence of 5% CO2 into the 4-well plates (Greiner) in complete RPMI medium supplemented with 5% FCS overnight. PEI/DNA complexes were generated by mixing LIN-PEI 25 kDa (10 μM, NrE = 5, 7 and 10, Polysciences) with 5 μg of pEGFP-L1 plasmid and pEGFP-N1 as a positive control in HBS buffer (HEPES buffered saline) and incubated at room temperature for 15 min. COS-7 cells were used as a negative control. PEI/DNA complexes were added to 0.5 × 105 COS-7 cells in serum-free media. The medium was replaced after 6 h incubation at 37°C with complete RPMI medium supplemented with 5% FCS. Transfection efficiency of each construct was determined by the fluorescence microscopy (Nikon, E200, Japan) and flow cytometry analysis (Partec GmbH) at 24 and 48 h after transfection.
Generation of Leishmania tarentolae expressing HPV16 L1 through homologous recombination
For the generation of L1-GFP-expressing plasmid [pLEXSY-L1-GFP], the L1-GFP gene (∼2265 bp) was subcloned from pEGFP-L1 into the XhoI (SlaI) and NotI cloning sites of pLEXSY-neo2 expression vector (Jena bioscience). DNA construct containing L1-GFP was purified in large-scale using Midi-kit (Qiagen). For gene integration in leishmania genome, approximately 8 μg of the expression plasmid was digested with SwaI restriction sites and the linearized product was gel purified (QIAquick gel extraction kit) for transfection in L.tarentolae. Briefly, 4 × 107 log-phase parasites re-suspended in ice-cold electroporation buffer (pH = 7.5) containing 5–6 μg of linearized pLEXSY-L1-GFP stored on ice for 10 min and electroporated using Bio-Rad Gene PulserEcell under conditions of 500 mF, 450 V and pulse time ∼5–6 ms. The electroporated promastigotes were plated on solid media containing 50 μg/ml of G418. Clones were selected on Noble agar plates and further propagated in liquid M199 10% medium in the absence of G418 16, 17,19 (LEXSYcon2 Expression Kit, Jena Bioscience). Expression of L1-GFP protein in promastigote stage of the recombinant Leishmania species was evaluated by Epi-fluorescent microscopy and flow cytometry. Integration of the linearized expression cassettes (pLEXSY-L1-GFP) into the ssu locus of genome was confirmed by diagnostic PCR using genomic DNA of wild type and transgenic strain of L. tarentolae (L.tar-L1) as template (LEXSYcon2 Expression Kit, Jena Bioscience). Furthermore, RNA samples were extracted from promastigote form of wild type and transgenic parasites using RNeasy mini-kit (Qiagen) according to the manufacturer's instructions. The cDNA synthesis was performed using Omniscript Reverse Transcriptase kit (Qiagen) from 1 μg of RNA. PCR analysis was performed to amplify L1 and L1-GFP under standard conditions.
For protein analysis, samples from both wild type and transgenic L. tarentolae were separated by SDS-PAGE in a 12.5% (w/v) polyacrylamide gel as previously described 16,17,19 Western blot analysis was performed to confirm L1-GFP protein expression using anti-HPV16 L1 monoclonal antibody (MD2H11, kindly provided by Prof. Martin Muller, German Cancer Research Center; 1:10000 v/v) or anti-GFP polyclonal antibody (1:5000 v/v; Acris antibodies GmbH) under standard procedures. The immunoreactive protein bands were visualized using peroxidase substrate named 3, 3′-diaminobenzidine (DAB, Sigma).
Generation of Pichia pastoris expressing HPV16 L1 through homologous recombination
HPV16 L1 gene was amplified by PCR from the plasmid vector pUF-L1 using Pfu DNA polymerase and the primer sets [F/ R1 and then F/R2] as following:

The resulting L1 fragment (∼1543 bp) was then cloned into the unique EcoRI and XbaI cloning sites of the linearized pPICZ-B vector (3270 bp, Invitrogen). Plasmid extraction was done by alkaline lysis and confirmed by PCR and digestion with EcoRI/XbaI. The accuracy of gene direction was confirmed using Bgl II enzyme by generating 2 fragments (∼2949 and ∼1864 bp). DNA sequence analysis was also performed using AOX1 primer set (AOX1-F & R; Pichia expression kit, Invitrogen) on an automated DNA sequencer using the dideoxy termination method. DNA construct containing HPV16 L1 was purified in large-scale using Midi-kit (Qiagen). After confirmation, the pPICZB-L1 construct was linearized by SacI for gene integration into Pichia genome. Seven micrograms of pPICZB/L1 was added to 80 μl of competent yeast cells and transferred into a 0.2 cm-gap electroporation cuvette that pre-cooled on ice. Pichia pastoris strains (GS115) were transformed by electroporation at 1.5 kV, 200 Ω and 25 μF with a Gene Pulser II system (Bio-Rad). After the pulse, 1 ml cold 1 M sorbitol was added and the suspension was transferred into a sterile 2 ml Eppendorf tube. Cells were incubated for 1–2 h at 30°C without shaking. Aliquots of 200 μl were spread onto agar plates containing YPD supplemented with 500 μg/ml zeocin and incubated for 3 days at 30°C (Pichia expression kit, Invitrogen). The transformants were cultured in selection medium YPD and screened for the existence of the L1 gene by PCR using AOX1 primers according to Pichia Expression Kit manual. Yeast colonies that were positive for L1 DNA were inoculated in 10 ml of YPD medium supplemented with 500 μg/ml zeocin and grown overnight with shaking (250 rpm) at 30°C. Fresh BMMY medium supplemented with 500 μg/ml zeocin was inoculated 1:10 with the overnight culture and grown at 30°C. Methanol was added to final concentration of 1% (v/v) to induce protein expression and cultures were grown at 30°C with shaking (250 rpm) for 3 days. To maintain the induction of the recombinant protein, 100% methanol was added every 24 h to the culture to a final concentration of 1%. Then, protein expression was performed by SDS-PAGE and Western blotting. Yeast cells were harvested by centrifugation at 5000 rpm for 5 min and re-suspended in 300 μl of breaking buffer. An equal volume of micro glass beads (0.5 mm) was added to the tubes and a total of 8 cycles of vortexing and incubation on ice were performed (30 s each). Samples were centrifuged at 5000 rpm for 10 min and the supernatant was transferred to a new microcentrifuge tube for protein analysis. Protein samples were analyzed by SDS-PAGE in a gel containing 12.5% (w/v) polyacrylamide for Pichia and Pichia-L1, followed by staining with Coomassie brilliant blue. For protein gel blot analysis, the proteins resolved on the gel were transferred onto protran nitrocellulose transfer membrane (Schleicher and Schuell Bioscience, Dassel, Germany). The membrane was pre-equilibrated with TBST solution [10 mM Tris-HCl (pH = 7.4), 150 mM NaCl and 0.1% Tween 20) containing 2.5% bovine serum albumin (BSA) for overnight and then reacted with anti-HPV16 L1 monoclonal antibody (MD2H11, Germany; 1:10000 v/v) for 2 h at room temperature. After three washes with TBST, the membrane was incubated with anti-mouse IgG-HRP (Sigma, 1:5000) for 1.5 h at room temperature. The immunoreactive protein bands were visualized using DAB substrate.
Purification and assay of the recombinant protein
For the production of recombinant GST and GST-L1 proteins, the bacterial cultures containing pGEX-L1 were grown to an optical density of 0.8–1 at 600 nm and protein expression was induced with 1 mM IPTG for 2 h at 37°C. Protein samples were analyzed by SDS-PAGE in 12.5% (W/V) polyacrylamide gel. The recombinant L1 protein (rL1) was purified by Zn+2 reverse staining method as described before.20 The purified protein fraction was concentrated by ultrafiltration and dialyzed against PBS. Protein concentration was measured using BCA assay kit (Pierce, Rockford, USA). The recombinant protein was kept at −70°C until use. For monitoring humoral and cellular immune responses, the purified rL1 protein and VLP (previously provided by Prof. Martin Muller, German Cancer Research Center 37) were used as a coated antigen in ELISA.
Preparation of killed yeast and parasites
After washing of Pichia-L1 and yeast (GS115 as a control) for 3 times with PBS, the cells were heat-killed at 60°C for 2 h and then stored at −20°C until use. For parasites, the promastigotes of wild (L.tar) and transgenic parasites (L.tar-L1) were harvested at stationary phase (7 days) and washed 2 times in sterile PBS. Parasites were killed by several approaches for selecting the best method: 1) repeated freeze-thawing (15 rounds); 2) heat-killing at 60°C for 2 h; 3) Autoclaving and 4) suspension in 0.1% formalin overnight and then washing 3 times in PBS.4 In each approach, the dead promastigotes were counted by haemocytometer and re-suspended in a concentration of 1 × 108 / mL in sterile PBS and kept at −70°C prior to use. SDS-PAGE and Western blot analyses were done using Balb/c serum (1: 200 dilution) twice injected by parasites with 3 week intervals as first antibody and anti-mouse IgG-HRP (Sigma, 1:5000) as a secondary antibody. Finally, total protein concentrations were measured using BCA assay kit.
Cells
C3 cancerous cell line (Kindly provided by Prof. Martin Kast, Departments of Molecular Microbiology & Immunology and Obstetrics & Gynecology, University of Southern California, USA), a cell line containing the entire HPV16 genome (confirmed for L1 and L2, Hitzeroth et al., 2009) was cultured in complete RPMI 1640 supplemented with 5% FCS, 2 mM glutamine, 10 mM HEPES and 40 μg/ml gentamycin. On the day of tumor challenge, C3 cells were harvested by trypsinization, counted (∼0.5 × 106) and finally re-suspended in PBS.
Mice immunization
Female C57BL/6 mice, 6–8-weeks old, were obtained from breeding stock maintained at the Pasteur Institute of Iran. All mice were maintained under specific pathogen-free conditions and all procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals. Six groups of 8 mice were immunized including: Pichia-L1, L.tar-L1, Gardasil, Pichia (control), L.tar (control), and PBS (control), respectively.
In vivo tumor protection
For the tumor protection experiment, C57BL/6 mice (n = 8) were subcutaneously vaccinated 3 times at 2-week intervals with 5 mg of Pichia-L1 and Pichia (groups 1 and 4), 100 μg of L.tar-L1 and L.tar (groups 2 and 5) as well as one fifth of a dose of Gardasil as a positive control (group 3). Group 6 was injected with PBS as a negative control. Two weeks after the last vaccination, mice were subcutaneously challenged in the right flank with 0.5 × 106 C3 tumor cells. Tumor growth and survival rates were monitored by palpation twice a week. At each time point, tumor size was determined by measuring the smallest diameter (a) and the biggest diameter (b) by caliper. Tumor volume was calculated using the formula: V = (a2b)/2.21
The doses of different regimens were chosen after a pilot study and also other studies15,30,38 In our preliminary studies, the tumor growth was measured after mice immunization with different doses of 2.5, 5 and 10 mg of Pichia-L1 and 50 and 100 μg of L.tar-L1. Then, the concentrations of interest were selected based on low toxicity and decrease of tumor growth.
Monitoring humoral immune response
Pooled sera were prepared after retro-orbital bleeding from the whole blood samples of each group after the second and third injections and post-challenge. L1-specific IgG1 and IgG2a in the sera were measured by enzyme-linked immunosorbent assay (ELISA). Briefly, a 96-well flat-bottom ELISA plate (Greiner) was coated overnight at 4°C with rL1 (5 μg/ml), rGST (5 μg/ml) or VLP L1 (0.2 ng/ml) proteins diluted in PBS (pH = 7.2). Then, the plate was rinsed with washing buffer (0.5% (v/v) Tween-20 in PBS), incubated with blocking buffer (1% BSA in PBS) for 2 h at 37°C. The pooled sera were diluted 1:50 in dilution buffer (0.5% (v/v) Tween-20 in blocking buffer), added to the plate and incubated for 2 h at 37°C. After rinsing with washing buffer, the plate was incubated with biotin-conjugated goat anti-mouse IgG1 or IgG2a (diluted 1:1000 in 1% BSA/PBS-Tween, Southern biotechnology Association Inc., USA) for 2 h at 37°C. Then, the plates were washed and incubated with streptavidin-horseradish peroxidase diluted in PBS (1:100,000; Sigma) at 37°C for 1 h. Detection was done with 100 μl of O-Phenylenediamine (Sigma) as the substrate in citrate phosphate buffer (pH = 4.5), followed by incubation for 30 min at 37°C. The enzyme reaction was stopped by 1 M H2SO4 and the absorbance was measured at 492 nm.
Cytokine assay
To determine the T-helper phenotype of lymphocytes activated by L1, GST and VLP antigens, we investigated the pattern of IFN-γ and IL-4 cytokine production in spleen. Two weeks after third injection (before C3 challenge), 2 mice from each group were killed and the spleens were removed. An amount of 2 × 106 cells/ml of red blood cell-depleted pooled splenocytes from immunized mice of each group were re-suspended in complete RPMI medium 1640 supplemented with 5% FCS, 2 mM glutamine, 5 × 10−5 mM mercaptoethanol (2-ME), 10 mM HEPES and 40 μg/ml gentamycin. Cells were incubated in U-bottomed, 96-well plates (Costar, USA) in the presence of 10 μg/ml of rL1, 10 μg/ml of rGST and 2 μg/ml of VLP L1 protein. RPMI 5% and 5 μg/ml of concanavalin A (ConA) were used as negative and positive controls, respectively. Cells were cultured for 3 days at 37°C and 5% CO2. Supernatants were then collected and frozen at −70°C, until the samples were analyzed. The presence of IFN-γ and IL-4 was measured using a DuoSet ELISA system (R&D Systems, USA) according to the manufacturer's instructions. All data were represented as mean ± SD of duplicate for each set of samples. The detection limit was 2 pg/ml for IFN-γ and 7 pg/ml for IL-4.
Statistical analysis
Statistical analysis was performed using Prism 5.0 software (GraphPad, San Diego, California, USA). One-way ANOVA and Student's t-test were performed to analyze cellular and humoral immune responses as well as tumor volume measurements among the various immunization groups and compare individual data points, respectively. In the tumor protection experiment, the percentage of tumor-free mice in different groups (Survival of animals) was analyzed with log-rank test on Kaplan-Meier curves. For all comparisons, p < 0.05 was considered statistically significant. Data are presented as mean ± standard deviation (SD).
Acknowledgments
The authors are grateful to the collaborators of “Molecular Immunology and Vaccine Research Lab, Pasteur Institute of Iran” for technical assistance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by Pasteur Institute of Iran for experimental works (ID#568) and also by Iran National Science Foundation (INSF) (ID#91060229).
References
- 1. Tobery TW, Smith JF, Kuklin N, Skulsky D, Ackerson C, Huang L, Chen L, Cook JC, McClements WL, Jansen KU. Effect of vaccine delivery system on the induction of HPV16L1-specific humoral and cell-mediated immune responses in immunized rhesus macaques. Vaccine 2003; 21:1539-47; PMID:12615451; http://dx.doi.org/ 10.1016/S0264-410X(02)00679-5 [DOI] [PubMed] [Google Scholar]
- 2. Fraillery D, Baud D, Pang SY, Schiller J, Bobst M, Zosso N, Ponci F, Nardelli-Haefliger D. Salmonella entrica Serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin Vaccine Immunol 2007; 14(10):1285-95; PMID:17687110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roy P, Noad R. Virus-like particles as a vaccine delivery system. Hum Vaccin 2008; 4(1):5-8; PMID:18438104; http://dx.doi.org/ 10.4161/hv.4.1.5559 [DOI] [PubMed] [Google Scholar]
- 4. Mutiso JM, Macharia JC, Mutisya RM, Taracha E. Subcutaneous immunization against Leishmania major-infection in mice: Efficacy of formalin-killed promastigotes combined with adjuvants. Rev Inst Med Trop 2010; 52(2):95-100; PMID:20464130 [DOI] [PubMed] [Google Scholar]
- 5. Bian G, Cheng Y, Wang Z, Hu Y, Zhang X, Wu M, Chen Z, Shi B, Sun S, Shen Y, et al. . Whole recombinant Hansenulapolymorpha expressing hepatitis B virus surface antigen (yeast-HBsAg) induces potent HBsAg-specific Th1 and Th2 immune responses. Vaccine 2010; 28:187-94. [DOI] [PubMed] [Google Scholar]
- 6. Bernstein MB, Chakraborty M, Wansley EK, Guo Z, Franzusoff A, Mostböck S, Sabzevari H, Schlom J, Hodge JW. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine 2008; 26(4):509-21; PMID:18155327 [DOI] [PubMed] [Google Scholar]
- 7. Franzusoff A, Duke RC, King TH, Lu Y, Rodell TC. Yeasts encoding tumor antigens in cancer immunotherapy. Expert Opin Biol Ther 2005; 5(4):565-75; PMID:15934834; http://dx.doi.org/ 10.1517/14712598.5.4.565 [DOI] [PubMed] [Google Scholar]
- 8. Gellissen G, Kunze G, Gaillardin C, Cregg JM, Berardi E, Veenhuis M, van der Klei I. New yeast expression platforms based on methylotrophic Hansenula polymorpha and Pichia pastoris and on dimorphic Arxula adeninivorans and Yarrowiali polytica-a comparison. FEMS Yeast Res 2005; 5(11):1079-96; PMID:16144775 [DOI] [PubMed] [Google Scholar]
- 9. Bazan SB, Chaves AAM, Aires KA, Cianciarullo AM, Garcea RL, Ho PL. Expression and characterization of HPV16 L1 capsid protein in Pichia pastoris. Arch Virol 2009; 154:1609-17; PMID:19756360; http://dx.doi.org/ 10.1007/s00705-009-0484-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phan HP, Sugino M, Niimi T. The production of recombinant human laminin-332 in a Leishmania tarentolae expression system. Protein Expr Purif 2009; 68: 79-84; PMID:19607924; http://dx.doi.org/ 10.1016/j.pep.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 11. Giancarlo B, Peticca M. Recombinant protein expression in Leishmania tarentolae. Mol Biotechnol 2009; 43:273-8; PMID:19779853; http://dx.doi.org/ 10.1007/s12033-009-9213-5 [DOI] [PubMed] [Google Scholar]
- 12. Bolhassani A, Zahedifard F. Therapeutic live vaccines as a potential anticancer strategy. Int J Cancer 2012; 131:1733-43; PMID:22610886; http://dx.doi.org/ 10.1002/ijc.27640 [DOI] [PubMed] [Google Scholar]
- 13. Okwor I, Liu D, Beverley SM, Uzonna JE. Inoculation of killed Leishmania major into immune mice rapidly disrupts immunity to a secondary challenge via IL-10-mediated process. PNAS 2009; 106(33):13951-6; PMID:19666482; http://dx.doi.org/ 10.1073/pnas.0905184106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayrink W, Pinto J, Costa CD, Toledo V, Guimarães T, Genaro O, Vilela L. Short report: evaluation of the potency and stability of a candidate vaccine against American cutaneous leishmaniasis. Am J Trop Med Hyg 2009; 61(2):294-5; PMID:10463682 [DOI] [PubMed] [Google Scholar]
- 15. Rhee EG, Mendez S, Shah JA, Wu CY, Kirman JR, Turon TN, Davey DF, Davis H, Klinman DM, et al. . Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4 +and CD8+ T cell responses and protection against Leishmania major infection. J Exp Med 2002; 195(12):1565-73; PMID:12070284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salehi M, Taheri T, Mohit E, Zahedifard F, Seyed N, Taslimi Y, Sattari M, Bolhassani A, Rafati S. Recombinant Leishmania tarentolae encoding the HPV type 16 E7 gene in tumor mice model. Immunotherapy 2012; 4(11):1107-20; PMID:23194361; http://dx.doi.org/ 10.2217/imt.12.110 [DOI] [PubMed] [Google Scholar]
- 17. Hosseinzadeh S, Bolhassani A, Rafati S, Taheri T, Zahedifard F, Daemi A, Taslimi Y, Hashemi M, Memarnejadian A. A non-pathogenic live vector as an efficient delivery system in vaccine design for the prevention of HPV16 E7-overexpressing cancers. Drug Deliv 2013; 20 (3-4):190-8; PMID:23745741 [DOI] [PubMed] [Google Scholar]
- 18. Basak A, Shervani NJ, Mbikay M, Kolajova M. Recombinant proproteinconvertase 4 (PC4) from Leishmania tarentolae expression system: Purification, biochemical study and inhibitor design. Protein Exp Purif 2008; 60:117-26; PMID:18485734; http://dx.doi.org/ 10.1016/j.pep.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 19. Bolhassani A, Taheri T, Taslimi Y, Zamanilui S, Zahedifard F, Seyed N, Torkashvand F, Vaziri B, Rafati S. Fluorescent Leishmania species: development of stable GFP expression and its application for invitro and in vivo studies. Exp Parasitol 2011; 127(3): 637-45; PMID:21187086; http://dx.doi.org/ 10.1016/j.exppara.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 20. Javanzad S, Bolhassani A, Doustdari F, Hashemi M, Movafagh A. Reverse staining method of polyacrylamide gels by imidazole-zinc salts for the detection and purification of L1 capsid protein in E.coli. J Paramed Sci 2013; 4(2):33-7. [Google Scholar]
- 21. Li Y, Subjeck J, Yang G, Repasky E, Wang XY. Generation of anti-tumor immunity using mammalian heat shock protein 70 DNA vaccines for cancer immunotherapy. Vaccine 2006; 24: 5360-70; PMID:16714072; http://dx.doi.org/ 10.1016/j.vaccine.2006.04.028 [DOI] [PubMed] [Google Scholar]
- 22. Kuck D, Leder C, Kern A, Müller M, Piuko K, Gissmann L, Kleinschmidt JA. Efficiency of HPV16L1/E7 DNA immunization: Influence of cellular localization and capsid assembly. Vaccine 2006; 24: 2952-65; PMID:16414157; http://dx.doi.org/ 10.1016/j.vaccine.2005.12.023 [DOI] [PubMed] [Google Scholar]
- 23. Thones N, Muller M. Oral immunization with different assembly forms of the HPV16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes. Virology 2007; 369:375-88; PMID:17822733; http://dx.doi.org/ 10.1016/j.virol.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 24. Lunn DP. Immunological basis of vaccination. A.A.E.P. Proceedings 2000; 46:1-9. [Google Scholar]
- 25. Foged C, Hansen J, Agger EM. License to kill: formulation requirements for optimal priming of CD8+ CTL responses with particulate vaccine delivery systems. European Journal of Pharmaceutical Sciences 2011; 1-10. [DOI] [PubMed] [Google Scholar]
- 26. Mani S, Tripathi L, Raut R, Tyagi P, Arora U, Barman T, Sood R, Galav A, Wahala W, de Silva A, et al. . Pichia pastoris-expressed Dengue 2 envelope forms virus-like particles without pre-membrane protein and induces high titer neutralizing antibodies. PLOS Oone 2013; 8(5):e64595; PMID:23717637; http://dx.doi.org/ 10.1371/journal.pone.0064595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dubensky TW, Skoble J, Lauer P, Brockstedt DG. Killed, but metabolically active vaccines. Curr Opin Biotechnol 2012; 23:917-23; PMID:22608846; http://dx.doi.org/ 10.1016/j.copbio.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 28. Shin SJ, Bae JL, Cho YW, Lee DY, Kim DH, Yang MS, Jang YS, Yoo HS. Induction of antigen-specific immune responses by oral vaccination with Saccharomyces cerevisiae expressing Actinobacillus pleuropneumoniae ApxIIA. FEMS Immunol Med Microb 2005; 43:155-64; PMID:15681145 [DOI] [PubMed] [Google Scholar]
- 29. Haller AA, Lauer GM, King TH, Kemmler C, Fiolkoski V, Lu Y, Bellgrau D, Rodell TC, Apelian D, Franzusoff A, et al. . Whole recombinant yeast-based immunotherapy induces potent T cell responses targeting HCV NS3 and Core proteins. Vaccine 2007; 25:1452-63; PMID:17098335; http://dx.doi.org/ 10.1016/j.vaccine.2006.10.035 [DOI] [PubMed] [Google Scholar]
- 30. Capilla J, Clemons KV, Liu M, Levine HB, Stevens DA. Saccharomyces cerevisiae as a vaccine against coccidioidomycosis. Vaccine 2009; 27(27): 3662-8; PMID:19464548 [DOI] [PubMed] [Google Scholar]
- 31. Hitzeroth II, Passmore JAS, Shephard E, Stewart D, Müller M, Williamson AL, Rybicki EP, Kast WM. Immunogenicity of an HPV16 L2 DNA vaccine. Vaccine 2009; 27:6432-4; PMID:19559114; http://dx.doi.org/ 10.1016/j.vaccine.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miblitz A, Mottram JC, Overath P, Aebischer T. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol Biochem Parasitol 2000; 107:251-61; PMID:10779601 [DOI] [PubMed] [Google Scholar]
- 33. Breton M, Zhao C, Ouellette M, Tremblay MJ, Papadopoulou B. A recombinant non-pathogenic Leishmania vaccine expressing human immunodeficiency virus 1 (HIV-1) Gag elicits cell-mediated immunity in mice and decreases HIV-1 replication in human tonsillar tissue following exposure to HIV-1 infection. J Gen Virol 2007; 88:217-25; PMID:17170454 [DOI] [PubMed] [Google Scholar]
- 34. Soleimani M, Mahboudi F, Davoudi N, Amanzadeh A, Azizi M, Adeli A, Rastegar H, Barkhordari F, Mohajer-Maghari B. Expression of human tissue plasminogen activator in the trypanosomatid protozoan Leishmania tarentolae. Biotechnol Appl Biochem 2007; 48:55-61; PMID:17371294 [DOI] [PubMed] [Google Scholar]
- 35. Okwor I, Liu D, Uzonna J. Qualitative differences in the early immune response to live and killed Leishmania major: Implications for vaccination strategies against Leishmaniasis. Vaccine 2009; 27:2554-62; PMID:19428861 [DOI] [PubMed] [Google Scholar]
- 36. Vendrell A, Gravisaco MJ, Pasetti MF, Croci M, Colombo L, Rodríguez C, Mongini C, Waldner CI. A novel Salmonella Typhi–based immunotherapy promotes tumor killing via an antitumor Th1-type cellular immune response and neutrophil activation in a mouse model of breast cancer. Vaccine 2011; 29:728-36; PMID:21095252 [DOI] [PubMed] [Google Scholar]
- 37. Muller M, Zhou J, Reed TD, Rittmüller C, Burger A, Gabelsberger J, Braspenning J, Gissmann L. Chimeric papillomavirus-like particles. Virology 1997; 234(1):93-111; PMID:9234950; http://dx.doi.org/ 10.1006/viro.1997.8591 [DOI] [PubMed] [Google Scholar]
- 38. Karanam B, Gambhira R, Peng S, Jagu S, Kim DJ, Ketner GW, Stern PL, Adams RJ, Roden RBS. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine 2009; 27:1040-9; PMID:19095032; http://dx.doi.org/ 10.1016/j.vaccine.2008.11.099 [DOI] [PMC free article] [PubMed] [Google Scholar]