Abstract
We investigated the etiology of reported sporadic suspected mumps cases with a negative RT-PCR result for the mumps virus in the Barcelona-South region in 2007–2011. Samples from mumps virus-negative patients presenting unilateral or bilateral parotitis or other salivary gland swelling were tested for Epstein-Barr virus (EBV) by real-time PCR and for respiratory viruses by two multiplex-PCR-based assays to detect parainfluenza virus (PIV) 1–4, influenza virus (InV) A, B and C, respiratory syncytial virus (RSV), enterovirus, coronavirus 229E, coronavirus OC43, and rhinovirus. 101 samples were analyzed in persons aged 8 months to 50 years. Oral samples were collected on the first day of glandular swelling in 53 patients (52.5%), and on the first two days in 74 patients (73.3%). Viruses were detected in 52 (51.5%) of samples: one virus (25 EBV, 8 PIV3, 4 adenovirus, 4 PIV2, 1 PIV1, 1 InVA, and 1 enterovirus) was detected in 44 patients (84.6%), two viruses in 7 patients, and three viruses in one patient. In 58 patients (57.5%) whose sample was collected in the first 2 days after onset of parotitis and had received two doses of MMR vaccine and in 15 patients (14.8%) whose sample was collected on the first day, it is very likely that the cause was not the mumps virus. This would mean that 72.3% (73/101) of the reported sporadic suspected mumps cases were not mumps cases. The timing of oral-sample collection is crucial to correctly interpret the negative results for mumps virus RNA, especially when suspected cases occur in vaccinated persons.
Keywords: mumps virus, parotitis, suspected cases, laboratory diagnosis, MMR vaccine, sample collection timing
Introduction
Mumps is an acute generalized infection caused by a virus of the Rubalavirus genus of the Paramyxoviridae family. Infection occurs mainly in school-aged children and adolescents and the most prominent clinical manifestation is nonsuppurative swelling and tenderness of the salivary glands, with one or both parotid glands involved in most cases.1
The mumps virus is the only cause of epidemic parotitis in humans. As in other viral infections, mumps is usually more severe after puberty than in children.1 Complications are uncommon and include orchitis (15–30%) and, less frequently, oophoritis, mastitis, pancreatitis, meningitis, encephalitis, myocarditis, nephritis and permanent hearing impairment.2
Some epidemiological studies suggest that mumps presents interepidemic periods of approximately 3 y3-5 and seasonality has been observed in temperate zones, with a peak incidence in winter and spring.2
By the end of 2006, 112 of the 193 WHO member states included the mumps vaccine in their national immunization schedules and the incidence of mumps has dropped markedly in countries where high levels of vaccine coverage have been maintained.6
In Catalonia, mumps vaccination was introduced in 1980 with one dose of the measles, mumps, rubella vaccine (MMR) at 12 mo of age. In 1988, the first dose was moved to 15 mo and a second dose was introduced at 11 y. To reduce the number of cohorts vaccinated with a single dose, the second dose of MMR was advanced to 4 y in 1998 and the first dose was changed back to 12 mo. A program aimed at eliminating indigenous mumps by the end of 2010, with enhanced disease surveillance reinforced by laboratory confirmation using a PCR test, was implemented.7
Cases with a negative laboratory result for mumps are usually classified as suspected cases of mumps, and a differential diagnosis with other infectious agents is not routine. Parotitis may be caused by the parainfluenza virus (PIV), Epstein-Barr virus (EBV), influenza virus (InV), rhinovirus, adenovirus or other viruses in addition to noninfectious causes such as drugs, immunologic diseases or obstruction of the salivary tract.8,9
The objective of this study was to investigate the possible infectious etiology of sporadic suspected cases of mumps with a negative mumps PCR result between 2007 and 2011.
Results
Patient characteristics
Study cases ranged in age from 8 mo to 50 y (median 6 y), and 64% were male. Parotitis was the presenting symptom in 99% of cases, and other salivary gland involvement in 1%. Most patients (87%) presented unilateral swelling lasting a mean of 4.3 days SD (± 1.8 d). Fever (>38 °C) was observed in 33.7% (34) of cases. No case presented complications or hospitalizations. A total of 88% (89/101) of cases had received MMR vaccination (27% one dose and 73% two doses). Only one case had received one of the two doses with a MMR vaccine containing the Rubini strain; the rest had received vaccines containing the Jeryl Lynn strain.
Table 1 shows the demographic and clinical characteristics and the vaccination status of study patients. One or more study virus was detected in 51.5% of cases. No statistically significant differences were observed in patients according to whether they had a positive or negative result for the study viruses.
Table 1. Patient characteristics of sporadic suspected cases of parotitis according to viral screening of salivary samples. Barcelona-South Health Region, 2007-2011.
| All patients (n = 101) | Virus detected (n = 52) | No virus detected (n = 49) | P | |
|---|---|---|---|---|
| Median Age (Range) | 6 y (8 mo-50 y) | 5.5 y (8 mo-38 y) | 6 y (1–50 y) | 0.3 |
| Male sex, No. (%) | 65 (64.4%) | 32 (61.5%) | 33 (67.3%) | 0.7 |
| Unilateral mumps or other salivary gland, No. (%) | 88 (87%) | 43 (82.7%) | 45 (91.8%) | 0.2 |
| Fever, No. (%) | 34 (33.7%) | 21 (40.4%) | 13 (26.5%) | 0.2 |
| Prodromal Symptoms, No. (%) | 0 (0%) | 0 (0%) | 0 (0%) | __ |
| Complications, No. (%) | 0 (0%) | 0 (0%) | 0 (0%) | __ |
|
Vaccination
0 MMR Doses, No. (%) 1 MMR Dose, No (%) 2 MMR Doses, No (%) Unknown MMR Doses, No (%) |
10 (9.9%) 24 (23.7%) 65 (64.4%) 2 (1.9%) |
4 (7.7%) 15 (28.8%) 31 (59.7%) 2 (3.8%) |
6 (12.2%) 9 (18.4%) 34 (69.4%) 0 (0) |
0.1 |
Viruses detected
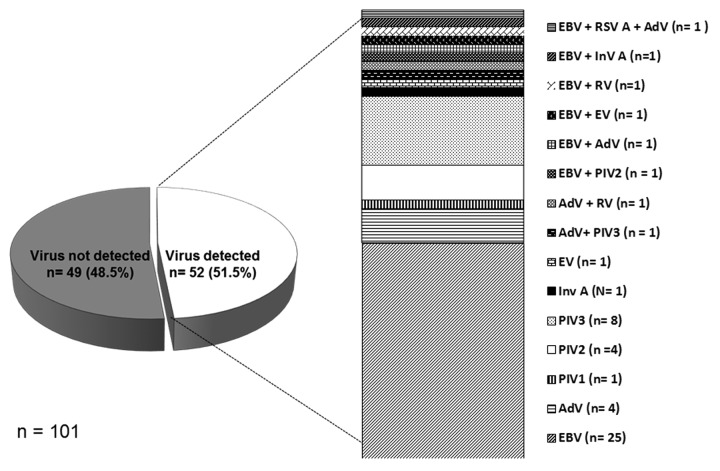
A total of different 15 viruses were detected in 52 (51.5%) oral samples collected. One virus (25 EBV, 8 PIV-3, 4 adenovirus, 4 PIV2, 1 PIV1, 1 InVA, and 1 enterovirus) was found in 84.6% (44) of samples with a positive result, two viruses in 7 samples, and three viruses in one sample (Fig. 1).
Figure 1. Distribution of viruses detected in samples with a negative result for mumps. Barcelona-South Health Region, 2007–2011.
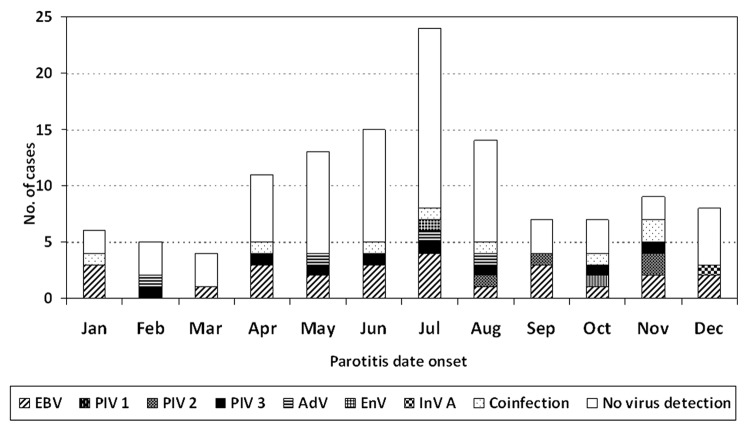
Virus detection was non-significantly lower in the typical mumps season (January to May) than in the remaining months (47.7% and 54.4%, respectively) (P = 0.6) (Fig. 2).
Figure 2. Distribution of viruses detected according to month of onset. Barcelona-South Health Region, 2007–2011.
Table 2 summarizes the characteristics of patients with a positive result for viruses. EBV was the most frequently detected virus (24.8%). Most cases were male (72%), with a median age of 6 y (range 1–38 y). Thirteen of the 21 patients (52%) had received two doses of MMR vaccine. Unilateral salivary gland swelling was the most frequent presentation (76%). No seasonal pattern was observed.
Table 2. Characteristics of suspected sporadic cases of parotitis according to the virus detected. Barcelona-South Health Region, 2007-2011.
| Virus detected | n | Median age (range) | Male sex, No. (%) | Unilateral mumps or other salivary gland, No. (%) | Fever, No. (%) | Seasonal presentation |
|---|---|---|---|---|---|---|
| EBV | 25 | 6 y (1–38 y) | 18 (72%) | 19 (76%) | 10 (40%) | No |
| PIV3 | 8 | 4.5 y (8 mo-13 y) | 3 (37.5%) | 8 (100%) | 3 (37.5%) | Yes Spring (4) Summer (4) |
| PIV2 | 4 | 9 y (5–10 y) | 3 (75%) | 4 (100%) | 1 (25%) | Yes Autumn |
| AdV | 4 | 6.5 y (3–12 y) | 2 (50%) | 3 (75%) | 2 (50%) | Yes Spring (2) Summer (2) |
| PIV1 | 1 | 5 y | 1 (100%) | 1 (100%) | 0 (0%) | Yes Autumn |
| InVA | 1 | 6 y | 1 (100%) | 1 (100%) | 1 (100%) | Yes Winter |
| EV | 1 | 3 y | 0 (0%) | 0 (0%) | 0 (0%) | Yes Summer |
PIV3 and PIV2 were detected in 8% and 4% of patients, respectively. The median age of children with PIV2 infection was higher than in children with PIV3 infection (9 y vs 4 y). Both infections presented unilateral swelling and seasonality.
Timing of oral sample collection
The median time from symptom onset to collection of oral swabs was 1 d (range 1 to 8 d). In 52.5% (53) of patients, the oral sample was collected on the first day after the swelling and in 73.3% (74) on the first 2 days. Of the viruses detected, 52.7% (39/74) corresponded to samples collected in the 2 first days and 41% (11/27) to samples collected later (3–8 d), but the difference was not statistically significant (P = 0.4) (Table 3).
Table 3. Distribution of suspected cases of parotitis according to the timing of collection of salivary samples and MMR vaccination status. Barcelona-South Health Region, 2007- 2011.
| 2 MMR doses (n = 65) | 1 MMR dose (n = 24) | Unvaccinated MMR (n = 10) | Total | ||||
|---|---|---|---|---|---|---|---|
| Timing of salivary sample collection | Virus detected | No virus detected | Virus detected | No virus detected | Virus detected | No virus detected | |
| First day | 17 | 21 | 7 | 3 | 3 | 2 | 53 |
| Second day | 7 | 6 | 4 | 2 | 1 | 1 | 21 |
| Third day | 7 | 5 | 2 | 2 | 0 | 0 | 16 |
| Fourth or more days | 0 | 2 | 2 | 2 | 0 | 3 | 9* |
In 2 additional cases the vaccination status was unknown. Both cases were negative for the viruses tested.
Discussion
Our results show that viruses other than the mumps virus were detected in 51.5% (95% CI: 41.7–61.2%) of reported cases of suspected mumps cases. In this type of case, the other viruses detected may be the cause of parotitis or may be co-circulating viruses in patients with mumps virus infection.
As reported by other authors,10-12 EBV was the most frequently detected virus (24.8%), followed by PIV 3 and 2, and adenovirus. These viruses have been implicated as a cause of parotitis in other studies based on serological findings or isolation of the virus.12-14
The timing of the sample collection and the MMR vaccination status are useful to evaluate the likelihood that a suspected sporadic case of mumps without laboratory confirmation is really a case of mumps or not.
Information on the timing of sample collection is crucial to the correct interpretation of the results of laboratory tests. RT-PCR is the most sensitive test for the mumps virus in oral samples (98%; 95% CI: 93.2–99.2%), and its specificity is also very high (100%; 95% CI: 85.1–100.0%).15,16 Rota et al.,17 found that detection of the mumps virus by RT-PCR decreases after 2 days from the onset of swelling, independently of the vaccination status. The sensitivity of RT-PCR is 87% in oral samples collected on the first day, 78% for the first 2 days and 41% for the first 3 d. Therefore, negative results in samples collected in the first 2 days suggest it is highly likely that the mumps virus can be ruled out as a cause of parotitis. In the present study, 74 (73.3%) oral samples were collected in the first 2 days and viruses were detected in 39, the most frequent: 21 EBV, 6 PIV3 and 3 PIV2. No virus was detected in the remaining 35 patients, possibly because the causal agent was not tested for or the sensitivity of the test used was not 100%.15-17 However, in 26 (74.3%) patients, the oral sample was collected on the first day of the onset of swelling.
In field studies, the effectiveness of two doses of the MMR vaccine containing the widely-used Jeryl Lynn strain varies from 88% (95%CI: 63–96%)18 to 95% (95%CI: 93–96%).19 Patients who have received two MMR doses containing this strain have a lower probability of presenting mumps than unvaccinated patients. In our study, 89 patients had received the MMR vaccine (24 one dose and 65 two doses). In 78.5% (51) of patients who had received two doses of MMR, the sample had been collected in the first 2 days after the onset of swelling. In the other 14 patients, the sample was collected 3 days after swelling onset and viruses were detected in 7 (6 EBV and 1 PIV3) of these patients. This suggests that 57.4% (58/101) of cases classified as suspected mumps cases were not caused by the mumps virus.
These criteria might also be used to assess patients who have received one dose of MMR vaccine and unvaccinated patients whose oral sample was collected on the first day after the onset of swelling (10 patients with 1 MMR dose and 5 unvaccinated). In this case, the probability that patients did not have mumps virus infection would increase to 72.3% (73/101).
It is possible that some study patients did have mumps, even though the RT-PCR result was negative. In 27 patients, saliva was collected ≥3 days after the onset of swelling and other viruses were detected in 11 of these patients (9 EBV, 1 PIV2 and 1 PIV3); no virus was detected in the remaining 16 patients. In these cases (16%) mumps cannot be ruled out and the cases should be classified as suspected cases of mumps with false negative RT-PCR results15,17 (Table 3).
Some authors have suggested that a loss of immunity could explain the presence of mumps in people vaccinated with the MMR vaccine.19-22 Our results show that 60% of patients were aged <8 y, were vaccinated at one year or after, and had no deficiencies in the humoral response.23 Therefore, it seems unlikely that secondary vaccine failure (waning of immunity) or primary vaccine failure could be associated with mumps virus infection.24
Finland is the only country where mumps has been eliminated using a two-dose MMR policy.25,26 However, mumps is endemic throughout the world, achieving elimination is considered difficult, and current goals center on reduction.27,28 To evaluate the objectives of control and/or elimination of mumps, appropriate and timely surveillance of reported cases, and scientifically-relevant homogeneous case definitions are necessary. At present, sporadic suspected cases of mumps are generally assumed to be caused by the mumps virus, even when laboratory tests are negative. It is crucial to distinguish between mumps and other diseases causing parotitis. Our results show that 72% of suspected cases were probably not mumps. If these data are extrapolated to the whole of Catalonia, 265 of the 367 cases classified as suspected mumps during the study period would probably not be true mumps infections.29
There are no unified criteria for the definition and classification of mumps cases in developed countries.30-33 The 2012 European Centre for Disease Prevention and Control definition incorporated the presence of fever in the case definition of mumps.33 In the present study, 63 cases of mumps were confirmed by RT-PCR during the study period, of which 49% (31/63) did not present fever. Therefore, these cases would not be considered true cases of mumps if this criterion were applied.
The strength of this study is that all reported cases were exhaustively investigated and no epidemiological link was found in any case: therefore, they may be considered true sporadic cases.
A limitation of the study is the possibility that other viruses which were not tested for could have contributed to the clinical manifestations in our patients, and that, in reality, the number of cases positive for viruses other than mumps may have been higher. Likewise, an etiologic relationship between all the viruses detected and parotitis should not be assumed, as the carriage rate among similar healthy individuals is unknown.5
In conclusion, in a high proportion of suspected sporadic cases of mumps reported to public health services a possible etiologic origin due to other viruses was found. To correctly rule out the etiology of mumps in sporadic cases with negative RT-PCR results, other viruses should be investigated when oral samples are collected after the first two days of swelling. This is especially important because most possible patients have received the MMR vaccine.
Materials and methods
Setting and study period
The study was performed in the Barcelona-South Health region between 2007 and 2011. The mean population in this period was 1 290 525. In the study period, 209 cases of mumps were reported, of which 85 were confirmed cases (63 laboratory confirmed and 22 epidemiologically linked to a confirmed case), and 124 were suspected cases. The median incidence rate was 2.4/100 000 persons-year; range 1.8 - 7.1/100 000 persons-year. In 2007, there was a mumps epidemic, while the years 2008–2011 were interepidemic years.
Cases of parotitis were investigated by field epidemiologists according to routine procedures for the investigation of mumps cases.30 The case definition of a suspected case of mumps was acute onset of unilateral or bilateral swelling of the parotid or other salivary glands lasting at least 2 d, without laboratory confirmation of mumps. Information on age, sex, onset date of parotitis, vaccination status, date of sample collection and diagnostic tests were collected as part of the epidemiological case study. A suspected case was considered sporadic if the patient was not epidemiologically linked to a laboratory-confirmed case or to another case of parotitis.
Patient selection
All suspected sporadic cases of mumps reported between 2007 and 2011 in the Barcelona-South Health region for which saliva samples were available were included in the study (n = 101). All cases included were negative for mumps RT-PCR in salivary samples.
Laboratory methods
Viral genomic RNA and DNA was extracted from a total volume of 200 μl of sample, by the guanidinium thiocyanate extraction method using an EasyMag extractor. The lysis buffer included an internal control in each reaction tube to exclude false-negative results due to non-specific inhibitors or extraction failure.
Mumps virus infection was assessed in oral samples by one-step RT-PCR, amplifying a region located in the fusion (F) gene, as described previously.15 The analytical sensitivity of this assay is, on average, 100-fold greater than the previously widely-used RT nested-PCR assay amplifying the SH gene.34
In cases where RT-PCR was negative for mumps, EBV and respiratory viruses were tested for. EBV was investigated by RT-PCR using the EBVQ-PCR Alert Amplimix Kit (Nanogen®). For respiratory viruses, two independent multiplex reverse transcription nested-PCR assays able to detect from 1 to 10 copies of viral genomes were performed as described previously.35,36 We used specific primers for InV types A, B and C, respiratory syncytial virus (RSV) type A and B, and adenovirus in one RT-PCR assay, and specific primers for PIV 1, 2, 3, and 4, coronavirus 229E and OC43, and for generic detection of rhinovirus and enterovirus in another RT-PCR assay. In each assay, negative (viral transport medium containing no nucleic acid) and positive controls (cDNA obtained from our viral lysates or from reference strains) were treated with the same procedure.
Statistical methods
Proportions were compared using the χ2 test or Fisher exact test (when indicated). Means were compared using the Student’s t test. The level of statistical significance was established as an α error of 0.05. The analyses were performed using the SPSS v18.0 for Windows and Epistat statistical packages.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
This work was partially funded by CIBER Epidemiología y Salud Pública (CIBERESP), Spain and by AGAUR (expedient number 2009/SGR 42).
The authors thank the reporting physicians of the Barcelona-South Health region and public health nurses of the Surveillance Unit, Teresa Tolo and Eva Donate, who were instrumental in collecting data and managing the cases.
Glossary
Abbreviations:
- AdV
adenovirus
- cDNA
complementary deoxyribonucleic acid
- d
day
- DNA
deoxyribonucleic acid
- EBV
Epstein-Barr Virus
- EV
enterovirus
- PIV
parainfluenza virus
- InV
influenza virus
- InVA
influenza virus type A
- MMR vaccine
measles, mumps and rubella vaccine
- mo
month
- RNA
ribonucleic acid
- RT-PCR
reverse-transcriptase polymerase chain reaction
- RSV
respiratory syncytial virus
- RSVA
respiratory syncytial virus type A
- RV
rhinovirus
- SH gene
small hydrophobic gene
- WHO
World Health Organization
- y
year
References
- 1.Litman N, Baum SG. Mumps virus. In: Mandell GL, Bennet JE, Dolin R, editors. Principle and Practice of Infectious Diseases. 7th ed. Philadelphia: Churchill Livingstone 2010: 2210-6. [Google Scholar]
- 2.Rubin SA, Plotkin SA. Mumps vaccine. In: Plotkin SA, Orentsin WA, Offit PA, editors. Vaccines 6th ed. Philadelphia: Elsevier Saunders, 2013: 419-46. [Google Scholar]
- 3.Nokes DJ, Wright J, Morgan-Capner P, Anderson RM.. Serological study of the epidemiology of mumps virus infection in north-west England. Epidemiol Infect 1990; 105:175 - 95; http://dx.doi.org/ 10.1017/S0950268800047762; PMID: 2384142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo M, Alia JM, Mateos ML, Carrasco JL, Ballesteros F, Lardinois R.. Natural immunity to measles, rubella and mumps among Spanish children in the pre-vaccination era. Int J Epidemiol 1986; 15:95 - 100; http://dx.doi.org/ 10.1093/ije/15.1.95; PMID: 3957548 [DOI] [PubMed] [Google Scholar]
- 5.Barskey AE, Glasser JW, LeBaron CW.. Mumps resurgences in the United States: A historical perspective on unexpected elements. Vaccine 2009; 27:6186 - 95; http://dx.doi.org/ 10.1016/j.vaccine.2009.06.109; PMID: 19815120 [DOI] [PubMed] [Google Scholar]
- 6.Heyman DL, ed. Control of communicable diseases manual. 19th edition. Washington: American Public Health Association 2008; 432-4. [Google Scholar]
- 7.Domínguez A, Oviedo M, Torner N, Carmona G, Costa J, Caylà J, Sala MR, Barrabeig I, Camps N, Minguell S, et al. , Mumps Control Working Group of Catalonia.. Mumps: a year of enhanced surveillance in Catalonia, Spain. Vaccine 2009; 27:3492 - 5; http://dx.doi.org/ 10.1016/j.vaccine.2009.03.022; PMID: 19460603 [DOI] [PubMed] [Google Scholar]
- 8.Fiebelkorn AP, Barskey A. Hickman, Bellini W. Mumps. In: Roush SW, McIntyre L, Baldy LM, editors. Vaccine Preventable Diseases Manual. 5th ed. 2012. Available at: http://www.cdc.gov/vaccines/pubs/surv-manual/index.html. Accessed 27 May 2014.
- 9.Rubin S, Carbone KM. Mumps. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison’s principles of internal medicine. 18th ed. New York: McGraw-Hill, 2012: 1608-10. [Google Scholar]
- 10.Barskey AE, Juieng P, Whitaker BL, Erdman DD, Oberste MS, Chern SW, Schmid DS, Radford KW, McNall RJ, Rota PA, et al.. Viruses detected among sporadic cases of parotitis, United States, 2009-2011. J Infect Dis 2013; 208:1979 - 86; http://dx.doi.org/ 10.1093/infdis/jit408; PMID: 23935203 [DOI] [PubMed] [Google Scholar]
- 11.Hatchette TF, Mahony JB, Chong S, LeBlanc JJ.. Difficulty with mumps diagnosis: what is the contribution of mumps mimickers?. J Clin Virol 2009; 46:381 - 3; http://dx.doi.org/ 10.1016/j.jcv.2009.09.024; PMID: 19828368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidkin I, Jokinen S, Paananen A, Leinikki P, Peltola H.. Etiology of mumps-like illnesses in children and adolescents vaccinated for measles, mumps, and rubella. J Infect Dis 2005; 191:719 - 23; http://dx.doi.org/ 10.1086/427338; PMID: 15688285 [DOI] [PubMed] [Google Scholar]
- 13.Meurman O, Vainionpää R, Rossi T, Hänninen P.. Viral etiology of parotitis. Scand J Infect Dis 1983; 15:145 - 8; PMID: 6308753 [DOI] [PubMed] [Google Scholar]
- 14.Lee AC, Lim WL, So KT.. Epstein-Barr virus associated parotitis. J Paediatr Child Health 1997; 33:177 - 8; http://dx.doi.org/ 10.1111/j.1440-1754.1997.tb01033.x; PMID: 9145372 [DOI] [PubMed] [Google Scholar]
- 15.Krause CH, Eastick K, Ogilvie MM.. Real-time PCR for mumps diagnosis on clinical specimens--comparison with results of conventional methods of virus detection and nested PCR. J Clin Virol 2006; 37:184 - 9; http://dx.doi.org/ 10.1016/j.jcv.2006.07.009; PMID: 16971175 [DOI] [PubMed] [Google Scholar]
- 16.Hatchette T, Davidson R, Clay S, Pettipas J, Leblanc J, Sarwal S, Smieja M, Forward K.. Laboratory diagnosis of mumps in a partially immunized population: The Nova Scotia experience. Can J Infect Dis Med Microbiol 2009; 20:e157 - 62; PMID: 21119794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rota JS, Rosen JB, Doll MK, McNall RJ, McGrew M, Williams N, Lopareva EN, Barskey AE, Punsalang A Jr., Rota PA, et al.. Comparison of the sensitivity of laboratory diagnostic methods from a well-characterized outbreak of mumps in New York city in 2009. Clin Vaccine Immunol 2013; 20:391 - 6; http://dx.doi.org/ 10.1128/CVI.00660-12; PMID: 23324519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marin M, Quinlisk P, Shimabukuro T, Sawhney C, Brown C, Lebaron CW.. Mumps vaccination coverage and vaccine effectiveness in a large outbreak among college students--Iowa, 2006. Vaccine 2008; 26:3601 - 7; http://dx.doi.org/ 10.1016/j.vaccine.2008.04.075; PMID: 18539365 [DOI] [PubMed] [Google Scholar]
- 19.Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, Brown D, Ramsay ME.. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerg Infect Dis 2007; 13:12 - 7; http://dx.doi.org/ 10.3201/eid1301.060649; PMID: 17370510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz NG, Bernard H, Melnic A, Bucov V, Caterinciuc N, an der Heiden M, Andrews N, Pebody R, Aidyralieva C, Hahné S.. Mumps outbreak in the Republic of Moldova, 2007-2008. Pediatr Infect Dis J 2010; 29:703 - 6; http://dx.doi.org/ 10.1097/INF.0b013e3181d743df; PMID: 20308934 [DOI] [PubMed] [Google Scholar]
- 21.Peltola H, Kulkarni PS, Kapre SV, Paunio M, Jadhav SS, Dhere RM.. Mumps outbreaks in Canada and the United States: time for new thinking on mumps vaccines. Clin Infect Dis 2007; 45:459 - 66; http://dx.doi.org/ 10.1086/520028; PMID: 17638194 [DOI] [PubMed] [Google Scholar]
- 22.Kontio M, Jokinen S, Paunio M, Peltola H, Davidkin I.. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. J Infect Dis 2012; 206:1542 - 8; http://dx.doi.org/ 10.1093/infdis/jis568; PMID: 22966129 [DOI] [PubMed] [Google Scholar]
- 23.Eriksen J, Davidkin I, Kafatos G, Andrews N, Barbara C, Cohen D, Duks A, Griskevicius A, Johansen K, Bartha K, et al.. Seroepidemiology of mumps in Europe (1996-2008): why do outbreaks occur in highly vaccinated populations?. Epidemiol Infect 2013; 141:651 - 66; http://dx.doi.org/ 10.1017/S0950268812001136; PMID: 22687578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heininger U, Bachtiar NS, Bahri P, Dana A, Dodoo A, Gidudu J, Santos EM.. The concept of vaccination failure. Vaccine 2012; 30:1265 - 8; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.048; PMID: 22197579 [DOI] [PubMed] [Google Scholar]
- 25.Peltola H, Heinonen OP, Valle M, Paunio M, Virtanen M, Karanko V, Cantell K.. The elimination of indigenous measles, mumps, and rubella from Finland by a 12-year, two-dose vaccination program. N Engl J Med 1994; 331:1397 - 402; http://dx.doi.org/ 10.1056/NEJM199411243312101; PMID: 7969278 [DOI] [PubMed] [Google Scholar]
- 26.Peltola H, Jokinen S, Paunio M, Hovi T, Davidkin I.. Measles, mumps, and rubella in Finland: 25 years of a nationwide elimination programme. Lancet Infect Dis 2008; 8:796 - 803; http://dx.doi.org/ 10.1016/S1473-3099(08)70282-2; PMID: 19022194 [DOI] [PubMed] [Google Scholar]
- 27.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS, Centers for Disease Control and Prevention.. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary secommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:RR-041 - 34; PMID: 23760231 [PubMed] [Google Scholar]
- 28.WHO.. Mumps virus vaccine. Wkly Epidemiol Rec 2007; 82:51 - 60 [PubMed] [Google Scholar]
- 29.Agència de Salut Pública de Catalunya. Pla d’eliminació de la parotiditis a Catalunya: avaluació i perspectives de futur. Butlletí Epidemiològic de CatalunyaGeneralitat 2011; 32:126-35. Available at: http://www20.gencat.cat/docs/canalsalut/Home%20Canal%20Salut/Professionals/Recursos/Butlletins_de_salut/PROMOCIO_I_PROTECCIO_DE_LA_SALUT/BEC_Butlleti_pidemiologic_de_Catalunya/2011/Arxius/octubre%202011.pdf Accessed 27 May 2014.
- 30.Centers for Disease Control and Prevention. Mumps. In: Manual for the surveillance of vaccine-preventable diseases. Centers for Disease Control and Prevention, Atlanta, GA, 5th Edition, 2012. Available at: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt09-mumps.pdf. Accessed 27 May 2014.
- 31.Canada Communicable Disease Report. Guidelines for the Prevention and Control of Mumps Outbreaks in Canada, 2010; 36(Suppl1): 6-7. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10vol36/36s1/index-eng.php. Accessed 27 May 2014. [DOI] [PMC free article] [PubMed]
- 32.Department of Health. Surveillance Case Definitions for the Australian National Notifiable Diseases Surveillance System. Australian Government. Available at:http://www.health.gov.au/internet/main/publishing.nsf/Content/cdna-casedefinitions.htm//$File/consolidated-case-definitions-may2014.pdf. Accessed 27 May 2014.
- 33.European Centre for Disease Prevention and Control. Case definitions for reporting communicable diseases to the Community network, 2012. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:262:0001:0057:EN:PDF. Accessed 27 May 2014.
- 34.Jin L, Brown DWG, Litton PA, White JM.. Genetic diversity of mumps virus in oral fluid specimens: application to mumps epidemiological study. J Infect Dis 2004; 189:1001 - 8; http://dx.doi.org/ 10.1086/382134; PMID: 14999602 [DOI] [PubMed] [Google Scholar]
- 35.Coiras MT, Pérez-Breña P, García ML, Casas I.. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested-PCR assay. J Med Virol 2003; 69:132 - 44; http://dx.doi.org/ 10.1002/jmv.10255; PMID: 12436489 [DOI] [PubMed] [Google Scholar]
- 36.Coiras MT, Aguilar JC, García ML, Casas I, Pérez-Breña P.. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J Med Virol 2004; 72:484 - 95; http://dx.doi.org/ 10.1002/jmv.20008; PMID: 14748074 [DOI] [PMC free article] [PubMed] [Google Scholar]