Abstract
Rotavirus is a major burden on the Spanish Healthcare System. Rotarix® and RotaTeq® were simultaneously commercialized in Spain by February, 2007. The objective is to analyze the incidence and seasonality of rotavirus hospitalizations (RH) in Castile-La Mancha (CLM), following the first 3 y of commercialization. A cross-sectional study of the hospital discharge registry's Minimum Basic Data Set (MBDS) in CLM between 2003 and 2009 was performed. We used the Poisson regression model to quantify the percentage of change in the rotavirus incidence rate (IR) for 2007–09 vs. 2003–05, adjusting for age, sex, and province. To analyze changes between epidemic seasons the chi-square test was used. The median IR in 2003–09 was 224.71 per 105 [interquartile range (IQR): 185.24–274.70], which represents 60% of hospital admissions due to infectious acute gastroenteritis. The median rate in 2007–09 decreased [incidence rate ratio (IRR): 0.86, 95% CI: 0.78–0.96], significantly in Toledo (IRR: 0.54, 95% CI: 0.39–0.75). An incipient decline at the beginning of the last cold season was observed, preceded by a significant decrease of 68% in the autumn season of 2009. Despite its limited coverage, the rotavirus vaccine may have contributed to decrease RH in CLM.
Keywords: Children, hospitalizations, MBDS, rotavirus, Spain, vaccines
Abbreviations
- AGE
Acute Gastroenteritis
- CLM
Castile-La Mancha
- MBDS
Minimum Basic Data Set
- ARGE
Acute Rotavirus Gastroenteritis
- IQR
Interquartile Range
- IR
Incidence Rate
- RH
rotavirus hospitalizations
- IRR
Incidence Rate Ratio
Introduction
Rotavirus is the most common infectious cause of acute gastroenteritis (AGE) in children under 5 y of age worldwide.1 While mortality from AGE in Spain is almost zero, the disease still poses a major burden for the healthcare system because of its high infectivity and morbidity.2 In fact, hospitalization costs due to rotavirus account for more than half the expenses caused by all AGE cases in children under 5.3 Indeed, the incidence of hospitalizations due to rotavirus for this age group in Europe ranges from 30 to 1,190 per 105 children per year (100–310 per 105 in Spain),4 with an estimated cost of 1,525–2,101 euros per admission.5 The disease costs the Spanish national health system EUR 28 million a year.6
The vaccines Rotarix® and RotaTeq® were first commercialized in Spain in July, 2006, and February, 2007, respectively.7. Although the Spanish Association of Pediatrics recommended vaccination at the time and again in 2013,8 these vaccines were never included in the calendar of the National Health System's Interregional Council because they were not considered cost-effective.7,9 Still, according to the number of doses distributed and assuming a complete vaccination schedule, coverage in Castile-La Mancha (CLM) went from 18% in 2007 up to 39% in 2008 and 44% in 2009, compared with 17%, 35%, and 38%, respectively, in Spain as a whole (data provided by Sanofi Pasteur MSD, SA). Despite these drugs are not subsidized by the government, recent studies have estimated a significant decrease in rotavirus hospitalizations in Spain since their use became more widespread in 2008.10-13 Other industrialized countries have already shown a reduction in hospital admissions after 1 to 2 y of a complete immunization schedule,14-16 with the vaccine remaining effective up to 3 y after the last dose.17,18
The aim of our research is to describe the burden borne by hospitals due to community-acquired rotavirus in CLM and its temporary and seasonal evolution between 2003 and 2009. We also analyze any possible changes that took place in the first triennium of the commercialization of the rotavirus vaccine in Spain (2007–09), compared to 2003–05.
Results
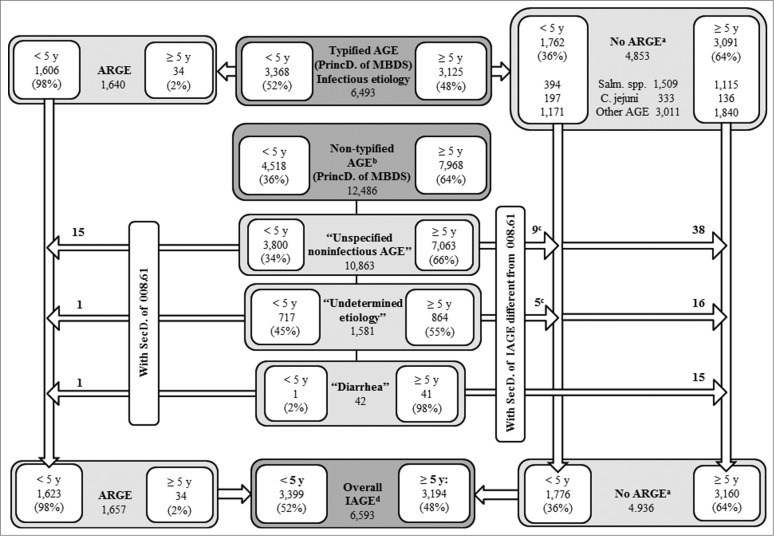
Eight records in the Minimum Basic Data Set (MBDS) with a diagnosis of localized salmonella infection (003.21–003.24) and 4 with a diagnosis of amebic abscess (006.3–006.6) were excluded, leaving 22,998 records. A total of 6,593 cases of infectious AGE were identified through the principal diagnosis, including undetermined or cause-unspecified AGE and diarrheal syndromes with a specific secondary diagnosis of infectious AGE (Fig. 1). The total estimated cases of acute rotavirus gastroenteritis (ARGE) consisted of 1,640 records with a 008.61 diagnosis, to which we added another 17 after assigning the 008.61 code to cases with an original principal diagnosis coded as 009.0, 558.9, or 787.91, but which were linked to a secondary diagnosis coded as 008.61 (Fig. 1).
Figure 1.
Counting of hospital admissions by IAGE of typified and non-typified etiology. Source: MBDS. CLM, Spain. 2003–09.*
We calculated that 25% (1,657/6,593) of all hospital admissions due to infectious AGE between 2003 and 2009 in CLM were caused by rotavirus. The median number of hospital discharges due to rotavirus was 203 (Interquartile range (IQR): 190–263) per year in children under 5 y of age, which represents 2% (1,623/85,042) of hospital admissions for any cause and 48% (1,623/3,399) of those caused by infectious AGE in this age group (Fig. 1 and Table 1). Salmonellosis accounted for 12% [(394+10)/3,399] and campylobacteriosis for 6% [(197+4)/3,399] of all hospitalizations coded as infectious AGE in children under 5 (Fig. 1). Ninety-eight percent of rotavirus hospitalizations occurred in this age group, with 84% occurring in children under 2 y of age (Fig. 1 and Table 1). The frequency of concomitant AGE in children under 5 was 2%; the most common cause was adenovirus (70%), followed by Campylobacter jejuni (19%).
Table 1.
Hospital admissions for rotavirus (per 105) compared to overall admissions for infectious AGE and admissions for non-typified AGE for the three-year periods 2003-05 and 2007-09. Source: MBDS. Castile-La Mancha, Spain*
| 2003–05 Age | Overall infectious AGEa | AGE for rotavirus | Non-typified AGEb | |||||
|---|---|---|---|---|---|---|---|---|
| N | Median rate | N | Median rate | % rota/overall AGE by group | % rota by group/total rota | N | Median rate | |
| 0–6 ms | 316 | 780.5 | 189 | 403.1 | 59.8 | 24.6 | 420 | 1037.3 |
| 7–11 ms | 276 | 939.9 | 212 | 701.6 | 76.8 | 27.6 | 370 | 1260.0 |
| < 1 y | 592 | 847.5 | 401 | 529.5 | 67.7 | 52.2 | 790 | 1131.1 |
| 1 y | 370 | 695.8 | 246 | 393.0 | 66.5 | 32.0 | 615 | 1156.6 |
| 2 y | 177 | 330.0 | 78 | 145.3 | 44.1 | 10.1 | 307 | 572.5 |
| 3 y | 74 | 136.3 | 19 | 38.5 | 25.7 | 2.47 | 212 | 390.7 |
| 4 y | 69 | 126.8 | 14 | 16.5 | 20.3 | 1.8 | 141 | 259.2 |
| < 5 y | 1282 | 594.8 | 758 | 224.7 | 59.1 | 98.6 | 2065 | 958.4 |
| ≥ 5 y | 1093 | 21.0 | 11 | 1.1 | 1.01 | 1.4 | 3209 | 61.7 |
| Total | 2375 | 43.9 | 769 | 11.3 | 32.4 | 100.0 | 5274 | 97.3 |
| 2007–09 | Overall infectious AGEa | AGE for rotavirus | Non-typified AGEb | |||||
| Age | N | Median rate | N | Median rate | % rota/overall AGE by group | % rota by group/total rota | N | Median rate |
| 0–6 ms | 248 | 642.0 | 154 | 356.8 | 62.1 | 23.3 | 387 | 1001.8 |
| 7–11 ms | 224 | 811.5 | 163 | 469.5 | 72.8 | 24.6 | 331 | 1199.1 |
| < 1 y | 472 | 712.6 | 317 | 403.7 | 67.2 | 47.9 | 718 | 1084.1 |
| 1 y | 323 | 534.8 | 231 | 343.2 | 71.5 | 34.9 | 528 | 874.0 |
| 2 y | 115 | 194.1 | 63 | 92.1 | 54.8 | 9.5 | 264 | 445.4 |
| 3 y | 60 | 102.3 | 24 | 35.6 | 40.0 | 3.6 | 153 | 260.8 |
| 4 y | 46 | 78.7 | 13 | 19.8 | 28.3 | 2.0 | 118 | 201.9 |
| < 5 y | 1016 | 429.1 | 648 | 184.5 | 63.8 | 97.9 | 1781 | 752.4 |
| ≥ 5 y | 864 | 15.3 | 14 | 1.1 | 1.6 | 2.1 | 3594 | 63.7 |
| Total | 1880 | 32.0 | 662 | 9.7 | 35.2 | 100.0 | 5375 | 91.5 |
*AGE: acute gastroenteritis; MBDS: Minimum Basic Data Set. Rota: rotavirus; ms: months; y: years old.
aPrincipal diagnosis of bacterial or parasitic AGE (001–005, 006–007, and 008.0–008.5) and viral AGE (008.6 to 008.8), excluding a principal diagnosis of localized salmonella infection (003.21–003.24) or amebic abscess (006.3–006.6). Also, non-typified AGE with a secondary diagnosis of infectious (typified) AGE.
bNon-typified AGE without a secondary diagnosis of infectious AGE: “AGE of undetermined cause” (009.0 to 009.3), “unspecified noninfectious AGE” (558.9), or “diarrhea” (787.91).
A total of 25 readmissions were due to “the same process” of ARGE, as defined in Methods section,19 with none occurring in 2008 or 2009. Only 5 readmissions due to different processes from the original diagnosis were documented; all occurred in different years. Mortality of infectious AGE requiring admission was 6‰ (38/6,593); no deaths were caused by rotavirus.
The median incidence rate (IR) of rotavirus hospitalizations (RH) in children under 5 y of age between 2003 and 2009 was 224.7 cases per 105 inhabitants (IQR: 185.2–274.7) and 11.30 per 105 (IQR: 9.8–12.8) for all ages. The mean age of this particular risk group was 14 months, with a standard deviation (SD) of 11 months; 56% were male. The highest incidence of RH occurred between 7 and 11 months, the group in which rotavirus represented 75% of hospitalizations for infectious AGE. Ciudad Real and Guadalajara showed an IR 6 times higher than that of Toledo; Cuenca and Albacete had IRs 3 and 2 times greater, respectively (Fig. 2).
Figure 2.
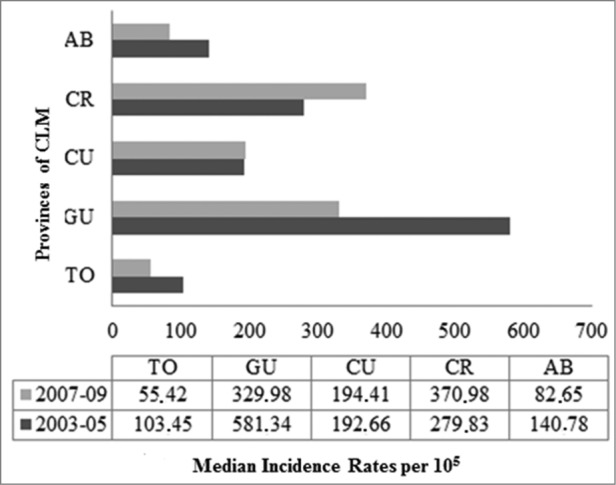
Distribution of triennial rates of acute rotavirus gastroenteritis by provinces. Children under 5 y of age. MBDS. CLM, Spain. 2003–05 and 2007–09. MBDS: Minimum Basic Data Set. CLM: Castile-La Mancha.
The median IR of hospitalization for infectious AGE under 5 y of age decreased 28% in the period after the vaccine was made available in comparison with the pre-commercialization triennium. The actual IR was estimated to be 594.8 per 105 (IQR: 513.0–676.6) in the period between 2003 and 2005, down to 429.1 (IQR: 367.7–490.5) between 2007 and 2009 (Table 1). Rotavirus incidence decreased by 18%, from 224.7 per 105 between 2003 and 2005 (IQR: 193.8–255.6) down to 184.5 (IQR: 158.1–210.9) between 2007 and 2009 (p<0.001). This decrease was greatest in children aged 2 years, 7–11 months, and 1 y of age (37%, 33%, and 13%, respectively) (Table 1). The rate of hospitalization for AGE of unknown etiology decreased significantly by 22% under 5 y of age. In this age group, the median incidence rate of ARGE in the period after introduction of the vaccine went down in Albacete, Guadalajara, and especially in Toledo. It increased in Ciudad Real and remained stable in Cuenca (Fig. 2).
The seasonal pattern observed coincided with that of temperate countries in general, with peaks during the winter months (December-February), accounting for 57% of annual RH, compared to 27% of hospitalizations for other infectious AGE of typified or non-typified etiology in that season (Fig. 3). The highest rates of RH were registered in December and January (92 and 93%, respectively), with only 5% occurring from June to August. Seasonal distribution of non-typified AGE in children under 5 shows a seasonal pattern similar to that of rotavirus, but different from that of Salmonella spp or Campylobacter jejuni, typical of the summer season (Fig. 3).
Figure 3.
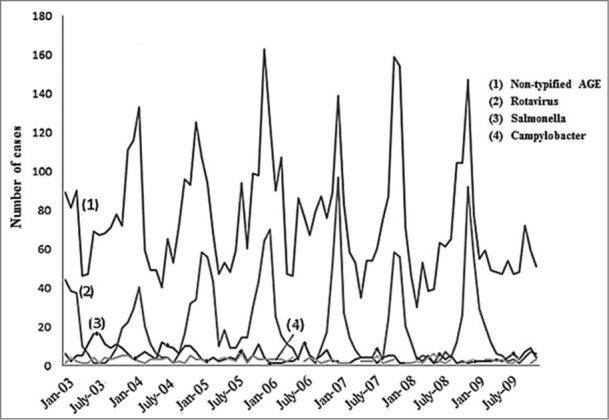
Seasonal distribution of the number of hospitalizations caused by rotavirus, Salmonella spp., Campylobacter jejuni and non-typified AGE. Children under 5 y of age. MBDS. CLM, Spain. 2003–09. AGE: acute gastroenteritis. MBDS: Minimum Basic Data Set. CLM: Castile-La Mancha.
The trend was upwards until 2007, with 2 peaks of high incidence, one in 2005 and another, albeit smaller, in 2007, which affected all age groups to a greater or lesser degree (Fig. 4). This trend then went steadily downwards in 2008 (by 39% compared to 2007) and 2009 (by 20% compared to 2008), p < 0.001. RH went down by 15% in the autumn of 2008 with respect to the previous autumn (p = 0 .825), but increased by 12% in the corresponding winter season (December 2008-February 2009) as compared to December 2007-February 2008 (p < 0.424). After a decrease of 68% in the fall of 2009 with regard to the same season in 2008 (p < 0.001), the trend looked downwards in the early winter of 2009 (Fig. 5).
Figure 4.
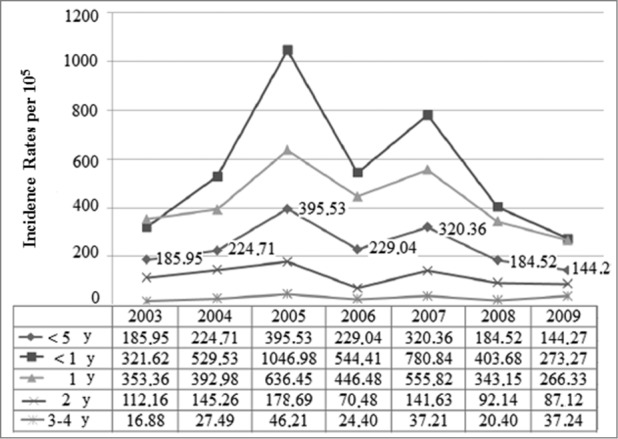
Distribution of incidence rates of rotavirus hospital discharges by year groups. Children under 5 y of age. MBDS. CLM, Spain. 2003–09. MBDS: Minimum Basic Data Set. CLM: Castile-La Mancha. Y: year/s.
Figure 5.
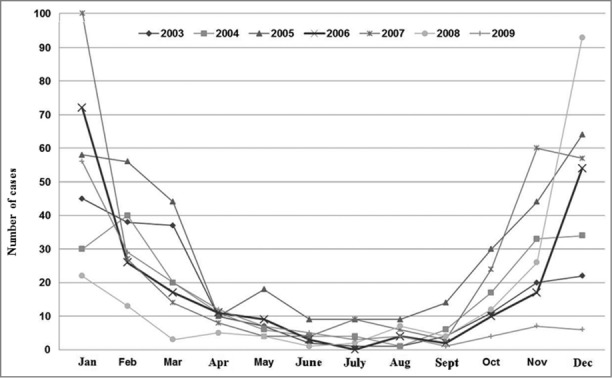
Distribution of rotavirus hospital discharge rates by year. Children under 5 y of age. MBDS. CLM, Spain. 2003–09. MBDS: Minimum Basic Data Set. CLM: Castile-La Mancha.
In the multivariate analysis we observed a 14% decline in the IR of ARGE in 2007–09 compared to 2003–05 (Incidence Rate Ratio (IRR): 0.86; 95% CI: 0.78–0.96) (Table 2). The decrease was seen in all age groups under 5 years, but was only significant for the 2 year-old group (IRR: 0.42; 95% CI: 0.23–0.79). Despite a noticeable decrease in IRs during the triennium after vaccine introduction in all provinces except Cuenca, only Toledo experienced a statistically significant decrease (IRR: 0.54, 95% CI: 0.39–0.75).
Table 2.
Multivariate analysis: comparison of the incidence rates of rotavirus hospital discharges in CLM in the triennium 2007–09 vs. the triennium 2003-05. Source: MBDS. Castile-La Mancha, Spain*
| Variables | IRR | CI 95% | P value |
|---|---|---|---|
| 2007–09 vs. 2003–05 trienniums | 0.86 | 0.78–0.96 | 0.006 |
| Age | |||
| < 1 y | 0.90 | 0.77–1.03 | 0.148 |
| 1 y | 0.87 | 0.62–1.20 | 0.395 |
| 2 y | 0.42 | 0.23–0.79 | 0.007 |
| 3-4 y | 0.72 | 0.33–1.55 | 0.399 |
| 5-9 y | 1.77 | 0.61–5.07 | 0.291 |
| ≥ 10 y | 3.87 | 0.64–23.47 | 0.141 |
| Sex | |||
| Male | 0.85 | 0.64–0.98 | 0.023 |
| Female | 0.88 | 0.75–1.03 | 0.121 |
| Provinces | |||
| Toledo | 0.54 | 0.39–0.75 | 0.001 |
| Ciudad Real | 0.89 | 0.77–1.04 | 0.146 |
| Guadalajara | 0.87 | 0.71–1.08 | 0.218 |
| Albacete | 0.78 | 0.57–1.07 | 0.118 |
| Cuenca | 1.32 | 0.93–1.89 | 0.124 |
*Poisson regression, adjusted for age, sex, and province.
MBDS: Minimum Basic Data Set.
IRR: Incidence Rate Ratio.
Y: years old.
Discussion
Our results show that in the 3 y following the commercial introduction of Rotarix® and RotaTeq® in Spain, the coding of hospital discharges for any type of AGE in general, and rotavirus in particular, fell by a quarter in the region of CLM.
We found wide variability in the rate of RH under 5 y of age (144.27–395.53 per 105), somewhat lower than that described for Spain as a whole between 2003–08 (105.31–462.11 per 105).20 In fact, the prevalence of RH compared to that of total hospitalizations for typified or non-typified AGE in children under 5 was 1.4 times lower than the national prevalence rates (19.2% vs. Twenty-eight.4%). In Europe, rotavirus represents 14% to 54% of all admissions for AGE in this group.4,6,21 This variability constitutes a limitation when making comparisons and is most likely due to differences in study design, coding methods, access to health care, disease management, data sources, coverage and representativeness of surveillance systems, and the years and geographic areas included. Moreover, rotavirus possesses significant genetic diversity that can cause changes in its antigenicity and pathogenic ability.22 Anyway, this observed variability, which was even further influenced by the introduction of the new vaccines,23 results in different clinical effects over the years in various geographic areas.24-27
The trend described in CLM resembles that noted for Spain as a whole.10-13 With the exception of gaps in both 2001 and 2006, the annual IR of RH in Spain increased progressively from the beginning of this century, rising from 103.9 per 105 in 1999 to 182 per 105 in 2005.28 Apart from the period between 2005 and 2006, when the median IR in children under 5 in CLM exceeded that in Spain overall,10 IR decreases in CLM were 1.5 and 2.1 times greater than the national average in 2008 and 2009, respectively.10,20 This most likely corresponds to the fact that vaccination coverage in this region was 4% higher than the national average in 2007–09. The downward trend in 2008 in CLM and in the country as a whole, coincides with the fact that vaccination coverage that year was twice as high as in 2007, both in CLM and at the national level.
The MBDS data revealed that Spain experienced a decrease in RH of 33.1% in 2008–09 with respect to 2005–06,10 compared with a decrease of 47.4% between the same periods in CLM. Given an average cost of 1,813 euros per hospital admission,5 the decrease in RH during 2007–09 resulted in a savings of approximately 200,000 euros for the healthcare system of CLM. In fact, subsidized vaccination programs in European countries have led to an annual decrease of 74% in the IR of RH under 18 y of age when comparing the post-vaccination period (2008–09) with the pre-vaccination period (2002–05).29,30 This means a 73% annual reduction of direct costs of RH. In European regions of low to moderate commercialization of the new vaccine, the reduction of IR under 2 y of age was 25–36% when comparing the periods 2008–11 and 2004–06,31 similar to the decrease in Spain.
As in Europe32-34 and the rest of the world,1,14 the risk of RH is greater under 2 y (80–89%, p <0.001). It is possible that cases in elderly patients are underestimated due to a less systematic search for rotavirus in the action protocol compared with that used in pediatrics, though the specific laboratory diagnosis does not change the treatment regimen nonetheless. The decrease in the 5–9 and 10–64 year-old age groups may actually indicate “herd immunity” for those living or in close contact with vaccinated children.15 In contrast, the rate increased by 20% in the 4 year-old age group, who were born before the vaccines availability. Unfortunately, the small number of patients over the age of 2 prevents us from making definitive conclusions.
This research was carried out from the MBDS, a nationwide database. This is a compulsory data source that is retrospectively constructed with every hospitalization episode in Spain. Many studies have been conducted from it. A major limitation is that MBDS is not intended for surveillance of infectious diseases. Although the main reason for hospital discharges has previously been used to assess the burden of disease, some studies show an underestimation when MBDS is used.2,35 In fact, 66% (12,486/18,979) of the records coded with a principal diagnosis of AGE correspond to non-typified AGE (57% under 5 years) (Fig. 1). The 558.9 code is usually used to encode non-typified diarrhea at discharge, even without knowing whether these cases are infectious. Similar results on incidence categorized by age group in ARGE and non-typified AGE (Table 1) described previously36 suggest that many cases of non-typified AGE could be attributed to rotavirus, a hypothesis bolstered by the similarities observed in the seasonality of AGE, which is different when caused by Salmonella or Campylobacter than when due to rotavirus (Fig. 3). In addition, in 2007–09, cases of non-typified AGE fell by 22% in children under 5 (p<0.001), a greater decrease than that of rotavirus. About 28% of ARGE is under-diagnosed through MBDS.37 Besides, microbiological data depends on the diagnostic practices and availability of techniques in hospitals and MBDS does not collect cases from the emergency room. Finally, MBDS does not include the patterns of administered vaccines.
Despite the modest diagnostic sensitivity of MBDS,35 it has proved its usefulness in monitoring the impact of vaccination, since coding defects and underestimation appear to be comparable in both the pre- and post-vaccination periods.10,35 By this way, a study revealed that rotavirus-coded hospitalization rates decreased by 50% in 2007–08, compared with prevaccine rates in 2001–06.38
To the defects and gaps in the information gathered from the MBDS, we have to add the aforementioned natural oscillations in the incidence of ARGE22,23 and the difficulty in observing the effects of low vaccination coverage (18% in 2007). As explained, the vaccine coverage was derived from distributed doses and no information is available on compliance of vaccine. Otherwise, the upward trend might simply involve a stronger coding culture of hospitalization processes over time. In addition, the decline in RH after the introduction of the vaccine may contribute to changes in the criterion of “need for hospitalization.”
The count of hospitalizations became insufficient to draw solid conclusions on the incidence decrease, especially, when stratifying by age group. Both the complete absence of cases in some age groups over 5 as well as low numbers of cases in other groups under and over 5 decrease the statistical power when stratifying by this variable. This would explain why the decrease in rates between the 2 time periods categorized by age groups was not statistically significant in the multivariate analysis except in the 2 year-old group (Table 2). It may also help explain why differences between the time periods did not prove to be significant when categorized by provinces, except in Toledo, the only province where a decline in rates in all groups under the age of 5 occurred.
In summary, analysis of a secondary data source has provided evidence about a decline of RH in CLM after a short period of low immunization. In view of the low number of hospitalizations obtained from the available data, mainly when stratified, the timeframe of observation should be broadened for future research. Even though it is not conclusive that such decrease may be related to the use of the self-funded vaccines, it seems appropriate that pediatricians continue encouraging community-based rotavirus vaccination at well-baby visits. Future research could clarify present findings.
Materials and Methods
Setting and data source
This is a cross-sectional study of ARGE requiring hospital admission in CLM from January 1, 2003, to December 31, 2009. The MBDS of the CLM health service was used as source of information along with the Statistics on Health Establishments with Inpatient Regimen, which together represent an average population coverage of 99.37%. These data were provided by the Service of Information and Sanitary Statistics of the Counseling of Health and Social Welfare in CLM.
Definition of the variable of interest
We requested information from the MBDS database (hospital discharges) about any records with a primary or secondary diagnosis of infectious AGE coded according to the Ninth Revision of the International Classification of Diseases, Clinical Modification (ICD-9-CM). This included bacterial or parasitic AGE (001–005, 006–007, and 008.0–008.5) and viral AGE (008.6 to 008.8), as well as a primary or secondary diagnosis of non-typified AGE (of unknown etiology): “AGE of undetermined cause” (009.0 to 009.3), “unspecified noninfectious AGE” (558.9), or “diarrhea” (787.91), according to that classification. Altogether, 23,006 records were retrieved.
All infectious cases of AGE leading to hospital admission which had been coded as the principal diagnosis were included to assess both the burden and seasonality of ARGE with respect to the rest of AGE. AGE of “undetermined etiology,” “cause-unspecified” AGE, and “diarrhea” were included as primary diagnoses because ARGE is not a notifiable disease in Spain and is thus not always diagnosed with a specific laboratory code. All records with a secondary diagnosis of infectious AGE were included with the aim of identifying rotavirus as a probable etiology of any hospital admissions in which a principal diagnosis of AGE was coded as “undetermined etiology,” “cause-unspecified,” or “diarrhea.”
Hospital readmission listed as due to “the same process” was defined as a hospitalization episode of the same patient occurring twice in a period of one month.19 The records compiled in Figure 1 correspond to new patients only, since readmissions “for the same process” were not included in the count.
Statistical Analysis
Frequency measures with their 95% Confidence Intervals (95% CI) were estimated for each year. To estimate IRs, population censuses from the Spanish National Institute of Statistics (INE) categorized by age, sex, and province of residence were used. Monthly disaggregated population data of children under one year of age were provided by the National Center for Epidemiology, also from census estimates.
Estimates of medians with interquartile ranges were then calculated for the 3 y prior to vaccine availability (2003–05) as well as for the subsequent triennium (2007–09). Data for the year 2006, which was when the first of the 2 vaccines became commercially available, was not taken into account, as it was considered to be a year of transition. Finally, variations in the IRs of RH in the latter period (2007–09) were quantified and compared to those in 2003–05 by means of IRR (CI 95%). To this end, a Poisson regression model was used, adjusting for the variables of age, sex, and province of hospital admission.
Seasonal patterns were described in accordance with monthly distribution of discharges per year. To investigate possible changes in the percentage of rotavirus in terms of annual discharges and in its epidemic seasons, the chi-square test was used. Analyses were performed using Statistical Software Stata 11 (StataCorp LP. 2009); the level of significance (p) was taken to be values less than 0.05.
Acknowledgments
The study was accomplished thanks to two collaborating institutions: the “La Mancha Centro General Hospital” of Alcázar de San Juan in Ciudad Real, Spain, and the National Center of Epidemiology in Madrid. We thank members of the “Service of Information and Sanitary Statistics of the Counseling of Health and Social Welfare” in Castile-La Mancha, Spain, who provided the MBDS information. We also thank our coworkers at the Research Support Unit in La Mancha Centro General Hospital, for their contribution in peer review of the final manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Pan American Health Organization/World Health Organization Epidemiological Surveillance of Diarrheal Diseases Due to Rotavirus - Field Guide. Scientific and Technical Publication No. 623; PAHO: Washington DC; 2010. [Internet]. [Accessed 23 oct 2013]. Available at: http://new.paho.org/hq/dmdocuments/2010/FieldGuide_Rotavirus_1stEd_e.pdf [Google Scholar]
- 2. Hernández Pezzi G, Varela Martínez MC. Vigilancia epidemiológica de las gastroenteritis agudas víricas. Laia Alemany Vilches L, Moraga Llop F, García Jiménez S. En: Bellido Blasco JB, coordinador. 6 monografía de la Sociedad Española de Epidemiología. EMISA; 2007. [Google Scholar]
- 3. Rivero MJ, Román E, García MI, Zafra M, Gil Á, González-Escalada A. Epidemiología de la gastroenteritis por rotavirus adquirida en la comunidad en el área de Fuenlabrada (Madrid). Enf Inf Microbiol Clin 2011; 29(6):432-4; http://dx.doi.org/ 10.1016/j.eimc.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 4. Ogilvie I, Khoury H, Goetghebeur MM, El Khoury AC, Giaquinto C. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: a scoping review. BMC Infect Dis 2012; 12:62; PMID:22429601; http://dx.doi.org/ 10.1186/1471-2334-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giaquinto C, Van Damme P, Huet F, Gothefors L, Van der Wielen M, REVEAL Study Group . Costs of community-acquired pediatric rotavirus gastroenteritis in 7 European countries: the REVEAL Study. J Infect Dis 2007; 195 Suppl 1:S36-S44; PMID:17539193; http://dx.doi.org/ 10.1086/516716 [DOI] [PubMed] [Google Scholar]
- 6. Álvarez Aldeán J, Aristegui J, López-Belmonte JL, et al. Economic and psychosocial impact of rotavirus infection in Spain: a literature review. Vaccine 2014 Jun; 32(30):3740-51; http://dx.doi.org/ 10.1016/j.vaccine.2014.04.058 [DOI] [PubMed] [Google Scholar]
- 7. Imaz Iglesia I, Cornejo Gutiérrez AM, Rubio González B, et al. “Análisis Coste-Utilidad de la Introducción de la Vacunación Universal frente al Rotavirus en España.” Madrid: Agencia de Evaluación de Tecnologías Sanitarias - Ministerio de Ciencia e Investigación - Instituto de Salud Carlos III, Madrid. Diciembre 2011 [Google Scholar]
- 8. Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Barrio Corrales F, Cilleruelo Ortega MJ, Corretger Rauet JM, et al. Calendario de vacunaciones de la Asociación Española de Pediatría: recomendaciones 2013. An Pediatr (Barc) 2013; 78(1):59.e1-59.e27. [DOI] [PubMed] [Google Scholar]
- 9. Pachón I, Martínez MV, Suárez B, Sánchez-Fouquier A, Salmerón F, Soler M, et al. Situación epidemiológica de la gastroenteritis producida por rotavirus. Recomendaciones de la vacunación frente al rotavirus. Documento enviado a la Ponencia de Programa y registro de vacunaciones. Ministerio de Sanidad y Consumo: Madrid; 2006. Madrid: Ministerio de Sanidad y Consumo [Google Scholar]
- 10. Gil-Prieto R, González-Escalada A, Álvaro-Meca A, García-García L, San-Martín M, González-López A, et al. Impact of non-routine rotavirus vaccination on hospitalizations for diarrhoea and rotavirus infections in Spain. Vaccine 2013 oct 9; 31(43):5000-4; http://dx.doi.org/ 10.1016/j.vaccine.2013.05.109 [DOI] [PubMed] [Google Scholar]
- 11. Castilla J, Beristain X, Martínez-Artola V, Navascués A, García Cenoz M, Álvarez N, et al. Effectiveness of rotavirus vaccines in preventing cases and hospitalizations due to rotavirus gastroenteritis in Navarre, Spain. Vaccine 2012 ene 11; 30(3):539-43; http://dx.doi.org/ 10.1016/j.vaccine.2011.11.071 [DOI] [PubMed] [Google Scholar]
- 12. Martinón-Torres F, Martinón-Torres N, Bouzón Alejandro M, Redondo Collazo L, Pértega-Díaz S, Seoane-Pillado MT, et al. Acute gastroenteritis hospitalizations among children aged < 5 years before and after introduction of rotavirus vaccines: a hospital-based surveillance study in Galicia, Spain. Hum Vaccin Immunother 2012 jul; 8(7):946-52; http://dx.doi.org/ 10.4161/hv.20178 [DOI] [PubMed] [Google Scholar]
- 13. García-Basteiro AL, Bosch A, Sicuri E, Bayas JM, Trilla A, Hayes EB. Hospitalizations due to rotavirus gastroenteritis in Catalonia, Spain, 2003-2008. BMC Res Notes 2011; 4:429; http://dx.doi.org/ 10.1186/1756-0500-4-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yen C, Tate JE, Wenk JD, et al. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics 2011. Ene; 127(1):e9-e15; PMID:21172995; http://dx.doi.org/ 10.1542/peds.2010-1393 [DOI] [PubMed] [Google Scholar]
- 15. Plosker GL. Pentavalent rotavirus vaccine (RotaTeq): a review of its use in the prevention of rotavirus gastroenteritis in Europe. Drugs 2010 Jun 18; 70(9):1165-1188; http://dx.doi.org/ 10.2165/11205030-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 16. Giaquinto C, Dominiak-Felden G, Van Damme P, Myint TTH, Maldonado YA, Spoulou V, et al. Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: a systematic review of the experience in industrialized countries. Hum Vaccin 2011 jul; 7(7):734-48; http://dx.doi.org/ 10.4161/hv.7.7.15511 [DOI] [PubMed] [Google Scholar]
- 17. Vesikari T, Karvonen A, Ferrante SA, et al. Sustained efficacy of the pentavalent rotavirus vaccine, RV5, up to 3.1 years following the last dose of vaccine. Pediatr Infect Dis J 2010 Oct; 29(10):957-963; http://dx.doi.org/ 10.1097/INF.0b013e3181e28e6e [DOI] [PubMed] [Google Scholar]
- 18. Vesikari T, Uhari M, Renko M, Hemming M, Salminen M, Torcel-Pagnon L, et al. Impact and Effectiveness of Rotateq® Vaccine Based on Three Years of Surveillance Following Introduction of a Rotavirus Immunization Program in Finland. Pediatr Infect Dis J 2013 Dec; 32(12):1365-73; http://dx.doi.org/ 10.1097/INF.0000000000000086 [DOI] [PubMed] [Google Scholar]
- 19. Agencia de Calidad del Sistema Nacional de Salud Instituto de Información Sanitaria. Metodología de Análisis de la Hospitalización en el Sistema Nacional de Salud. Ministerio de Sanidad y Consumo. Modelo de indicadores basado en el Registro de Altas (CMBD). Documento base. de Sanidad y Consumo: Madrid; 2007. [Internet]. [Accessed 15 dec 2013]. Available at: http://www.msssi.gob.es/estadEstudios/estadisticas/docs/metod_modelo_cmbd_pub.pdf [Google Scholar]
- 20. Centro Nacional de Epidemiología Informe sobre la situación de las infecciones por rotavirus en España. Años 1999-2009. Madrid: Instituto de Salud Carlos III; 2010 [Google Scholar]
- 21. The Pediatric ROTavirus European CommitTee (PROTECT) . The paediatric burden of rotavirus disease in Europe. Epidemiol Infect 2006; 134:908-16; PMID:16650331; http://dx.doi.org/ 10.1017/S0950268806006091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirkwood CD. Genetic and antigenic diversity of human rotaviruses: potential impact on vaccination programs. J Infect Dis 2010; 202 Suppl:S43-48; PMID:20684716; http://dx.doi.org/ 10.1086/653548 [DOI] [PubMed] [Google Scholar]
- 23. Patton JT. Rotavirus Diversity and Evolution in the Post-Vaccine World. Discov Med 2012; 13(68):85-97; PMID:22284787 [PMC free article] [PubMed] [Google Scholar]
- 24. Roué J-M, Nowak E, Le Gal G, Lemaitre T, Oger E, Poulhazan E, et al. Impact of rotavirus vaccine in premature infants. Clinical and Vaccine Immunology 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uhlig U, Kostev K, Schuster V, et al. Impact of Rotavirus Vaccination in Germany: Rotavirus Surveillance, Hospitalization, Side Effects and Comparison of Vaccines. Pediatr Infect Dis J 2014; 33(11):e299-304. [DOI] [PubMed] [Google Scholar]
- 26. Panozzo CA, Becker-Dreps S, Pate V, Weber DJ, Jonsson Funk M, Stürmer T, et al. Direct, indirect, total, and overall effectiveness of the rotavirus vaccines for the prevention of gastroenteritis hospitalizations in privately insured US children, 2007-2010. Am J Epidemiol 2014. abr 1; 179(7):895-909; PMID:24578359; http://dx.doi.org/ 10.1093/aje/kwu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang W-C, Yen C, Wu F-T, Huang Y-C, Lin J-S, Huang F-C, et al. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J 2014 mar; 33(3):e81-86; http://dx.doi.org/ 10.1097/INF.0000000000000105 [DOI] [PubMed] [Google Scholar]
- 28. López-de-Andrés A, Jiménez-García R, Carrasco-Garrido P, Alvaro-Meca A, Galarza PG, de Miguel AG. Hospitalization sassociated with rotavirus gastroenteritis in Spain, 2001-2005. BMC Public Health 2008; 8:109; http://dx.doi.org/ 10.1186/1471-2458-8-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paulke-Korinek M, Kollaritsch H, Aberle SW, Zwazl I, Schmidle-Loss B, Vécsei A, et al. Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine 2013 may 31; 31(24):2686-91; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 30. Zlamy M, Kofler S, Orth D, Würzner R, Heinz-Erian P, Streng A, et al. The impact of Rotavirus mass vaccination on hospitalization rates, nosocomial Rotavirus gastroenteritis and secondary blood stream infections. BMC Infect Dis 2013; 13:112; PMID:23452879; http://dx.doi.org/ 10.1186/1471-2334-13-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dudareva-Vizule S, Koch J, An der Heiden M, Oberle D, Keller-Stanislawski B, Wichmann O. Impact of rotavirus vaccination in regions with low and moderate vaccine uptake in Germany. Hum Vaccin Immunother 2012 oct; 8(10):1407-15; http://dx.doi.org/ 10.4161/hv.21593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Forster J, Guarino A, Parez N, et al. Hospital-Based Surveillance to Estimate the Burden of Rotavirus Gastroenteritis Among European Children Younger Than 5 Years of Age. Pediatrics. 2009 Mar 1; 123(3):e393-400; http://dx.doi.org/ 10.1542/peds.2008-2088 [DOI] [PubMed] [Google Scholar]
- 33. Parez N, Mory O, Pozzetto B, Garbag-Chenon A, Pillet S, Texier N, et al. Impact of Rotavirus gastroenteritis requiring hospitalization or presenting to emergency room among children less than 5 years in France. Pathol. Biol. 2012 oct; 60(5):275-81; http://dx.doi.org/ 10.1016/j.patbio.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 34. Standaert B, Gomez JA, Raes M, Debrus S, Velázquez FR, Postma MJ. Impact of rotavirus vaccination on hospitalisations in Belgium: comparing model predictions with observed data. PLoS ONE 2013; 8(1):e53864; PMID:23349754; http://dx.doi.org/ 10.1371/journal.pone.0053864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jayasinghe S, Macartney K. Estimating rotavirus gastroenteritis hospitalisations by using hospital episode statistics before and after the introduction of rotavirus vaccine in Australia. Vaccine Thirty de enero de 2013; 31(6):967-72; PMID:23246261; http://dx.doi.org/ 10.1016/j.vaccine.2012.11.099 [DOI] [PubMed] [Google Scholar]
- 36. Gil A, Carrasco P, Jiménez R, San-Martín M, Oyagüez I, González A. Burden of hospitalizations attributable to rotavirus infection in children in Spain, period 1999-2000. Vaccine 2004; 22:2221-5; PMID:15149780; http://dx.doi.org/ 10.1016/j.vaccine.2003.11.037 [DOI] [PubMed] [Google Scholar]
- 37. Mast TC, Walter EB, Bulotsky M, Khawaja SS, Di Stefano DJ, Sandquist MK, et al. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J 2010 Feb; 29(2):e19-25; http://dx.doi.org/ 10.1097/INF.0b013e3181ca7e2e [DOI] [PubMed] [Google Scholar]
- 38. Leshem E, Moritz RE, Curns AT, Zhou F, Tate JE, Lopman BA, et al. Rotavirus Vaccines and Health Care Utilization for Diarrhea in the United States (2007–2011). Pediatrics 2014 Jul; 134(1):15-23; http://dx.doi.org/ 10.1542/peds.2013-3849 [DOI] [PMC free article] [PubMed] [Google Scholar]