Abstract
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by the presence of the Philadelphia (Ph) chromosome resulting from the reciprocal translocation t(9;22)(q34;q11). The molecular consequence of this translocation is the generation of the BCR–ABL fusion gene, which encodes a constitutively active protein tyrosine kinase. The oncogenic protein tyrosine kinase, which is located in the cytoplasm, is responsible for the leukemia phenotype through the constitutive activation of multiple signaling pathways involved in the cell cycle and in adhesion and apoptosis. Avascular necrosis of the femoral head (AVNFH) is not a specific disease. It occurs as a complication or secondary to various causes. These conditions probably lead to impaired blood supply to the femoral head. The diagnosis of AVNFH is based on clinical findings and is supported by specific radiological manifestations. We reported a case of a 34-year-old Sudanese female with CML who developed AVNFH after receiving dasatinib as a second-line therapy. Though the mechanism by which dasatinib can cause avascular necrosis (AVN) is not clear, it can be postulated because of microcirculatory obstruction of the femoral head. To the best of our knowledge and after extensive literature search, this is the first reported case of AVNFH induced by dasatinib in a patient with CML.
Keywords: dasatinib, CML, avascular necrosis of the femoral head (AVNFH)
Background
Chronic myelogenous leukemia (CML) is a chronic myelo-proliferative disorder with an initially chronic course lasting for 3–5 years. It eventually transforms into accelerated or blastic phases, which are generally fatal. CML was one of the first diseases in which a specific chromosomal abnormality was identified, a t(9;22)(q34;q11) or Philadelphia (Ph) chromosome.
Avascular necrosis of the femoral head (AVNFH) occurs as a complicated traumatic or nontraumatic disorder. Most cases of AVNFH are nontraumatic and occur secondary to excessive corticosteroid use and alcohol abuse.1 Other causes include coagulopathies, hemoglobinopathies (eg, sickle cell disease), chronic liver disease, gout, idiopathic hyperlipidemia, metabolic bone disorders, pregnancy, radiation, chemotherapy, smoking, systemic lupus erythematosus, and vasculitis syndromes. Intravascular coagulation appears to be the central event associated with nontraumatic AVNFH.2 Coagulation may occur secondary to extravascular compression (eg, marrow fat enlargement), vessel wall injury (eg, chemotherapy, radiation), or a thromboembolic event (eg, fat emboli). In addition, ischemic insult to the femoral head may result in subchondral bone infarction. In this situation, weakened and unrepaired necrotic bony trabeculae fail under a compressive load, leading to subchondral collapse (eg, crescent sign), and ultimately, articular collapse.3 Traumatic causes of femoral head avascular necrosis (AVN) include femoral neck fractures, hip dislocation, and slipped capital femoral epiphysis.4
AVNFH can be presenting manifestation for a patient with CML. We did literature review and came with number of cases reporting AVNFH as an initial presentation of CML, illustrated in Table 1.
Table 1.
CML presenting with AVNFH.
| PATIENT | AGE | GENDER | SITE | NOTE | YEAR | REFERENCE |
|---|---|---|---|---|---|---|
| 1 | 24 | Male | Rt Femoral Head | Leukocyte count 96,800/mm3, Platelets count 684,000/mm3, and Hemoglobin 10.4 g/dL | 2005 | Moon JY, et al5 |
| 2 | 15 | Female | Rt Femoral Head | Leukocyte count of 290 × 109/L, Platelet count 250 × 109/L, Hemoglobin 10.8 g/dL | 2003 | Gupta D, et al6 |
| 3 | 17 | Male | Rt Femoral Head | Unknown | 1984 | Gibson J, et al7 |
| 4 | 9 | Female | Lt Femoral Head | Leukocyte count 359,000/mm3, Platelets count 809,000/mm3 | 1988 | Salimi Z, et al8 |
| 5 | 17 | Male | Rt Femoral Head | Unknown | 1996 | Leone J, et al9 |
| 6 | 12 | Female | Rt Femoral Head | Unknown | 2013 | Leone J, et al10 |
AVNFH has been reported as an initial presentation in few cases with CML by many authors (Table 1).
Case Presentation
A 34-year-old Sudanese female with the diagnosis of CML was started on imatinib as a first-line therapy, but she failed the first-line therapy as per European LeukemiaNet guidelines 2010. She was referred to the hematology service at the National Center for Cancer Care & Research (NCCCR) for further evaluation. Her clinical examination was unremarkable, and her work-up was repeated, including a complete blood count, cytogenetics, and BCR/ABL by PCR plain radiograph before treatment was available and she was reported as normal. Her work-up revealed a chronic phase of CML in failing first-line therapy. She was started on dasatinib 100 mg PO once daily as a second-line therapy with which she achieved CHR, CCYR, and MMR at 18 months. After 18 months on therapy with dasatinib, she presented with severe pain in her left groin with limping. Her complete blood count (CBC) was within normal limit—WBC 6000, Hb 13 g/dL, and Plts 235,000. Her peripheral smear reported as normal, and her disease revaluated at the molecular level and showed major molecular response. Radiological evaluation, including pelvic radiography (see Fig. 1A and B) and magnetic resonance imaging (MRI) showed grade 3–4 AVNFH (see Fig. 2A–E).
Figure 1.
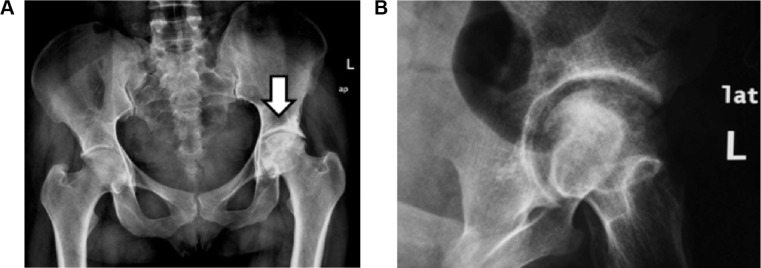
(A) Plain radiography (anteroposterior (AP) view of both hip joints) and (B) pelvic radiography (close oblique view of the left hip).
Notes: Plain radiography of the hip joints: AP view and oblique view of the left hip showing heterogeneous matrix of the left femoral head giving geographical appearance with mixed sclerosis and lucent areas as well as relative mild narrowing of the ipsilateral joint space representing AVN-related changes.
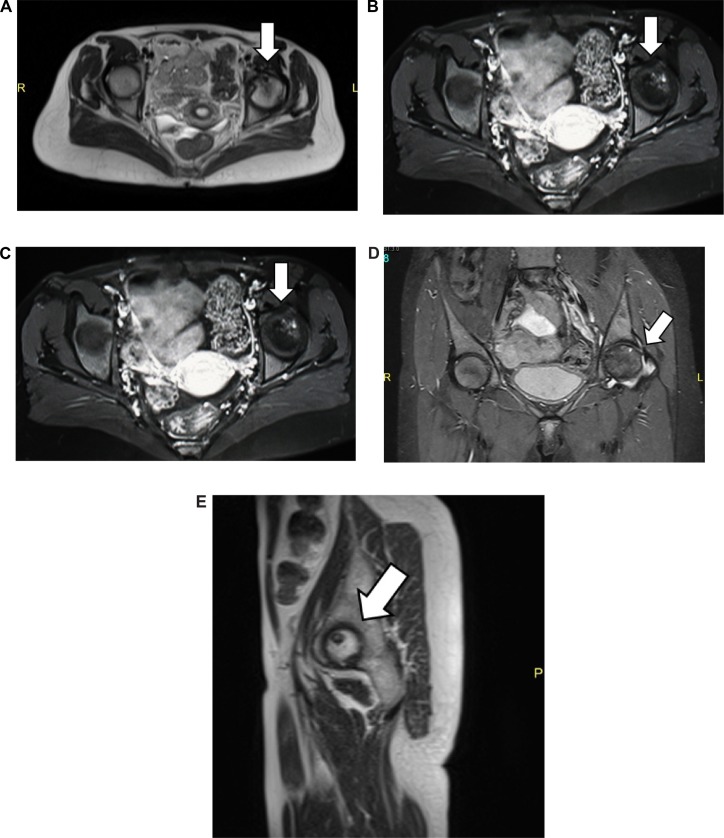
Figure 2.
MRI of both hip joints: (A) axial view of the T2WI sequence, (B) axial view of the T1WI sequence post-contrast (gadolinium DTPA) intravenous administration, (C) coronal view of the T2WI sequence, (D) coronal T2 STIR sequence, and (E) sagittal view of the left hip joint T2WI sequence.
Notes: MRI examination of the hip joints before and after intravenous administration of gadolinium. Axial, coronal, and sagittal views of the hip joints T1, T2, and STIR showing multiple subarticular areas of abnormal signal intensity within the head left femur, mainly at the superoanterior medial aspect showing low T1 signal intensity and non-homogenous mixed T2-fat suppression with mild enhancement. Associated mild degenerative changes and thickened synovium are noted, consistent with stage III–IV AVN. No signs of AVN on the right hip were detected.
She underwent total hip replacement, which was successful.
Discussion
AVNFH is a recognized complication for a group of traumatic and nontraumatic disorders. The incidence of AVNFH in CML is not identified. This is because of the limited number of reports and absence of prospective studies evaluating this issue in this situation. In addition, whether the disease itself and/or the treatment encourage the development of AVNFH is not known. Post-contrast material-enhanced MRI with its inherent high spatial resolution capabilities is considered an excellent diagnostic tool for detecting and staging of femoral head AVN. It is considered the preferred method for diagnosis of occult AVN, since it is more sensitive than bone scan or plain films. Owing to the high incidence of bilateral AVN, MRI may pick up AVN in opposite asymptomatic hip. An AVN lesion is typically a well-demarcated epiphyseal area of altered and variable signal intensity (Figs. 1 and 2). In its early stages, T2-weighted images and STIR short tau-inversion recovery can help in detection of necrotic tissue in some unusual AVN lesions that mostly showed ill-delimited edema-like marrow changes.11,12 MRI has 90–100% sensitivity for symptomatic disease. In some cases, contrast-enhanced MRIs may increase diagnostic confidence by showing homogeneous hypervascularization in bone marrow edema lesions and by depicting hypovascular marrow areas in AVN lesions. Also the MRI can discriminate between the AVN and transient marrow edema.11,13 Sequential and follow-up MRI is considered valuable in the assessment of equivocal femoral head lesions, especially in its early stages where the findings are usually trivial.12,13
Treatment historically has passed through two eras. The era of interferon: there is a limited data that interferon alfa 2a can cause AVNFH. There are no reports in the literature about AVNFH with other therapeutic uses of interferon alfa. Therefore, the occurrence of AVNFH in patients with CML on interferon treatment may be the result of an interaction between CML and interferon alfa therapy. Interferon alfa can inhibit angiogenesis, which may cause AVN, and the stress of weight bearing may make the femoral head, particularly, vulnerable.14 The second era for CML treatment started with using tyrosine kinase inhibitor (TKI) Table 3. Few cases of AVNFH have been reported at disease presentation (CML) as well as with the use of first-generation TKIs imatinib (Glivec®). Though the mechanism by which dasatinib can cause AVN is not clear, it can be postulated because of micro-circulatory obstruction of the femoral head.
Table 3.
Patients treated with TKIs.
| SUMMARY OF PATIENTS WITH CHRONIC MYELOID LEUKEMIA AND ASSOCIATED AVNFH | |||||||
|---|---|---|---|---|---|---|---|
| PATIENT | PATIENT (Yrs) | GENDER | INTERVAL FROM CML dx TO DEVELOPMENT OF AVN | PLATELET AND WBC COUNTS AT TIME OF AVN dx | TKI DOSE | OTHER Rx | COMMENT |
| 1 | 12 | Male | 8 years | WBC 5600/mm3 | Start dose 400 mg/d; escalated to 600 mg/d to achieve complete cytogenetic response | Dx as CML (chronic phase in 2005; started on imatinib 400 mg (340 mg/m2) after 20 months dose escalated to 600 mg/day (continued for 1 yr)16 | |
| 2 | 34 | Female | 3 years | WBC 6000, Hb 13 g/dl, and Plts 235/l | Failed imatinib 400 mg then shifted to Dasatinib 100 mg | Developed AVNFH 18 months after dastinib in CHR, CCR, MMR17 | |
Era of Interferon-alfa15 AVN in CML patients treated with interferon. Table 2 illustrates that patients with CML developed AVNFH while on interferon alfa.
Table 2.
Patients treated with interferon-alfa.
| SUMMARY OF PATIENTS WITH CHRONIC MYELOID LEUKEMIA AND ASSOCIATED AVNFH | |||||||
|---|---|---|---|---|---|---|---|
| PATIENT | PATIENT (Yrs) | GENDER | INTERVAL FROM CML dx TO DEVELOPMENT OF AVNFH | PLATELET AND WBC COUNTS AT TIME OF AVNFH dx | IFNα DOSE | OTHER Rx | COMMENT |
| 1 | 22 | Male | 18 | Platelets, 61–140 ×109/L; WBC, 2.5–3.5 ×109/L | 5MU/q.o.d. to2MU 2×/week | HU pegylated IFN, steroids × 1 week, anagrelide | |
| 2 | 45 | Female | 54 | Platelets, 120–210 × 109/L; WBC, 15×109/L | Varied from 10 MU/day to 5 MU/day | HU, busulfan, ara-C (3 mos) | |
| 3 | 46 | Female | 6 | Platelets, 160–220 × 109/L; WBC, 8.4–18 ×109/L | 10 MU/day with concurrent ATRA and ara-C | HU | |
| 4 | 17 | Male | Presenting symptom and symptoms recurred 1 month after starting IFNa and ara-c | Platelets, 895×109/L; WBC, 167×109/L | Unknown | HU | HU cytoreduction a/w clinical and radiographic improvement of ANFH |
| 5 | 25 | Female | 4 yrs | Platelets, 1200×109/L; WBC49×109/L | Unknown | HU | ANFH developed when CML entered accelerated phase after 4 yrs of IFNα therapy |
Abbreviations: CML, chronic myeloid leukemia; dx, diagnosis; AVN, avascular necrosis; WBC, leukocyte; IFNa, interferon-a; HU, hydroxyurea; ara-C, cytosine arabinoside; ATRA, all-trans retinoic acid; MU, million unit; N/A, not applicable; a/w, associated with; AVNFH, avascular necrosis of the femoral head.
Conclusion
The above mentioned review of literature states that six patients with CML presented with AVNFH as the initial presentation prior to any therapy, five in the era of interferon and two in the era with TKIs, and one with imatinib and the other with dasatinib treatment. There are two issues to be considered: either the condition is rare or there is underreporting of this side effect. Observational studies with proper reporting are required to accurately measure the incidence of this complication, which could significantly affect patients’ safety and quality of life.
Footnotes
ACADEMIC EDITOR: Robert E. Richard, Editor in Chief
FUNDING: This research was conducted as part of a Qatar National Research Fund-sponsored project titled Novel Approach in molecular pathophysiology of myeloprofilerative neoplasms: what determines phenotypes of JAK2 mutations (Qatari prospective). NPRP No. 4-471-3-148. MAY is the lead PI of the project. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: MAY. Analyzed the data: MAY, AHM. Wrote the first draft of the manuscript: MAY, AHM. Contributed to the writing of the manuscript: MAY, AHM. Agree with manuscript results and conclusions: MAY, AHM, AJN, ATS, HE, SFM, DSM, SE, DRA, HLGG, RMH, MAM, SK, NAD, AE. Jointly developed the structure and arguments for the paper: MAY, AHM. Made critical revisions and approved final version: MAY, AHM, AJN, ATS, HE, SFM, DSM, SE, DRA, HLGG, RMH, MAM, SK, NAD, AE. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77(3):459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;386:173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am AcadOrthop Surg. 1999;7(4):250–261. doi: 10.5435/00124635-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Arlet J, Ficat P. [Non-traumatic avascular femur head necrosis. New methods of examination and new concepts] [Polish] Chir Narzadow Ruchu Ortop Pol. 1977;42(3):269–276. [PubMed] [Google Scholar]
- 5.Moon JY, Kim BS, Yun HR, et al. Rheology of leukocytes, leukocyte suspensions, and blood in leukemia possible relationship to clinical manifestations. Korean J Intern Med. 2005;20(3):255–259. [Google Scholar]
- 6.Gupta D, Gaiha M, Siddaraju N, Daga MK, Anuradha S. Chronic myeloid leu-kemia presenting with avascular necrosis of femur head. J Assoc Physicians India. 2003;51:214–215. [PubMed] [Google Scholar]
- 7.Gibson J, Joshua DE, Collis D, Kronenberg H. Chronic myeloid leukaemia presenting as femoral head necrosis. Scand J Haematol. 1984;32:376–378. doi: 10.1111/j.1600-0609.1984.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 8.Salimi Z, Vas W, Sundaram M. Avascular bone necrosis in an untreated case of chronic myelogenous leukemia. Skeletal Radiol. 1988;17:353–355. doi: 10.1007/BF00367182. [DOI] [PubMed] [Google Scholar]
- 9.Leone J, Vilque JP, Pignon B, et al. Avascular necrosis of the femoral head as a complication of chronic myelogenous leukemia. Skeletal Radiol. 1996;25:696–698. doi: 10.1007/s002560050163. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Bansal D, Prakash M, Sharma P. Avascular necrosis of femoral head as the initial manifestation of CML. Pediatric Hematol Oncol. 2014;31(6):568–573. doi: 10.3109/08880018.2013.831961. [DOI] [PubMed] [Google Scholar]
- 11.Vande Berg, BE Malghem, JJ Labaisse, MA Noel, HM Maldague, BE Vande, Berg BE. MR imaging of avascular necrosis and transient marrow edema of the femoral head. Radiographics. 1993;13:501–520. doi: 10.1148/radiographics.13.3.8316660. [DOI] [PubMed] [Google Scholar]
- 12.Karantanas AH, Drakonaki EE. The role of MR imaging in avascular necrosis of the femoral head. Semin Musculoskelet Radiol. 2011;15(3):281–300. doi: 10.1055/s-0031-1278427. [DOI] [PubMed] [Google Scholar]
- 13.Sansgiri RK, Neel MD, Soto-Fourier M, Kaste SC. Unique MRI findings as an early predictor of osteonecrosis in pediatric acute lymphoblastic leukemia. AJR Am J Roentgenol. 2012;198(5):W432–W439. doi: 10.2214/AJR.11.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith DW. Is avascular necrosis of the femoral head the result of inhibition of angiogenesis? Med Hypotheses. 1997;49(6):497–500. doi: 10.1016/s0306-9877(97)90067-0. [DOI] [PubMed] [Google Scholar]
- 15.Kozuch P, Talpaz M, Faderl S, O’Brien S, Freireich EJ, Kantarjian H. Avascular necrosis of the femoral head in chronic myeloid leukaemia patients treated with interferon-alpha: a synergistic correlation? Cancer. 2000;89(7):1482–1489. doi: 10.1002/1097-0142(20001001)89:7<1482::aid-cncr10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Nataraj V, Kandasamy D, Bakhshi S. Imatinib-induced avascular necrosis of femur in childhood chronic myeloid Leukemia. Pediatr Hematol Oncol. 2014;31:268–270. doi: 10.3109/08880018.2013.862588. [DOI] [PubMed] [Google Scholar]
- 17.Yassin MA. Dasatinib-induced avascular necrosis of femoral head in adult patient with chronic myeloid leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apperley JF. Chronic myeloid leukaemia. Lancet. 2015;385(9976):1447–1459. doi: 10.1016/S0140-6736(13)62120-0. Epub 2014 Dec 5. [DOI] [PubMed] [Google Scholar]
- 19.Vail TP, Covington DB. The incidence of osteonecrosis. In: Urbaniak JR, Jones JR, editors. Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont: American Academy of Orthopedic Surgeons; 1997. pp. 43–49. [Google Scholar]