Abstract
Introduction
Universal varicella vaccination in Sicily was introduced in infant population since 2003, with a rapidly increasing coverage. Aim of the present study was to analyze changes in the epidemiology of varicella since the introduction of universal vaccination.
Methods
The study was performed by analyzing Sicilian administrative/clinical data on varicella case notifications and hospitalizations from 2003 to 2012 (ICD-9-CM discharge diagnosis codes 052 and 052.×). MMR+V and V coverage were also calculated for each birth cohort. Moreover, blood samples drawn in 2013/2014 from general population stratified by age were tested for varicella antibodies.
Results
From 2003 to 2012, 15 433 varicella cases were notified with a decreasing temporal trend (1.1/1000 population in 2003 to 0.1/1000 in 2012) (P < 0.001). In the same period, a total of 1145 patients were hospitalized with a diagnosis of varicella, with a 6-fold reduced risk of hospitalization over time (from 4.8 to 0.8/100 000 population per year; P < 0.001). Varicella vaccination coverage rates increased from 40% (2001 birth cohort) to 85% (2010 birth cohort), and inversely correlated with both notification and hospitalization rates (P < 0.001). Finally, 80.0% of subjects enrolled in the seroepidemiological survey were positive for varicella and seroprevalence increased significantly with age in both sexes (P < 0.001).
Discussion
The results show the impact of infant universal varicella vaccination in Sicily. Noteworthy, notifications and hospitalizations for varicella have significantly decreased after the introduction of universal vaccination, confirming the effectiveness of the adopted strategy.
Keywords: epidemiology, varicella, universal mass vaccination, coverage, notifications, hospitalizations, Sicily
Introduction
Varicella is an infectious disease caused by the Varicella zoster virus (VZV), a DNA virus member of the herpesvirus group. Varicella transmission is mainly person-to-person by airborne respiratory droplets, but also by direct contact with vesicle fluid of subjects suffering from varicella or herpes zoster. Although varicella is a disease with a low mortality, it is characterized by a high morbidity, and in the absence of vaccination strategies, more than 90% of subjects will be affected by varicella within the first 12 y of age.1 Only occasionally varicella can have serious complications, that are more common among adolescents and adults or in immunosuppressed subjects. Varicella during pregnancy can be associated with severe illnesses for both the mother and neonate (congenital varicella).2 Finally, VZV can persist in a latent form within sensory ganglia and, in 10–30% of cases, it can reactivate later (usually in the elderly and in immunosuppressed subjects) to cause herpes zoster.3 In Italy, notification of varicella is mandatory.4
Although immunization represents the best strategy to reduce the epidemiological impact of the disease, the associated complications and its attributable costs, this vaccine is not yet included in the national immunization programme and only few regions have actively offered it, in the last years, as pivotal programmes.5 Sicily, was the first region, in 2003, to offer varicella vaccination free of charge to all children in their 13th–15th month of life and to all susceptible adolescents in their 12th year of age.6 Since 2010, the second dose of varicella vaccine on the 5th year of life was added in the regional immunization program as well as the possibility of vaccinating with tetravalent vaccine (MMR-V).6
The present paper aims to describe the epidemiology of varicella in Sicily, before and after the introduction of universal mass vaccination against varicella, assessing vaccination coverage over time, evaluating the burden of the disease (notifications and hospitalizations) and, finally, estimating the prevalence of varicella seropositive subjects in a sample of the general population.
Results
As shown in Figure 1A, from 2003 (birth cohort 2001) to 2012 (birth cohort 2010) varicella vaccination coverage increased from 40.4% to 84.5% (P < 0.001). During the same period, varicella notifications decreased by more than 95% (from 5290 cases in 2003 to 207 cases in 2012; P < 0.001) (Fig. 1B). Overall, from 2007 notification rates were lower than 0.1 cases per 1000 inhabitants per year. The reduction was particularly evident for subjects aged 0–14 y, who represented about 94% of all notifications (data not shown).
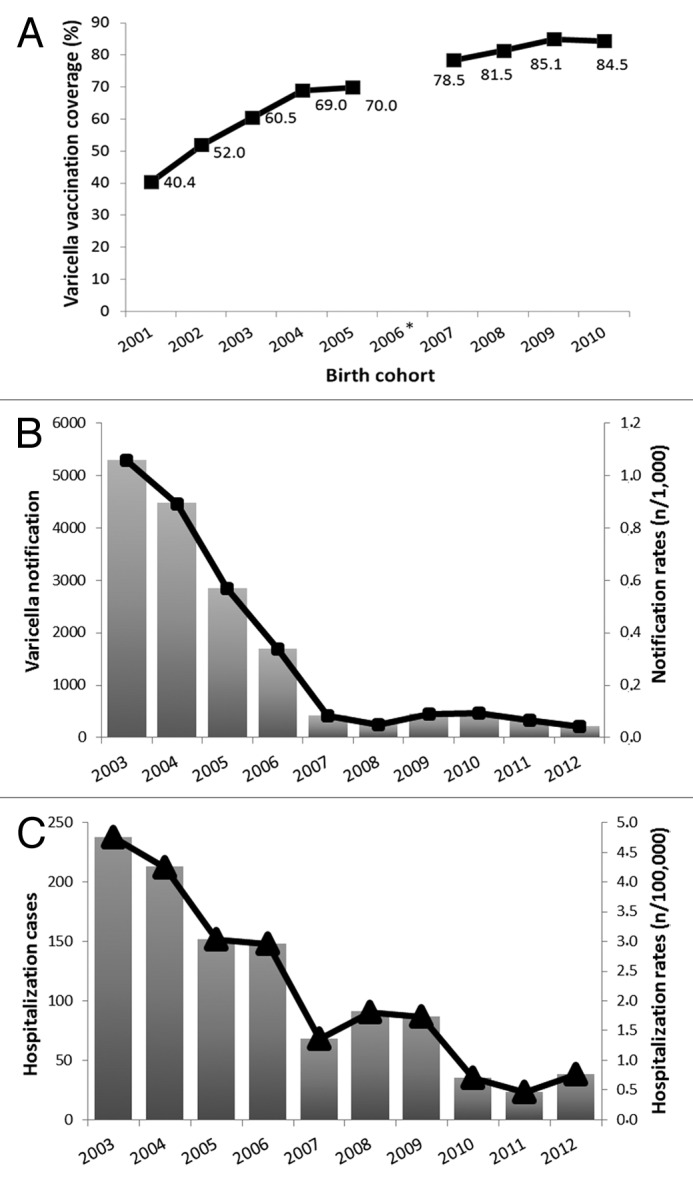
Figure 1. (A) Varicella vaccination coverage in Sicily by birth cohort (*data in 2006 birth cohort were lacking); (B) Notifications for varicella in Sicily, 2003–2012; (C) Hospitalization with a diagnosis of varicella in Sicily, 2003–2012.
According to the notification trend, the number of varicella hospitalizations decreased from 238 cases in 2003 to 38 cases in 2012 (from 4.8 cases/100 000 to 0.8 cases/100 000; P < 0.001) (Fig. 1C). Median age at hospital admission was positively correlated with year of hospital admission, ranging from 5 y of age (IQR = 11.5) in 2003 to 20 y of age (IQR = 23) in 2009 (P < 0.001). Direct hospitalization costs decreased from 485,000 euro in 2003 to about 82,000 euro in 2012, with a spare of more than 80%.
Finally, the general characteristics of subjects enrolled in the seroepidemiological study were reported in Table 1. Overall, the final sample accounted for 175 subjects (80 males and 95 females) with a median age of 19 y (IQR = 28). Fifty-seven (57) out of 175 (32.9%) study participants reported previous varicella vaccination and subjects aged 1–6 y and 7–14 y showed higher coverage rates (76.5% and 66.7% among males and 77.8% and 68.4% among females, respectively). One-hundred and 40 out of 175 (80.0%) subjects were seropositive for varicella and seroprevalence increased significantly with age in both sexes (P < 0.001).
Table 1. General characteristics, varicella vaccination status and seropositivity of the 175 subjects recruited in the seroepidemiological study.
| Total | Vaccinated* | Seropositive | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Total, n (% by row) | 80 (45.7) | 95 (54.3) | 26 (32.9) | 31 (33.0) | 62 (77.5) | 78 (82.1) |
| Age, n (% by row) | ||||||
| <13 mo | 3 (50) | 3 (50) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) |
| 13 mo to 6 y | 17 (48.6) | 18 (51.5) | 13 (76.5) | 14 (77.8) | 10 (58.8) | 12 (66.7) |
| 7 to 14 y | 16 (45.7) | 19 (54.3) | 10 (66.7) | 13 (68.4) | 10 (62.5) | 14 (73.7) |
| 15 to 25 y | 15 (45.5) | 18 (54.5) | 2 (13.3) | 2 (11.8) | 12 (80.0) | 16 (88.9) |
| >25 y | 29 (43.9) | 37 (56.1) | 1 (3.4) | 2 (5.4) | 29 (100.0) | 36 (97.3) |
As stated by study participants or their legal guardians.
Discussion
Sicily was the first Italian region to introduce varicella vaccination in its regional immunization program in 2003. Ten years after its introduction, the epidemiology of varicella in Sicily seems to be profoundly changed with a marked decrease of the disease and economic burden attributable to this infection. Intriguingly, it should be noted that the epidemiologic changes associated with varicella vaccination become particularly evident after 2007 and 2008. This “breakpoint period” is consistent with the consideration that, in these years, vaccination coverage increased to more than 70% and, thus, the decrease of the susceptible population has probably reduced the active circulation of the virus because of the herd effect.
The reduction of varicella morbidity was particularly evident for notifications (< 95%), whereas resulted less significant for severe cases requiring hospitalization (≈84%). Although this difference may be theoretically due to under-reporting or under-diagnosis, it should be considered that both the passive surveillance systems analyzed have not undergone to significant changes during the study period. Moreover, the minor impact of vaccination on hospitalizations may be explained by considering the overall increased age at hospital admission observed among Sicilian subjects over time. In fact, it is well known that the risk of complications due to varicella increases proportionally with age at infection and several authors have hypothesized an increased risk of morbidity due to shifts in the age at infection, when vaccination coverage is between 30–70%, as observed in Sicily during the first five years after the introduction of varicella vaccination.7,8 The World Health Organization (WHO) has recently suggested that this risk may be present at coverage rates below 80%.9
Noteworthy, our seroepidemiological results have confirmed that a relatively high proportion of subjects aged 15–25 y (≈15%) were seronegative. This age-group is of paramount importance because it is at higher risk of having complications, but also because about 11% of females in this group were seronegative and, thus, susceptible of contracting varicella during pregnancy.
Seronegative subjects were also relatively common among children aged 2–14 y (≈35%). These results are quite similar to those observed in Italy before varicella vaccination introduction, with the difference that in our case seropositivity has been attributable to varicella vaccination instead of natural infection and disease.10 Although a higher seropositive rate could be expected in this last age-group, this finding could be explained by considering both the sub-optimal vaccination coverage and the one-dose varicella immunization strategy present in Sicily before 2010. Some authors have hypothesized that in similar epidemiological context “post-honeymoon” epidemics may occur with both natural and breakthrough varicella cases.11 Despite of this consideration, to date varicella in Sicily has been characterized by an endemic trend with decreasing incidence and no epidemics were observed in the study period. Further studies and long-term surveillance are required in order to evaluate the effectiveness of the immunization program over time. Moreover, according to the previously discussed results, the potential benefits of a possible progressive introduction of booster doses in adolescents and young adults should be evaluated in our geographical setting, also for reducing the probability of acquiring varicella infection in an age at higher risk for complications.9
Another potential risk that should be monitored is the possible increase in the incidence of herpes zoster in the elderly. In this sense, the international literature suggests that the decreased VZV circulation resulting from mass vaccination may reduce the exposure to natural boosters, potentially increasing the number of zoster cases in first decades of a universal mass vaccination program.12 Finally, the present study has some possible limitations that should be taken into account. First, notifications and hospital discharge databases could be affected by underreporting and underdiagnosis. However, when the rate of underreporting/underdiagnosis is stable over time, trends can be informative and accurately estimated. Second, the relatively small seroepidemiological sample size and the recruitment of subjects requiring diagnostic health analysis could have reduced both precision and generalizability of our results. Nevertheless our data highlight that the first ten years of universal varicella vaccination have allowed to significantly reduce morbidity attributable to varicella, as shown by the very low incidence and hospitalization rates recently observed in the Sicilian population.
Despite of these encouraging findings, as suggested by several authors, there is a clear need to implement an active surveillance of varicella cases (including those due to breakthrough infections) and complications, to monitor varicella vaccination uptake and to evaluate alternative immunization strategies as well as appropriate administration of booster doses in particular age or risk groups.13
Material and Methods
The present paper analyzes data obtained from 4 different sources, as schematically reported below:
Varicella vaccination coverage rates collected by the Region of Sicily’s Health Commission from 2003 to 2012 (data about 2008 were missing).
Varicella notifications collected by the Italian National Surveillance System of Infectious Diseases (SIMI) and the Region of Sicily’s Health Commission from 2003 to 2012.14
Administrative and clinical data of hospitalized patients, resident of Sicily, from 2003 to 2012. All data included in the analyses were obtained by the Region of Sicily’s Health Commission, which routinely collects hospital discharge records (HDRs) from all Sicilian hospitals. The Regional Hospital Discharge Database, which was established in 1994, collects complete data on hospitalizations from both public and private hospitals. Each HDR includes demographic information (birthplace, residence, gender, and date of birth), admission and discharge dates, discharge status (categorized as “discharged/transferred” or “dead”), and up to six discharge diagnoses (1 principal and 5 secondary diagnoses) coded according to International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9 CM).
A seroepidemiological study, was performed in 2013/2014, on subjects who attended two main hospitals located in Palermo for routine laboratory analyses. Subjects were selected from the laboratory register, after random stratified sampling, with proportional allocation by age and sex. Before enrollment, each subject was informed about the study and an informed consent was obtained from each patient or from the patient’s legal guardian. Participants with a diagnosis of current immunodepression and those who have had a blood transfusion in the previous 180 d were excluded. For each enrolled subject data on sex, age, previous varicella vaccination, and date of each dose, were collected.
All subjects discharged with the ICD-9-CM code 052.×, on any diagnosis position, have been considered as varicella cases. Hospitalization of Sicilian residents that occurred outside the region was also included in the analysis, while multiple hospitalizations due to transfers were combined. Neither ethics committee approval nor individual written consent were required according to the Italian law (anonymous aggregated data).
All the blood samples were centrifuged at 1500 × g for 15 min, and sera were aliquoted and stored at −20 °C until analyses. Serological laboratory analyses were performed at the national reference laboratory of the University of Salento, Lecce. Varicella antibodies were quantified using an immunoenzymatic micro-method (Enzygnost anti-VZV-virus/IgG, Dade Behring GmbH), which has a high sensitivity and specificity (respectively, 99.3% and 100%). In accordance with the international standard for varicella-zoster immunoglobulin of the World Health Organization, the cut-off for the test was set at 50 mIU/ml.
The study was conducted in compliance with the Helsinki Declaration and with the Law Decree n. 196/2003, article 24 (Code for the protection of personal data) and approved by the Institutional Review Board of the Azienda Ospedaliero Universitaria Policlinico “P. Giaccone” of Palermo, Italy.
Statistical analyses were performed by using EpiInfo ver. Seven and R software. Population data per year, age group and sex were collected from the DemoIstat.15 Vaccination coverage was calculated as the number of children vaccinated with at least 1 dose of varicella divided by the total number of children aged 12–24 mo per each year. Annual notification (per 1000 inhabitants) and hospitalization rates (per 100 000 inhabitants) were calculated.
Categorical variables were summarized as frequency (percent) and compared by using Pearson χ2 test, χ2 test for trend or Fisher exact test as appropriate. Median and interquartile range (IQR) was used for summarizing not normally distributed quantitative variables. Correlation between age and year of hospital admission was evaluated by Spearman rank correlation. All reported p-values were two-sided and P < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- MMR
measles-mumps-rubella
- V
varicella
- VZV
Varicella zoster virus
- SIMI
National Surveillance System of Infectious Diseases
- HDRs
hospital discharge records
- ICD-9 CM
International Classification of Disease, Ninth Revision, Clinical Modification
- mIU
milli-international units
- IQR
interquartile range
- WHO
World Health Organization
References
- 1.Varicella: The Pink Book:Course Textbook-12th Edition Second Printing (May 2012). Available at: http://www.cdc.gov/vaccines/pubs/pinkbook.varicella.html Last access on April 2014.
- 2.Wharton M.. The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am 1996; 10:571 - 81; http://dx.doi.org/ 10.1016/S0891-5520(05)70313-5; PMID: 8856352 [DOI] [PubMed] [Google Scholar]
- 3.Thomas SL, Hall AJ.. What does epidemiology tell us about risk factors for herpes zoster?. Lancet Infect Dis 2004; 4:26 - 33; http://dx.doi.org/ 10.1016/S1473-3099(03)00857-0; PMID: 14720565 [DOI] [PubMed] [Google Scholar]
- 4.Italian Health Ministry decree. Published on the Official Bulletin 1991; 6:8 - 18 [Google Scholar]
- 5.WHO Position paper. Available at http://www.who.int/immunization/wer7332varicella_Aug98_position_paper.pdf.
- 6.Sicilian Health Commission. Decree 22 July 2002, n. 1087. Regional Official Bulletin, 16 August 2002 [Google Scholar]
- 7.Skull SA, Wang EE.. Arch Dis Child. Varicella vaccination - a critical review of the evidence. 2001; 85:83 - 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisson M, Edmunds WJ, Gay NJ, Law B, De Serres G.. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol Infect 2000; 125:651 - 69; http://dx.doi.org/ 10.1017/S0950268800004714; PMID: 11218215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brisson M, Racine E, Drolet M. The potential impact of Varicella vaccination in Low to Middle Income Countries: A feasibility modeling study. Report to the SAGE working group on Varicella and Herpes Zoster vaccines, Geneva, April 2014. available at http://www.who.int/immunization/sage/meetings/2014/april/5_The_potential_impact_Varicella_vaccination_Low_Middle_Income_Countries_feasibility_modeling.pdf
- 10.Gabutti G, Rota MC, Guido M, De Donno A, Bella A, Ciofi degli Atti ML, Crovari P, Seroepidemiology Group.. The epidemiology of Varicella Zoster Virus infection in Italy. BMC Public Health 2008; 8:372; http://dx.doi.org/ 10.1186/1471-2458-8-372; PMID: 18954432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edmunds WJ, Brisson M, European Centre for Disease Prevention and Control.. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect 2002; 44:211 - 9; http://dx.doi.org/ 10.1053/jinf.2002.0988; PMID: 12099726 [DOI] [PubMed] [Google Scholar]
- 12.Schmid DS, Jumaan AO.. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev 2010; 23:202 - 17; http://dx.doi.org/ 10.1128/CMR.00031-09; PMID: 20065330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrieri MP, Salmaso S, Bella A, D’Ancona F, Demicheli V, Marongiu C, Niglio T, Sellitri C.. Evaluation of the SIMI system, an experimental computerised network for the surveillance of communicable diseases in Italy. Eur J Epidemiol 2000; 16:941 - 7; http://dx.doi.org/ 10.1023/A:1011094116944; PMID: 11338126 [DOI] [PubMed] [Google Scholar]
- 14.ISTAT – National Institute for Statistics. Available at http://demo.istat.it/.


