Abstract
Bacterial DNA comprising palindromic sequences and containing unmethylated CpG is recognized by toll-like receptor 9 of plasmacytoid dendritic cells (pDCs) and induces the production of interferon-α and chemokines, leading to the activation of a Th1 immune response. Therefore, synthetic equivalents of bacterial DNA (CpG oligodeoxynucleotides) have been developed for clinical applications. They are usually phosphorothioated for in vivo use; this approach also leads to adverse effects as reported in mouse models.Mucosal vaccines that induce both mucosal and systemic immunity received substantial attention in recent years. For their development, phosphodiester-linked oligodeoxynucleotides, including the sequence of a palindromic CpG DNA may be advantageous as adjuvants because their target pDCs are present right there, in the mucosa of the vaccination site. In addition, the probability of adverse effects is believed to be low. Here, we review the discovery of such CpG oligodeoxynucleotides and their possible use as mucosal adjuvants.
Keywords: CpG, IgG1, IgG2a/c, mucosal adjuvant, palindrome, phosphodiester, pDC, phylaxis, secretory IgA, Th1
Abbreviations
- pDCs
plasmacytoid dendritic cells
- ODNs
oligodeoxynucleotides
- TLR
toll-like receptor
- IFN
interferon
- sIgA
secretory IgA
- rCTB
recombinant cholera toxin B subunit
- DT
diphtheria toxoid
- BCG
Mycobacterium bovis Bacillus Calmette-Guerin
- G
guanine
- PPD
purified protein derivative
- PBMCs
peripheral blood mononuclear cells
- Ab
antibody
- DTH
delayed-type hypersensitivity
Advantages of a Mucosal Adjuvant that Activates Plasmacytoid Dendritic Cells
Improvement of vaccines for the prevention of infectious diseases remarkably progressed in recent years. From the standpoint of protective immunity, mucosal immunization procedures attracted a substantial interest in recent years because they can stimulate both mucosal and systemic immunity.1-3 Moreover, the procedure is simpler, faster, more reliable, and cheaper, and needles are not required. However, the immunogenicity of synthetic proteins or peptide antigens is generally weak, and non-living vaccine administered to a mucosal site may be ineffective and can even lead to mucosal tolerance. Therefore, an adjuvant that potentiates the induction of appropriate immune responses to such antigens in the mucosa and in the organs is urgently required for the development of mucosal vaccines. Cholera toxin and Escherichia coli-derived heat-labile toxin are well known as the most potent mucosal adjuvants,4-6 but they are not currently available for human use because of their toxicity.7 Therefore, nontoxic recombinant cholera toxin B subunit (rCTB) is an attractive candidate as a safe and potent mucosal adjuvant because it induces protective responses against many microbial infections.8 However, Maeyama et al. found that IL-12 production by activated macrophages is inhibited by rCTB.9 Current promising candidates are microorganism-derived compounds, nucleic acid-based adjuvants, toll-like receptor (TLR) ligands, and cytokines, among others.10 Their adjuvanticity has been thoroughly studied.
Plasmacytoid dendritic cells (pDCs) are a unique population exhibiting plasmacytoid morphology. Their distinctive biological feature is that they produce a large amount of interferon (IFN)-α through the ligation of TLR7 or TLR9, which recognize single-stranded RNA and CpG DNA, respectively. The activated pDCs migrate and cluster in the lymph node, where they stimulate adaptive immune responses. This process is driven by IFN-α secreted by pDCs; with the secretion of Th1-promoting chemokines, such as CXCL10, pDCs contribute to the induction of Th1 immunity. Further, the activation of pDCs and IFN-α production were recently shown to be important for the immunogenicity of vaccines against influenza, measles/mumps/rubella, or rabies.11,12 CpG oligodeoxynucleotides (ODNs) that activate pDCs and stimulate IFN-α production could be advantageous as a vaccine adjuvant. In this article, we review evidence that phosphodiester ODNs containing a palindromic CpG DNA sequence (hereafter called PO-palCpG) are promising mucosal adjuvants.
Development of Synthetic CpG ODNs
Originally identified as antitumor BCG DNA by Tokunaga et al.,13 DNA composed of unmethylated CpG was later identified as a major component of microbial DNA. The latter is responsible for the activation of the mammalian immune system through TLR9. Therefore, synthetic ODNs containing CpG DNA are considered to be promising immunomodulators.14 The intensive research efforts by many investigators led to the development of several types of CpG ODNs in order to advance their practical use. The structures that determine the immunogenicity of CpG ODNs demonstrate some differences among the target cells and species. The active sequences inducing IFN-α production in pDCs comprise a palindromic structure containing CpG,15,16 but the ones inducing B-cell activation are nonpalindromic sequences containing CpG in a particular sequence context;16,17 these types of ODNs were later classified as A-class (D-type) CpG ODNs and B-class (K-type) CpG ODNs, respectively. More practical C-class CpG ODNs have been recently discovered have the properties of A- and B-CpG ODN;18 however, their IFN-α-inducing activities are weaker than A-CpG ODN. Another new class, P-class CpG ODN,19 which contains 2 palindromic sequences, has an even higher ability to induce IFN-α production than that of class C.
Development of IFN-Inducing CpG ODNs
Yamamoto and Tokunaga, et al. were the first to report that the IFN-inducing DNA sequences in the BCG genome have a self-complementary hexamer palindrome containing 5′-CpG-3′ motif(s), such as AACGTT or CGATCG.20 Their subsequent studies demonstrated that these sequences are widely present in DNA from other types of bacteria, viruses, and invertebrate animas but are rarely present in vertebrate or plant DNA.21 Therefore, palindromic sequences with CpG motif(s) are foreign DNA for mammalian immunocompetent cells. These studies were performed between the mid 80s and early 90s,22 and this field markedly advanced by many researchers, such as Dr. Krieg et al., who established the immunological concept of CpG DNA.23
ODNs are usually required to contain poly-guanine (G) to facilitate their entry into cells in higher-order structures. Therefore, Yamamoto and Tokunaga et al. analyzed the length of palindromic CpG DNA and the location in ODNs; these researchers determined that the length should be ≥18 bases and that a G nucleotide should be present outside the palindromic sequence to induce the production of IFNs.24,25 Among those ODNs, the most prominent was the 10-mer palindromic sequence GACGATCGTC linked with a 10-mer oligoG on both sides (5′ and 3′).
From the practical point of view, Kitagawa and Iho, et al. identified the ODN that efficiently induces the production of IFN-α in human peripheral blood mononuclear cells (PBMCs) as described below. This research is based on the idea that a bigger difference in the number of G nucleotides on both sides (5′ and 3′) may make the self-complementary structure of GACGATCGTC more stable (Japan Patent 4415200, June 2007; US Patent 7,718,623B2, May 2010). The researchers compared the IFN-α-inducing properties of the 20-mer phosphodiester ODN sequences, where a 10-mer oligoG was added at the 5′ and 3′ ends of GACGATCGTC in various combinations. As a result, GACGATCGTC with a 9-mer oligoG on the 5′ side and a single G on the 3′ side (GGGGGGGGG-GACGATCGTC-G) was found to be a potent inducer of IFN-α in human PBMCs.26 We call this CpG ODN “G9.1.”
The activity of G9.1 is comparable to that of A-class CpG ODN2216 in its ability to induce IFN-α production and CD80 expression (Fig. 1); this ODN also contains the sequence GACGATCGTC but is linked to PO/PS-G 4- and 6-mers at its 5′ and 3′ ends, respectively, for protection from nuclease degradation. G9.1 also induces the expression of CXCL10. These physiological reactions induced by G9.1 are dependent only on the CpG dinucleotide present within its palindromic sequence. In addition, G9.1 allows pDCs to kill tumor cells in a manner independent of IFN-α (unpublished data).
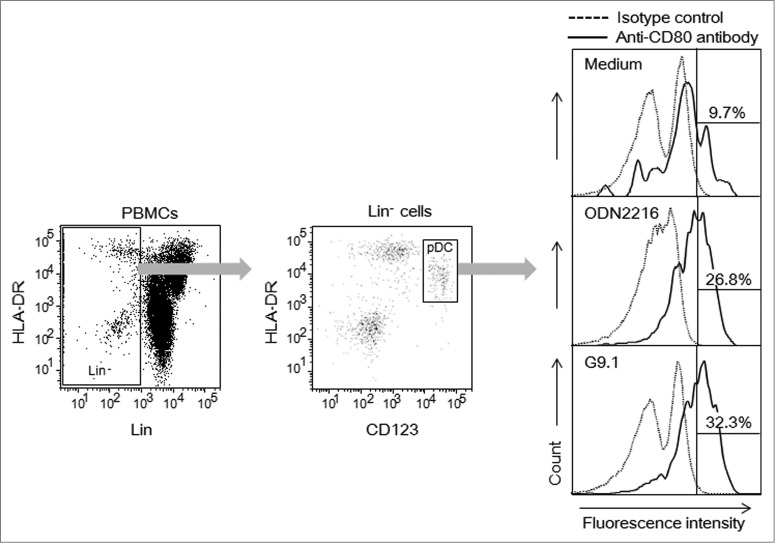
Figure 1.
G9.1 causes CD80 expression in pDCs. Human peripheral blood mononuclear cells (PBMCs) were cultured with medium alone, or with 0.8 μM of G9.1, a negative control of G9.1 (negG9.1), ODN2216, or a negative control of ODN2216 (negODN2216) for 19 hours. The numbers in the right panels represent % of CD80+ cells among plasmacytoid dendritic cells (pDCs), which were detected as CD123+HLA-DR+ cells (middle) among lineage-negative cells (left). Co-culture with negG9.1 and with negODN2216 resulted in the percentages of CD80+ cells to be 8.3% and 16.8%, respectively.
Suitability of PO-palCpG as a Mucosal Adjuvant
In general, the phosphodiester linkage is considered unsuitable for in vivo use because of the degradation by DNases; thus, this approach is inefficient for delivery to target cells. Therefore, the phosphorothioate modification is indispensable. Although clinical trials of such CpG ODNs have demonstrated their safety, there is still a possibility that practically usable phosphorothioate-linked CpG ODNs may cause adverse effects, such as lymphoid follicle destruction or immunosuppression, as shown in mice.14,27 Meanwhile, delivery systems for phosphodiester ODNs have also been developed and involve packaging an ODN in viruslike particles28 or conjugating it to nanoparticles.29 However, if PO-palCpG is used as a mucosal adjuvant, such delivery methods will become unnecessary because pDCs are present in the mucosa. G9.1, even in its naked form, exhibits adjuvanticity during nasal vaccination in mice.26 In those mice, an excess production of proinflammatory and immunosuppressive cytokines is not induced. Further, repetitive administration does not cause problems in the major organs, and IgE antibody (Ab) production is not induced but may be suppressed in a mouse model of ovalbumin-induced allergy. These data suggest that the incidence of adverse effects may be avoided when PO-palCpG is used as a mucosal adjuvant.
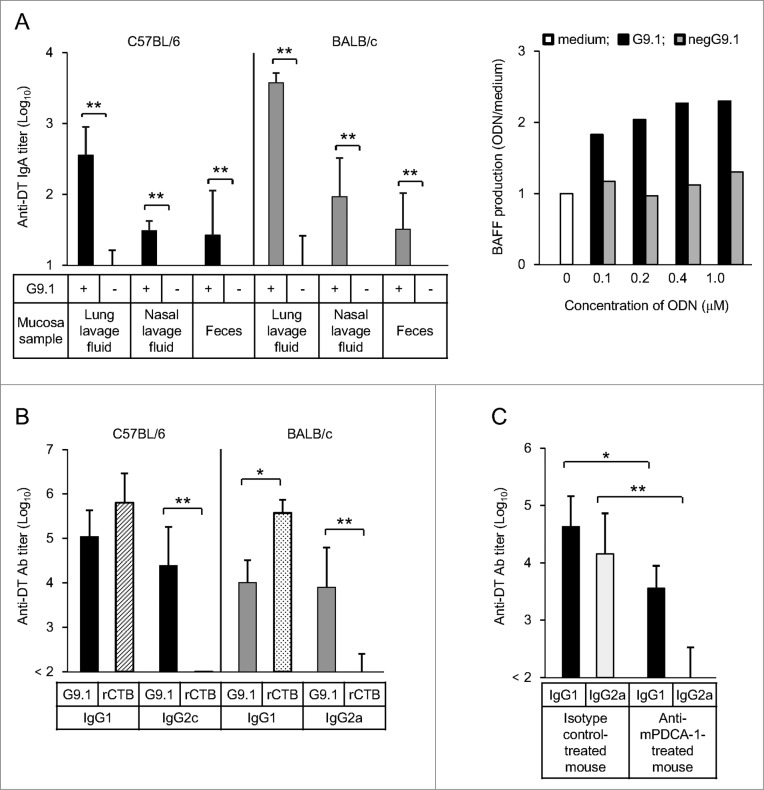
The results of our studies support the feasibility of the clinical application of PO-palCpG as a mucosal adjuvant.26 In mice, a nasal administration of G9.1 with diphtheria toxoid (DT) induces the production of secretory IgA (sIgA) Ab specific to DT. This Ab was detected in the lungs, nasal lavage fluids, and feces, in conjunction with the increase of BAFF (B cell activating factor belonging to the tumor necrosis factor family) production by splenocytes. A protective immune response to DT was also observed, and the DT-specific IgG1 and IgG2a/c Abs were both detected in sera. The Ab titers for IgG2a/c are substantially higher in mice receiving G9.1 than in those receiving rCTB. These findings are consistent with the strong production of IFN-γ in mice treated with G9.1 but not with rCTB.
The induction of Th1 immunity by G9.1 was completely dependent on pDCs as evidenced by the inhibition of IgG2a Ab production in pDC-depleted BALB/c mice. These data are summarized in Figure 2. In vitro activation of pDCs induces the production of IFN-α and CXCL10 and increases expression of T-bet relative to GATA-3 by enhancing T-bet expression in both mouse splenocytes and human PBMCs. No induction of Th17-related cytokines and no upregulation of proinflammatory cytokines was observed.
Figure 2.
Nasally administered G9.1 stimulates mucosal and systemic immune responses in mice. The administration of diphtheria toxoid (DT) and G9.1 induced secretory IgA (sIgA) antibody (Ab) production in the mucosa, in conjunction with the increase of BAFF (B cell activating factor belonging to the tumor necrosis factor family) production in splenocytes (A). IgG1 and IgG2a/c Abs were also detected in serum samples, but the latter was detected only with G9.1 and not with rCTB (B). The effect of G9.1 was pDC-dependent (C). *P < 0.05 and **P < 0.01 in analysis of variance or t tests; n = 5 in (A) and (B) and n = 4 in (C). Reproduced with permission from Maeyama et al.26
As with other CpG ODNs, the activity of PO-palCpG is species-specific (probably because of the diversity of target cells in mice). Although G9.1 is effective both in mice and in humans, ODN composed of G12-AACGTT-G12 preferentially causes rodents to activate immune responses. This type of ODNs was discovered by Yamamoto et al. in their earlier studies (called OligoB; which is also phosphodiester-linked). Maeyama et al.30 demonstrated that an intranasal administration of OligoB enhances not only IgG1 but also IgG2a/c and sIgA Ab responses and delayed-type hypersensitivity to a purified protein derivative caused by the simultaneous administration of DT and BCG (Fig. 3), respectively. This observation suggests that the mucosal adjuvanticity of PO-palCpG does not depend on the type of antigen; it may be applicable when used with particles of a live antigen and a soluble protein antigen.
Figure 3.
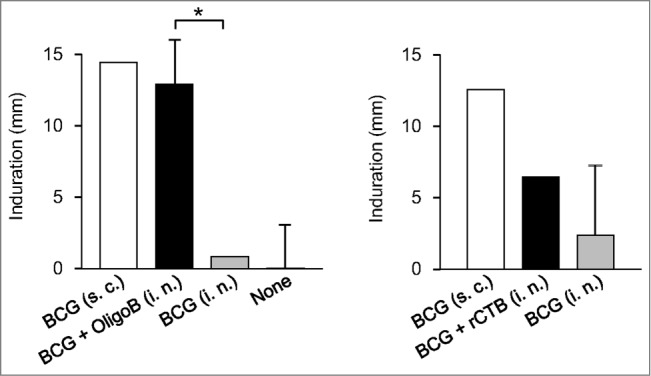
Nasally administered OligoB enhances DTH reaction to PPD. Guinea pigs were nasally immunized with BCG + OligoB or BCG + rCTB or subcutaneously immunized with BCG alone. After six weeks, a delayed-type hypersensitivity (DTH) reaction to a purified protein derivative (PPD) was evaluated. The two panels are from independent experiments. The DTH reaction induced by BCG + OligoB immunization was significantly stronger than that after immunization with BCG alone, reaching the level induced by the subcutaneous immunization with BCG. *P < 0.05 in analysis of variance followed by post hoc Tukey's test (n = 5). Reproduced with permission from Maeyama et al.30,33
Unique Mechanisms Behind the Action of PO-palCpG
Using phosphodiester-linked G10-GACGATCGTC-G10, Osawa and Iho, et al.31 demonstrated that PO-palCpG induces the expression of a gene of interferon regulatory factor-7 (IRF-7, a master transcription factor of the IFN-α gene) in a manner independent of the activation of the IFN-α/β receptor. The mechanism involves upregulating the NF-κB p65 and p50 subunits, which are constitutively expressed in pDCs. In contrast, for the expression of IFN-α, the signal(s) generated upstream of the TLR9 activation of NF-κB is also required, most likely to activate both constitutively expressed and de novo-synthesized IRF-7. Furthermore, G9.1 induces IFN-α production in a manner similar to that of G10-GACGATCGTC-G10; a large amount of IFN-α was produced even by blocking the IFN-α/β receptor (Fig. 4). An autonomous induction of IFN-α by PO-palCpG (approximately 70%) may be responsible for its immunostimulatory effects.
Figure 4.
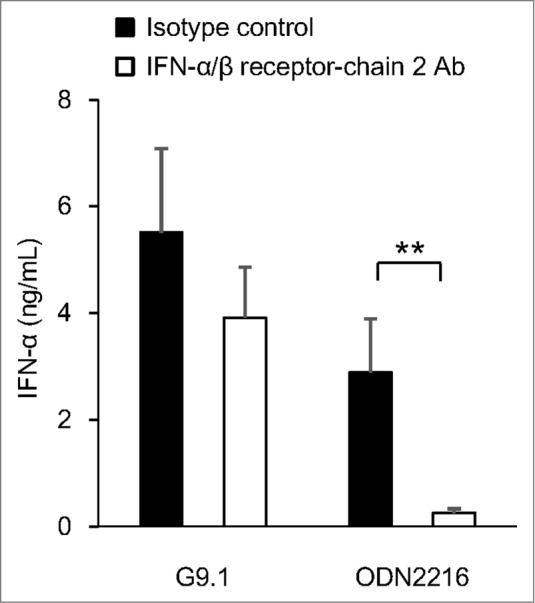
G9.1 generates a stronger IFN-α/β receptor-independent IFN-α production than ODN2216. Peripheral blood mononuclear cells (PBMCs) were cultured with 1 μM G9.1 or ODN2216 in the presence of anti-human interferon (IFN)-α/β receptor chain 2 antibody (Ab) or an isotype control (4 μg/mL, PBL Interferon Source, NJ, USA). The amount of IFN-α in the culture supernatant was measured using a multi-subtype ELISA Kit (PBL Interferon Source). **P < 0.01 in the paired t test (n = 6). Reproduced with permission from Maeyama et al.26
Taniguchi and Takii et al.32 have recently demonstrated the booster effect of the OligoB on aged mice (89-week-old: equivalent to the late 50s in humans). These mice were primarily vaccinated with BCG at the age of 4 weeks but showed decreased protection from Mycobacterium tuberculosis infection with aging. Subcutaneous boosting with OligoB increased the number of central memory-type T cells and enhanced the protection from infection with M. tuberculosis H37Rv. PO-palCpG may facilitate the restimulation and expansion of the memory cells prepared by various vaccination methods.
Issues to be Resolved for the Clinical Use of PO-palCpG
The chronic activation of TLR9 in mice induces autoimmune disease, and certain infections are exacerbated by the CpG ODN-mediated immune activation.14 Although the frequent and long-term vaccination is usually unnecessary, a prudent use of PO-palCpG may be required for patients in whom the acceleration of immune responses is detrimental. In addition, a delineation of the optimal storage conditions may be required for a commercial product based on PO-palCpG.
Conclusions and Perspectives
Palindromic CpG ODNs exhibit adjuvanticity, even in the phosphodiester form, when administered nasally. Because of the phylaxis involving Ab responses and the Th1-enhancing effects demonstrated in mice/human PBMCs, we believe that G9.1 is a promising mucosal adjuvant relevant to the development of novel vaccines against emerging and re-emerging infectious diseases. The mechanisms of adjuvanticity and optimal methods for mucosal vaccination warrant further research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful to all the coinvestigators.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research [22591105 and 19591962] from the JSPS; A-STEP [AS231Z03382G, AS2314067G, AS2121188G, 09-08 and 06-057] from the JST; a grant for Research on Publicly Essential Drugs and Medical Devices [KHC1021 and SH54411] from The Japan Health Sciences Foundation; and the Smoking Research Foundation. The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 1992; 10:75-88; PMID:1539467; http://dx.doi.org/ 10.1016/0264-410X(92)90021-B [DOI] [PubMed] [Google Scholar]
- 2. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11:S45-53; PMID:15812489; http://dx.doi.org/ 10.1038/nm1213 [DOI] [PubMed] [Google Scholar]
- 3. Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6:148-58; PMID:16491139; http://dx.doi.org/ 10.1038/nri1777 [DOI] [PubMed] [Google Scholar]
- 4. Elson CO, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol 1984; 132:2736-41; PMID:6233359 [PubMed] [Google Scholar]
- 5. Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology 1986; 59:301-8; PMID:3021614 [PMC free article] [PubMed] [Google Scholar]
- 6. Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 1988; 6:269-77; PMID:3048010; http://dx.doi.org/ 10.1016/0264-410X(88)90223-X [DOI] [PubMed] [Google Scholar]
- 7. Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med 2004; 350:896-903; PMID:14985487; http://dx.doi.org/ 10.1056/NEJMoa030595 [DOI] [PubMed] [Google Scholar]
- 8. Isaka M, Yasuda Y, Kozuka S, Taniguchi T, Matano K, Maeyama J, Komiya T, Ohkuma K, Goto N, Tochikubo K. Induction of systemic and mucosal antibody responses in mice immunized intranasally with aluminium-non-adsorbed diphtheria toxoid together with recombinant cholera toxin B subunit as an adjuvant. Vaccine 1999; 18:743-51; PMID:10547435; http://dx.doi.org/ 10.1016/S0264-410X(99)00258-3 [DOI] [PubMed] [Google Scholar]
- 9. Maeyama J, Isaka M, Yasuda Y, Matano K, Kozuka S, Taniguchi T, Ohkuma K, Tochikubo K, Goto N. Cytokine responses to recombinant cholera toxin B subunit produced by Bacillus brevis as a mucosal adjuvant. Microbiol Immunol 2001; 45:111-7; PMID:11293476; http://dx.doi.org/ 10.1111/j.1348-0421.2001.tb01276.x [DOI] [PubMed] [Google Scholar]
- 10. Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine 2007; 25:3752-62; PMID:17336431; http://dx.doi.org/ 10.1016/j.vaccine.2007.01.111 [DOI] [PubMed] [Google Scholar]
- 11. Koyama S, Aoshi T, Tanimoto T, Kumagai Y, Kobiyama K, Tougan T, Sakurai K, Coban C, Horii T, Akira S, et al. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med 2010; 2:25ra24; PMID:20424013; http://dx.doi.org/ 10.1126/scitranslmed.3000759 [DOI] [PubMed] [Google Scholar]
- 12. de Vries IJ, Tel J, Benitez-Ribas D, Torensma R, Figdor CG. Prophylactic vaccines mimic synthetic CpG oligonucleotides in their ability to modulate immune responses. Mol Immunol 2011; 48:810-7; PMID:21257206; http://dx.doi.org/ 10.1016/j.molimm.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 13. Tokunaga T, Yamamoto H, Shimada S, Abe H, Fukuda T, Fujisawa Y, Furutani Y, Yano O, Kataoka T, Sudo T, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst 1984; 72:955-62; PMID:6200641 [PubMed] [Google Scholar]
- 14. Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov 2006; 5:471-84; PMID:16763660; http://dx.doi.org/ 10.1038/nrd2059 [DOI] [PubMed] [Google Scholar]
- 15. Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol 2001; 31:2154-63; PMID:11449369; http://dx.doi.org/ 10.1002/1521-4141(200107)31:7%3c2154::AID-IMMU2154%3e3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- 16. Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human Peripheral Blood Cells Differentially Recognize and Respond to Two Distinct CpG Motifs. J Immunol 2001; 166:2372-7; PMID:11160295; http://dx.doi.org/ 10.4049/jimmunol.166.4.2372 [DOI] [PubMed] [Google Scholar]
- 17. Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol 2000; 164:944-53; PMID:10623843; http://dx.doi.org/ 10.4049/jimmunol.164.2.944 [DOI] [PubMed] [Google Scholar]
- 18. Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood 2004; 103:3058-64; PMID:15070685; http://dx.doi.org/ 10.1182/blood-2003-08-2972 [DOI] [PubMed] [Google Scholar]
- 19. Samulowitz U, Weber M, Weeratna R, Uhlmann E, Noll B, Krieg AM, Vollmer J. A novel class of immune-stimulatory CpG oligodeoxynucleotides unifies high potency in type I interferon induction with preferred structural properties. Oligonucleotides 2010; 20:93-101; PMID:20384481; http://dx.doi.org/ 10.1089/oli.2009.0210 [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J Immunol 1992; 148:4072-6; PMID:1376349 [PubMed] [Google Scholar]
- 21. Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, Tokunaga T. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol 1992; 36:983-97; PMID:1281260; http://dx.doi.org/ 10.1111/j.1348-0421.1992.tb02102.x [DOI] [PubMed] [Google Scholar]
- 22. Tokunaga T, Yamamoto T, Yamamoto S. How BCG led to the discovery of immunostimulatory DNA. Jpn J Infect Dis 1999; 52:1-11; PMID:10808252 [PubMed] [Google Scholar]
- 23. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995; 74(6522):546-9; PMID:7700380 [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto T, Yamamoto S, Kataoka T, Tokunaga T. Ability of oligonucleotides with certain palindromes to induce interferon production and augment natural killer cell activity is associated with their base length. Antisense Res Dev 1994; 4:119-22; PMID:7950298 [DOI] [PubMed] [Google Scholar]
- 25. Kuramoto E, Yano O, Kimura Y, Baba M, Makino T, Yamamoto S, Yamamoto T, Kataoka T, Tokunaga T. Oligonucleotide sequences required for natural killer cell activation. Jpn J Can Research 1992; 83:1128-31; PMID:1483927; http://dx.doi.org/ 10.1111/j.1349-7006.1992.tb02734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maeyama J, Takatsuka H, Suzuki F, Kubota A, Horiguchi S, Komiya T, Shimada I, Murata E, Osawa Y, Kitagawa H, et al. A palindromic CpG-containing phosphodiester oligodeoxynucleotide as a mucosal adjuvant stimulates plasmacytoid dendritic cell-mediated TH1 immunity. PLoS One 2014; 9:e88846; PMID:24586411; http://dx.doi.org/ 10.1371/journal.pone.0088846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, Zinkernagel R, Aguzzi A. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med 2004; 10:187-92; PMID:14745443; http://dx.doi.org/ 10.1038/nm987 [DOI] [PubMed] [Google Scholar]
- 28. Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol 2004; 172:1777-85; PMID:14734761; http://dx.doi.org/ 10.4049/jimmunol.172.3.1777 [DOI] [PubMed] [Google Scholar]
- 29. Hanagata N. Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system. Int J Nanomedicine 2012; 7:2181-95; PMID:22619554; http://dx.doi.org/ 10.2147/IJN.S30197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maeyama JI, Komiya T, Takahashi M, Isaka M, Goto N, Yamamoto S. The mucosal adjuvanticity of the oligonucleotides containing a non-methylated CpG motif on BCG and diphtheria toxoid. Vaccine 2009; 27:1166-73; PMID:19136040; http://dx.doi.org/ 10.1016/j.vaccine.2008.12.025 [DOI] [PubMed] [Google Scholar]
- 31. Osawa Y, Iho S, Takauji R, Takatsuka H, Yamamoto S, Takahashi T, Horiguchi S, Urasaki Y, Matsuki T, Fujieda S. Collaborative action of NF-kappaB and p38 MAPK is involved in CpG DNA-induced IFN-alpha and chemokine production in human plasmacytoid dendritic cells. J Immunol 2006; 177:4841-52; PMID:16982926; http://dx.doi.org/ 10.4049/jimmunol.177.7.4841 [DOI] [PubMed] [Google Scholar]
- 32. Taniguchi K, Takii T, Yamamoto S, Maeyama J, Iho S, Maruyama M, Iizuka N, Ozeki Y, Matsumoto S, Hasegawa T, et al. Reactivation of immune responses against Mycobacterium tuberculosis by boosting with the CpG oligomer in aged mice primarily vaccinated with Mycobacterium bovis BCG. Immun Ageing 2013; 10:25; PMID:23799936; http://dx.doi.org/ 10.1186/1742-4933-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maeyama JI. Mucosal adjuvants. BCG −Vaccine and Adjuvant−. Takii T, Maeyama JI, Yamamoto S, editors. Tokyo: JATA 2011. 177-92. [Google Scholar]